Abstract
We previously reported that the activation of histamine H3 receptors (H3Rs) selectively counteracts the facilitatory action of adenosine A2A receptors (A2ARs) on GABA release from rat globus pallidus (GP) isolated nerve terminals (synaptosomes). In this work, we examined the mechanisms likely to underlie this functional interaction. Three possibilities were explored: (a) changes in receptor affinity for agonists induced by physical A2AR/H3R interaction, (b) opposite actions of A2ARs and H3Rs on depolarization-induced Ca2+ entry, and (c) an A2AR/H3R interaction at the level of adenosine 3′,5′-cyclic monophosphate (cAMP) formation. In GP synaptosomal membranes, H3R activation with immepip reduced A2AR affinity for the agonist 2-p-(2-carboxyethyl)phenethylamino-5′-N-ethylcarboxamidoadenosine hydrochloride hydrate (CGS-21680) (Ki control 4.53 nM; + immepip 9.32 nM), whereas A2AR activation increased H3R affinity for immepip (Ki control 0.63 nM; + CGS-21680 0.26 nM). Neither A2AR activation nor H3R stimulation modified calcium entry through voltage-gated calcium channels in GP synaptosomes, as evaluated by microfluorometry. A2AR-mediated facilitation of depolarization-evoked [2,3-3H]-γ-aminobutyric acid ([3H]-GABA) release from GP synaptosomes (130.4 ± 3.6% of control values) was prevented by the PKA inhibitor H-89 and mimicked by the adenylyl cyclase activator forskolin or by 8-Bromo-cAMP, a membrane permeant cAMP analogue (169.5 ± 17.3 and 149.5 ± 14.5% of controls). H3R activation failed to reduce the facilitation of [3H]-GABA release induced by 8-Bromo-cAMP. In GP slices, A2AR activation stimulated cAMP accumulation (290% of basal) and this effect was reduced (− 75%) by H3R activation. These results indicate that in striato-pallidal nerve terminals, A2ARs and H3Rs interact at the level of cAMP formation to modulate PKA activity and thus GABA release.
Keywords: Adenosine A2A receptor, Histamine, Histamine H3 receptor, Globus pallidus, Basal ganglia, GABA release
Introduction
Adenosine is an important modulator of the function of the mammalian central nervous system (CNS) [1]. Adenosine is produced by the ectoenzymatic breakdown of ATP co-released with several classical neurotransmitters and neuromodulators, such as acetylcholine, noradrenaline, γ-amino butyric acid (GABA), glutamate, and dopamine [2]. In addition, neurons and astrocytes can directly release adenosine formed intracellularly via nucleoside transporters [3]. Four G protein-coupled receptors (A1, A2A, A2B, and A3) mediate the actions of adenosine in the CNS [4], and high expression of A2A receptors (A2ARs) is found in the striatum, nucleus accumbens, globus pallidus (GP), and olfactory tubercle, with low expression levels elsewhere in the brain [1].
The GP belongs to the basal ganglia, a group of sub-cortical neuronal nuclei involved in the control of motor behavior, among other functions [5]. The GP has therefore been implicated in the pathophysiology of motor disorders, for example Parkinson’s disease, in which alterations in the pattern and synchrony of discharge of pallidal neurons have been reported [6]. The main synaptic input to the GP is provided by a sub-population of GABAergic striatal neurons that preferentially express dopamine D2 receptors and enkephalins [5]. These neurons also express high levels of A2ARs [7], coupled to Gαs proteins and thus to the adenosine 3′,5′-cyclic monophosphate (cAMP)/PKA pathway [8], and whose activation facilitates GABA release in rat GP [9–13].
The GP is innervated by histaminergic fibers [14] and striato-pallidal neurons express a high density of histamine H3 receptors (H3Rs) both on their bodies [15] and nerve terminals [16]. In a previous study, we showed that the activation of H3Rs, coupled to Gαi/o proteins, selectively counteracted the facilitatory action of A2AR stimulation on depolarization-evoked [2,3-3H]-γ-aminobutyric acid ([3H]-GABA) release from rat GP isolated nerve terminals (synaptosomes) [16], and in this work, we have examined the mechanisms likely to underlie the functional interaction reported. Three possibilities were addressed: (a) the modulation by H3R activation of A2AR affinity for agonists, due to a physical A2AR/H3R interaction; (b) opposite actions of A2ARs and H3Rs on depolarization-induced Ca2+ entry through voltage-activated channels; and (c) a functional A2AR/H3R interaction at the level of cAMP formation.
A preliminary account of this work was presented in the abstract form to the European Histamine Research Society [17].
Methods
Animals
Rats (males, Wistar strain, 250–300 g), bred in the Cinvestav facilities, were used in the experiments. All procedures were approved by the Cinvestav Animal Care Committee and followed the guidelines for the care and use of laboratory animals issued by the National Institutes of Health (NIH Publications No. 8023, revised 1978) and the Mexican Council for Animal Care (NOM-062-ZOO-1999). All efforts were made to minimize animal suffering and to use only as many animals were required for proper statistical analysis.
Preparation of slices and synaptosomes
Animals were decapitated, the brain was quickly removed from the skull, and the forebrain was cut and immersed in ice-cold Krebs-Henseleit solution. Coronal slices (300 μm thick) were then obtained with a vibratome (World Precision Instruments, Sarasota, FL), and the pallidal tissue was carefully dissected from the slices [16]. The composition of the Krebs-Henseleit solution was (mM) NaCl 116, KCl 3, MgSO4 1, KH2PO4 1.2, NaHCO3 25, and d-glucose 11 (pH 7.4 after saturation with O2/CO2, 95:5% v/v). This solution did not contain CaCl2 in order to reduce excitotoxicity. Synaptosomes were prepared from GP slices (seven rats per experiment) as described previously [16].
Radioligand binding to rat GP synaptosomal membranes
The synaptosomal pellet was resuspended in 20 ml of a hypotonic solution (10 mM Tris-HCl, 1 mM EGTA, pH 7.4, 4 °C). After 20 min at 4 °C, the suspension was centrifuged (32,000×g, 20 min, 4 °C) and the pellet (synaptosomal membranes) was resuspended in incubation buffer (50 mM Tris-HCl, 5 mM MgCl2 pH 7.4). Protein contents were determined by the bicinchoninic acid assay (BCA; Pierce, Rockford, IL), using bovine serum albumin (BSA) as standard.
Binding of N-α-[methyl-3H]-histamine ([3H]-NMHA) to H3Rs present in synaptosomal membranes (~ 50 μg protein aliquots) was performed as described in detail elsewhere [18]. For [3H]-CGS-21680 binding to A2ARs, saturation analysis was performed in 100 μl buffer containing [3H]-CGS-21680 (0.01–12 nM) and ~ 50 μg protein. Nonspecific binding was determined as that insensitive to the A2AR antagonist ZM-241385 (10 μM). For inhibition experiments, membranes were pre-incubated (15 min, 30 °C) with adenosine deaminase (2 U/ml) to eliminate the endogenous adenosine and then incubated with [3H]-CGS-21680 (~ 4 nM) and increasing concentrations (10−10–10−5 M) of unlabelled 2-p-(2-carboxyethyl)phenethylamino-5′-N-ethylcarboxamidoadenosine hydrochloride hydrate (CGS-21680). After 2 h at 25 °C, incubations were terminated by filtration through Whatman GF/B glass fiber paper, pre-soaked in 0.3% polyethyleneimine. Filters were soaked in 3-ml scintillator and the tritium content was determined by scintillation counting.
Saturation binding data were fitted to a hyperbola by nonlinear regression with GraphPad Prism 5 (Graph Pad Software, San Diego, CA). Inhibition curves were fitted to a logistic (Hill) equation and values for inhibition constants (Ki) were calculated according to the equation [19]: Ki = IC50 / 1 + {[D] / Kd, where [D] is the concentration of radioligand present in the assay and Kd the mean value for the equilibrium dissociation constant estimated from the corresponding saturation analysis.
Depolarization-evoked [3H]-GABA release from GP synaptosomes
Experiments were performed as described in detail elsewhere [16]. Briefly, synaptosomes were suspended in Krebs-Ringer-Hepes solution supplemented with 10 μM aminooxyacetic acid, 2 U/ml adenosine deaminase, and a mixture of [3H]-GABA/GABA (80 nM/3 μM). After incubation for 30 min at 37 °C, the synaptosomal suspension was apportioned randomly between the chambers of a superfusion apparatus (15 chambers in parallel; 100 μl per chamber) and perfused (1 ml/min) with Krebs-Ringer-Hepes solution. The composition of this solution was (mM) NaCl 113, NaHCO3 25, KCl 4.7, MgCl2 1.2, KH2PO4 1.2, CaCl2 1.8, d-glucose 15, and Hepes 20, at pH 7.4 with NaOH.
Synaptosomes were perfused for 20 min before the collection of 17 fractions of 1 ml (1 min) each. [3H]-GABA release was stimulated by switching to a Krebs-Ringer-Hepes solution containing high K+ (20 mM, KCl substituted for NaCl) for fractions 4 and 13, returning to normal solution between these fractions and after the second K+ stimulus. Drugs under test were present 5 min (CGS-21680, 8-Br-cAMP, and forskolin) or 8 min (immepip and H-89) before and throughout the second K+ stimulus (i.e., fractions 8–13 for CGS-21680, 8-Br-cAMP and forskolin, and fractions 5–13 for immepip and H-89). The double-pulse protocol allows for the same synaptosomal sample being the control for the effect of drugs under test.
To allow for variations between chambers, fractional values were transformed to a percentage of the first fraction. To test for statistical differences between treatments, after subtraction of basal release, the area under the release curve for six fractions after the change to high K+ (i.e., fractions 3–8 and 12–17) was determined for each individual chamber and the ratio of the second over the first K+ stimuli (S2/S1) was calculated.
cAMP accumulation assay
GP punches (2 mm diameter) were obtained from brain coronal slices (300 μm thick) in Krebs-Henseleit solution with no CaCl2 added. After equilibrium for 30 min at 37 °C in the same solution containing 1.8 mM CaCl2, punches were placed in plastic tubes (two per tube) and incubated (15 min, 37 °C) in 200 μl Krebs-Henseleit solution containing adenosine deaminase (0.5 U/ml) and 1 mM 3-isobutyl-1-methylxantine (IBMX). Drugs under test were added in a 10-μl volume and incubations were continued for 30 min. Reactions were stopped by adding 1 ml of ice-cold Krebs-Henseleit solution, tubes were placed on ice, and the solution was aspirated before adding 25 μl of ice-cold HCl (1 M). After 30 min at 4 °C, samples were neutralized (25 μl 1 M NaOH and 100 μl 1 M Tris-HCl pH 7.0) and centrifuged (15,000×g, 3 min, 4 °C).
Endogenous cAMP was determined by a competition assay [20]. Briefly, samples of the extracts (50 μl) were mixed with 75 μl incubation buffer containing a crude supernatant from bovine adrenal medulla and [2,8-3H]-adenosine 3′,5′-cyclic phosphate ([3H]-cAMP; 10 nM). After 2.5 h at 4 °C, incubations were terminated by filtration over GF/B filters pre-soaked in 0.3% polyethylenimine followed by three washes with 1-ml ice-cold deionized water. Retained radioactivity was determined by liquid scintillation, and the amount of cAMP present in each sample was calculated by extrapolation to a standard cAMP curve (10−12–10−5 M). The composition of the incubation buffer was 50 mM Tris-HCl, 100 mM NaCl, 5 mM EDTA, and 5 mg/ml BSA, pH 7.0 at 4 °C.
Microfluorometry
Synaptosomes were resuspended in Krebs-Ringer-Hepes solution containing the fluorescent Ca2+ indicator dye Fura 2-AM (1 μM) and 3% BSA and plated on recording plastic chambers previously coated with concanavalin A (2 mg/ml). After 60 min at 37 °C in the dark, the synaptosomes were rinsed with Krebs-Ringer-Hepes solution. The recording chamber was positioned on a TMD inverted microscope (Nikon, Japan) coupled to an RF-F3010 microfluorometer (Photon Technology International, South Brunswick, NJ). Changes in the [Ca2+]i were determined by measuring the fluorescence ratio (510 nm) after excitation with lights of either 340 or 380 nm wavelengths. Fura 2 recordings were acquired at a frequency of 20 Hz and the background fluorescence at 340 and 380 nm was determined from synaptosome-free areas of the chamber.
Synaptosomes were perfused with Krebs-Ringer-Hepes solution at a rate of 1 ml/min. For two-pulse experiments, drugs or KCl (25 mM, substituted for NaCl) were applied in the perfusion solution. To test for drug effects, after subtraction of basal fluorescence, the area under the curve for the K+-induced increase in fluorescence was determined and the ratio of the second over the first K+ stimuli (S2/S1) was calculated.
Statistical analysis
Data are presented are means ± standard error (SEM), unless otherwise indicated. Statistical comparisons were performed with Student’s t test or one-way ANOVA and post hoc Dunnett’s or Tukey’s tests (GraphPad Prism 5.0) as appropriate. Statistical significance was set at P ≤ 0.05.
Drugs
The following drugs were purchased from Sigma-Aldrich (St. Louis, MO): adenosine deaminase (from bovine spleen), aminooxyacetic acid hemihydrochloride, cAMP, CGS-21680, histamine dihydrochloride, IBMX, and immepip dihydrobromide. Fura 2-AM was from Molecular Probes (Thermo Fisher Scientific, Waltham, MA). [3H]-GABA (82 Ci/mmol), N-α-[methyl-3H]-histamine (83.4 Ci/mmol), [3H]-cAMP (34 Ci/mmol), and [3H]-CGS-21680 (35.2 Ci/mmol) were from PerkinElmer (Boston, MA). For the cAMP accumulation assay, a crude supernatant from bovine adrenal medulla was used.
Results
[3H]-CGS-21680 binding to GP synaptosomal membranes
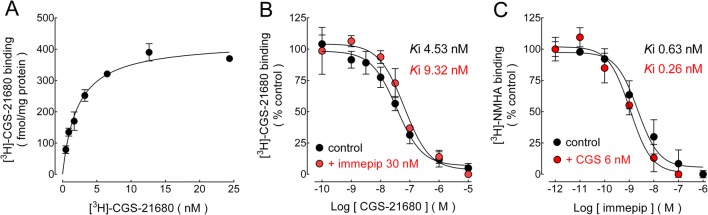
Saturation binding of the A2AR agonist [3H]-CGS-21680 [21] to membranes from GP synaptosomes (Fig. 1a) yielded maximum specific binding (Bmax) 454 ± 77 fmol/mg protein (mean ± SEM, five experiments) and equilibrium dissociation constant (Kd) 3.98 nM (pKd 8.40 ± 0.08), similar to the Ki obtained in homologous inhibition experiments (see below).
Fig. 1.
In GP synaptosomal membranes, H3R activation decreases A2AR affinity for the agonist CGS-21680, whereas A2AR activation increases H3R affinity for the agonist immepip. a Saturation binding of [3H]-CGS-21680 to A2ARs. Specific receptor binding was determined by subtracting the binding in the presence of the A2AR antagonist ZM-241385 (10 μM) from total binding. Points are means ± range from duplicates from a single experiment, which was repeated a further four times. The line drawn is the best fit to a hyperbola. Best-fit values for the equilibrium dissociation constant (Kd) and maximum binding (Bmax) are given in the text. b The H3R agonist immepip (30 nM) decreases the potency of CGS-21680 to inhibit the specific binding of [3H]-CGS-21680 to A2ARs. c The A2AR agonist CGS-21680 (6 nM) increases the potency of immepip to inhibit [3H]-NMHA binding to H3Rs. For panels b and c, values are expressed as the percentage of control binding and correspond to means ± SEM from three replicates from representative experiments. The line drawn is the best fit to a logistic equation for a one-site model. The analysis of the Ki and pKi values calculated from the best-fit IC50 estimates is presented in the text
Specific [3H]-CGS-21680 binding was inhibited in a concentration-dependent manner by unlabelled CGS-21680 (Fig. 1b), with pKi value (−log Ki) 8.34 ± 0.10 (Ki 4.53 nM; three experiments). The selective H3R agonist immepip (30 nM) reduced modestly but significantly A2AR affinity for CGS-21680 (Ki 9.32 nM; pKi 8.03 ± 0.07; P = 0.002, Student’s t test, four experiments).
A high density of H3Rs was previously detected in GP synaptosomal membranes (maximum [3H]-NMHA binding, 1327 ± 79 fmol/mg protein) [16]. The A2AR agonist CGS-21680 (3 and 6 nM) increased in a modest but significant manner: the Ki of the H3R agonist immepip from 0.63 nM (pKi 9.20 ± 0.01) to 0.50 and 0.26 nM (pKi 9.30 ± 0.02; P < 0.05 and 9.58 ± 0.02; P < 0.01; one-way ANOVA and Tukey’s test; three experiments). Figure 1c illustrates the effect of 6 nM CGS-21680.
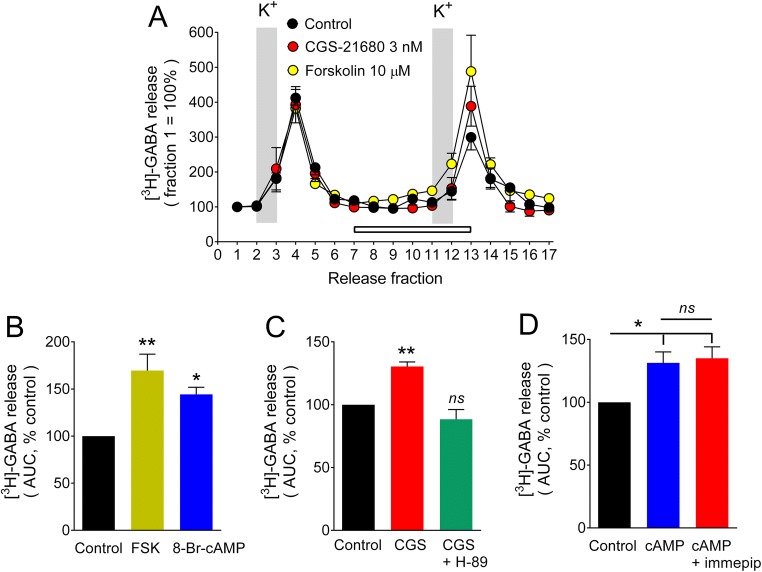
The stimulatory effect of A2AR activation on depolarization-evoked [3H]-GABA release from GP synaptosomes involves the cAMP/PKA pathway
The Ca2+-dependent [3H]-GABA release from GP synaptosomes induced by depolarization with 20 mM KCl is enhanced by A2AR activation [16]. In this study, the facilitatory effect of CGS-21680 (3 nM, 130.4 ± 3.6% of control values) was mimicked by forskolin (10 μM), a direct adenylyl cyclase activator, and by 8-Bromo-cAMP (500 μM), a membrane permeant cAMP analogue (Fig. 2a, b). In GP slices, electrophysiological studies showed A2AR-mediated facilitation of GABA release to depend on the cAMP/PKA pathway [11, 22], and in GP synaptosomes, the PKA inhibitor H-89 (10 μM) prevented the effect of A2AR activation (Fig. 2c). Together, these data indicated that the cAMP/PKA pathway underlies the enhancing effect of A2AR activation on depolarization-evoked [3H]-GABA release from GP synaptosomes.
Fig. 2.
Involvement of the cAMP/PKA pathway in depolarization-evoked [3H]-GABA release from rat globus pallidus synaptosomes. a Illustration of a representative experiment. Labeled synaptosomes were perfused with Krebs-Ringer-Hepes solution and [3H]-GABA release was evoked by raising the K+ concentration from 4 to 20 mM for the periods indicated by the vertical gray bars. Drugs under test were present for the period indicated by the open bar. Values are expressed as a percentage of [3H]-GABA release in fraction 1 and represent means ± SEM from four to six replicates. b Forskolin and 8-Br-cAMP enhance depolarization-evoked [3H]-GABA release. After subtraction of basal release, the area under the release curves for fractions 3–8 (S1) and 12–17 (S2) was determined for each individual chamber and the ratio S2/S1 was calculated. Values are expressed as a percentage of control [3H]-GABA release (no drugs added) and are means ± SEM from three experiments with four to six replicates. The statistical analysis was performed with ANOVA and Dunnett’s test. c The PKA antagonist H-89 (10 μM) prevented the facilitatory effect of the A2AR agonist CGS-21680 (3 nM) on K+-evoked [3H]-GABA release. Values are means ± SEM from four experiments. d The H3R agonist immepip (100 nM) did not inhibit the facilitatory effect of 8-Br-cAMP (500 μM) on depolarization-evoked [3H]-GABA release. Values are means ± SEM from five experiments. For panels b and c, the statistical analysis was performed with ANOVA and Dunnett’s test; ns, no significantly different, *P < 0.05, **P < 0.01 versus control values. For panel d, values were compared with ANOVA and Tukey’s test; ns, no significantly different, *P < 0.05
To test whether the inhibitory action of H3R activation on the facilitation of [3H]-GABA release induced by the cAMP/PKA pathway was exerted at or downstream cAMP formation, the effect of 8-Bromo-cAMP was evaluated in the presence or absence of the H3R agonist immepip. Figure 2d shows that H3R activation had no significant effect on the facilitatory action of 8-Bromo-cAMP on depolarization-evoked [3H]-GABA release from GP synaptosomes.
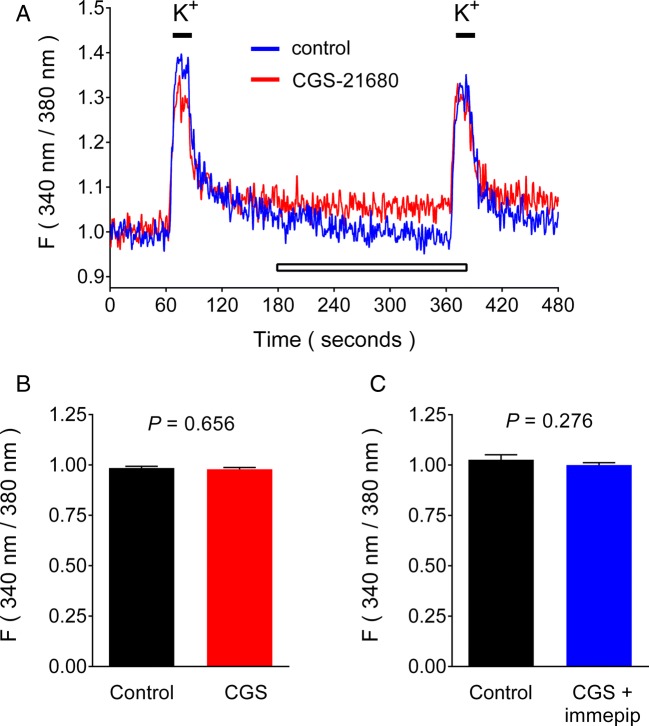
Effect of A2AR and H3R activation on depolarization-induced increase in the intracellular Ca2+ concentration ([Ca2+]i) of GP synaptosomes
Increasing the concentration of K+ ions in the perfusing solution from 4.7 to 25 mM resulted in an increase in the [Ca2+]i of GP synaptosomes. Figure 3 shows that in the two-pulse protocol, perfusion with the A2AR agonist CGS-21680 (10 nM) had no effect on the S2/S1 ratio (control 0.985 ± 0.007; CGS-21680 0.979 ± 0.008; P = 0.656). The co-perfusion of CGS-21680 and the H3R agonist immepip (100 nM) also failed to modify the S2/S1 ratio (control 1.023 ± 0.003; CGS-21680/immepip 1.001 ± 0.010; P = 0.2759). These results indicated that the effects of A2AR or H3R activation on GABA release did not involve modulatory actions at voltage-activated Ca2+ channels.
Fig. 3.
Lack of effect of A2AR and H3R activation on the depolarization-induced increase in the intracellular Ca2+ concentration ([Ca2+]i) in globus pallidus synaptosomes. a Representative traces. Synaptosomes loaded with Fura 2-AM were perfused with Krebs-Ringer-Hepes solution (1 ml/min), and changes in fluorescence were determined by microfluorometry. Drugs under test were present in the solution (open bar) before and during the second depolarization stimulus (25 mM KCl, 20 s, black bars). b Lack of effect of the A2AR agonist CGS-21680 (10 nM). The ratios of the second over the first K+ stimuli (S2/S1) were calculated as described in “Methods” and expressed as means ± SEM from four experiments. AUC, area under the curve. c Lack of effect of CGS-21680 (10 nM) and the H3R agonist immepip (100 nM). Values are means ± SEM from four experiments. The statistical analysis was performed with Student’s t test
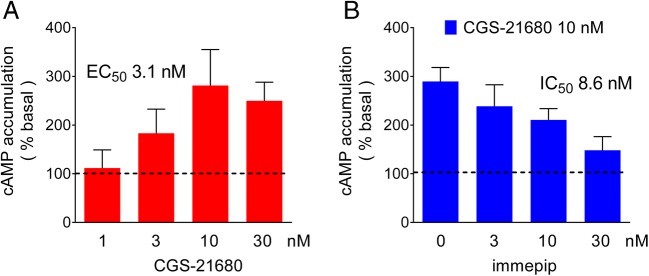
Effect of H3R activation on A2AR-induced increases in cAMP accumulation in GP slices
Figure 4a shows that in GP slices, the A2AR agonist CGS-21680 stimulated cAMP accumulation in a concentration-dependent manner (EC50 3.1 nM; maximum effect 281% of basal accumulation at 10 nM). In a different series of experiments, the effect of CGS-21680 (10 nM, 290% of basal accumulation) was reduced by the H3R agonist immepip (75% inhibition at 100 nM, IC50 8.6 nM; Fig. 4b).
Fig. 4.
Effect of A2AR and H3R activation on cAMP accumulation in rat globus pallidus slices. a Stimulation by the A2AR agonist CGS-21680. Slices were incubated with CGS-21680 for 30 min in Krebs-Henseleit solution (1 mM IBMX). Values are means ± SEM from five experiments. b Inhibition by H3R activation of A2AR-induced cAMP accumulation. Slices were incubated for 30 min with CGS-21680 (10 nM) in the absence or presence of the indicated concentrations of the H3R agonist immepip, added 5 min before CGS-21680. Values for the half-stimulatory (EC50, a) or half-inhibitory concentrations (IC50, b) were obtained by nonlinear regression with GraphPad Prism 5
Discussion
In GP slices, A2AR activation facilitates GABA release [9, 12], and electrophysiological studies showed this effect to depend on the cAMP/PKA pathway [11, 22]. We previously reported that H3Rs are present at high density on GP synaptosomes, where their activation selectively counteracted the facilitatory action of A2AR stimulation on depolarization-evoked [3H]-GABA release [16]. In this work, we analyzed three mechanisms that could underlie the H3R effect and show that it most likely relies on the inhibition of cAMP formation.
Effect of H3R activation on the affinity of A2ARs for the agonist CGS-21680
In transfected cells, H3Rs form heteromers with dopamine D1 and D2 receptors, and in striatal membranes, H3R activation decreases the affinity of D2 receptors for agonists [23], whereas in SK-N-MC cells shifts the coupling of D1 receptors from Gαs to Gαi/o proteins and therefore from stimulation to inhibition of cAMP formation [24]. We recently showed that endogenous and transfected H3 and A2A receptors form heterodimers [25], which could therefore underlie the interaction H3R/A2AR in GABA release.
Our results show that H3R activation reduces A2AR affinity for the agonist CGS-21680 and, conversely, that A2AR activation increases H3R affinity for the agonist immepip. Although these data support H3R/A2AR dimerization in rat GP nerve terminals, the reduction in A2AR affinity (~ 2-fold) implies a modest effect on receptor occupancy by adenosine, particularly at high concentrations of the modulator.
Fast-scan cyclic voltammetry showed that in anesthetized rats spontaneous adenosine transients yielded 170 and 190 nM for the striatum and the prefrontal cortex [26], whereas in slices, these transients yielded 110, 160, and 240 nM for prefrontal cortex, thalamus, and hippocampus, respectively [27]. A2AR affinity for adenosine approximates 150 nM [1]. Assuming that in GP spontaneous adenosine transients yield the value reported for the striatum (170 nM), A2AR occupancy by adenosine would be 53% and the twofold decrease in affinity for agonists induced by H3R activation would decrease receptor occupancy to 36%. As mentioned above, this effect would be reduced and eventually abolished at high concentrations of adenosine. This point is illustrated by Fig. 1b, where the shift to the right in the concentration-response curve is observed for concentrations of CGS-21680 between 1 and 30 nM to disappear at concentrations of 100 nM and above. Thus, dimerization alone appears not sufficient to explain the functional interaction between H3Rs and A2ARs.
Lack of effect of A2AR activation on depolarization-induced increase in [Ca2+]i
The coupling of H3Rs to Gαi/o proteins makes likely that their inhibitory effect on neurotransmitter release involves a decrease in depolarization-induced Ca2+ entry through N- and P/Q-type voltage-operated channels [28–30]. The A2AR-mediated enhancement of both acetylcholine release from striatal synaptosomes and GABA release from hippocampal synaptosomes involves, at least partially, the cAMP/PKA pathway and P-type Ca2+ channels [31, 32], and D1 receptor-mediated facilitation and H3R-mediated inhibition of GABA release from striatal terminals appear to converge at P-type Ca2+ channels [33, 34]. One plausible explanation for the interaction H3R/A2AR in [3H]-GABA release from striato-pallidal terminals was thus that Gβγ complexes released from Gαi/o proteins upon H3R activation inhibited voltage-activated Ca2+ channels whose opening was facilitated by A2AR stimulation, presumably by PKA-mediated phosphorylation. However, in our experiments, the activation of A2ARs failed to enhance the increase in the [Ca2+]i induced by depolarization in GP synaptosomes, indicating that their facilitatory action on GABA release and therefore the modulatory effect of H3Rs do not take place at voltage-activated Ca2+ channels, at least under the experimental conditions employed in this study. This conclusion is in agreement with the electrophysiological data of Shindou et al. [11], who showed that blocking Ca2+ channels with CdCl2 did not prevent the A2AR-mediated facilitation of GABA release in GP slices.
[3H]-GABA release and modulation of the cAMP/PKA pathway by A2ARs and H3Rs
Shindou et al. [11, 22] showed that the A2AR modulatory effect on GABA release observed in GP slices depended on the activation of the cAMP/PKA pathway, because the adenylyl cyclase activator forskolin increased GABA release and both the adenylyl cyclase inhibitor SQ22536 and the PKA inhibitor H-89 prevented the increase in the frequency of GABAA receptor-mediated miniature inhibitory postsynaptic currents (mIPSCs). With a neurochemical approach, we show here that forskolin and 8-Bromo-cAMP mimicked the A2AR-mediated enhancement of depolarization-induced [3H]-GABA release from GP synaptosomes and that PKA inhibition prevented the A2AR effect (Fig. 2c), supporting the participation of the cAMP/PKA pathway.
The facilitatory effect of A2A receptors on GABA release has been also reported for hippocampal synaptosomes, where CGS-21680 (1–10 nM; apparent EC50 3 nM) enhanced K+-evoked [3H]-GABA release with maximal facilitation of 34 ± 4% at 10 nM. This effect was mimicked by forskolin (but not by its inactive analogue 1,9-dideoxyforskolin), the cAMP analogues dibutyryl-cAMP and 8-bromo-cAMP, and the phosphodiesterase inhibitor rolipram. Furthermore, the effect of a submaximal concentration of CGS-21680 (1 nM) was significantly occluded by dibutyryl-cAMP, 8-bromo-cAMP, and forskolin and was potentiated by the phosphodiesterase inhibitor rolipram [32]).
In regard to other neurotransmitters, for the neuromuscular transmission, the facilitation by methylprednisolone of exocytosis and [3H]-acetylcholine release involves the activation of presynaptic A2A receptors by endogenous adenosine leading to synaptic vesicle redistribution, and the methylprednisolone effect was markedly reduced by PKA inhibition [35]. In differentiated human neuroblastoma SH-SY5Y cells, the activation of endogenous A2A receptors facilitates depolarization-evoked [3H]-noradrenaline release, and this effect was also prevented by PKA inhibition [36]. This information supports that the facilitatory effect of A2A receptors on neurotransmitter release is mainly mediated by the cAMP/PKA pathway.
In this work, H3R activation failed to inhibit the effect of 8-Bromo-cAMP on GABA release (Fig. 2d), indicating that the H3R action on A2AR-mediated enhancement of GABA release is exerted at the level of cAMP formation. This hypothesis is supported by the H3R-mediated inhibition of A2AR-induced cAMP accumulation observed in GP slices (Fig. 4). Because A2AR activation had no effect on the [Ca2+]i in GP synaptosomes (Fig. 3), the cAMP/PKA pathway may thus act downstream Ca2+ entry, for example on exocytosis proteins such as synapsin 1, SNAP-25, rabfilin 3A, syntaxin, and RIM1a/2a, whose phosphorylation by PKA leads to redistribution of synaptic vesicles [35, 37, 38]. In this regard, in neurons of rat medulla oblongata in primary culture, A2AR activation enhanced exocytosis and the phosphorylation of synapsin I and the latter effect was prevented by the PKA inhibition [38].
Conclusion
The GP has emerged as a key point in the control of the basal ganglia motor output [5, 6]. In this work, we showed that the opposite effects of A2ARs and H3Rs on GABA release from striato-pallidal afferents rely on the stimulation and inhibition of the cAMP/PKA pathway, respectively. Recent work shows that cAMP formation in striato-pallidal neurons increases their spiking activity leading to inhibition of the spontaneous firing of GP neurons and reduced motor activity [39]. Through the activation of presynaptic H3Rs, histamine could therefore contribute to the regulation of the activity of GP neurons and thus of basal ganglia function.
Acknowledgements
G.-E.M.-F. and N.R.R. held Conacyt pre-doctoral scholarships.
Abbreviations
- A2AR
Adenosine A2A receptor
- GABA
γ-Aminobutyric acid
- GP
Globus pallidus
- H3R
Histamine H3 receptor
Author contributions
G.-E.M.-F., E.J.G., and J.-A.A.-M. designed the study. G.-E.M.-F., J.E.-S., R.G.-P., U.G.-H., and N.R.-R. conducted experiments. G.-E.M.-F. and J.-A.A.-M. performed data analysis. G.-E.M.-F. and J.-A.A.-M. wrote the manuscript.
Funding
This study was supported by Cinvestav and Conacyt (grant 220448 to J.-A.A.-M.).
Conflict of interest
The authors declare they do not have any actual or potential conflict of interest.
Ethical approval
All procedures were approved by the Cinvestav Animal Care Committee and followed the guidelines for the care and use of laboratory animals issued by the National Institutes of Health (NIH Publications No. 8023, revised 1978) and the Mexican Council for Animal Care (NOM-062-ZOO-1999).
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Stockwell J, Jakova E, Cayabyab FS. Adenosine A1 and A2A receptors in the brain: current research and their role in neurodegeneration. Molecules. 2017;22:676. doi: 10.3390/molecules22040676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burnstock G. Historical review: ATP as a neurotransmitter. Trends Pharmacol Sci. 2006;27:166–176. doi: 10.1016/j.tips.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 3.Wall MJ, Dale N. Auto-inhibition of rat parallel fibre-Purkinje cell synapses by activity-dependent adenosine release. J Physiol. 2007;581:553–565. doi: 10.1113/jphysiol.2006.126417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fredholm BB, IJzerman AP, Jacobson KA, Linden J, Müller CE. International Union of Basic and Clinical Pharmacology. LXXXI. Nomenclature and classification of adenosine receptors-an update. Pharmacol Rev. 2011;63:1–34. doi: 10.1124/pr.110.003285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bolam JP, Hanley JJ, Booth PA, Bevan MD. Synaptic organisation of the basal ganglia. J Anat. 2000;196:527–542. doi: 10.1046/j.1469-7580.2000.19640527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan CS, Surmeier DJ, Yung WH. Striatal information signaling and integration in globus pallidus: timing matters. Neurosignals. 2005;14:281–289. doi: 10.1159/000093043. [DOI] [PubMed] [Google Scholar]
- 7.Bogenpohl JW, Ritter SL, Hall RA, Smith Y. Adenosine A2A receptor in the monkey basal ganglia: ultrastructural localization and colocalization with the metabotropic glutamate receptor 5 in the striatum. J Comp Neurol. 2012;520:570–589. doi: 10.1002/cne.22751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fredholm BB, IJzerman AP, Jacobson KA, Klotz K-N, Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev. 2001;53:527–552. [PMC free article] [PubMed] [Google Scholar]
- 9.Mayfield R, Suzuki F, Zahniser NR. Adenosine A2A receptor modulation of electrically evoked endogenous GABA release from slices of rat globus pallidus. J Neurochem. 1993;60:2334–2337. doi: 10.1111/j.1471-4159.1993.tb03526.x. [DOI] [PubMed] [Google Scholar]
- 10.Dayne Mayfield R, Larson G, Orona RA, Zahniser NR. Opposing actions of adenosine A2a and dopamine D2 receptor activation on GABA release in the basal ganglia: evidence for an A2a/D2 receptor interaction in globus pallidus. Synapse. 1996;22:132–138. doi: 10.1002/(SICI)1098-2396(199602)22:2<132::AID-SYN6>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 11.Shindou T, Nonaka H, Richardson PJ, Mori A, Kase H, Ichimura M. Presynaptic adenosine A2A receptors enhance GABAergic synaptic transmission via a cyclic AMP dependent mechanism in the rat globus pallidus. Br J Pharmacol. 2002;136:296–302. doi: 10.1038/sj.bjp.0704702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Floran B, Gonzalez B, Floran L, Erlij D, Aceves J. Interactions between adenosine A2A and dopamine D2 receptors in the control of [3H]GABA release in the globus pallidus of the rat. Eur J Pharmacol. 2005;520:43–50. doi: 10.1016/j.ejphar.2005.06.035. [DOI] [PubMed] [Google Scholar]
- 13.Diao HL, Xue Y, Han XH, Wang SY, Liu C, Chen WF, Chen L. Adenosine A2A receptor modulates the activity of globus pallidus neurons in rat. Front Physiol. 2017;8:897. doi: 10.3389/fphys.2017.00897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Panula P, Pirvola U, Auvinen S, Airaksinen MS. Histamine-immunoreactive nerve fibers in the rat brain. Neuroscience. 1989;28:585–610. doi: 10.1016/0306-4522(89)90007-9. [DOI] [PubMed] [Google Scholar]
- 15.Pillot C, Heron A, Cochois V, Tardivel-Lacombe J, Ligneau X, Schwartz JC, Arrang JM. A detailed mapping of the histamine H3 receptor and its gene transcripts in rat brain. Neuroscience. 2002;114:173–193. doi: 10.1016/S0306-4522(02)00135-5. [DOI] [PubMed] [Google Scholar]
- 16.Morales-Figueroa GE, Márquez-Gómez R, González-Pantoja R, Escamilla-Sánchez J, Arias-Montaño JA. Histamine H3 receptor activation counteracts adenosine A2A receptor-mediated enhancement of depolarization-evoked [3H]-GABA release from rat globus pallidus synaptosomes. ACS Chem Neurosci. 2014;5:637–645. doi: 10.1021/cn500001m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morales-Figueroa GE, González-Pantoja R, Escamilla-Sánchez J, Arias-Montaño JA (2017) Functional interaction between histamine H3 receptors and adenosine A2A receptors in rat striato-pallidal nerve terminals. 46th Annual Meeting, European Histamine Research Society. Inflamm Res 66 (Suppl. 1), S21
- 18.Osorio-Espinoza A, Alatorre A, Ramos-Jimenez J, Garduño-Torres B, García-Ramírez M, Querejeta E, Arias-Montaño JA. Pre-synaptic histamine H3 receptors modulate glutamatergic transmission in rat globus pallidus. Neuroscience. 2011;176:20–31. doi: 10.1016/j.neuroscience.2010.12.051. [DOI] [PubMed] [Google Scholar]
- 19.Cheng Y, Prusoff WH. Relationship between the inhibition constant (KI) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- 20.Flores-Clemente C, Osorio-Espinoza A, Escamilla-Sánchez J, Leurs R, Arias JM, Arias-Montaño JA. A single-point mutation (Ala280Val) in the third intracellular loop alters the signalling properties of the human histamine H3 receptor stably expressed in CHO-K1 cells. Br J Pharmacol. 2013;170:127–135. doi: 10.1111/bph.12257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alexander SPH, Christopoulos A, Davenport AP, Kelly E, Marrion NV, Peters JA. The concise guide to PHARMACOLOGY 2017/2018: G protein-coupled receptors. Br J Pharmacol. 2017;174:S17–S129. doi: 10.1111/bph.13878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shindou T, Mori A, Kase H, Ichimura M. Adenosine A2A receptor enhances GABAA-mediated IPSCs in the rat globus pallidus. J Physiol. 2001;532:423–434. doi: 10.1111/j.1469-7793.2001.0423f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferrada C, Ferré S, Casadó V, Cortés A, Justinova Z, Barnes C, Canela EI, Goldberg SR, Leurs R, Lluis C, Franco R. Interactions between histamine H3 and dopamine D2 receptors and the implications for striatal function. Neuropharmacology. 2008;55:190–197. doi: 10.1016/j.neuropharm.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferrada C, Moreno E, Casadó V, Bongers G, Cortés A, Mallol J, Canela EI, Leurs R, Ferré S, Lluís C, Franco R. Marked changes in signal transduction upon heteromerization of dopamine D1 and histamine H3 receptors. Br J Pharmacol. 2009;157:64–75. doi: 10.1111/j.1476-5381.2009.00152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Márquez-Gómez R, Robins MT, Gutiérrez-Rodelo C, Arias JM, Olivares-Reyes JA, van Rijn RM, Arias-Montaño JA. Functional histamine H3 and adenosine A2A receptor heteromers in recombinant cells and rat striatum. Pharmacol Res. 2018;129:515–525. doi: 10.1016/j.phrs.2017.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nguyen MD, Lee ST, Ross AE, Ryals M, Choudhry VI, Venton BJ. Characterization of spontaneous, transient adenosine release in the caudate-putamen and prefrontal cortex. PLoS One. 2014;9:e87165. doi: 10.1371/journal.pone.0087165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee ST, Venton BJ. Regional variations of spontaneous, transient adenosine release in brain slices. ACS Chem Neurosci. 2018;9:505–513. doi: 10.1021/acschemneuro.7b00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takeshita Y, Watanabe T, Sakata T, Munakata M, Ishibashi H, Akaike N. Histamine modulates high-voltage-activated calcium channels in neurons dissociated from the rat tuberomammillary nucleus. Neuroscience. 1998;87:797–805. doi: 10.1016/S0306-4522(98)00152-3. [DOI] [PubMed] [Google Scholar]
- 29.Molina-Hernandez A, Nunez A, Sierra JJ, Arias-Montano JA. Histamine H3 receptor activation inhibits glutamate release from rat striatal synaptosomes. Neuropharmacology. 2001;41:928–934. doi: 10.1016/S0028-3908(01)00144-7. [DOI] [PubMed] [Google Scholar]
- 30.De Waard M, Hering J, Weiss N, Feltz A. How do G proteins directly control neuronal Ca2+ channel function? Trends Pharmacol Sci. 2005;26:427–436. doi: 10.1016/j.tips.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 31.Gubitz AK, Widdowson L, Kurokawa M, Kirkpatrick KA, Richardson PJ. Dual signalling by the adenosine A2a receptor involves activation of both N- and P-type calcium channels by different G proteins and protein kinases in the same striatal nerve terminals. J Neurochem. 1996;67:374–381. doi: 10.1046/j.1471-4159.1996.67010374.x. [DOI] [PubMed] [Google Scholar]
- 32.Cunha RA, Ribeiro JA. Purinergic modulation of [3H]GABA release from rat hippocampal nerve terminals. Neuropharmacology. 2000;39:1156–1167. doi: 10.1016/S0028-3908(99)00237-3. [DOI] [PubMed] [Google Scholar]
- 33.Arias-Montano J-A, Floran B, Garcia M, Aceves J, Young J-M. Histamine H3 receptor-mediated inhibition of depolarization-induced, dopamine D1 receptor-dependent release of [3H]-gamma-aminobutyric acid from rat striatal slices. Br J Pharmacol. 2001;133:165–171. doi: 10.1038/sj.bjp.0704053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arias-Montano J-A, Floran B, Floran L, Aceves J, Young J-M. Dopamine D1 receptor facilitation of depolarization-induced release of gamma-amino-butyric acid in rat striatum is mediated by the cAMP/PKA pathway and involves P/Q-type calcium channels. Synapse. 2007;61:310–319. doi: 10.1002/syn.20372. [DOI] [PubMed] [Google Scholar]
- 35.Oliveira L, Costa AC, Noronha-Matos JB, Silva I, Cavalcante WL, Timóteo MA, Corrado AP, Dal Belo CA, Ambiel CR, Alves-do-Prado W, Correia-de-Sá P. Amplification of neuromuscular transmission by methylprednisolone involves activation of presynaptic facilitatory adenosine A2A receptors and redistribution of synaptic vesicles. Neuropharmacology. 2015;89:64–76. doi: 10.1016/j.neuropharm.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 36.Ibrisimovic E, Drobny H, Yang Q, Höfer T, Boehm S, Nanoff C, Schicker K. Constitutive activity of the A2A adenosine receptor and compartmentalised cyclic AMP signalling fine-tune noradrenaline release. Purinergic Signal. 2012;8:677–692. doi: 10.1007/s11302-012-9298-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seino S, Shibasaki T. PKA-dependent and PKA-independent pathways for cAMP-regulated exocytosis. Physiol Rev. 2005;85:1303–1342. doi: 10.1152/physrev.00001.2005. [DOI] [PubMed] [Google Scholar]
- 38.Matsumoto JP, Almeida MG, Castilho-Martins EA, Costa MA, Fior-Chadi DR. Protein kinase a mediates adenosine A2a receptor modulation of neurotransmitter release via synapsin I phosphorylation in cultured cells from medulla oblongata. Neurosci Res. 2014;85:1–11. doi: 10.1016/j.neures.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 39.Bouabid S, Zhou FM. Cyclic AMP-producing chemogenetic activation of indirect pathway striatal projection neurons and the downstream effects on the globus pallidus and subthalamic nucleus in freely moving mice. J Neurochem. 2018;145:436–448. doi: 10.1111/jnc.14331. [DOI] [PMC free article] [PubMed] [Google Scholar]