Abstract
The aging-related decline in fertility is an increasingly pressing medical and economic issue in modern society where women are delaying family building. Increasingly sophisticated, costly, and often increasingly invasive, assisted reproductive clinical protocols and laboratory technologies (ART) have helped many older women achieve their reproductive goals. Current ART procedures have not been able to address the fundamental problem of oocyte aging, the increased rate of egg aneuploidy, and the decline of developmental potential of the eggs. Oocyte maturation, which is triggered by luteinizing hormone (LH) in vivo or by injection of human chorionic gonadotropin (hCG) in an in vitro fertilization (IVF) clinic, is the critical stage at which the majority of egg aneuploidies arise and when much of an egg’s developmental potential is established. Our proposed strategy focuses on improving egg quality in older women by restoring a robust oocyte maturation process. We have identified putrescine deficiency as one of the causes of poor egg quality in an aged mouse model. Putrescine is a biogenic polyamine naturally produced in peri-ovulatory ovaries. Peri-ovulatory putrescine supplementation has reduced egg aneuploidy, improved embryo quality, and reduced miscarriage rates in aged mice. In this paper, we review the literature on putrescine, its occurrence and physiology in living organisms, and its unique role in oocyte maturation. Preliminary human data demonstrates that there is a maternal aging-related deficiency in ovarian ornithine decarboxylase (ODC), the enzyme responsible for putrescine production. We argue that peri-ovulatory putrescine supplementation holds great promise as a natural and effective therapy for infertility in women of advanced maternal age, applicable in natural conception and in combination with current ART therapies.
Keywords: Putrescine, Ornithine decarboxylase, Oocyte maturation, Aneuploidy, Infertility, Aging, Embryo development
Introduction
Immature mammalian oocytes are maintained in meiotic prophase I by intricate cell-cell contact and multiple signaling pathways, ultimately leading to sustained and high levels of cAMP in the oocytes (Fig. 1). The LH surge (or the injection of an ovulatory dose of hCG) triggers multiple signaling pathways, leading to reduction of cAMP and oocyte maturation. Oocyte maturation determines egg quality in two critical ways. First, regardless of maternal age, aneuploidy is the result of a chromosome mis-segregation in meiosis, especially premature separation of sister chromatids in meiosis I during oocyte maturation [2, 52, 58, 63]. Second, oocyte maturation is critical for determining the cytoplasmic capacity to support embryonic development [37]. This aspect of oocyte maturation is often referred to as cytoplasmic maturation [14]. The critical determinants of oocyte maturation ensuring normal developmental potential of the eggs have been published in many recent and excellent reviews [10, 35]. However, no clear candidate therapy has yet emerged.
Fig. 1.
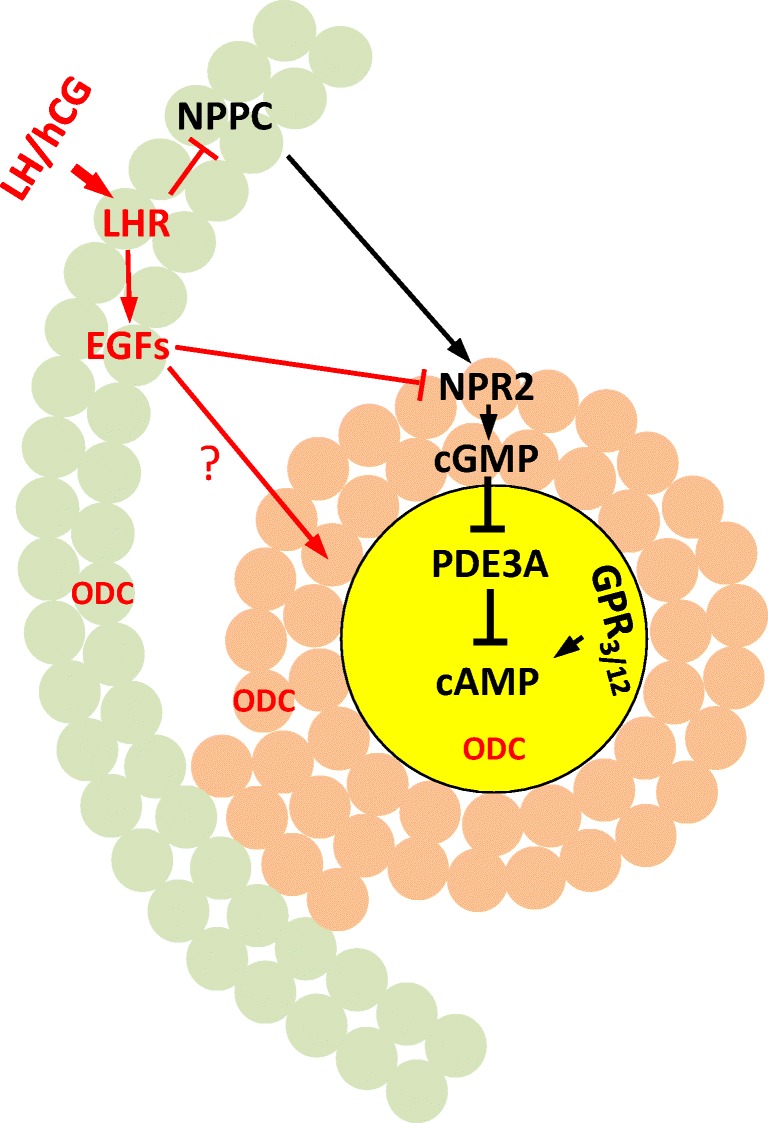
Mammalian oocyte meiosis arrest and oocyte maturation. A section of antral follicle including oocyte (yellow), cumulus cells (brown), and mural granulosa cells (green). Prophase maintenance signaling pathways are depicted in black and LH/hCG-induced signaling in red. Oocyte cAMP is produced by constitutively active Gs-coupled receptors GPR3 and GPR12. Cyclic AMP-specific phosphodiesterase, PDE3A, is kept inactive by cGMP, produced by a paracrine signaling involving mural granulosa cell-derived natriuretic peptide precursor type C (NPPC) and its receptor NPR2 (which is a guanylyl cyclase) and transported to the enclosed oocyte through gap junctions. Luteinizing hormone (LH) activates LH receptor in mural granulosa cells leading to the expression of several EGF-like peptides, which in turn cause rapid inactivation of NPR2 ending cGMP production. The lack of cGMP releases PDE3A to hydrolyze cAMP in the oocytes. LH surge also inhibits NPPC expression, thus shutting down cGMP production permanently. LH surge also triggers a brief (lasting several hours in rats and mice) and robust expression of ornithine decarboxylase (ODC) in all three components of the antral follicles [3, 58], producing high levels of putrescine in the ovaries
It has been known for almost five decades that the LH surge also triggers a brief but robust expression of ornithine decarboxylase (ODC) and production of putrescine in the ovaries of several mammalian and non-mammalian species (Fig. 1). More recent studies have demonstrated that aging mouse ovaries exhibit peri-ovulatory ODC deficiency [58], and putrescine supplementation greatly improves egg quality and reproductive efficiency in aged mice [32, 57]. Peri-ovulatory putrescine supplementation could be the “fountain of youth” for aged human eggs.
ODC and putrescine
ODC and cellular polyamines
ODC catalyzes decarboxylation of L-ornithine, producing putrescine (Fig. 2). Putrescine is the precursor for spermidine (a triamine), which serves as the precursor for spermine (a tetraamine) [18]. This synthetic pathway, from putrescine to spermine, is essential in all eukaryotic cells (see below).
Fig. 2.
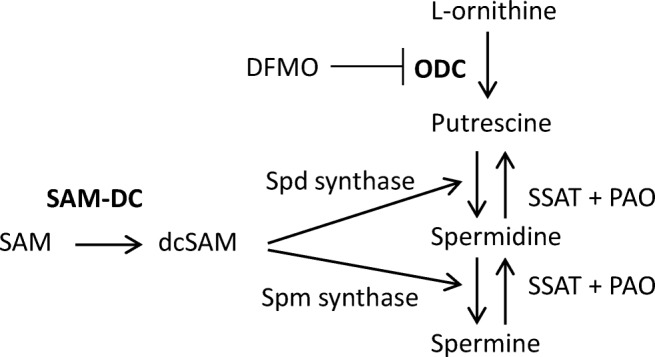
Cellular polyamine pathways. ODC catalyzes decarboxylation of L-ornithine to produce putrescine [NH2(CH2)4NH2]. Two other enzymes are involved in spermidine (spd) synthesis. First, S-adenosylmethionine decarboxylase (SAM-DC) converts SAM to decarboxylated SAM (dcSAM). Spermidine (spm) synthase then transfers an amino propyl radical (NH2-CH2-CH2-CH2-) from dcSAM to putrescine to produce spermidine [NH2(CH2)3NH(CH2)4NH2]. Similarly, spermine synthase transfers an amino propyl radical from dcSAM to spermidine to produce spermine [NH2(CH2)3NH(CH2)4NH-(CH2)3NH2]. In the catabolic pathway, spermidine/spermine N1-acetyltransferase (SSAT) first converts spermine to N1, N12-diacetylspermine, which is then oxidized to spermidine by polyamine oxidase (PAO). Similarly, SSAT converts spermidine to N1-acetylspermidine, which is then oxidized to putrescine by PAO. The two rate-limiting enzymes in (forward) polyamine synthesis are in bold
A catabolic pathway also exits to convert spermine to spermidine, and spermidine to putrescine (Fig. 2) [18]. The physiological significance of this back-conversion is not clear, since deletion of SSAT in mice results in no changes in polyamine levels, nor any phenotypic abnormalities [41]. Interestingly, putrescine is undetectable in fruit fly but it is present in high concentration (100 nmol/g; comparable to therapeutic putrescine concentrations in the ovaries, Fig. 4) in flies fed with spermidine [12]. This raises the question if putrescine has a role in promoting longevity.
Fig. 4.
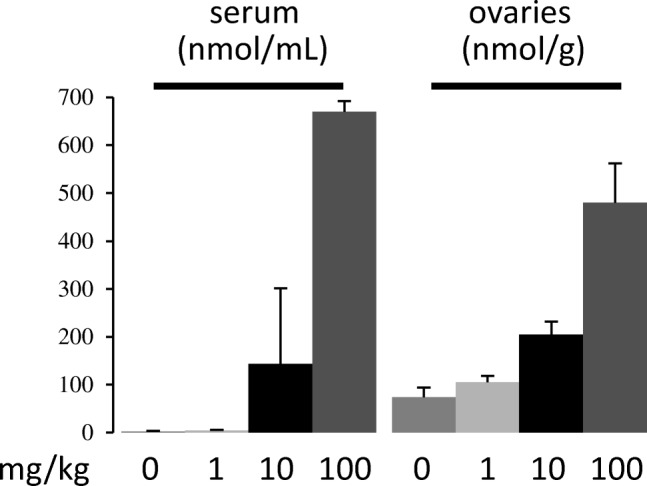
Putrescine supplementation. CF1 mice were injected subcutaneously with phosphate buffer saline (0) or the indicated doses of putrescine dihydrochloride in phosphate buffer saline. Twenty minutes after injection, mice were killed. Serum and ovaries were collected for polyamine analyses. Only putrescine concentrations are shown. N = 3 mice for each treatment
ODC is the rate-limiting enzyme in polyamine synthesis (Fig. 2). In most tissues, ODC activity is between 1/10 and 1/100 that of spermidine synthase or spermine synthase [51]. This is correlated with the typically very low-putrescine levels in most tissues, compared to the much higher (typically > 100-fold) levels of spermidine and spermine [42, 60]. Another factor contributing to this disparity is the short half-life of putrescine (e.g., 2 h in regenerating rat liver), as opposed to that of spermidine (4 days) [9]. Similarly, ODC is among the eukaryotic proteins with the shortest in-cell half-life [47]. ODC is degraded by proteasome in a fashion that is independent of polyubiquitination [39] but, instead, is facilitated by the cellular inhibitor of ODC, ODC antizyme (AZ) [64]. AZ synthesis is stimulated by high concentrations of polyamines, providing a feedback mechanism to inhibit and degrade ODC. Adding to this complexity, antizyme inhibitor is a catalytically inactive ODC homolog which binds AZ tighter than ODC, hence releasing ODC from AZ inhibition [46].
ODC is an essential gene from yeast [53] to mammals [48], indicating that polyamines are required for cell proliferation [18]. Consistent with this role, during mouse embryogenesis the expression of ODC and SAM-DC increases sharply after implantation (E6-E8), with corresponding increase of putrescine and spermidine but not spermine [16]. Similar increase of putrescine and spermidine is observed during emergence of the embryos after oligate diapause in minks [29]. Administering an ODC inhibitor D,L-α-difluoromethylornithine [DFMO [36]] during E5-E8 prevents putrescine and spermidine increase and causes immediate developmental arrest and embryo death [16]. DFMO treatment of emerging mink embryos after obligate diapause similarly reduces the two polyamines and causes embryo rearrest [29].
A unique role of putrescine in animal reproduction and its deficiency in aging ovaries
Putrescine appears to have a unique role in the peri-ovulatory period of animal reproduction. It was first reported in 1971 that rat ovaries exhibit a transient rise of ODC activity in the evening of proestrus day, peaking at levels greater than tenfold of the rest of the 4-day estrus cycle [27]. The ODC activity rise is dependent on LH or hCG, and requires transcription and translation [26, 27, 34]. In contrast, ovarian SAM-DC activity remains low and unchanged [34]. Correspondingly, the peri-ovulatory ovaries produce high levels of putrescine (250–500 nmol/g), with no change in spermidine and spermine concentrations [17, 57], in contrast to the post-implantation uterus where both ODC and SAM-DC increase resulting in a modest putrescine increase (to ~ 100 nmol/g) and a robust increase in spermidine [16].
Similar hCG-dependent ODC activity increase have been found in mice [3, 58] and hamster [49]. In mouse ovaries, a significant increase in putrescine is observed between 2 and 4 h afte hCG injection, peaking 5 h post-hCG, and followed by a slower decrease such that at time of ovulation (14-h post-hCG), putrescine levels are still higher than before the hCG injection [57]. Within the ovaries, ODC activity is found mainly in antral follicles [23], both in the granulosa cells (mural granulosa and cumulus cells) and in the oocytes [3, 58].
It has been suggested that the transient rise of ODC and putrescine in the ovaries is required for luteinization of the granulosa cells, since mice treated with DFMO contain corpora lutea with fewer blood vessels and produce less progesterone [3]. Similar DFMO treatment in rats [17] and in mice (Y. Tao, unpublished) do not influence the number of implantation sites. Furthermore, oocyte maturation in Xenopus laevis, a species lacking luteinzation or implantation, is also accompanied by significant and transient rise in ODC activity and putrescine in the oocytes [56, 62, 65]. In Xenopus oocytes, ODC activity increase is the result of protein translation following cytoplasmic polyadenylation of ODC mRNA [65]. When the translation is inhibited via ODC-specific antisense morpholino oligos, the maturing oocytes exhibit elevated levels of reactive oxygen species (ROS) followed by clear apoptotic events in mature eggs: release of mitochondrial cytochrome c, elevated caspase activity, and disruption of metaphase II spindle [65]. Putrescine supplementation in the oocyte maturation medium or injection of catalase into the oocytes suppresses the excess ROS and prevents apoptosis [65].
In mice, the peri-ovulatory ODC/putrescine rise is concurrent with oocyte maturation in vivo [13]. Administration of DFMO during ovulation diminishes ovarian ODC activity [3, 58], preventing putrescine accumulation without affecting the levels of spermidine and spermine [57]. DFMO does not affect ovulation [58] in mice, similarly to rats [17]. However, DFMO increases aneuploidy rates in ovulated eggs from 1.3 to 7.7% [58]. Complete inhibition of ODC results in only modest (7.7%) aneuploidy rate, with the majority harboring a single chromosome error [58], suggesting that the effect of putrescine on chromosome segregation might be indirect (i.e., unlike disruption of microtubules or preventing cohesin degradation). No significant difference was observed between control and DFMO-treated young mice in pregnancy rates or the number of implantations per pregnancy (Tao et al., unpublished results) consistent with the finding in rats [17]. The lack of acute reproductive failure in peri-ovulatory ODC deficiency in young rats and mice suggest the presence of compensatory mechanisms in young animals.
Aged mice exhibit significantly reduced levels of peri-ovulatory ODC [58] and putrescine, but unaltered spermidine and spermine [57]. A combination of peri-ovulatory putrescine supplementation in mouse drinking water and in IVM (in vitro maturation) medium reduces egg aneuploidies of aged oocytes [58]. The reproductive benefit of peri-ovulatory putrescine supplementation in aged mice is remarkable. Supplementation increases blastocyst cell numbers, reduces embryo resorption rates, and doubles the number of live pups [57]. Putrescine shows no toxicity to mothers and fetuses even if the supplementation is extended beyond peri-ovulatory period; mice born after peri-ovulatory putrescine supplementation grow up normal and fertile [57]. These studies suggest that putrescine supplementation in aged mice reduces egg aneuploidy and, more importantly, improves the developmental potential of the eggs.
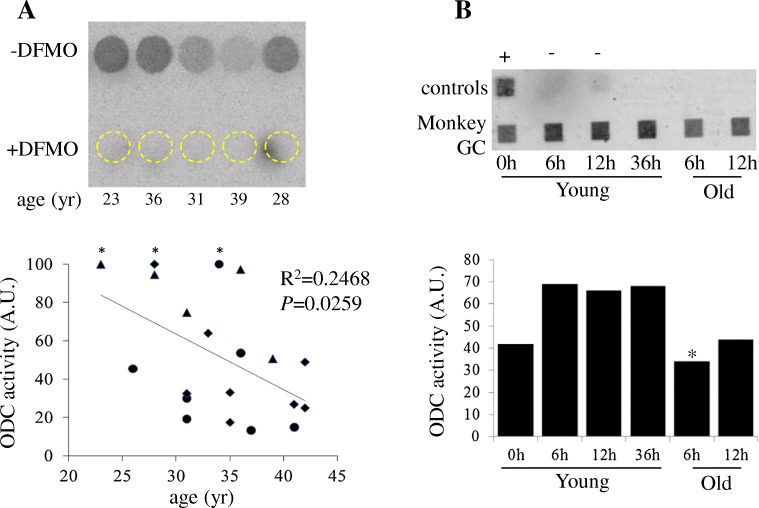
Since rodent ovaries exhibit elevated ODC activity and putrescine levels at the time of ovulation [17, 57], we sought to determine if surplus granulosa cells from IVF patients at the time of egg retrieval (~ 36 h after hCG injection) similarly exhibit ODC activity. Human granulosa cells were donated by IVF patients at Ottawa Fertility Center with written informed consent. Controlled ovarian stimulation was performed using either a long pituitary down-regulation protocol with GnRH agonist Suprefact (Sanofi, Canada), Lupron or Depo Lupron (AbbVie, Canada) or a short GnRH antagonist protocol with Cetrotide (EMD Serono, Canada). The follicles were stimulated using recFSH (EMD Serono, Canada) or recFSH and recLH (Luveris, EMD Serono, Canada). Final follicular maturation was triggered with recHCG Ovidrel (EMD Serono, Canada) when at least three follicles reached > 17 mm in diameter. Cumulus oocyte complexes were retrieved transvaginal using a single lumen ultrasound-guided needle (Cook, Australia) at 36 h post HCG. Following ovum pick-up, granulosa cells (cell layers and big chunks) were collected from all dishes from the same patient and transfer to a new dish containing 5 mL of buffered 0.9% saline solution. The pooled cells were transferred to an Eppendorf tube containing 1 mL of buffered saline solution. The cells were collected by low-speed centrifugation (3000×g for 5 min) as a pellet and flash frozen in liquid nitrogen within 1 hour of follicle aspiration. Cell extracts were prepared and assayed for ODC activity according to Tao and Liu [58]. These experiments indicated that human granulosa cells exhibit ODC activity, which is inhibited by DFMO (Fig. 3a, b). Furthermore, the levels of granulosa cell ODC activity appears to inversely correlate with donor age (Fig. 3a). These results support the notion that advanced maternal age is associated with physiologic ovarian putrescine deficiency in humans. Similarly, granulosa cells isolated from peri-ovulatory young and old rhesus monkeys exhibited ODC activity (Fig. 3b). Further work will be required to determine if aging-related reduction of ovarian ODC activity also occurs in monkeys.
Fig. 3.
Granulosa cell ODC activity in human and monkey. a An inverse relationship between granulosa cell ODC activity and maternal age. The image shows the result of the first assay where the granulosa cell extract from each patient was split equally into two, analyzed in the absence or the presence of DFMO (ODC inhibitor). Dark circles depict PhosphorImager scan of 14CO2 release from decarboxylation of 14C-ornithine (-DFMO). Corresponding positions of the reaction in the presence DFMO are depicted by dashed circles. The graph summarizes data of three experiments (triangle, diamond, or circle), with a total of 20 patients aged 23 to 42. ODC activities are expressed in arbitrary unit, normalized by total protein concentrations in the cell lysates in each of the three experiments, and with the highest ODC activity sample (marked with a star) in each experiment arbitrarily set as 100. Data are analyzed using GraphPad Prism 6.02. b Monkeys underwent controlled ovarian stimulation [55]. At the indicated time following hCG injection, follicle aspiration was carried out. After oocyte removal, granulosa cells (GC) were purified using Percoll gradient centrifugation [50], counted, and snap-frozen in liquid N2. Cell extracts were prepared and subjected to ODC activity assay, each reaction representing 1 million cells with the exception of the indicated (*) reaction (830,000; max available). The image shows PhosphorImager scan of the single assay, with each sample representing a single aspiration (both ovaries) at the indicated time after hCG injection. The graph shows relative ODC activity in arbitrary units. +, Xenopus egg extract; −, buffer only
A potential role for exogenous putrescine in assisted reproduction?
Natural sources for putrescine
Putrescine is produced by the decarboxylation of the non-protein amino acid L-ornithine (Fig. 2). Despite its name, putrescine is ubiquitous in living organisms. Putrescine is present in most food [42] and, more importantly, because many gut bacteria produce a large amount of putrescine, can be reabsorbed, and distributed to the rest of the body [46]. The highest concentration of putrescine is found in fresh green pepper measuring ~ 2000 nmol per g [42], compared to 250-500nmol/g in peri-ovulatory ovaries [17,57]. Interestingly, high putrescine concentrations in green pepper and other fresh food (orange, mango and zucchini) are thought to be important for keeping food fresh during storage, likley by enhancing cellular antioxidant mechanisms [Wang Y, Zhou F, Zuo J, Zheng Q, Gao L, Jiang A. Pre-storage treatment of mechanically-injured green pepper (Capsium annuum L.) fruit with putrescine reduces adverse physiological responses. Postharvent Biol Technol 2018;145:239-246; and references therein], similarly to the mechanism found in Xenopus oocytes [65]. Putrescine has very low toxicity, with LD50 in rats being 2000 mg/kg [59]. By comparison, the estimated therapeutic dose of putrescine is 10 mg/kg by subcutaneous injection, increasing ovarian putrescine by ~ 130 nmol/g (Fig. 4), similar to the putrescine increase found in the ovaries of older mice in our peri-ovulatory putrescine supplementation experiments [57].
In our experiments, significant putrescine supplementation could not be achieved by consuming a putrescine-rich food such as green pepper. Putrescine in fresh green pepper appears to be poorly bioavailable and/or poorly absorbed in the gut (Y. Tao, unpublished).
Putrescine supplementation in other systems
In addition to our work demonstrating that putrescine supplementation improves egg quality of aged mice [31, 57, 58], putrescine supplementation has been applied effectively in many other animal and cell models. Most of these likely relates to the function of ODC and putrescine in promoting cell proliferation [16, 48]. For example, putrescine supplementation (1 mM) in vitro reactivates mink blastocysts from obligate embryonic diapause [15]. Similarly, putrescine supplementation (200 μM) in vitro rescues blastocyst development in mouse embryos deficient in the pluripotent Mga transcription factor [61]. Putrescine (25 μM) stimulates protein translation and cell proliferation in porcine trophectoderm cells in vitro [28]. Putrescine supplementation (0.1%) in drinking water promotes neurogenesis in adult insects [8]. Simultaneous putrescine supplementation (100 mM solution delivered through controlled release Alzet minipumps) improves the outcome of Schwann cell implantation in spinal cord–injured rats [24]. Putrescine supplementation of milk-soy diet (25 g per kg of food) has been used for newborn calves and piglets to help small intestinal mucosal growth and nutrient uptake [19, 20].
Brain injuries followed by ischemia and epileptic seizures are accompanied by increase of ODC activity and putrescine in the brain [38, 45]. This prompted studies which demonstrated that putrescine supplementation protected animals from chemically and electrically induced seizures. It remains uncertain if the neuro-protective role of putrescine is due to its conversion to GABA [4, 22].
Peri-ovulatory putrescine supplementation in humans
Our animal studies indicate that exogenous-administered putrescine has a half-life of less than 1 h in the blood and less than 2 h in the ovaries [57]. In mouse experiments, putrescine was given in mouse drinking water providing continuous supplementation owing to the almost constant water intake by lab mice, especially during the night (100–200 licks/30 min) [25] when the natural putrescine concentration rises in the ovaries occurs [17, 31].
Given the transient rise of putrescine during ovulation [17, 27, 57], putrescine supplementation needs to take place only between the start of the LH surge and the end of ovulation. This period lasts about 14 h in mice and 36 h in humans. In the fertility clinic, the timing of administration could be conveniently accomplished since the start of LH surge is replaced with injection of a bolus hCG or GnRH agonist. Putrescine supplementation would be terminated at the time of egg retrieval 36 h later. Based on a review of the literature, there have been no studies of pure putrescine supplementation in humans and no pharmacokinetic data are available in humans. Given the short half-life of putrescine in live animals [9, 57], it is likely that multiple injections will be required over the 36-h period to maintain elevated human ovarian putrescine levels. Alternatively, a controlled release formulation could be developed to deliver constant putrescine in the circulation.
In natural conception, the precise timing of ovulation is more difficult to pinpoint. Fortunately, prolonged putrescine supplementation for several days is unlikely to have a negative impact. An earlier mouse study [48] has suggested that the presence of 0.5–1 mM putrescine in the drinking water after placentation is harmful to pregnancy. They attribute toxicity to the possible oxidation of putrescine by placenta-resident diamine oxidase [7] to produce excess ROS [48]. In our laboratory, we did not find similar toxicity in mouse experiments, even with higher putrescine concentrations in mouse drinking water over the entire gestational period [57]. In the proposed peri-ovulatory putrescine supplementation therapy, fetal toxicity is unlikely given the gap of at least 1 week between ovulation and the beginning of placentation and diamine oxidase expression (in mice) [30] and the short-half-life of exogenous putrescine [9, 57].
Putrescine supplementation could be used to facilitate in IVM of oocytes in the fertility clinic [11]. In a proof of principle study we tested the ability of putrescine supplementation during IVM to improve the developmental potential of aged mouse oocytes. Cumulus-oocyte complexes (COCs) of aged mice were subjected to IVM with or without the addition of 0.5 mM putrescine. This concentration is based on the physiological concentration we have observed (500 nmol putrescine/g ovarian tissues) [31, 57]. The mature eggs were fertilized in vitro followed by in vitro culture to blastocyst stage. Analyses of the blastocyst embryos indicated that the putrescine group exhibited significantly better embryo qualities: higher proportion exhibiting top-grade morphology, greater total cell number, and higher proportion having octamer-binding transcription factor 4 (OCT4)-positive inner cell mass [31].
IVM is a laboratory technique for ART that has been advocated for patients with a high-ovarian reserve and who are at high risk of severe hyperstimulation syndrome [43] following gonadotropin stimulation. IVM use is controversial and of variable clinical efficacy. It has been advocated for patients with polycystic ovaries or polycystic ovarian syndrome [21, 44]. In some clinics, including at UVZBrussel, germinal vesicle (GV) stage oocytes are aspirated from patients not previously injected with hCG and the entire oocyte maturation, from GV to metaphase II, is carried out in vitro. This approach [11] is physiologically identical to IVM practice in mice [32] and domestic animal species [6] and may likely benefit from putrescine supplementation.
Concluding remarks
There are compelling reasons to believe that peri-ovulatory putrescine supplementation will prove to be a successful therapy for older infertile women. First, it corrects aging-related ODC deficiency in peri-ovulatory ovaries. Secondly, it targets oocyte maturation with a very brief intervention (at most days), in contrast to other proposed interventions (such as Co-enzyme Q10, omega-3 fatty acids, calorie-restriction, and antioxidant) which are mid- to long-term in nature, and are not necessarily specific to fertility [5, 33, 40, 54]. Thirdly, it is equally applicable in IVF patients and in natural conception. Finally, the ubiquitous nature of putrescine in living organisms, its chemical stability, and its rapid distribution and clearance are attractive features in its consideration as a therapeutic intervention to improve live birthrates in older women, especially those over the age of 37 [1].
Abbreviations
- AZ
Antizyme
- COC
Cumulus-oocyte complex
- dcSAM
Decarboxylased SAM
- DFMO
D,L-α-Difluoromethylornithine
- GABA
Gamma-aminobutyric acid
- GV
Germinal vesicle or oocyte nucleus
- hCG
Human chorionic gonadotropin
- IVM
Oocyte in vitro maturation
- IVF
In vitro fertilization
- LH
Luteinizing hormone
- ODC
Ornithine decarboxylase
- ROS
Reactive oxygen species
- PDE3A
Phosphodiesterase 3A
- SAM
S-adenosylmethionine
- SAM-DC
SAM decarboxylase
- SSAT
Spermidine/spermine N1 acetyltransferase
Funding information
Supported by a research grant from March of Dimes (6-FY13-126) (to XJL), and by the Office of the Director, National Institutes of Health under Award Number P51OD011092 (to ONPRC). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.American College of Obstetricians and Gynecologists Committee on Gynecologic Practice and Practice Committee Female age-related fertility decline. Committee opinion no. 589. Fertil Steril. 2014;101:633–634. doi: 10.1016/j.fertnstert.2013.12.032. [DOI] [PubMed] [Google Scholar]
- 2.Angell RR. Predivision in human oocytes at meiosis I: a mechanism for trisomy formation in man. Hum Genet. 1991;86:383–387. doi: 10.1007/BF00201839. [DOI] [PubMed] [Google Scholar]
- 3.Bastida CM, Cremades A, Castells MT, Lopez-Contreras AJ, Lopez-Garcia C, Tejada F, Penafiel R. Influence of ovarian ornithine decarboxylase in folliculogenesis and luteinization. Endocrinology. 2005;146:666–674. doi: 10.1210/en.2004-1004. [DOI] [PubMed] [Google Scholar]
- 4.Bell MR, Belarde JA, Johnson HF, Aizenman CD. A neuroprotective role for polyamines in a Xenopus tadpole model of epilepsy. Nat Neurosci. 2011;14:505–512. doi: 10.1038/nn.2777. [DOI] [PubMed] [Google Scholar]
- 5.Ben-Meir A, Burstein E, Borrego-Alvarez A, Chong J, Wong E, Yavorska T, Naranian T, Chi M, Wang Y, Bentov Y, et al. Coenzyme Q10 restores oocyte mitochondrial function and fertility during reproductive aging. Aging Cell. 2015;14:887–895. doi: 10.1111/acel.12368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown HM, Dunning KR, Sutton-McDowall M, Gilchrist RB, Thompson JG, Russell DL. Failure to launch: aberrant cumulus gene expression during oocyte in vitro maturation. Reproduction. 2017;153:R109–R120. doi: 10.1530/REP-16-0426. [DOI] [PubMed] [Google Scholar]
- 7.Bruun L, Houen G. In situ detection of diamine oxidase activity using enhanced chemiluminescence. Anal Biochem. 1996;233:130–136. doi: 10.1006/abio.1996.0017. [DOI] [PubMed] [Google Scholar]
- 8.Cayre M, Strambi C, Charpin P, Augier R, Strambi A. Specific requirement of putrescine for the mitogenic action of juvenile hormone on adult insect neuroblasts. Proc Natl Acad Sci U S A. 1997;94:8238–8242. doi: 10.1073/pnas.94.15.8238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen SS. A guide to the polyamines. Oxford: Oxford University Press; 1998. Pathways of polyamine metabolism in animals; pp. 208–230. [Google Scholar]
- 10.Conti M, Franciosi F. Acquisition of oocyte competence to develop as an embryo: integrated nuclear and cytoplasmic events. Hum Reprod Update. 2018;24:245–266. doi: 10.1093/humupd/dmx040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Vos M, Smitz J, Thompson JG, Gilchrist RB. The definition of IVM is clear-variations need defining. Hum Reprod. 2016;31:2011–2015. doi: 10.1093/humrep/dew208. [DOI] [PubMed] [Google Scholar]
- 12.Eisenberg T, Knauer H, Schauer A, Buttner S, Ruckenstuhl C, Carmona-Gutierrez D, Ring J, Schroeder S, Magnes C, Antonacci L, et al. Induction of autophagy by spermidine promotes longevity. Nat Cell Biol. 2009;11:1305–1314. doi: 10.1038/ncb1975. [DOI] [PubMed] [Google Scholar]
- 13.Eppig JJ. The relationship between cumulus cell-oocyte coupling, oocyte meiotic maturation, and cumulus expansion. Dev Biol. 1982;89:268–272. doi: 10.1016/0012-1606(82)90314-1. [DOI] [PubMed] [Google Scholar]
- 14.Eppig JJ, Schultz RM, O'Brien M, Chesnel F. Relationship between the developmental programs controlling nuclear and cytoplasmic maturation of mouse oocytes. Dev Biol. 1994;164:1–9. doi: 10.1006/dbio.1994.1175. [DOI] [PubMed] [Google Scholar]
- 15.Fenelon JC, Banerjee A, Lefevre P, Gratian F, Murphy BD. Polyamine-mediated effects of prolactin dictate emergence from mink obligate embryonic diapause. Biol Reprod. 2016;95:6. doi: 10.1095/biolreprod.116.139204. [DOI] [PubMed] [Google Scholar]
- 16.Fozard JR, Part ML, Prakash NJ, Grove J, Schechter PJ, Sjoerdsma A, Koch-Weser J. L-ornithine decarboxylase: an essential role in early mammalian embryogenesis. Science. 1980;208:505–508. doi: 10.1126/science.6768132. [DOI] [PubMed] [Google Scholar]
- 17.Fozard JR, Prakash NJ, Grove J. Ovarian function in the rat following irreversible inhibition of L-ornithine decarboxylase. Life Sci. 1980;27:2277–2283. doi: 10.1016/0024-3205(80)90395-1. [DOI] [PubMed] [Google Scholar]
- 18.Gerner EW, Meyskens FL., Jr Polyamines and cancer: old molecules, new understanding. Nat Rev Cancer. 2004;4:781–792. doi: 10.1038/nrc1454. [DOI] [PubMed] [Google Scholar]
- 19.Grant AL, Holland RE, Thomas JW, King KJ, Liesman JS. Effects of dietary amines on the small intestine in calves fed soybean protein. J Nutr. 1989;119:1034–1041. doi: 10.1093/jn/119.7.1034. [DOI] [PubMed] [Google Scholar]
- 20.Grant AL, Thomas JW, King KJ, Liesman JS. Effects of dietary amines on small intestinal variables in neonatal pigs fed soy protein isolate. J Anim Sci. 1990;68:363–371. doi: 10.2527/1990.682363x. [DOI] [PubMed] [Google Scholar]
- 21.Guzman L, Ortega-Hrepich C, Albuz FK, Verheyen G, Devroey P, Smitz J, De VM. Developmental capacity of in vitro-matured human oocytes retrieved from polycystic ovary syndrome ovaries containing no follicles larger than 6 mm. Fertil Steril. 2012;98:503–507. doi: 10.1016/j.fertnstert.2012.01.114. [DOI] [PubMed] [Google Scholar]
- 22.Halonen T, Sivenius J, Miettinen R, Halmekyto M, Kauppinen R, Sinervirta R, Alakuijala L, Alhonen L, MacDonald E, Janne J, et al. Elevated seizure threshold and impaired spatial learning in transgenic mice with putrescine overproduction in the brain. Eur J Neurosci. 1993;5:1233–1239. doi: 10.1111/j.1460-9568.1993.tb00978.x. [DOI] [PubMed] [Google Scholar]
- 23.Icekson I, Kaye AM, Lieberman ME, Lamprecht SA, Lahav M, Lindner HR. Stimulation by luteinizing hormone of ornithine decarboxylase in rat ovary: preferential response by follicular tissue. J Endocrinol. 1974;63:417–418. doi: 10.1677/joe.0.0630417. [DOI] [PubMed] [Google Scholar]
- 24.Iorgulescu JB, Patel SP, Louro J, Andrade CM, Sanchez AR, Pearse DD. Acute putrescine supplementation with Schwann cell implantation improves sensory and serotonergic axon growth and functional recovery in spinal cord injured rats. Neural Plast. 2015;2015:186385. doi: 10.1155/2015/186385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson RF, Beltz TG, Thunhorst RL, Johnson AK. Investigations on the physiological controls of water and saline intake in C57BL/6 mice. Am J Physiol Regul Integr Comp Physiol. 2003;285:R394–R403. doi: 10.1152/ajpregu.00130.2003. [DOI] [PubMed] [Google Scholar]
- 26.Kaye AM, Icekson I, Lamprecht SA, Gruss R, Tsafriri A, Lindner HR. Stimulation of ornithine decarboxylase activity by luteinizing hormone in immature and adult rat ovaries. Biochemistry. 1973;12:3072–3076. doi: 10.1021/bi00740a020. [DOI] [PubMed] [Google Scholar]
- 27.Kobayashi Y, Kupelian J, Maudsley DV. Ornithine decarboxylase stimulation in rat ovary by luteinizing hormone. Science. 1971;172:379–380. doi: 10.1126/science.172.3981.379. [DOI] [PubMed] [Google Scholar]
- 28.Kong X, Wang X, Yin Y, Li X, Gao H, Bazer FW, Wu G. Putrescine stimulates the mTOR signaling pathway and protein synthesis in porcine trophectoderm cells. Biol Reprod. 2014;91:106. doi: 10.1095/biolreprod.113.113977. [DOI] [PubMed] [Google Scholar]
- 29.Lefevre PL, Palin MF, Chen G, Turecki G, Murphy BD. Polyamines are implicated in the emergence of the embryo from obligate diapause. Endocrinology. 2011;152:1627–1639. doi: 10.1210/en.2010-0955. [DOI] [PubMed] [Google Scholar]
- 30.Liang XH, Zhao ZA, Deng WB, Tian Z, Lei W, Xu X, Zhang XH, Su RW, Yang ZM. Estrogen regulates amiloride-binding protein 1 through CCAAT/enhancer-binding protein-beta in mouse uterus during embryo implantation and decidualization. Endocrinology. 2010;151:5007–5016. doi: 10.1210/en.2010-0170. [DOI] [PubMed] [Google Scholar]
- 31.Liu D, Mo G, Tao Y, Wang H, Liu XJ. Putrescine supplementation during in vitro maturation of aged mouse oocytes improves the quality of blastocysts. Reprod Fertil Dev. 2017;29:1392–1400. doi: 10.1071/RD16061. [DOI] [PubMed] [Google Scholar]
- 32.Liu D, Mo G, Tao Y, Wang H, Liu XJ. Putrescine supplementation during in vitro maturation of aged mouse oocytes improves the quality of blastocysts. Reprod Fertil Dev. 2017;29:1392–1400. doi: 10.1071/RD16061. [DOI] [PubMed] [Google Scholar]
- 33.Liu M, Yin Y, Ye X, Zeng M, Zhao Q, Keefe DL, Liu L. Resveratrol protects against age-associated infertility in mice. Hum Reprod. 2013;28:707–717. doi: 10.1093/humrep/des437. [DOI] [PubMed] [Google Scholar]
- 34.Maudsley DV, Kobayashi Y. Induction of ornithine decarboxylase in rat ovary after administration of luteinizing hormone or human chorionic gonadotrophin. Biochem Pharmacol. 1974;23:2697–2703. doi: 10.1016/0006-2952(74)90040-9. [DOI] [PubMed] [Google Scholar]
- 35.Meldrum DR, Casper RF, Diez-Juan A, Simon C, Domar AD, Frydman R. Aging and the environment affect gamete and embryo potential: can we intervene? Fertil Steril. 2016;105:548–559. doi: 10.1016/j.fertnstert.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 36.Metcalf BW, Bey P, Danzin C, Jung MJ, Casara P, Vevert JP. Catalytic irreversible inhibition of mammalian ornithine decarboxylase (E.C.4.1.1.17) by substrate and product analogues. J Am Chem Soc. 1978;100:2551–2553. doi: 10.1021/ja00476a050. [DOI] [Google Scholar]
- 37.Moor RM, Dai Y, Lee C, Fulka J., Jr Oocyte maturation and embryonic failure. Hum Reprod Update. 1998;4:223–236. doi: 10.1093/humupd/4.3.223. [DOI] [PubMed] [Google Scholar]
- 38.Muller M, Cleef M, Rohn G, Bonnekoh P, Pajunen AE, Bernstein HG, Paschen W. Ornithine decarboxylase in reversible cerebral ischemia: an immunohistochemical study. Acta Neuropathol. 1991;83:39–45. doi: 10.1007/BF00294428. [DOI] [PubMed] [Google Scholar]
- 39.Murakami Y, Matsufuji S, Kameji T, Hayashi S, Igarashi K, Tamura T, Tanaka K, Ichihara A. Ornithine decarboxylase is degraded by the 26S proteasome without ubiquitination. Nature. 1992;360:597–599. doi: 10.1038/360597a0. [DOI] [PubMed] [Google Scholar]
- 40.Nehra D, Le HD, Fallon EM, Carlson SJ, Woods D, White YA, Pan AH, Guo L, Rodig SJ, Tilly JL, et al. Prolonging the female reproductive lifespan and improving egg quality with dietary omega-3 fatty acids. Aging Cell. 2012;11:1046–1054. doi: 10.1111/acel.12006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Niiranen K, Keinanen TA, Pirinen E, Heikkinen S, Tusa M, Fatrai S, Suppola S, Pietila M, Uimari A, Laakso M, et al. Mice with targeted disruption of spermidine/spermine N1-acetyltransferase gene maintain nearly normal tissue polyamine homeostasis but show signs of insulin resistance upon aging. J Cell Mol Med. 2006;10:933–945. doi: 10.1111/j.1582-4934.2006.tb00536.x. [DOI] [PubMed] [Google Scholar]
- 42.Nishimura K, Shiina R, Kashiwagi K, Igarashi K. Decrease in polyamines with aging and their ingestion from food and drink. J Biochem (Tokyo) 2006;139:81–90. doi: 10.1093/jb/mvj003. [DOI] [PubMed] [Google Scholar]
- 43.Nogueira D, Sadeu JC, Montagut J. In vitro oocyte maturation: current status. Semin Reprod Med. 2012;30:199–213. doi: 10.1055/s-0032-1311522. [DOI] [PubMed] [Google Scholar]
- 44.Ortega-Hrepich C, Stoop D, Guzman L, Van LL, Tournaye H, Smitz J, De VM. A “freeze-all” embryo strategy after in vitro maturation: a novel approach in women with polycystic ovary syndrome? Fertil Steril. 2013;100:1002–1007. doi: 10.1016/j.fertnstert.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 45.Paschen W, Csiba L, Rohn G, Bereczki D. Polyamine metabolism in transient focal ischemia of rat brain. Brain Res. 1991;566:354–357. doi: 10.1016/0006-8993(91)91726-H. [DOI] [PubMed] [Google Scholar]
- 46.Pegg AE, Casero RA., Jr Current status of the polyamine research field. Methods Mol Biol. 2011;720:3–35. doi: 10.1007/978-1-61779-034-8_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pegg AE, McGovern KA, Wiest L. Decarboxylation of alpha-difluoromethylornithine by ornithine decarboxylase. Biochem J. 1987;241:305–307. doi: 10.1042/bj2410305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pendeville H, Carpino N, Marine JC, Takahashi Y, Muller M, Martial JA, Cleveland JL. The ornithine decarboxylase gene is essential for cell survival during early murine development. Mol Cell Biol. 2001;21:6549–6558. doi: 10.1128/MCB.21.19.6549-6558.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Persson L, Isaksson K, Rosengren E, Sundler F. Distribution of ornithine decarboxylase in ovaries of rat and hamster during pro-oestrus. Acta Endocrinol. 1986;113:403–409. doi: 10.1530/acta.0.1130403. [DOI] [PubMed] [Google Scholar]
- 50.Quinn MC, McGregor SB, Stanton JL, Hessian PA, Gillett WR, Green DP. Purification of granulosa cells from human ovarian follicular fluid using granulosa cell aggregates. Reprod Fertil Dev. 2006;18:501–508. doi: 10.1071/RD05051. [DOI] [PubMed] [Google Scholar]
- 51.Raina A, Eloranta T, Kajander O. Biosynthesis and metabolism of polyamines and S-adenosylmethionine in the rat. Biochem Soc Trans. 1976;4:968–971. doi: 10.1042/bst0040968. [DOI] [PubMed] [Google Scholar]
- 52.Sakakibara Y, Hashimoto S, Nakaoka Y, Kouznetsova A, Hoog C, Kitajima TS. Bivalent separation into univalents precedes age-related meiosis I errors in oocytes. Nat Commun. 2015;6:7550. doi: 10.1038/ncomms8550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schwartz B, Hittelman A, Daneshvar L, Basu HS, Marton LJ, Feuerstein BG. A new model for disruption of the ornithine decarboxylase gene, SPE1, in Saccharomyces cerevisiae exhibits growth arrest and genetic instability at the MAT locus. Biochem J. 1995;312(Pt 1):83–90. doi: 10.1042/bj3120083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Selesniemi K, Lee HJ, Muhlhauser A, Tilly JL. From the cover: prevention of maternal aging-associated oocyte aneuploidy and meiotic spindle defects in mice by dietary and genetic strategies. Proc Natl Acad Sci U S A. 2011;108:12319–12324. doi: 10.1073/pnas.1018793108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stouffer RL, Zelinski-Wooten MB. Overriding follicle selection in controlled ovarian stimulation protocols: quality vs quantity. Reprod Biol Endocrinol. 2004;2:32. doi: 10.1186/1477-7827-2-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sunkara PS, Wright DA, Nishioka K. An essential role for putrescine biosynthesis during meiotic maturation of amphibian oocytes. Dev Biol. 1981;87:351–355. doi: 10.1016/0012-1606(81)90158-5. [DOI] [PubMed] [Google Scholar]
- 57.Tao Y, Liu D, Mo G, Wang H, Liu XJ. Peri-ovulatory putrescine supplementation reduces embryo resorption in older mice. Hum Reprod. 2015;30:1867–1875. doi: 10.1093/humrep/dev130. [DOI] [PubMed] [Google Scholar]
- 58.Tao Y, Liu XJ. Deficiency of ovarian ornithine decarboxylase contributes to aging-related egg aneuploidy in mice. Aging Cell. 2013;12:42–49. doi: 10.1111/acel.12016. [DOI] [PubMed] [Google Scholar]
- 59.Til HP, Falke HE, Prinsen MK, Willems MI. Acute and subacute toxicity of tyramine, spermidine, spermine, putrescine and cadaverine in rats. Food Chem Toxicol. 1997;35:337–348. doi: 10.1016/S0278-6915(97)00121-X. [DOI] [PubMed] [Google Scholar]
- 60.Wagner J, Claverie N, Danzin C. A rapid high-performance liquid chromatographic procedure for the simultaneous determination of methionine, ethionine, S-adenosylmethionine, S-adenosylethionine, and the natural polyamines in rat tissues. Anal Biochem. 1984;140:108–116. doi: 10.1016/0003-2697(84)90140-4. [DOI] [PubMed] [Google Scholar]
- 61.Washkowitz AJ, Schall C, Zhang K, Wurst W, Floss T, Mager J, Papaioannou VE. Mga is essential for the survival of pluripotent cells during peri-implantation development. Develop. 2015;142:31–40. doi: 10.1242/dev.111104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Younglai EV, Godeau F, Mester J, Baulieu EE. Increased ornithine decarboxylase activity during meiotic maturation in Xenopus laevis oocytes. Biochem Biophys Res Commun. 1980;96:1274–1281. doi: 10.1016/0006-291X(80)90089-3. [DOI] [PubMed] [Google Scholar]
- 63.Yun Y, Lane SI, Jones KT. Premature dyad separation in meiosis II is the major segregation error with maternal age in mouse oocytes. Develop. 2014;141:199–208. doi: 10.1242/dev.100206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang M, Pickart CM, Coffino P. Determinants of proteasome recognition of ornithine decarboxylase, a ubiquitin-independent substrate. EMBO J. 2003;22:1488–1496. doi: 10.1093/emboj/cdg158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhou Y, Ma C, Karmouch J, Katbi HA, Liu XJ. Antiapoptotic role for ornithine decarboxylase during oocyte maturation. Mol Cell Biol. 2009;29:1786–1795. doi: 10.1128/MCB.01815-08. [DOI] [PMC free article] [PubMed] [Google Scholar]