Abstract
Hen's egg is the most common allergen in IgE-mediated food allergy among children in Japan. Although the majority of patients with egg allergy can eat heated egg yolk safely because of its low allergenicity, severely allergic patients show an immediate-type reaction to heated egg yolk. We hypothesized that patients with hyperresponsiveness to boiled egg yolk may have difficulty in acquiring tolerance to egg. The purpose of this study was to examine the prognosis of patients with hyperresponsiveness to boiled egg yolk. Data from 121 patients with egg allergy who underwent oral food challenge (OFC) with boiled egg yolk between January 2012 and December 2013 were analyzed retrospectively. The proportion of patients who could consume heated whole egg 3 years after OFC was 15.4% in the OFC-positive group and 75.8% in the OFC-negative group. Hyperresponsiveness to boiled egg yolk in early life might lead to prolonged egg allergy in children. This finding might aid in the selection of an appropriate population requiring practical immunotherapy.
Keywords: Egg hyperresponsiveness, egg yolk, egg white, pediatrics, prognosis
INTRODUCTION
Hen's egg is a common allergen in IgE-mediated food allergy in childhood. The reported prevalence of egg allergy in children ranges from 0.5% to 2.5% in meta-analyses.1 Although the natural history of egg allergy is favorable, with a tolerance ratio of 49% by the age of 4 years and 66% by 6 years, egg allergy persists into adolescence in some patients.2 For those patients, treatments, such as oral immunotherapy (OIT), could be administered to decrease reactivity to the allergen.3 Care should be taken in selecting patients for OIT because OIT is often associated with immediate allergic symptoms including anaphylaxis.4
The egg proteins recognized as allergens are mostly contained in egg white (EW).5 Among these proteins, ovomucoid (OVM) is stable against heat and digestive enzymes, so that EW is considered to be mainly responsible for IgE-mediated heated egg allergy.6 Some allergen components such as chicken serum albumin (Gal d 5), a major allergen in bird-egg syndrome, are found in egg yolk (EY), but they are so heat-labile that hard-boiled EY is well tolerated.7 By using enzyme-linked immunosorbent assay, only 7 mg of whole egg protein (contents including ovalbumin, ovotransferrin, and OVM) was detected in boiled one EY, while boiled whole egg contains 6,749.2 mg.8 For these reasons, boiled EY can be ingested more safely than heated EW by patients with egg allergy. Patients who are positive for oral food challenge (OFC) to boiled EY may have high sensitivity to a small amount of EW contained in the yolk.
The clinical implications of OFC to heated EY are unclear, and little attention has been paid to the prognosis of EY-reactive patients. We retrospectively focused on patients who had hyperresponsiveness to boiled EY and assessed whether the results of OFC to boiled EY could predict their prognosis of egg allergy. If hyperresponsiveness to boiled EY in early life leads to difficulty in acquiring tolerance to egg, the results of OFC to boiled EY might support the appropriate adoption of active intervention as OIT.
MATERIALS AND METHODS
This retrospective study was based on data from patients who underwent OFC to boiled EY in Miyagi Children's Hospital (Sendai, Japan) from January 2012 to December 2013. The patients had been diagnosed with egg allergy on the basis of their symptoms immediately after consumption of egg and having positive specific IgE (sIgE) to EY and/or EW. The symptoms were any allergic reactions of the skin, gastrointestinal tract, respiratory tract, neurological system, cardiovascular system, and anaphylaxis occurring within a few hours after consumption of heated egg. Patients whose medical records could not be followed up because of changes of their address or refusal to visit the hospital were excluded. We also excluded patients who had undergone a previous OFC to boiled EY or administered OIT during the course. Serum levels of sIgE to EY, EW, and OVM were measured within 6 months before OFC to boiled EY by ImmunoCAP® assay (Phadia, Thermo Fisher Scientific, Uppsala, Sweden).
All challenges were performed in the hospital by the open method. Boiled EYs were strictly separated from EW immediately after boiling at 100ºC for 20 minutes. The starting dose of OFC was one-tenth, followed by three-tenths and six-tenths, of the total challenge dose at intervals of 30 minutes. An OFC was regarded as positive if grade 2 or higher severe allergic reactions according to Sampson's grading score9 were found within 5 hours after intake. If an adverse reaction occurred, the patient was observed carefully, and, if necessary, antihistamines, inhaled beta2-agonists, and intramuscular adrenaline were administered.
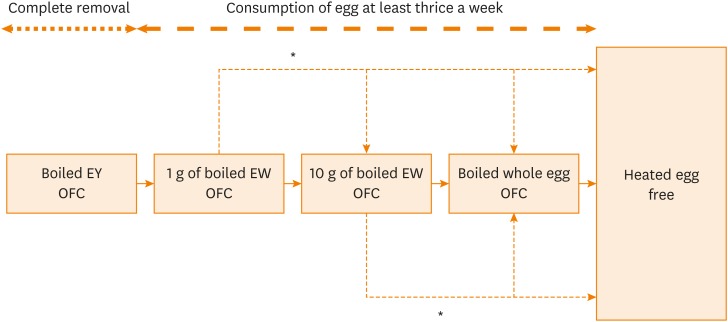
After the first OFC, the patients received dietary instruction and underwent the next OFCs (Figure). OFCs were performed according to the following steps in order: boiled whole EY, 1 g of boiled EW, 10 g of boiled EW, and boiled whole egg. If a safe dose was discovered by accidental ingestion without any allergic symptoms, the lower step of OFC could be omitted. Patients who were positive for OFC to boiled whole EY could undergo the same step of OFC after complete avoidance of egg for at least 6 months. After OFC, the patients were advised to consume the safe dose of egg at least thrice a week at home; the patients then underwent the following step of OFC. Gradually increasing dose (10% to 20% of regular eating dose every 2 weeks) at home was allowed if adverse reactions did not appear because some patients could not underwent all steps of OFC for familial or economic reasons. Each step of OFC could be omitted if gradually increasing dose reached the amount of the next step. Intervals of each step were determined by the attending physicians and different in patients; the presence or absence of adverse reactions at home, past histories of immediate reactions, and familial circumstances were comprehensively taken into consideration. The patients visited the hospital approximately every 3 months, and the amount of heated EW that the patients could eat at a single dose without allergic reaction was recorded by the attending physicians.
Figure. Flow of egg allergy management in the study. After passing each step of OFC to boiled EW, patients were advised to consume the same dose at least thrice a week. Some patients were allowed to increase gradually at home if adverse symptoms did not occur (*). If patients were positive for OFC, the majority of them underwent the same step of OFC after at least 6 months. Each step of OFC could be omitted if gradually increasing dose reached the amount of the next step.
EW, egg white; EY, egg yolk; OFC, oral food challenge.
The data were analyzed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is designed as a graphic user interface for R (The R Foundation for Statistical Computing, Version 3.3.1, Vienna, Austria). The Mann-Whitney U test was used to compare age and levels of sIgE to egg components between the OFC-positive and OFC-negative groups. Fisher's exact test was used to compare characteristics between the OFC-positive and OFC-negative groups. The logistic regression analysis was performed to assess the association between the results of first OFC and the amount of heated EW tolerated 3 years after first OFC. P values of <0.05 were considered to indicate statistical significance.
This study was approved by the Miyagi children's hospital Ethics Committee (approval number 377). Written informed consent for OFC was obtained from all parents or guardians of the participating children. The information disclosure document of this study has been published on the hospital website. Parents or guardians were informed about the study protocol and were given the option to opt out of the study at any time. Patient anonymity was preserved using methods approved by the Ethics Committee.
RESULTS
Of 156 patients with egg allergy who underwent OFC to boiled EY, 20 were excluded from analysis because they underwent OIT and 15 were excluded because of changes of their address or refusal to visit the hospital. The proportion of excluded patients did not significantly differ between the OFC-positive and OFC-negative groups. As a result, we analyzed 121 patients whose baseline clinical characteristics are shown in Table 1. All patients had immediate allergic symptoms and were diagnosed with egg allergy. Age, sex ratio, past anaphylactic history, and allergic anamnesis were not significantly different between the OFC-positive and OFC-negative groups. The levels of sIgE to egg components were significantly higher in the OFC-positive group.
Table 1. Characteristics of the study population who had first OFC to boiled egg yolk.
| OFC to boiled egg yolk | Positive | Negative | P value |
|---|---|---|---|
| Total | 26 | 95 | |
| Age, (yr) | 2.3 (1.8–3.5) | 2.5 (1.8–3.9) | 0.260 |
| Sex (male/female) | 21/5 | 60/35 | 0.100 |
| History of anaphylaxis due to egg | 3 | 12 | 1.000 |
| History of atopic dermatitis | 15 | 49 | 0.660 |
| History of bronchial asthma | 15 | 39 | 0.180 |
| Total IgE level at OFC, IU/mL | 209 (10.1–4,853) | 321 (11.5–10,600) | 0.380 |
| Egg yolk sIgE level, UA/mL | 7.63 (0.58–67.9) | 3.87 (0.34–155) | 0.007 |
| Egg white sIgE level, UA/mL | 40.5 (2.09–856) | 14.9 (0.7–487) | 0.004 |
| Ovomucoid sIgE level, UA/mL | 19.9 (0.34–687) | 6.78 (0.34–488) | 0.003 |
Values are presented as median (IQR) or number (range).
IQR, interquartile range; OFC, oral food challenge; sIgE, specific IgE.
Table 2 shows the consumption status of heated EW 3 years after OFC to boiled EY. The proportion of patients who could consume heated whole egg 3 years after first OFC was 15.4% in the OFC-positive group and 75.8% in the OFC-negative group (P < 0.001). Similarly, the proportion of patients who could not consume any egg 3 years after the first OFC was higher in the OFC-positive group than in the OFC-negative group. The prolonged intolerance of boiled whole egg 3 years after first OFC in OFC-positive group was also demonstrated in EW sIgE level-adjusted odds ratio as 19.0 (95% CI, 5.63-64.2). This suggests that the results of OFC to boiled EY might predict the future clinical course of egg allergy.
Table 2. Patients classified according to the consumption status of heated EW 3 years after OFC to boiled EY.
| The consumption status of heated EW 3 years after OFC to boiled EY | OFC to boiled EY | P value | ||
|---|---|---|---|---|
| Positive | Negative | |||
| Complete restriction | 5 (19.2) | 1 (1.1) | 0.002 | |
| Partial restriction | ||||
| < 10 g | 12 (46.2) | 14 (14.7) | 0.002 | |
| > 10 g | 5 (19.2) | 8 (8.4) | 0.150 | |
| Whole egg tolerance | 4 (15.4) | 72 (75.8) | < 0.001 | |
| Total | 26 (100) | 95 (100) | ||
Values are presented as number (%).
EW, egg white; EY, egg yolk; OFC, oral food challenge.
DISCUSSION
Not much attention has been paid to OFC to heated EY, possibly because heated EY contains much less allergen leading to immediate allergic symptoms than heated EW. Only 1 study has shown the safety and feasibility of heated EY challenge, and most patients could consume heated EY safely.8 Okada et al.10 reported that patients with hyperresponsiveness to EY cake slightly contaminated with EW achieved a tolerance rate (66.7%) 3 years after OFC, which is greater than our result of 15.4%. The amount of egg protein in EY cake slightly contaminated with EW was 209 mg,10 while that of boiled one EY was approximately 7 mg.8 Therefore, patients in our study were considered to have higher sensitivity to egg than those in the previous study. This difference probably arises because our recipe of boiled EY aimed to remove EW protein more completely by separating the EW from the EY just after boiling. OFC-positive patients might react to either EY protein or a very slight contamination with EW protein. Our results suggest that patients with hyperresponsiveness to boiled EY might have difficulty in acquiring tolerance to egg and might be recommended to undergo OIT, despite its accompanying risks.
The limitations of this study are, first, that the data were obtained in only 1 central institution specialized for food allergy, and the patients may have been more severely allergic than those in general hospitals or clinics. Secondly, this was retrospective study which might include selection bias and the timing when the first OFC should be performed was not determined previously.
In conclusion, hyperresponsiveness to boiled EY in early life might lead to prolonged egg allergy in children. This finding might aid in the selection of an appropriate population requiring practical OIT.
ACKNOWLEDGMENTS
The authors would like to thank Ms. Miho Shikama for assistance in OFC and dietary instruction to the patients, and Enago (www.enago.jp) for the English language review.
Footnotes
Disclosure: There are no financial or other issues that might lead to conflict of interest.
References
- 1.Rona RJ, Keil T, Summers C, Gislason D, Zuidmeer L, Sodergren E, et al. The prevalence of food allergy: a meta-analysis. J Allergy Clin Immunol. 2007;120:638–646. doi: 10.1016/j.jaci.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 2.Ohtani K, Sato S, Syukuya A, Asaumi T, Ogura K, Koike Y, et al. Natural history of immediate-type hen's egg allergy in Japanese children. Allergol Int. 2016;65:153–157. doi: 10.1016/j.alit.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 3.Ibáñez MD, Escudero C, Sánchez-García S, Rodríguez del Río P. Comprehensive review of current knowledge on egg oral immunotherapy. J Investig Allergol Clin Immunol. 2015;25:316–328. [PubMed] [Google Scholar]
- 4.Feuille E, Nowak-Wegrzyn A. Allergen-specific immunotherapies for food allergy. Allergy Asthma Immunol Res. 2018;10:189–206. doi: 10.4168/aair.2018.10.3.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernhisel-Broadbent J, Dintzis HM, Dintzis RZ, Sampson HA. Allergenicity and antigenicity of chicken egg ovomucoid (Gal d III) compared with ovalbumin (Gal d I) in children with egg allergy and in mice. J Allergy Clin Immunol. 1994;93:1047–1059. doi: 10.1016/s0091-6749(94)70054-0. [DOI] [PubMed] [Google Scholar]
- 6.Caubet JC, Wang J. Current understanding of egg allergy. Pediatr Clin North Am. 2011;58:427–443. doi: 10.1016/j.pcl.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quirce S, Marañón F, Umpiérrez A, de las Heras M, Fernández-Caldas E, Sastre J. Chicken serum albumin (Gal d 5*) is a partially heat-labile inhalant and food allergen implicated in the bird-egg syndrome. Allergy. 2001;56:754–762. doi: 10.1034/j.1398-9995.2001.056008754.x. [DOI] [PubMed] [Google Scholar]
- 8.Yanagida N, Sato S, Asaumi T, Ogura K, Borres MP, Ebisawa M. Safety and feasibility of heated egg yolk challenge for children with egg allergies. Pediatr Allergy Immunol. 2017;28:348–354. doi: 10.1111/pai.12705. [DOI] [PubMed] [Google Scholar]
- 9.Sampson HA. Anaphylaxis and emergency treatment. Pediatrics. 2003;111:1601–1608. [PubMed] [Google Scholar]
- 10.Okada Y, Yanagida N, Sato S, Ebisawa M. Heated egg yolk challenge predicts the natural course of hen's egg allergy: a retrospective study. World Allergy Organ J. 2016;9:31. doi: 10.1186/s40413-016-0121-4. [DOI] [PMC free article] [PubMed] [Google Scholar]