Abstract
Purpose
Neutrophils are considered key effector cells in the pathogenic mechanisms of airway inflammation in asthma. This study assessed the activation status of neutrophils in adult asthmatics, and the therapeutic potential of FTY720, a synthetic sphingosine-1-phosphate analog, on activated neutrophils using an in vitro stimulation model.
Methods
We isolated peripheral blood neutrophils (PBNs) from 59 asthmatic patients (including 20 aspirin-exacerbated respiratory disease [AERD] and 39 aspirin-tolerant asthma [ATA] groups). PBNs were stimulated with N-formyl-methionyl-leucyl-phenylalanine (fMLP) or lipopolysaccharide (LPS) and their activation status was determined based on reactive oxygen species (ROS) production, cell surface expression of CD11b, interleukin (IL)-8 and matrix metallopeptidase (MMP)-9 release. PBNs were primed with FTY720 to evaluate its anti-inflammatory action.
Results
In vitro PBN stimulation with fMLP or LPS induced a significant increase in ROS/CD11b/IL-8/MMP-9 levels (P < 0.05 for all). In asthmatics, fMLP-induced ROS level was significantly correlated with values of forced expiratory volume in 1 second/forced vital capacity (r = −0.278; P = 0.036), maximal mid-expiratory flow (r = −0.309; P = 0.019) and PC20 methacholine (r = −0.302; P = 0.029). In addition, ROS levels were significantly higher in patients with AERD and in those with severe asthma than in those with ATA or non-severe asthma (P < 0.05 for all). FTY720 treatment could suppress ROS/CD11b levels, and LPS-induced IL-8 and MMP-9 levels (P < 0.05 for all). Responders to FTY720 treatment had significantly higher neutrophil counts in sputum (P = 0.004).
Conclusions
Our findings suggest a useful in vitro PBN stimulation model for evaluating the neutrophil functional status and the therapeutic potentials of neutrophil-targeting candidates in asthmatics.
Keywords: Neutrophils, asthma, reactive oxygen species, FTY720
INTRODUCTION
Asthma is a heterogeneous, respiratory disease with various endotypes that combine clinical phenotypes with a distinct pathological mechanism.1 To better understand the pathophysiology of asthma, studies have examined the functions of immune cells such as lymphocytes, mast cells, eosinophils and neutrophils in allergic inflammation, and targeted them for the development of novel therapeutics in asthmatics. Asthmatics with eosinophilic inflammation show favorable responses to corticosteroid therapy, while asthmatics with neutrophilic inflammation show poor responses to corticosteroid therapy.2 A specific treatment targeting for a specific endotype of asthma has been generally recognized as the most effective therapy in asthmatics.1,3
There is increasing evidence of a pathologic role of neutrophil-mediated inflammation and clinical benefits of neutrophil-targeting therapies in asthma.4,5 Neutrophilic asthma, which is defined by sputum neutrophil counts, shows distinct pathological features. Sputum neutrophil counts are associated with persistent airflow limitation and asthma severity in adult asthmatics.6,7 Neutrophil-mediated airway inflammation in severe asthma (SA) persists despite treatment with high-dose glucocorticoids.2 These findings strengthen the rationale for targeting neutrophils as an immunotherapeutic target for severe asthma. A number of' anti-inflammatory therapeutics such as C-X-C Motif Chemokine Receptor 2 (CXCR2) antagonists and anti-interleukin (IL)-17 receptor alpha monoclonal antibodies have been examined in non-eosinophilic asthma8,9; however, they do not appear to have significant therapeutic effects on asthma. Therefore, neutrophils are still of primary importance in the therapeutic strategy for severe asthma. Nevertheless, there is little information on who would benefit the most from neutrophil-targeting therapy. In a recent study, aberrant neutrophil processes such as autophagy and the neutrophil extracellular DNA trap enhanced the inflammatory responses of human airway epithelial cells and eosinophils in severe asthma.10,11 In addition, increased leukotriene B4 generation due to impaired granulocyte function was noted in patients with aspirin-exacerbated respiratory disease (AERD) along with a positive correlation with platelet-adherent neutrophils.12
Here, we evaluated the functional status of neutrophils in asthmatics using an in vitro stimulation model of human peripheral blood neutrophils (PBNs) by measuring reactive oxygen species (ROS) production, CD11b cell surface expression, and the release of IL-8 and granular enzyme matrix metallopeptidase (MMP)-9. Along with changes in lung function and airway hyperresponsiveness, we compared neutrophil functional status according to the phenotype of asthma. Considering the multicellular effect of sphingosine-1-phosphate (S1P) signaling in airway inflammation, we also investigated the potential therapeutic benefit of FTY720, a S1P functional antagonist.13 S1P signaling is associated with airway responsiveness, bronchoconstriction and airway remodeling via the regulation of target cell proliferation in asthma.14,15 Moreover, S1P can further enhance neutrophil activation (i.e., Fcγ receptor-mediated calcium influx16 and N-formyl-methionyl-leucyl-phenylalanine [fMLP]-induced ROS generation).17 Therefore, we hypothesized that FTY720 is a potential target for neutrophilic inflammation in adult asthmatic patients.
MATERIALS AND METHODS
Materials
S1P was purchased from Enzo Life Sciences, Inc. (Farmingdale, NY, USA). FTY720 (2-amino-2-[2-(4-octyl-phenyl)-ethyl]-propane-1,3-diol hydrochloride), fMLP, lipopolysaccharides (LPSs) and ethylenediaminetetraacetic were obtained from Sigma-Aldrich (St. Louis, MO, USA). 2′,7′-dichloroflourescin diacetate (H2DCFDA) was purchased from ThermoFisher Scientific (Waltham, MA, USA).
Study subjects
This study enrolled 59 asthmatic patients (20 AERD, and 39 aspirin-tolerant asthma [ATA] patients) from Ajou University Hospital (Suwon, Korea). Written informed consent was obtained from each subject and the study was approved by the Institutional Review Board of Ajou University Hospital (AJIRB-GEN-SMP-13–108). The clinical history and chest radiographs of all asthmatic patients were evaluated. Subjects underwent spirometry to record forced expiratory volume in 1 second (FEV1)% predicted value, forced vital capacity (FVC)% predicted value, FEV1/FVC and maximal mid-expiratory flow (MMEF)% predicted value. Asthma was diagnosed based on recurrent episodes of wheezing, dyspnea, cough and evidence of airway hyper-responsiveness to methacholine (a decrease in FEV1 [%] of 20% on methacholine challenge test [PC20]).18 All of the subjects had been maintained on ICS and a long-acting β2 agonist to control their asthma symptoms according to the Global Initiative for Asthma guidelines.19 The severity of asthma and control status were defined according to the International Guidelines of the European Respiratory Society/American Thoracic Society.20 Aspirin hypersensitivity was identified using the L-lysine aspirin provocation test (Lys-ASA BPT).21 A diagnosis of AERD was established based on 1) the clinical history of hyper-reactivity against aspirin or other nonsteroidal anti-inflammatory drugs and 2) the positive results to Lys-ASA BPT (a decrease in FEV1 [%] of more than 15% after the challenge). As a control group, asthmatic subjects who were able to tolerate to aspirin were classified as ATA. Subjects with comorbidities or using systemic corticosteroids, other immunosuppressants or biologics were excluded.
Atopy was identified as 1 or more positive reactions on skin prick test results.22 Serum total immunoglobulin E was measured using the ImmunoCAP system (ThermoFisher Scientific), with the range of detection 2-5,000 kU/L. Neutrophil and eosinophil counts in sputum were evaluated as described in a previous study.23
Isolation of PBN from asthmatic patients
Peripheral blood from all study subjects was collected into BD vacutainer® tubes containing acid citrate dextrose solution (BD Biosciences, Franklin Lakes, NJ, USA) and PBN isolation was processed within 2 hours after collection as described in a previous study.10 PBNs were resuspended in RPMI-1640 medium supplemented with 2% heat-inactivated fetal bovine serum, penicillin (100 IU/mL) and streptomycin (50 µg/mL) (all were obtained from Gibco, Grand Island, NY, USA). Cell viability (> 98%) was assessed using trypan blue exclusion assay (Sigma-Aldrich). Cell purity (>95%) was assessed using flow cytometry based on CD11b expression.
In vitro PBN stimulation assay
Freshly isolated PBNs (5 × 105 cells/well) were stimulated with 1 µM fMLP (or 1 µM LPS) for 1 hour at 37°C under 5% CO2. To examine the inhibitory action of FTY720 on PBN activation, PBNs were primed with 1 µM FTY720 for 10 minutes at 37°C under 5% CO2 and then stimulated with fMLP (or LPS) for 1 hour.
Measurement of ROS in PBNs
The ROS-sensitive dye H2DCFDA was added to the PBNs at a final concentration of 10 µM for 30 minutes in the dark at 37°C under 5% CO2. A gate of the single PBNs was set and the DCF fluorescence intensity of PBNs was measured by flow cytometry using a BD FACSCanto II (BD Biosciences, East Rutherford, NJ, USA). To quantify the ROS production in PBNs, the mean fluorescence intensity (MFI) of the DCF signals from PBNs were recorded for statistical analysis.
Evaluation of CD11b expression on PBN
CD11b expression on PBNs was quantified by flow cytometric analysis. After stimulation, PBNs were stained with phycoerythrin-labeled anti-human CD11b (activation epitope) antibody (eBioscience Inc., San Diego, CA, USA) for 20 minutes, at room temperature. Cells were analyzed immediately by BD FACSCanto II (BD Bioscience) and at least 10,000 events were recorded for each condition. The CD11b+ cells were defined by comparing to the isotype control. Given the limited numbers of PBNs, we were only able to perform CD11b labeling on a limited number of subjects.
Measurement of inflammatory mediators
Cell supernatants were collected and stored at −20°C for later enzyme-linked immunosorbent assay (ELISA) analysis. IL-8 and MMP-9 levels were measured using ELISA, according to the manufacturer's instructions (R&D systems, Minneapolis, MN, USA).
Statistical analysis
Statistical analysis was performed using SPSS for Windows version 20.0 (SPSS Inc., Chicago, IL, USA) and GraphPad Prism 6.02 (GraphPad Software, San Diego, CA, USA). All analyses were performed at the 0.05 level, and 95% confidence intervals were two-sided intervals. Student's t test was used to compare the data for continuous variables; Pearson's χ2 or Fisher's exact tests were used for categorical variables. General linear regression analysis was performed to adjust for confounding factors, sex and age to compare ROS and cytokine levels between two groups (AERD vs. ATA, severe asthma vs. non-severe asthma). Paired t test was used to compare paired continuous variables. Pearson's correlation coefficient was used to identify the associations between continuous variables.
RESULTS
Clinical characteristics of the study subjects
The study enrolled 59 asthma patients. Table 1 summarizes their clinical characteristics. The mean age of the asthmatic patients was 49.53 ± 14.36 years and 64.41% were female. Atopy was observed in 68.97% of the patients. Subjects were stratified by disease severity and aspirin hypersensitivity. Patients with severe asthma showed significantly reduced pulmonary functions; lower baseline levels of FEV1% predicted value, FVC % predicted value, and MMEF % predicted value (P < 0.05 for all). The patients with AERD had a higher frequency of rhinosinusitis than those with ATA (77.78% vs. 50%, P = 0.044).
Table 1. Clinical characteristics of the study subjects.
| Clinical characteristics | Asthma (n = 59) | SA (n = 9) | NSA (n = 50) | P value (SA vs. NSA) | AERD (n = 20) | ATA (n = 39) | P value (AERD vs. ATA) | |
|---|---|---|---|---|---|---|---|---|
| Demographics | ||||||||
| Sex (female), No. (%) | 38/59 (64.41) | 3/9 (33.33) | 35/50 (70) | 0.044 | 13/20 (65) | 25/39 (64.1) | 0.590 | |
| Age (yr) | 49.53 ± 14.36 | 56 ± 12.53 | 48.35 ± 14.47 | 0.143 | 53.85 ± 11.92 | 47.26 ± 15.15 | 0.076 | |
| Atopy (presence, %) | 40/58 (68.97) | 5/9 (55.56) | 35/49 (71.43) | 0.282 | 10/19 (52.63) | 30/39 (76.92) | 0.059 | |
| Total IgE | 342.27 ± 352.41 | 413.78 ± 546.84 | 328.28 ± 307.65 | 0.660 | 297.11 ± 326.95 | 364.24 ± 366.46 | 0.512 | |
| Pulmonary function | ||||||||
| FEV1 (% predicted) | 91.02 ± 13.89 | 78.38 ± 21.52 | 93.04 ± 11.31 | 0.005 | 89.33 ± 17.58 | 91.91 ± 11.67 | 0.506 | |
| FVC (% predicted) | 92.57 ± 11.97 | 84.64 ± 18.58 | 93.84 ± 10.26 | 0.042 | 92.81 ± 14.3 | 92.45 ± 10.75 | 0.914 | |
| FEV1/FVC | 82.45 ± 8.59 | 77.94 ± 12.64 | 83.17 ± 7.69 | 0.110 | 79.56 ± 9.3 | 83.98 ± 7.9 | 0.062 | |
| MMEF (% predicted) | 69.51 ± 23.5 | 52.03 ± 20.69 | 72.31 ± 22.87 | 0.022 | 66.6 ± 25.59 | 71.04 ± 22.52 | 0.498 | |
| PC20 (mg/mL) | 5.22 ± 7.28 | 3.72 ± 6.88 | 5.38 ± 7.38 | 0.633 | 3.74 ± 6.22 | 5.94 ± 7.73 | 0.312 | |
| Sputum cell differential count (%) | ||||||||
| Eosinophils | 32.78 ± 32.42 | 41 ± 38.01 | 31.75 ± 32.2 | 0.598 | 27.77 ± 34.11 | 35.61 ± 31.85 | 0.494 | |
| Neutrophils | 58.69 ± 32.81 | 57 ± 38.38 | 58.9 ± 32.75 | 0.915 | 65.83 ± 33.52 | 54.96 ± 32.55 | 0.360 | |
| Relevant comorbidities, No. (%) | ||||||||
| Rhinosinusitis | 33/56 (58.93) | 7/9 (77.78) | 26/47 (55.32) | 0.190 | 14/18 (77.78) | 19/38 (50) | 0.044 | |
| Nasal polyps | 15/20 (75) | 1/1 (100) | 14/19 (73.68) | 0.750 | 9/12 (75) | 6/8 (75) | 0.704 | |
Asthma phenotypes and severity were defined as in the Materials and Methods sections. The continuous data are presented as the means ± standard deviation. The dichotomous data are presented as numbers (%). The data were analyzed with Student's t test and Pearson's χ2 test. The values in bold indicate significant P value.
SA, severe asthma; NSA, non-severe asthma; AERD, aspirin-exacerbated respiratory disease; ATA, aspirin-tolerant asthma; IgE, immunoglobulin E; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; MMEF, maximal mid-expiratory flow; PC20, a decrease in FEV1 (%) of 20% on methacholine challenge test.
Functional evaluation of neutrophil status using an in vitro model
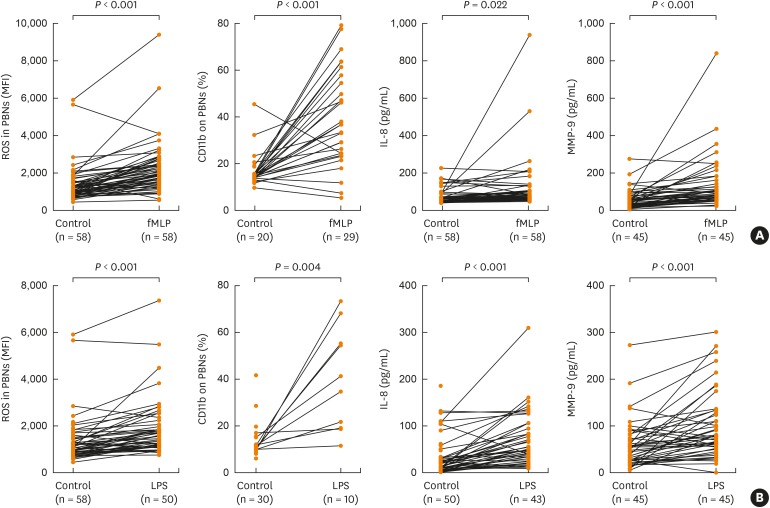
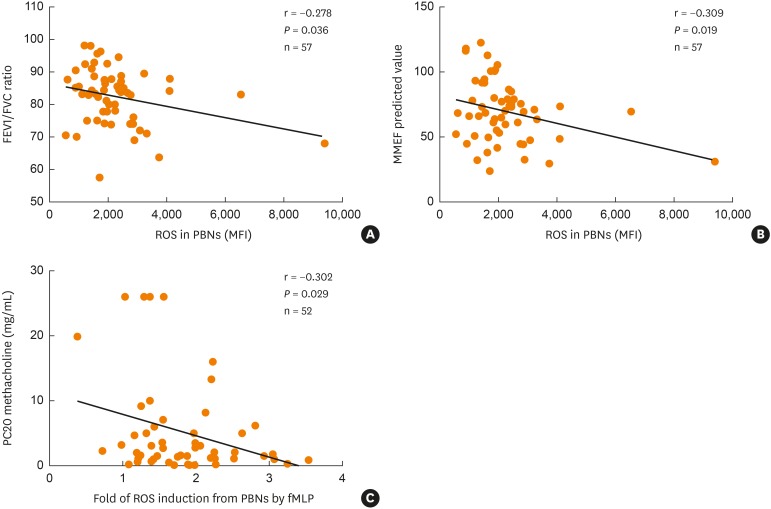
To examine the functional status of neutrophils in asthmatics, we isolated human PBNs, stimulated them with fMLP or LPS for 1 hour, and then measured biomarkers representing neutrophil functions (i.e., ROS production and CD11b cell surface expression on PBNs, IL-8 [a pro-inflammatory cytokine] and MMP-9 [a granular enzyme] release). fMLP and LPS stimulation on PBNs significantly increased ROS production and CD11b, IL-8 and MMP-9 levels (P < 0.05 for all, Fig. 1). Furthermore, the fold induction of ROS production after fMLP stimulation as compared to baseline was significantly associated with decreased MMEF % (r = −0.309; P = 0.019), decreased FEV1/FVC ratio (r = −0.278; P = 0.036), and increased bronchial hyper-reactivity to methacholine (r = −0.302; P = 0.029) (Fig. 2).
Fig. 1. In vitro PBN activation under fMLP or LPS stimulation. PBNs were isolated from asthmatics, stimulated under (A) fMLP stimulation or (B) LPS stimulation in 1 hour. The MFI of DCF fluorescence of PBNs and CD11b expression percentage were measured by flow cytometry; IL-8 and MMP-9 in the supernatants were measured by enzyme-linked immunosorbent assay. Data are presented as means ± standard deviation. P values were analyzed by paired t-test.
fMLP, N-formyl-methionyl-leucyl-phenylalanine; LPS, lipopolysaccharide; PBN, peripheral blood neutrophil; IL, interleukin; MMP, matrix metallopeptidase; ROS, reactive oxygen species.
Fig. 2. Correlations of PBN-derived ROS production with the severity of airway obstruction and bronchial hyper-reactivity. PBNs from asthmatics were stimulated with fMLP and measured for the DFC fluorescence intensity. Correlations of ROS and (A) FEV1/FVC; (B) MMEF (%) predicted values; (C) PC20. P values were analyzed by Pearson correlation coefficient analysis.
FEV1, forced expiratory volume in 1 second; FEV1/FVC ratio, a ratio of forced expiratory volume in 1 second to forced volume vital; MMEF, maximal mid-expiratory flow; PC20 methacholine, methacholine provocative concentration causing a 20% drop in FEV1; ROS, reactive oxygen species; MFI, mean fluorescence intensity.
Neutrophil functional status according to the phenotype of asthma
Next, we stratified the patients according to the phenotype and then compared the functional status of neutrophils. ROS production with simulation was further increased in asthmatics as the symptoms got worse (Table 2). fMLP-induced ROS production in the severe asthma group was significantly higher than that in the non-severe asthma group (P = 0.011). AERD patients showed significantly higher ROS production stimulated by both fMLP and LPS than ATA patients (P < 0.01 for all). Subjects with uncontrolled asthma had significantly increased ROS production under LPS stimulation compared to subjects with partly controlled or controlled asthma (P = 0.027, Supplementary Table S1).
Table 2. PBN-derived ROS production under stimulation according to the phenotype of asthma.
| Stimulation | SA (n = 9) | NSA (n = 49) | P value* (SA vs. NSA) | Asthma (n = 58) | AERD (n = 20) | ATA (n = 38) | P value* (AERD vs. ATA) | |
|---|---|---|---|---|---|---|---|---|
| fMLP stimulation | ||||||||
| Con | 1,642.33 ± 1,673.77 | 1,292.9 ± 808.57 | 0.151 | 1,347.12 ± 979.81 | 2,031.85 ± 1,399.63 | 986.74 ± 293.6 | < 0.001 | |
| fMLP | 3,235.44 ± 2,868.46 | 2,059.61 ± 806.32 | 0.011 | 2,242.07 ± 1,373.59 | 3,073.9 ± 1,935.96 | 1,804.26 ± 641.18 | 0.001 | |
| fMLP + FTY | 2,491 ± 1,947.11 | 1,699.79 ± 681.63 | 0.030 | 1,824.72 ± 1,008.1 | 2,433.11 ± 1,430.3 | 1,520.53 ± 507.75 | 0.002 | |
| LPS stimulation | ||||||||
| Con | 1,642.33 ± 1,673.77 | 1,292.9 ± 808.57 | 0.151 | 1,347.12 ± 979.81 | 2,031.85 ± 1,399.63 | 986.74 ± 293.6 | < 0.001 | |
| LPS | 2,541.38 ± 2,262.17 | 1,758.19 ± 897.58 | 0.100 | 1,883.5 ± 1,220.37 | 2,800.07 ± 1,696.19 | 1,527.06 ± 741.39 | 0.002 | |
| LPS + FTY | 1,944.5 ± 1,969.66 | 1,498.95 ± 639.46 | 0.189 | 1,570.24 ± 961.04 | 2,329.07 ± 1,482.62 | 1,275.14 ± 395.82 | 0.001 | |
Data are presented as means ± standard deviation of the mean fluorescence intensity values.
SA, severe asthma; NSA, non-severe asthma; AERD, aspirin-exacerbated respiratory disease; ATA, aspirin-tolerant asthma; Con, mock-treated cells; fMLP, N-formyl-methionyl-leucyl-phenylalanine, LPS, lipopolysaccharide; FTY, FTY720.
*P value was calculated by general linear regression analysis. The values in bold indicate significant P values.
Evaluation of the therapeutic potential of FTY720 using an in vitro model
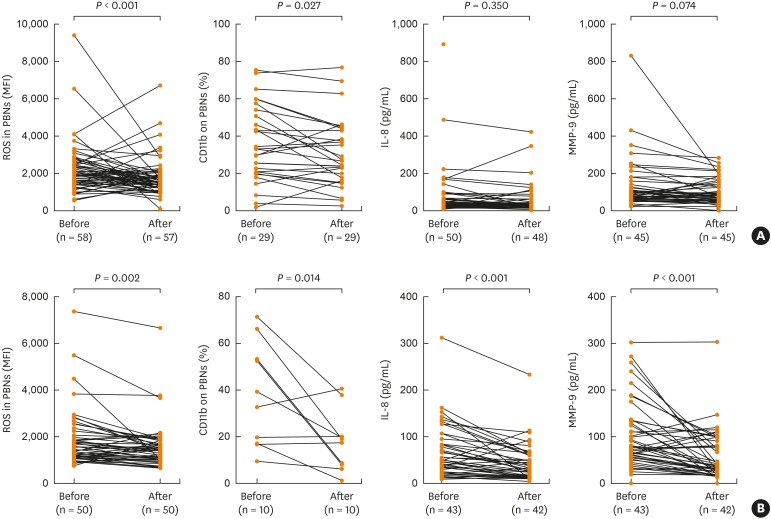
To examine the clinical relevance of this in vitro PBN stimulation model for the evaluation of the therapeutic potential of FTY720, we compared the effects of FTY720 on the fMLP- (and LPS) stimulated neutrophil functions. ROS production and CD11b surface expression induced by fMLP or LPS stimulation were significantly decreased in the presence of FTY720 (P < 0.05 for all, Fig. 3). LPS-induced IL-8 and MMP-9 levels were also significantly decreased in the presence of FTY720 (P < 0.05, Fig. 3), while the inhibitory effect of FTY720 on fMLP-mediated IL-8 and MMP-9 release did not reach a statistical significance (Fig. 3). To compare the clinical features of responders and non-responders to the FTY720 treatment, asthmatic patients were stratified by the reduction ratio of ROS production in the presence of FTY720. Responders were defined as patients who showed more than 10% ROS reduction after the FTY720 treatment. As shown in Table 3, the sputum neutrophil counts were significantly higher in responders than in non-responders (P = 0.004). There were no significant differences in % of FTY720 responders according to the phenotype of asthma such as aspirin hypersensitivity, the disease severity and the control status (P = 0.502; P = 1.000; P = 0.791, Table 3). However, degree of increment of ROS production under fMLP stimulation was significantly decreased with FTY720 treatment (Supplementary Table S2).
Fig. 3. Inhibitory action of FTY720 on neutrophil activation in asthmatics. PBNs were isolated from asthmatics, treated with FTY720 and stimulated under (A) fMLP stimulation or (B) LPS stimulation in 1 hour. P values were analyzed by paired t test.
fMLP, N-formyl-methionyl-leucyl-phenylalanine; LPS, lipopolysaccharide; PBN, peripheral blood neutrophil; IL, interleukin; MMP, matrix metallopeptidase; ROS, reactive oxygen species.
Table 3. Clinical characteristics of responders and non-responders to the FTY720 treatment.
| Clinical characteristics | Responders (n = 40) | Non-responders (n = 17) | P value | ||
|---|---|---|---|---|---|
| Demographics | |||||
| Sex (female), No. (%) | 30/40 (75) | 8/17 (47.1) | 0.043 | ||
| Age (yr) | 48.7 ± 14.7 | 48.8 ± 14.6 | 0.979 | ||
| Atopy (presence, %) | 26/40 (65) | 12/14 (85.7) | 0.454 | ||
| Total IgE | 337.3 ± 354.7 | 331.9 ± 362.1 | 0.960 | ||
| Pulmonary function | |||||
| Baseline FEV1 (% predicted) | 91.5 ± 13.3 | 89.8 ± 16.2 | 0.678 | ||
| Baseline FVC (% predicted) | 93.2 ± 11.4 | 91.1 ± 14 | 0.557 | ||
| Baseline FEV1/FVC | 82.3 ± 7.6 | 83 ± 11.1 | 0.796 | ||
| PC20 (mg/mL, methacholine) | 5.2 ± 7.2 | 5.5 ± 7.9 | 0.881 | ||
| Sputum cell differential count (%) | |||||
| Eosinophils | 28.4 ± 32.4 | 47.1 ± 29.7 | 0.124 | ||
| Neutrophils | 67.7 ± 29.7 | 33.4 ± 26.9 | 0.004 | ||
| Asthma phenotype | |||||
| Aspirin hypersensitivity | 0.502 | ||||
| AERD | 13/37 (35.1) | 6/17 (35.3) | |||
| ATA | 27/37 (73.0) | 11/17 (64.7) | |||
| Severity | 1.000 | ||||
| SA | 6/40 (15.0) | 3/17 (17.6) | |||
| NSA | 34/40 (85.0) | 14/17 (82.4) | |||
| Control status by GINA guideline, No. (%) | 0.791 | ||||
| Controlled | 20/40 (50.0) | 8/17 (47.1) | |||
| Partly controlled | 15/40 (37.5) | 8/17 (47.1) | |||
| Uncontrolled | 5/40 (12.5) | 1/17 (5.9) | |||
Responders were defined as patients who show ≥10% reactive oxygen species reduction in the presence of FTY720 under N-formyl-methionyl-leucyl-phenylalanine stimulation. Asthma control status and inflammatory subtype of asthma were defined as described in Materials and Methods section. The data are presented as means ± standard deviation for continuous variables and percentage for categorical variables. The data were analyzed with Student's t-test and Pearson's χ2 test.
FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; PC20, a decrease in FEV1 (%) of 20% on methacholine challenge test; AERD, aspirin-exacerbated respiratory disease; ATA, aspirin-tolerant asthma; SA, severe asthma; NSA, non-severe asthma.
DISCUSSION
Although the clinical relevance of neutrophilic inflammation to the phenotype of severe asthma is increasing, the functional status of neutrophils in asthmatic airway remains insufficiently understood. In the present study, we assessed the functional status of neutrophils using an in vitro PBN stimulation model along with clinical parameters of adult asthmatics and evaluated the therapeutic potential of FTY720 using an in vitro model.
Sputum neutrophil count allows for the evaluation of neutrophil numbers in the airway lumen and is associated with poor asthma control. 6,24 However, it's difficult to evaluate pre-activated or primed neutrophils in peripheral blood homing to the lungs and their contribution to exacerbating the airway inflammation. Consequently, we hypothesized that biomarkers of primed neutrophils in an in vitro model using PBNs of asthmatics would serve as an early read-out for assessing neutrophilic airway inflammation. Stimuli such as the neutrophil chemotactic factor fMLP and a bacterial endotoxin, LPS, can effectively induce ROS production in human PBNs.25,26 ROS is a key signaling molecule that stimulates pro-inflammatory cytokine production and promotes allergic airway inflammation through airway smooth muscle contraction and bronchial hyperresponsiveness.27,28,29 In the present study, ROS production under fMLP stimulation increased in adult asthmatics in association with the disease severity, particularly in patients with severe asthma, although the number of severe asthma patients is not enough to draw conclusion. This could be explained by significant correlations between fMLP-induced ROS production with decreased lung function, and increased bronchial hyper-reactivity to methacholine. These results are consistent with those of previous studies that found a negative correlation between lung function parameters and sputum neutrophil counts in asthmatics.6,24 These findings demonstrate that measurement of PBN-derived ROS production may be a useful in vitro model for assessing the functional status of neutrophils in severe asthma.
AERD has been considered the severe form of adult-onset eosinophilic asthma, which is comorbid with chronic rhinosinusitis and nasal polyps.30 Due to increased baseline levels of prostaglandin D2 and cysteinyl leukotrienes,31 both eosinophils and mast cells are targeted as the major effector cells in the pathogenesis of AERD. However, little is known about the effector function of neutrophils in the airway inflammation in AERD patients. Increased neutrophil chemotactic activity in serum has been noted during early and late asthmatic response after Lys-ASA BPT in AERD patients.32 The production of leukotriene B4, a chemoattractant to neutrophils, is also increased in AERD patients, similar to leukotriene E4,33 which can be explained by impaired granulocyte functions leading to leukotriene B4 production.12 In addition, we compared sputum inflammatory cell profiles among the 4 subtypes of AERD and found high neutrophil counts as well as eosinophils in sputum samples of subtypes 1 and 2 (having more severe clinical outcomes), suggesting that neutrophils are involved in the severity of airway inflammation in AERD.34 In the present study, when we compared the functional status of PBNs between the AERD and ATA groups in an in vitro, more activated neutrophils were noted in the AERD group than in the ATA group (i.e., the increased fold induction of ROS production after fMLP stimulation as compared to baseline). However, we could not find direct correlations between the ROS and cytokine production under stimulation with the % fall of FEV1 after Lys-ASA BPT. Taken together, these results suggest that the in vitro PBN stimulation model described herein may be useful for evaluating the functional status of neutrophils in AERD patients.
The functional status of neutrophils can be further examined by measurement of inflammatory cytokines and cytolytic granular enzymes released from neutrophils. IL-8 is a key cytokine in neutrophil recruitment, survival and inflammation,35 and plays an important pathogenic role in airway inflammation of asthma; IL-8 levels are increased in both bronchoalveolar lavage fluid (BALF) and induced sputum of asthmatics.36 Increased levels of granular enzymes, such as MMP-9 and myeloperoxidase (MPO), have also been found in the BALF and sputum of asthmatics in correlation with asthma severity.37,38 The functional relevance of MPO seems to be similar to that of MMP-9; the levels were increased in both the BALF and sputum of asthmatics and negatively correlated with lung functions.39 Neutrophil elastase (NE) may contribute to the development of asthma by inducing epithelial damage and enhancing bronchial hyper-reactivity.40 Elevated NE levels have also been observed during asthma exacerbation and negatively correlated with FEV1 in asthmatics.41 In the present study, we measured the pro-inflammatory cytokine (IL-8) and 3 cytolytic granular enzymes (MMP-9, MPO and NE) using the in vitro PBN stimulation model. Asthmatic patients showed an increased release of IL-8 and MMP-9 under stimulation. However, we failed to measure MPO and NE because the baseline level of MPO was too high to compare, while the baseline level of NE was less than the detection limit, therefore, they were excluded in comparisons. The expression of CD11b, a neutrophil activation marker, was also increased under stimulation, although the number of study subjects was limited. These findings suggest that measurement of IL-8 and MMP-9 released from PBNs are practical parameters to evaluate neutrophil functions using in vitro stimulation models.
The functional status of neutrophils could also be used for evaluating therapeutic potential of neutrophil-targeting candidates. In this study, FTY720 was targeted due to its antagonizing effect on S1P signaling, which may represent a novel therapeutic candidate for the management of asthma.13,14,15 S1P signaling can directly effect on neutrophil functions via S1P receptor type 1 (S1PR1)-mediated ROS generation under fMLP stimulation.17 Previously, we reported an altered sphingolipid metabolic pathway in AERD (i.e., increased serum level of S1P during Lys-ASA BPT in AERD patients).42 Therefore, we hypothesized that the inhibitory action of FTY720 on S1P signaling could be a biological target for controlling neutrophilic inflammation to attenuate neutrophil activation in asthmatics. In the present study, when we examined the therapeutic potential of FTY720 using the in vitro PBN stimulation model, the activated status of neutrophils was decreased significantly by FTY720 in the aspects of ROS production, CD11b expression, and IL-8/MMP-9 release. These findings suggest that FTY720 may modulate neutrophilic airway inflammation by reducing ROS production and inflammatory cytokine/cytolytic granular enzyme release. In addition, when we compared the clinical features between responders and non-responders to FTY 720 treatment, a favorable response was suggested in patients with higher sputum neutrophil counts. These inhibitory actions of FTY720 on ROS production seem more prominent in patients with AERD. Degree of increment of ROS production under fMLP stimulation was significantly decreased with FTY720 treatment. Taken together, these results suggest that modulation of ROS production is the major anti-inflammatory mechanism of FTY720 on activated neutrophils in asthmatic airway. Further interventions are essential to evaluate whether FTY720 may serve as a therapeutic option for patients with severe asthma or AERD.
The present study has limitations. First, we used neutrophil-enriched granulocytes with cell purity > 95% for examination of the functional status of neutrophils; however, this mixture may contain eosinophils (especially in atopic asthmatics). Mixed populations of eosinophils and neutrophils may have different in-vitro characteristics than pure neutrophils. Secondly, we could not detect NE, within 1 hour of stimulation, a biomarker to represent the effector functions of neutrophil enhancing epithelial damage and bronchial hyper-reactivity. Despite the limitation, ROS measurement after 1 hour stimulation would be appropriate for clinical utility. Thirdly, the detail signaling mechanism of FTY720 on the neutrophil function was not fully examined. Further studies are required to better understand the therapeutic effects of FTY720 on neutrophilic inflammation in asthmatics.
In conclusion, this study demonstrated that the activated status of neutrophils in adult asthmatics, particularly in severe asthma and AERD. The in vitro PBN stimulation model described herein may be useful for assessing the functional status of neutrophils, predicting the severity of airway inflammation, and screening potential therapeutics for asthmatic patients.
ACKNOWLEDGMENTS
This research was supported by the National Research Foundation of Korea (NRF) grants, funded by the Korean government (Ministry of Science, ICT and Future Planning [MSIP]) (grant numbers: 2015R1C1A2A01053492, 2018R1A2B6004905)
Footnotes
Disclosure: There are no financial or other issues that might lead to conflict of interest.
SUPPLEMENTARY MATERIALS
Peripheral blood neutrophil-derived reactive oxygen species production under stimulation according to asthma control status
The degree of increment of ROS production (ROS in a stimulated neutrophil status − ROS in a baseline neutrophil status) according to AERD
References
- 1.Chung KF, Adcock IM. How variability in clinical phenotypes should guide research into disease mechanisms in asthma. Ann Am Thorac Soc. 2013;10(Suppl):S109–S117. doi: 10.1513/AnnalsATS.201304-087AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wenzel SE, Szefler SJ, Leung DY, Sloan SI, Rex MD, Martin RJ. Bronchoscopic evaluation of severe asthma. Persistent inflammation associated with high dose glucocorticoids. Am J Respir Crit Care Med. 1997;156:737–743. doi: 10.1164/ajrccm.156.3.9610046. [DOI] [PubMed] [Google Scholar]
- 3.Fajt ML, Wenzel SE. Development of new therapies for severe asthma. Allergy Asthma Immunol Res. 2017;9:3–14. doi: 10.4168/aair.2017.9.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bruijnzeel PL, Uddin M, Koenderman L. Targeting neutrophilic inflammation in severe neutrophilic asthma: can we target the disease-relevant neutrophil phenotype? J Leukoc Biol. 2015;98:549–556. doi: 10.1189/jlb.3VMR1214-600RR. [DOI] [PubMed] [Google Scholar]
- 5.Pelaia G, Vatrella A, Busceti MT, Gallelli L, Calabrese C, Terracciano R, et al. Cellular mechanisms underlying eosinophilic and neutrophilic airway inflammation in asthma. Mediators Inflamm. 2015;2015:879783. doi: 10.1155/2015/879783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shaw DE, Berry MA, Hargadon B, McKenna S, Shelley MJ, Green RH, et al. Association between neutrophilic airway inflammation and airflow limitation in adults with asthma. Chest. 2007;132:1871–1875. doi: 10.1378/chest.07-1047. [DOI] [PubMed] [Google Scholar]
- 7.Uddin M, Nong G, Ward J, Seumois G, Prince LR, Wilson SJ, et al. Prosurvival activity for airway neutrophils in severe asthma. Thorax. 2010;65:684–689. doi: 10.1136/thx.2009.120741. [DOI] [PubMed] [Google Scholar]
- 8.Nair P, Gaga M, Zervas E, Alagha K, Hargreave FE, O'Byrne PM, et al. Safety and efficacy of a CXCR2 antagonist in patients with severe asthma and sputum neutrophils: a randomized, placebo-controlled clinical trial. Clin Exp Allergy. 2012;42:1097–1103. doi: 10.1111/j.1365-2222.2012.04014.x. [DOI] [PubMed] [Google Scholar]
- 9.Busse WW, Holgate S, Kerwin E, Chon Y, Feng J, Lin J, et al. Randomized, double-blind, placebo-controlled study of brodalumab, a human anti-IL-17 receptor monoclonal antibody, in moderate to severe asthma. Am J Respir Crit Care Med. 2013;188:1294–1302. doi: 10.1164/rccm.201212-2318OC. [DOI] [PubMed] [Google Scholar]
- 10.Pham DL, Ban GY, Kim SH, Shin YS, Ye YM, Chwae YJ, et al. Neutrophil autophagy and extracellular DNA traps contribute to airway inflammation in severe asthma. Clin Exp Allergy. 2017;47:57–70. doi: 10.1111/cea.12859. [DOI] [PubMed] [Google Scholar]
- 11.Mukherjee M, Nair P. Autoimmune responses in severe asthma. Allergy Asthma Immunol Res. 2018;10:428–447. doi: 10.4168/aair.2018.10.5.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laidlaw TM, Cutler AJ, Kidder MS, Liu T, Cardet JC, Chhay H, et al. Prostaglandin E2 resistance in granulocytes from patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2014;133:1692–1701.e3. doi: 10.1016/j.jaci.2013.12.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ryan JJ, Spiegel S. The role of sphingosine-1-phosphate and its receptors in asthma. Drug News Perspect. 2008;21:89–96. doi: 10.1358/dnp.2008.21.2.1188195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roviezzo F, Sorrentino R, Bertolino A, De Gruttola L, Terlizzi M, Pinto A, et al. S1P-induced airway smooth muscle hyperresponsiveness and lung inflammation in vivo: molecular and cellular mechanisms. Br J Pharmacol. 2015;172:1882–1893. doi: 10.1111/bph.13033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fuerst E, Foster HR, Ward JP, Corrigan CJ, Cousins DJ, Woszczek G. Sphingosine-1-phosphate induces pro-remodelling response in airway smooth muscle cells. Allergy. 2014;69:1531–1539. doi: 10.1111/all.12489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Florey O, Haskard DO. Sphingosine 1-phosphate enhances Fc γ receptor-mediated neutrophil activation and recruitment under flow conditions. J Immunol. 2009;183:2330–2336. doi: 10.4049/jimmunol.0901019. [DOI] [PubMed] [Google Scholar]
- 17.Wang Z, Fan H, Xie R, Yang J, Ren Y, Yang Y, et al. The effect of sphingosine 1-phosphate/sphingosine 1-phosphate receptor on neutrophil function and the relevant signaling pathway. Acta Haematol. 2015;134:49–56. doi: 10.1159/000369291. [DOI] [PubMed] [Google Scholar]
- 18.Hwang EK, Jin HJ, Nam YH, Shin YS, Ye YM, Nahm DH, et al. The predictors of poorly controlled asthma in elderly. Allergy Asthma Immunol Res. 2012;4:270–276. doi: 10.4168/aair.2012.4.5.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Global Initiative for Asthma. Global strategy for asthma management and prevention [Internet] Fontana (WI): Global Initiative for Asthma; 2017. [cited 2017 Dec 10]. Available from: http://ginasthma.org/ [Google Scholar]
- 20.Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43:343–373. doi: 10.1183/09031936.00202013. [DOI] [PubMed] [Google Scholar]
- 21.Park HS. Early and late onset asthmatic responses following lysine-aspirin inhalation in aspirin-sensitive asthmatic patients. Clin Exp Allergy. 1995;25:38–40. doi: 10.1111/j.1365-2222.1995.tb01000.x. [DOI] [PubMed] [Google Scholar]
- 22.Pham D, Kim MA, Yoon MG, Lee SI, Shin YS, Park HS. Serum specific IgG response to toluene diisocyanate-tissue transglutaminase conjugate in toluene diisocyanate-induced occupational asthmatics. Ann Allergy Asthma Immunol. 2014;113:48–54. doi: 10.1016/j.anai.2014.04.022. [DOI] [PubMed] [Google Scholar]
- 23.Pham DL, Kim SH, Losol P, Yang EM, Shin YS, Ye YM, et al. Association of autophagy related gene polymorphisms with neutrophilic airway inflammation in adult asthma. Korean J Intern Med. 2016;31:375–385. doi: 10.3904/kjim.2014.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Green RH, Brightling CE, Woltmann G, Parker D, Wardlaw AJ, Pavord ID. Analysis of induced sputum in adults with asthma: identification of subgroup with isolated sputum neutrophilia and poor response to inhaled corticosteroids. Thorax. 2002;57:875–879. doi: 10.1136/thorax.57.10.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Woloszynek JC, Hu Y, Pham CT. Cathepsin G-regulated release of formyl peptide receptor agonists modulate neutrophil effector functions. J Biol Chem. 2012;287:34101–34109. doi: 10.1074/jbc.M112.394452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gomes NE, Brunialti MK, Mendes ME, Freudenberg M, Galanos C, Salomão R. Lipopolysaccharide-induced expression of cell surface receptors and cell activation of neutrophils and monocytes in whole human blood. Braz J Med Biol Res. 2010;43:853–858. doi: 10.1590/s0100-879x2010007500078. [DOI] [PubMed] [Google Scholar]
- 27.Fialkow L, Wang Y, Downey GP. Reactive oxygen and nitrogen species as signaling molecules regulating neutrophil function. Free Radic Biol Med. 2007;42:153–164. doi: 10.1016/j.freeradbiomed.2006.09.030. [DOI] [PubMed] [Google Scholar]
- 28.Lavinskiene S, Jeroch J, Malakaskas K, Bajoriuniene I, Jackute J, Sakalauskas R. Peripheral blood neutrophil activity during Dermatophagoides pteronyssinus-induced late-phase airway inflammation in patients with allergic rhinitis and asthma. Inflammation. 2012;35:1600–1609. doi: 10.1007/s10753-012-9475-0. [DOI] [PubMed] [Google Scholar]
- 29.Hosoki K, Itazawa T, Boldogh I, Sur S. Neutrophil recruitment by allergens contribute to allergic sensitization and allergic inflammation. Curr Opin Allergy Clin Immunol. 2016;16:45–50. doi: 10.1097/ACI.0000000000000231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mascia K, Haselkorn T, Deniz YM, Miller DP, Bleecker ER, Borish L. Aspirin sensitivity and severity of asthma: evidence for irreversible airway obstruction in patients with severe or difficult-to-treat asthma. J Allergy Clin Immunol. 2005;116:970–975. doi: 10.1016/j.jaci.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 31.Higashi N, Taniguchi M, Mita H, Yamaguchi H, Ono E, Akiyama K. Aspirin-intolerant asthma (AIA) assessment using the urinary biomarkers, leukotriene E4 (LTE4) and prostaglandin D2 (PGD2) metabolites. Allergol Int. 2012;61:393–403. doi: 10.2332/allergolint.11-RA-0403. [DOI] [PubMed] [Google Scholar]
- 32.Kim SS, Park HS, Yoon HJ, Lee YM, Lee SK, Nahm DH. Enhanced serum neutrophil chemotactic activity was noted in both early and late asthmatic responses during lysine-aspirin bronchoprovocation test in ASA-sensitive asthmatic patients. J Korean Med Sci. 2003;18:42–47. doi: 10.3346/jkms.2003.18.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mita H, Higashi N, Taniguchi M, Higashi A, Akiyama K. Increase in urinary leukotriene B4 glucuronide concentration in patients with aspirin-intolerant asthma after intravenous aspirin challenge. Clin Exp Allergy. 2004;34:1262–1269. doi: 10.1111/j.1365-2222.2004.02034.x. [DOI] [PubMed] [Google Scholar]
- 34.Lee HY, Ye YM, Kim SH, Ban GY, Kim SC, Kim JH, et al. Identification of phenotypic clusters of nonsteroidal anti-inflammatory drugs exacerbated respiratory disease. Allergy. 2017;72:616–626. doi: 10.1111/all.13075. [DOI] [PubMed] [Google Scholar]
- 35.Silvestri M, Bontempelli M, Giacomelli M, Malerba M, Rossi GA, Di Stefano A, et al. High serum levels of tumour necrosis factor-α and interleukin-8 in severe asthma: markers of systemic inflammation? Clin Exp Allergy. 2006;36:1373–1381. doi: 10.1111/j.1365-2222.2006.02502.x. [DOI] [PubMed] [Google Scholar]
- 36.Gosset P, Tillie-Leblond I, Malaquin F, Durieu J, Wallaert B, Tonnel AB. Interleukin-8 secretion in patients with allergic rhinitis after an allergen challenge: interleukin-8 is not the main chemotactic factor present in nasal lavages. Clin Exp Allergy. 1997;27:379–388. [PubMed] [Google Scholar]
- 37.Cundall M, Sun Y, Miranda C, Trudeau JB, Barnes S, Wenzel SE. Neutrophil-derived matrix metalloproteinase-9 is increased in severe asthma and poorly inhibited by glucocorticoids. J Allergy Clin Immunol. 2003;112:1064–1071. doi: 10.1016/j.jaci.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 38.Hoshino M, Nakamura Y, Sim J, Shimojo J, Isogai S. Bronchial subepithelial fibrosis and expression of matrix metalloproteinase-9 in asthmatic airway inflammation. J Allergy Clin Immunol. 1998;102:783–788. doi: 10.1016/s0091-6749(98)70018-1. [DOI] [PubMed] [Google Scholar]
- 39.Monteseirín J, Bonilla I, Camacho J, Conde J, Sobrino F. Elevated secretion of myeloperoxidase by neutrophils from asthmatic patients: the effect of immunotherapy. J Allergy Clin Immunol. 2001;107:623–626. doi: 10.1067/mai.2001.113566. [DOI] [PubMed] [Google Scholar]
- 40.Amitani R, Wilson R, Rutman A, Read R, Ward C, Burnett D, et al. Effects of human neutrophil elastase and Pseudomonas aeruginosa proteinases on human respiratory epithelium. Am J Respir Cell Mol Biol. 1991;4:26–32. doi: 10.1165/ajrcmb/4.1.26. [DOI] [PubMed] [Google Scholar]
- 41.Monteseirín J, Bonilla I, Camacho MJ, Chacón P, Vega A, Chaparro A, et al. Specific allergens enhance elastase release in stimulated neutrophils from asthmatic patients. Int Arch Allergy Immunol. 2003;131:174–181. doi: 10.1159/000071483. [DOI] [PubMed] [Google Scholar]
- 42.Trinh HK, Kim SC, Cho K, Kim SJ, Ban GY, Yoo HJ, et al. Exploration of the sphingolipid metabolite, sphingosine-1-phosphate and sphingosine, as novel biomarkers for aspirin-exacerbated respiratory disease. Sci Rep. 2016;6:36599. doi: 10.1038/srep36599. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Peripheral blood neutrophil-derived reactive oxygen species production under stimulation according to asthma control status
The degree of increment of ROS production (ROS in a stimulated neutrophil status − ROS in a baseline neutrophil status) according to AERD