Abstract
Outcomes of nonmyeloablative (NMA) haploidentical (haplo) blood or marrow transplant (BMT) with post transplantation cyclophosphamide (PTCy) using non-first-degree relatives are unknown. We evaluated 33 consecutive adult patients (median age, 56 years) with hematologic malignancies who underwent NMA haplo T cell-replete BMT with PTCy at Johns Hopkins using second- or third-degree related donors. Donors consisted of 10 nieces (30%), 9 nephews (27%), 7 first cousins (21%), 5 grandchildren (15%), and 2 uncles (6%). Thirty-one patients (94%) reached full donor chimerism by day 60. The estimated cumulative incidence (Cul) of grades II to IV acute graft-versus-host disease (aGVHD) at day 180 was 24% (90% confidence interval [CI], 9% to 38%). Only 1 patient experienced grades III to IV aGVHD. At 1 year the CuI of chronic GVHD was 10% (90% CI, 0% to 21%). The CuI of nonrelapse mortality at 1 year was 5% (90% CI, 0% to 14%). At 1 year the probability of relapse was 31% (90% CI, 12% to 49%), progression-free survival 64% (90% CI, 48% to 86%), and overall survival 95% (90% CI, 87% to 100%). The 1-year probability of GVHD-free, relapse-free survival was 57% (90% CI, 41% to 79%). NMA haplo BMT with PTCy from non-first-degree relatives is an acceptably safe and effective alternative donor platform, with results similar to those seen with first-degree relatives.
Keywords: Haploidentical, Bone marrow transplant, Post-transplant, cyclophosphamide, Second-degree relatives
INTRODUCTION
Post-transplant cyclophosphamide (PTCy) has emerged as an effective intervention for graft-versus-host disease (GVHD) prophylaxis after allogeneic blood or marrow transplantation (BMT) [1].When given at a dose of50 mg/kg/dayon days 3 and 4 after BMT from first-degree related HLA haploidentical (haplo) donors, PTCy results in rates of GVHD, nonrelapse mortality (NRM), and overall survival (OS) similar to those seen with matched donor transplants [1–6].
Despite the success ofhaplo-BMT with PTCy and other alternative donor platforms [4,7–9], some patients still lack a suitable donor. For many of these patients a second- or third- degree relative may be an HLA haplo match. No studies have yet evaluated the safety profile of BMT with PTCy from haplo non-first-degree related donors.
METHODS
A prospective clinical trial of non-first-degree relative haplo BMT with PTCywas conducted at the Johns Hopkins Hospital (NCT01203722). This protocol also included a previously published cohort of mismatched unrelated donortransplants [10]. Ofthe 33 consecutive patients undergoing BMT from haplo non-first-degree related donors with PTCy, 15 were on the clinical trial. An additional 18 were treated off-protocol because of a lack of insurance benefits for clinical trial coverage. Both the prospective clinical trial and the retrospective analysis of additional patients, treated exactly the same, were approved by the Johns Hopkins Institutional Review Board.
Eligible patients were ages 18 to 75 years, lacked at least a, HLA haplo first-degree related donor or a matched unrelated donor, and had adequate organ function as previously published [10]. All donors were second-or third-degree relatives who shared 1 inherited haplotype with the patient. Desensitization for donor-specific antibodies was permitted and occurred in 2 patients using previously published methods [11].
All patients received T cell-replete bone marrow grafts between September 2012 and September 2017. Conditioning included fludarabine/ cyclophosphamide/total body irradiation [10]. GVHD prophylaxis consisted of high-dose PTCy (50 mg/kg i.v. daily on days +3 and +4) with mesna [1], mycophenolate mofetil from days +5 to +35, and either sirolimus (24 patients) ortacrolimus (9 patients) from days +5 to +180[10]. Filgrastim began on day +5.
Neutrophil recovery was defined as the first of 3 consecutive days with an absolute neutrophil count ≥ .5 × 109/L. Platelet recovery was defined as a platelet count ≥ 20 × 109/L without platelet transfusion in the preceding 7 days. Progression-free survival (PFS), OS, and GVHD-free, relapse-free survival (GRFS) [12] were estimated by the Kaplan-Meier method. Median follow-up was calculated by the reverse Kaplan-Meier method. Estimated cumulative incidences (CuI) of relapse, NRM, count recovery, and GVHD were estimated with competing risk methods [10,13,14]. PFS failures and graft failure were considered competing risks for GVHD. NRM was the competing risk for relapse. Graft failure was defined as ≤5% donor chimerism in peripheral blood or bone marrow after day 60 in the absence of bone marrow relapse. Donor chimerism was evaluated at approximately days 30, 60, 180, and 365. The database was locked on October 10, 2017.
RESULTS
Baseline patient characteristics are shown in Table 1. Donors consisted of10 nieces (30%), 9 nephews (27%), 7 first cousins (21%), 5 grandchildren (15%), and 2 uncles (6%). Two patients (6%) had received prior BMTs. Patients treated on-protocol were more likely to receive sirolimus for GVHD prophylaxis than those off-protocol. Otherwise, there were no significant differences between the 2 groups. The estimated median follow-up was 7.9 months.
Table 1.
Patient and Transplant Characteristics
| Baseline Characteristics | Off-Protocol | On-Protocol | Combined |
|---|---|---|---|
| (n = 18) | (n = 15) | (n = 33) | |
| Median patient age, yr (range) | 56(33–74) | 57(32–70) | 56(32–74) |
| Median donor age, yr (range) | 31(16–58) | 28(17–69) | 31(16–69) |
| BMI, kg/m2 | 26.8 | 25.7 | 26.4 |
| Gender | |||
| Female | 8(44) | 7(47) | 15(45) |
| Male | 10(56) | 8(53) | 18(55) |
| Donor–recipient gender | |||
| Same | 9(50) | 3(20) | 12(36) |
| Female to male | 5(28) | 6(40) | 11(33) |
| Male to female | 4(22) | 6(40) | 10(30) |
| Diagnosis | |||
| AML | 11(61) | 6(40) | 17(52) |
| ALL | 3(17) | 5(33) | 8(24) |
| Aggressive NHL | 3(17) | 3(20) | 6(18) |
| Hodgkin lymphoma | 1(6) | 0(0) | 1(3) |
| MDS | 0(0) | 1(7) | 1(3) |
| CR at transplant | |||
| No | 3(17) | 2(13) | 5(15) |
| Yes | 15(83) | 13(87) | 28(85) |
| Patient CMV status | |||
| Positive | 8(44) | 6(40) | 14(42) |
| Negative | 10(56) | 9(60) | 19(58) |
| Donor–patient CMV mismatch | |||
| No | 8(44) | 11(73) | 19(58) |
| Yes | 10(56) | 5(27) | 14(42) |
| ABO incompatibility | |||
| Major | 2(11) | 4(27) | 6(18) |
| Minor | 6(33) | 4(27) | 10(30) |
| Both | 1(6) | 1(7) | 2(6) |
| Refined disease risk index | |||
| Low | 1(6) | 1(7) | 2(6) |
| Intermediate | 11(65) | 13(87) | 24(75) |
| High/very high | 5(29) | 1(7) | 6(19) |
| HLA match | |||
| 5/10 | 14(78) | 9(60) | 23(70) |
| 6/10 | 2(11) | 4(27) | 6(18) |
| 7/10 | 2(11) | 1(7) | 3(9) |
| 8/10 | 0 | 1(7) | 1(3) |
| 9–10/10 | 0 | 0 | 0 |
| GVHD prophylaxis | |||
| Tacrolimus | 9(50) | 0(0) | 9(27) |
| Sirolimus | 9(50) | 15(100) | 24(73) |
| Median graft dose infused (25th-75th percentile) | |||
| TNCs, ×108/kg | 6.79(5.09–8.06) | 4.98(4.36–5.35) | 5.37(4.59–7.16) |
| CD34, ×106/kg | 9.4(4.3–10.0) | 5.2(4.2–5.7) | 5.5(4.2–9.9) |
| CD3, ×107/kg | 23.8(5.0–34.8) | 4.4(3.6–5.7) | 5.7(4.1–25.3) |
Values are n (%; representing column-wise percentages) unless otherwise defined. BMI indicates body mass index; AML, acute myeloid leukemia; ALL, acute lymphoid leukemia; NHL, non-Hodgkin lymphoma; MDS, myelodysplastic syndrome; CR, complete remission; CMV, cytomegalovirus; TNCs, total nucleated cells.
Thirty-two patients (97%) engrafted, and 2 patients (6%) developed secondary graft failure. Of the 3 patients with graft failure, all recovered with autologous hematopoiesis and remained in complete remission at a mean follow-up of 11.6 ± 1.9 months (range, 8 to 14.6). By day 60, 31 patients (94%) had reached full donor chimerism. All patients achieved neutrophil recovery by day 30 with a median time to recovery of 17 days (Figure 1A). The median time to platelet recovery was 25 days, with 1 patient (3%) failing to recover platelet production (Figure 1A).
Figure 1.
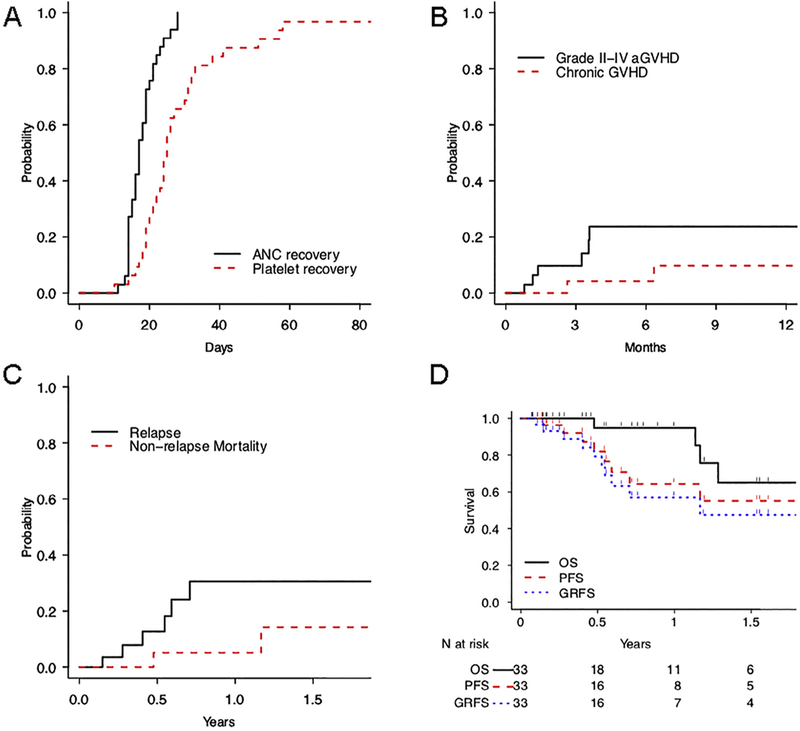
Outcomes after non–first-degree related haplo BMT. (A) Rate of neutrophil and platelet recovery. (B) CuI of grades II to IV aGVHD and chronic GVHD. (C) Probabilities of NRM and relapse. (D) Kaplan-Meier estimates for OS, PFS, and GRFS
The Cul of grades II to IV acute GVHD (aGVHD) at day 100 was 14% (90% confidence interval [Cl], 3% to 25%) and at day 180 was 24% (90% Cl, 9% to 38%) (Figure 1B).This was the same (24%) in patients whose donors were matched at 5/10 HLA antigens as those whose donors matched at >5/10 antigens. Of 5 patients with grade ll aGVHD, 3 had skin and ungradable visceral involvement, whereas 2 had skin involvement only. One patient (3%) experienced grades III to IV aGVHD involving the gut. By 1 year the CuI of chronic GVHD was 10% (90% CI, 0% to 21%) (Figure 1B). In total, 2 patients have developed any chronic GVHD, including 1 with extensive chronic GVHD affecting the skin and mouth. The CuI of immunosuppression use for treatment of any GVHD by 1 year was 19% (90% CI, 6% to 32%).
The CuI of NRM was 5% at 1 year (90% CI, 0% to 14%) (Figure 1C). Of 2 patients experiencing NRM, 1 died of sepsis at day 174 and 1 developed sarcoma of the hip 9 months after BMT and succumbed to this disease on day 426. By day 100, 18 patients (55%) required unplanned admission for toxicity, including neutropenic fever, infection, heart failure, and encephalopathy. Two patients experienced sirolimus intolerance, 1 with pneumonitis and 1 with headaches. The CuI of relapse was 31% (90% CI, 12% to 49%) (Figure 1C) at 1 year, with 6 patients (4 acute myeloid leukemia, 1 acute lymphoid leukemia, and 1 Hodgkin lymphoma) experiencing relapse thus far.
Survival outcomes are shown in Figure 1D. The 1-year probability of GRFS was 57% (90% CI, 41% to 79%). Median GRFS was 1.2 years. At 1 year PFS was 64% (90% CI, 48% to 86%). Median PFS was not reached. The median OS was 2.0 years. Five patients have died (3 from relapse), with an OS at 1 year of 95% (90% CI, 87% to 100%).
DISCUSSION
Prior literature shows the safety and efficacy of BMT with PTCy after first-degree related haplo, MUD, and even mismatched unrelated donor transplant BMT [14–18]. However, some patients lack an eligible first-degree related haplo donor. In this study BMT from non-first-degree related haplo donors with PTCy resulted in rates of GVHD and NRM that were comparable with first-degree related donor BMT. These results extend the observation of safety and efficacy to related haplo BMT donors that are not first-degree relatives.
Given the success of the PTCy platform at mitigating GVHD, our institutional standard is now to use nonmyeloablative BMT with PTCy using at least a haplo donor chosen on the basis of age, ABO compatibility, and cytomegalovirus status rather than the best HLA match. Including second-and third-degree relatives increases the likelihood of optimizing these donor selection criteria and may improve post-BMT outcomes. Similar to prior data with NMA BMT with PTCy, rates of relapse are the primary driver of mortality [19,20]. Strategies to reduce relapse, such as shortening the duration of immunosuppression, optimizing killer cell immunoglobulinlike receptor types, and adding post-BMT maintenance are under investigation.
The patients on the clinical trial received sirolimus in combination with PTCy and mycophenolate mofetil, whereas some treated off-study received tacrolimus. Our original PTCy regimen included tacrolimus for GVHD prophylaxis [1]. We substituted sirolimus in our regimen because preclinical data suggest synergism with PTCy and because it has been associated with a lower incidence of posterior reversible encephalopathy syndrome in our prior studies in sickle cell patients [21,22]. Additionally, renal transplant data have confirmed lower rates of nephrotoxicity with sirolimus [23]. Still, sirolimus has disadvantages, such as it can only be given orally and can cause pulmonary toxicity [24–26]. Here, sirolimus was well tolerated, with only 2 patients experiencing significant side effects, both with recovery after discontinuing the drug. Although tacrolimus and sirolimus have never been directly compared as part of PTCy GVHD prophylaxis, they generally appear to be comparable for all BMT outcomes in our experience. Thus, we consider sirolimus and tacrolimus equivalent options that can be interchanged based on toxicity considerations.
These data indicate that haplo BMT with PTCy from non- first-degree related donors is equivalent to haplo BMT with first-degree related donors. It is appropriate to consider aunts, uncles, cousins, nieces, nephews, and grandchildren in the search for a related donor when using the PTCy regimen, thus extending donor availability to nearly every patient in need of BMT.
ACKNOWLEDGMENTS
The authors thank Janice Davis Sproul and the Johns Hopkins Cell Therapy Laboratory for the graft data, Maria Bettinotti for HLA analysis, Amanda Stevens for regulatory assistance, and Judy Baker for data management.
Financial disclosure: This work was supported by National Institutes of Health grant P01 CA15396 (to R.J.J.).
Footnotes
Conflict ofintereststatement: There are no conflicts of interest to report.
REFERENCES
- 1.Luznik L, O’Donnell P, Symons H, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14(6):641–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kanakry C, Ganguly S, Zahurak M, et al. Aldehyde dehydrogenase expression drives human regulatory T cell resistance to posttransplantation cyclophosphamide. Sci Transl Med. 2013;5:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DiStasi A, Milton DR, Poon L, et al. Similar transplantation outcomes for acute myeloid leukemia and myelodysplastic syndrome patients with haploidentical versus 10/10 human leukocyte antigen-matched unrelated and related donors. Biol Blood Marrow Transplant. 2014;20:1975–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ciurea S, Zhang M, Bacigalupo A, et al. Haploidentical transplant with post-transplant cyclophosphamide versus matched unrelated donor transplant foracute myeloid leukemia. Blood. 2015;126:1033–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brunstein C, Fuchs E, Carter S, et al. Alternative donor transplantation after reduced intensity conditioning: results of parallel phase 2 trials using partially HLA-mismatched related bone marrow or unrelated double umbilical cord blood grafts. Blood. 2011;118:282–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCurdy S, Kasamon Y, Kanakry C, et al. Comparable composite endpoints after HLA-matched and HLA-haploidentical transplantation with post-transplantation cyclophosphamide. Haematologica. 2017;102:391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grager L, Eapen M, Williams E, et al. HLA Match likelihoods for hematopoietic stem-cell grafts in the U.S. registry. N Engl J Med. 2014;371:339–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saber W, Opie S, Rizzo J, Zhang M, Horowitz M, Schriber J. Outcomes after matched unrelated donor versus identical sibling hematopoietic cell transplantation in adults with acute myelogenous leukemia. Blood. 2012;119:3908–3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Milano F, Gooley T, Wood B, et al. Cord-blood transplantation in patients with minimal residual disease. N Engl J Med. 2016;375:944–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kasamon Y, Ambinder R, Fuchs E, et al. Prospective study of nonmyeloablative, HLA-mismatched unrelated BMT with high-dose posttransplantation cyclophosphamide. Blood Adv. 2017;1:288–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gladstone D, Zachary A, Fuchs E, et al. Partially mismatched transplantation and human leukocyte antigen donor-specific antibodies. Biol Blood Marrow Transplant. 2013;19:647–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holtan S, DeFor T, Lazaryan A, et al. Composite end point of graft-versus- host disease-free, relapse-free survival after allogeneic hematopoietic cell transplantation. Blood. 2015;125:1333–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fine J, Gray R. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 14.Kasamon Y, Bolanos-Meade J, Prince G, et al. Outcomes of nonmyeloablative HLA-haploidentical blood or marrow transplantation with high-dose post-transplantation cyclophosphamide in older adults. J Clin Oncol. 2015;33:3152–3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kanakry C, Fuchs E, Luznik L. Modern approaches to HLA-haploidentical blood or marrow transplantation. Nat Rev Clin Oncol. 2016;13:10–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanakry C,Tsai H, Bolanos-Meade J, et al. Single-agent GVHD prophylaxis with posttransplantation cyclophosphamide after myeloablative, HLA-matched BMT for AML, ALL, and MDS. Blood. 2014;124:3817–3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCurdy S, Kanakry J, Showel M, et al. Risk-stratified outcomes of nonmyeloablative HLA-haploidentical BMT with high-dose posttransplantation cyclophosphamide. Blood. 2015;125:3024–3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elmariah H, Pratz K. Role of alternative donorallogeneic transplants in the therapy of acute myeloid leukemia. J Natl Cancer Center Netw. 2017;15:959–966. [DOI] [PubMed] [Google Scholar]
- 19.Aoudjhane M, Labopin M, Gorin N, et al. Comparative outcome of reduced intensity and myeloablative conditioning regimen in HLA identical sibling allogeneic haematopoietic stem cell transplantation for patients older than 50 years of age with acute myeloblastic leukaemia: a retrospective survey from the Acute Leukemia Working Party (ALWP) of the European Group for Blood and Marrow Transplantation (EBMT). Leuloemia. 2005;19:2304–2312. [DOI] [PubMed] [Google Scholar]
- 20.Scott BL, Pasquini MC, Logan BR, et al. Myeloablative versus reduced- intensity hematopoietic cell transplantation for acute myeloid leukemia and myelodysplastic syndromes. JClin Oncol. 2017;35:1154–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bolanos-Meade J, Fuchs E, Luznik L, et al. HLA-haploidentical bone marrow transplantation with posttransplant cyclophosphamide expands the donor pool for patients with sickle cell disease. Blood. 2012;120:4285–4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fitzhugh C, Weitzel R, Hsieh M, et al. Sirolimus and post-transplant Cy synergistically maintain mixed chimerism in a mismatched murine model. Bone Marrow Transplant. 2013;48:1335–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Watson C, Williams F, Bradley J, et al. A randomized controlled trial of late conversion from CNI-based to sirolimus-based immunosuppression following renal transplantation. Am J Transplant. 2005;5:2496–2503. [DOI] [PubMed] [Google Scholar]
- 24.Morelon E, Stern M, Israël-Biet D, et al. Characteristics of sirolimus- associated interstitial pneumonitis in renal transplant patients. Transplantation. 2001;72:787–790. [DOI] [PubMed] [Google Scholar]
- 25.Garrean S, Massad M, Tshibaka M, Hanhan Z, Caines A, Benedetti E. Sirolimus-associated interstitial pneumonitis in solid organ transplant recipients. Clin Transplant. 2005;19:698–703. [DOI] [PubMed] [Google Scholar]
- 26.Patel A, Hahn T, Bogner P, et al. Fatal diffuse alveolar hemorrhage associated with sirolimus after allogeneic hematopoietic cell transplantation. Bone Marrow Transplant 2010;45:1363–1364. [DOI] [PubMed] [Google Scholar]


