Abstract
Tumor lymphangiogenesis has been previously documented to predict regional lymph node metastasis and promote the spread to distant organs. However, the underlying mechanism initiating tumor lymphangiogenesis remains unclear. Here we described a novel role of tumor cell-derived Lysyl Oxidase-like protein 2 (LOXL2) in promoting lymphangiogenesis and lymph node metastasis in breast cancer. Immunohistochemistry (IHC) analysis of samples from breast cancer patients showed that the expression of LOXL2 was positively correlated with lymphatic vessel density and breast cancer malignancy. In animal studies, LOXL2-overexpressing breast cancer cells significantly increased lymphangiogenesis and lymph node metastasis, whereas knockdown of LOXL2 suppressed both processes. In order to study the mechanisms of lymphangiogenesis progression, we performed further in vitro investigations and the data revealed that LOXL2 significantly enhanced lymphatic endothelial cells (LECs) invasion and tube formation through directly activation of the Akt-Snail and Erk pathways. Moreover, LOXL2 also stimulated fibroblasts to secrete high level of pro- lymphangiogenic factors VEGF-C and SDF-1α. Taken together, our study elucidates a novel function of tumor cell secreted LOXL2 in lymphangiogenesis and lymph node metastasis, demonstrating that LOXL2 serves as a promising target for anti-lymphangiogenesis and anti-metastasis therapies for breast cancer.
Abbreviations: α-SMA, α-Smooth muscle actin; CAF, Cancer associated fibroblast; CM, Conditioned medium; Erk, Extracellular regulated protein kinase; ECM, Extracellular matrix; HIF-1α, Hypoxia inducible factor-1α; IF, Immunofluorescent; IgG, Immunoglobulin G; IHC, Immunohistochemistry; LEC, Lymphatic endothelial cell; LOXL2, Lysyl oxidase-like protein 2; LYVE-1, Lymphatic vessel endothelial hyaluronan receptor 1; shRNA, Short hairpin RNA
Introduction
Metastasis accounts for more than 90% of cancer patients' mortality [1]. Although there are multiple mechanisms governing tumor metastasis, the main pathways for tumor cells gaining accesses to circulation are through the blood and lymphatic vessels [2]. Unlike blood vessels, lymphatic vessels are constructed by a single layer of endothelial cells, lacking basement membrane, pericytes or smooth muscle cells, which makes them easier for tumor cells intravasation [3]. Evidenced by numerous preclinical researches and clinicopathological studies, most epithelial cancers, especially breast cancers, preferentially use lymphatic vessels as the initial route for metastasis to adjacent lymph nodes [4]. Recent studies have demonstrated that tumor lymphangiogenesis is correlated with the incidence of primary tumor metastasis to the sentinel lymph node, and thereby facilitates metastasis to distant organs [5]. Therefore, inhibition of tumor lymphangiogenesis has been considered as a promising therapy for blocking tumor metastasis [6].
Lymphangiogenesis, the generation of new vessels from preexisting lymphatic vessels, involves the proliferation, migration, and sprouting of lymphatic endothelial cells (LECs) [7]. The best-known pro-lymphangiogenic factors are vascular endothelial growth factor-C and -D (VEGF-C and VEGF-D), which can bind to vascular endothelial growth factor receptor 3 (VEGFR3) on the LECs surface, and significantly enhance lymphangiogenic activities of LECs [8], [9]. Overexpression of VEGF-C in transgenic mice is accompanied by specific hyperplasia of the lymphatic network [10]. In addition, other factors have also been found to facilitate lymphangiogenesis, such as VEGF-A [11] and stromal cell derived factor-1 (SDF-1α) [12]. Nevertheless, the molecular mechanisms of tumor lymphangiogenesis remain largely elusive and merit further investigations.
Lysyl oxidase-like protein 2 (LOXL2) belongs to the lysyl oxidase (LOX) family, which is composed of five members (LOX and four related enzymes, LOXL1–4) [13]. All the members in this family can be secreted to the extracellular milieu, where they catalyze the covalent cross-linking of collagen and elastin, and contribute to the extracellular matrix synthesis and stabilization [14]. Many studies showed that LOXL2 expression is upregulated in invasive cancer cells in vitro and associated with poor overall survival in breast cancer, gastric cancer, skin cancer, and colon carcinoma [15], [16], [17], [18], [19]. LOXL2 promotes tumor invasion and metastasis through multiple ways, including epithelial-mesenchymal transitions [19], [20], [21], regulating cellular polarity [22], and establishing premetastatic niches by inducing the deposition of collagen and accelerating recruitment of bone marrow derived cells [23]. Neufeld and his colleagues reported that overexpression of LOXL2 in MCF-7 breast cancer cells induces a shift from non-invasive to invasive phenotype, accompanied by extensive deposition of collagen fibers in tumors [15]. Barkan and colleagues demonstrated that LOXL2 endows dormant tumor cells with a stem-like phenotype and mediates their transition to proliferative state [24].
Since both LOXL2 and lymphangiogenesis are crucial players in the dissemination of cancer cells and associated with a poor prognosis, we are prompted to investigate whether LOXL2 could contribute to the sophisticated coordination of lymphangiogenesis. In this study, we demonstrated the roles of LOXL2 as a novel pro- lymphangiogenic regulator in breast cancer and revealed that the expression of LOXL2 was positively correlated with lymphatic vessel density and lymph node metastasis. Our work provides new insights into the development of novel drugs targeting LOXL2.
Methods
Breast Cancer Tissue Microarray
Breast cancer tissue microarray (BR1006a) was purchased from Alenabio (Xi'an, China), which contains 50 clinical patient specimens including cancer adjacent normal breast tissues, benign breast tumor tissues, malignant breast cancer tissues.
Breast cancer tissue microarray (BR2161) contains 216 clinical female specimens including normal breast tissues, cancer adjacent normal breast tissues and malignant tissues with different staging. This microarray was purchased from Alenabio (Xi'an, China).
Briefly, tissue sections were immunostained with anti-human LYVE-1 and LOXL2 antibodies. The levels of LYVE-1 and LOXL2 on each specimen were scored as 0, 1, 2, 3 (0 = negative, 1 = low, 2 = moderate and 3 = high) according to their staining intensities.
Cell Culture, Lentivirus Infection
Primary mouse lymphatic endothelial cells (mLECs) were isolated and cultured as previously described [12], [25]. Human dermal lymphatic endothelial cells (hLECs) purchased from ScienCell Research Laboratories were cultured according to the manufacturer's instructions. MDA-MB-231 breast cancer cell lines were obtained from the American Type Culture Collection (Manassas, VA, USA), and maintained in RPMI1640 media supplemented with 10% fetal bovine serum (Gibco BRL, Grand Island, NY, USA). MCF-7 breast cancer cell lines, MRC-5 human fibroblast cell lines and 3 T3 mouse fibroblast cell lines were purchased from the Cell Resource Centre, China Infrastructure of Cell Line Resources, and were cultured according to their guidelines. All cells were maintained in a 37°C humidified incubator containing 5% CO2. MCF-7 cells were infected with recombinant lentiviruses carrying human LOXL2 cDNA (LV-LOXL2), or their negative controls LV-Vector (GenePharma, Shanghai, China) and MDA-MD-231 cells were infected with small hairpin RNA targeting LOXL2 (MDA231-shLOXL2), their negative controls were shControl (GenePharma, Shanghai, China). MCF7-LOXL2, MCF7-LV, MDA231-shLOXL2 and MDA231-LV cell lines were tested and authenticated by Western blotting and quantitative real-time PCR (qRT-PCR) for stably expressing exogenous LOXL2 or silencing endogenous LOXL2.
Reagents and Antibodies
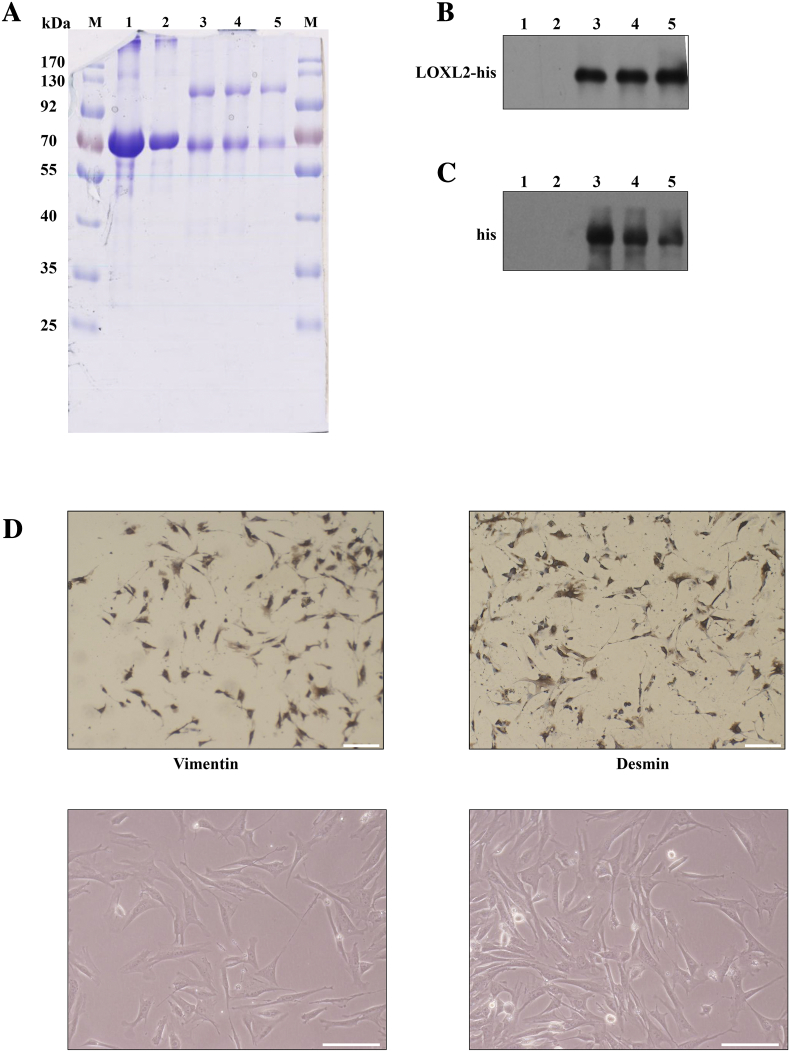
VEGF-C (ab97415) was purchased from Abcam (Cambridge, MA, USA), Matrigel (354230) and Rat tail collagen I (354236) was purchased from BD Biosciences (San Jose, CA). Inhibitor LY294002 (9901S), U0126 (9903S) were purchased from CST (Beverley, MA, United States). PP1 (S7060) and SAR131675 were from Selleck Chemicals (Houston, TX, USA). Ni-NTA Agarose was purchased from QIAGEN (Hilden, Germany). LOXL2 was expressed and purified from MCF7-LOXL2-His cells conditioned medium as previously described [26], [27]. Details of identification of LOXL2 protein was provided in Figure S1A-C.
Figure S1.
The identification of purified LOXL2 protein and fibroblasts. (A) SDS-PAGE showing the purity of the proteins used in this study. The marker is denoted by M, and molecular weights are labeled next to corresponding band. Lane 1 is BSA, lane 2 is BSA, lane 3 is LOXL2-1, lane 4 is LOXL2-2, and lane 5 is LOXL2-3 (the number represents the batch of the purified LOXL2 protein). (B) Western blot of proteins shown in A probed with anti-LOXL2 antibody. Lane 1 is BSA, lane 2 is BSA, lane 3 is LOXL2-1, lane 4 is LOXL2-2, and lane 5 is LOXL2-3. (C) Western blot of the proteins shown in A probed with anti-histidine tag antibody. Lane 1 is BSA, lane 2 is BSA, lane 3 is LOXL2-1, lane 4 is LOXL2-2, and lane 5 is LOXL2-3. (D) All cultivated fibroblasts were positive for expression of vimentin and desmin (top). The morphology of fibroblasts isolated from tumors (bottom). Scale bar = 200 μm.
Antibodies against LOXL2 (sc-48,724) and Hsp90 (sc-27,987) were from Santa Cruz Biotechnology (Santa Cruz, CA, United States). Antibodies against Erk1/2 (4695), p-Erk1/2 (4377), Akt(9272), p-Akt (4060), Src (2123), p-Src (6943), VEGFR3 (3408), α-SMA (19245) and HIF-1α (14179) were from Cell Signaling Technology. LYVE-1 (ab14917), Podoplanin (ab109059), LOXL2 (ab96233), Snail (ab53519), His-tag (ab5000) and β-actin (ab8227) antibodies were from Abcam (Cambridge, UK). FITC-linked anti-mouse (ZF-0312), FITC-linked anti-rabbit (ZDR-5209), TRITC-linked anti-mouse (ZF-0313) and IgG (ZDR-5006) antibodies were from Beijing ZSGB-BIO (Beijing, China).
Small Interfering RNA (siRNA) Transfection
siRNAs targeting HIF-1α, Snail and negative control scramble siRNAs were purchased from GenePharma and the sequence was described in Supplementary material, Table S1. According to the manufacturer's procedure, LECs transfected with siRNAs were performed with Lipofectamine 3000 (L3000001, Invitrogen, Carlsbad, CA, USA). Knockdown efficiencies were identified by qPCR and immunoblotting 24 h after transfection.
Protein and RNA analysis
Western blotting was conducted to analyze the protein levels. Cell samples were harvested, denatured and separated by SDS-PAGE gel and transferred to a PVDF membrane. After blocking in milk-based buffer, the membrane was incubated with indicated primary antibodies at 4°C overnight, washed three times with TBST, and subsequently incubated with corresponding horseradish peroxidase (HRP)-conjugated secondary antibodies (Abcam) for 1 h at room temperature. Finally, proteins on the membrane were detected with eluminous reaction (Santa Cruz Western blotting luminol reagents) following the manufacturer's protocol.
Total RNA was extracted with TRIzol reagent (Invitrogen), and cDNA was synthesized using the First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, Waltham, MA US). Quantitative PCR (qPCR) was adopted to assess the mRNA levels with TransStart Green qPCR SuperMix (TransGen Biotech, Beijing, China). The reaction was run on the Mx3000P system (Stratagene, La Jolla, CA, United States). Results were normalized to β-actin with the 2–ΔΔCt method and relative to control samples.
ELISA Assay
Levels of SDF-1α and VEGF-C were assessed respectively with commercially available ELISA kits (Dakewe Bio-engineering Co., LTD, China) according to the manufacturer's instructions.
Cell Invasion Assay
Cell invasion capacities in vitro were assessed with Matrigel (BD) coated transwell inserts. Briefly, 5 × 104 LECs were seeded in the upper chamber of 8 μm Millicell coated with Matrigel. Proteins including LOXL2, VEGF-C, LOXL2 antibody or IgG were added to the medium containing concentrated breast cancer cells conditioned medium (CM) or 1% FBS in the lower chamber to induce cell invasion for the appropriate time. Migrated cells were counted randomly in 8 independent fields at 40 × magnification under an Olympus IX71 optical microscope. The relative invasion activity was measured by normalizing the mean of migrated cells/per field in the testing groups to that in the control groups.
Tube Formation Assay
The tubule formation assay was performed as previously described [28]. Briefly, the pre-thawed Matrigel formed a solid structure in the bottom of a 48-well plate. LECs pretreated with or without CM or LOXL2 were seeded to the plate. After incubation for 4 h, the plate was observed for the tubular structure. Five independent fields per well were captured under an Olympus IX71 optical microscope. Quantification was conducted with the Image-Pro Plus 6.0 software (Media Cybernetics). The relative total tube length of the LECs was defined by normalizing the mean of the total tube length/per field of the testing groups to that of the control groups.
Matrigel Plug Assay
All animal studies were approved by the Institutional Animal Care and Use Committee of Tsinghua University (F16–00228; A5061–01). Lymphatic vessel formation in vivo was evaluated by the Matrigel plug assay conducted as previously described [12]. In brief, Matrigel containing PBS, CM, LOXL2 (50 nM), VEGF-C (500 ng/ml), or antibodies (100 μg/ml) was subcutaneously inoculated into BALB/c mice (6 mice per group). After 8 days, plugs were dissected and applied to immunofluorescent analysis. Evaluation of the lymphatic vessel density was assessed in 6 independent fields imaged by the Nikon A1 laser scanning confocal microscope using Nikon image software (NIS-Elements AR 3.0).
Immunofluorescence and Immunohistochemistry Assay
For immunofluorescence staining, frozen sections of Matrigel plugs, xenograft tumors and lymph nodes were fixed with cold acetone. Then these samples were blocked with 10% goat serum and stained with primary antibodies overnight at 4°C followed by the appropriate secondary fluorescently labeled antibodies. Nuclei were counterstained with DAPI (Thermo Fisher Scientific). Images were photographed and analyzed with the Nikon A1 Confocal Microscope.
Immunohistochemistry assay was conducted as described previously [29]. To detect the expression levels of LYVE-1 and LOXL2 on formalin-fixed, paraffin-embedded human breast tissue specimens were stained with primary antibodies (1:100) overnight at 4°C. After washed with PBS, these sections were incubated with HRP-conjugated secondary antibody. DAB substrate (ST033, Beyotime, China) was used to perform the chromogenic reaction. The levels of LYVE-1 and LOXL2 on each specimen were scored as 0, 1, 2 and 3 according to the staining intensities.
Orthotopic Breast Cancer Model
All animal studies, conducted as previously described [12], [30], [31]. In brief, Female 4- to 6-week-old nude mice were injected with 106 MCF7-LOXL2 and MCF7-LV cells (1:1 in Matrigel; BD Biosciences) into their left lower abdominal mammary fat pads. The mice were divided randomly into 4 groups (6 mice per group). MCF7-LV control group was injected with PBS via the tail vein twice weekly for 3 weeks. MCF7-LOXL2 groups were injected with PBS, LOXL2 antibody (0.5 mg/kg) or IgG via the tail vein twice weekly for 3 weeks. 5 × 106 MDA231-shLOXL2 and MDA231-LV tumor cells (1:1 in Matrigel; BD Biosciences) were inoculated into the left lower abdominal mammary fat pad of nude mice (female, 4–6 weeks). The mice were divided randomly into 4 groups (6 mice per group). MDA231-LV control group was injected with PBS via the tail vein twice weekly for 3 weeks. MDA231-shLOXL2 groups were injected with PBS, LOXL2 (0.25 mg/kg) or BSA via the tail vein twice weekly for 3 weeks. Once tumor was formed, subsequent tumor growth curve was monitored. The tumor volume (mm3) was calculated by the formula 1/2 × (length) × (width)2. After animal sacrifice, the tumors and lymph nodes were isolated, weighted and examined by IF staining. Area of lymphatic vessels in tumor tissues and GFP-positive signals in lymph nodes were assessed in at least 6 independent fields in different sections using Nikon image software (NIS-Elements AR 3.0).
Isolation and Culture of Fibroblasts
We established fibroblast cell lines from breast tumor tissues resected from orthotopic breast cancer mice. The technical procedure was similar to previous reports [32], [33], [34]. Briefly, we resected breast tumors from mice mammary fat pads, and used tumor dissociation kit (130–096-730, Miltenyi Biotec, Germany) to obtain cells. Then the cells were cultivated in DMEM containing 20% FBS at 37°C overnight. Tumor cells were marked by GFP, negative-GFP cells were screened by flow cytometry and then incubated. To confirm that the cultivated cells were fibroblasts, the cells were immunostained for vimentin and desmin. All the cells were positive for fibroblast makers (Figure S1D) and their third through sixth passage were used in experiments.
Statistical Analysis
All the values were expressed as mean ± standard deviations (SDs). Comparisons were conducted using two-tailed Student's t tests or χ2 test and P values <.05 were considered statistically significant.
Results
Elevated Expression of LOXL2 is Associated with Lymphangiogenesis in Malignant Breast Cancer Tissues
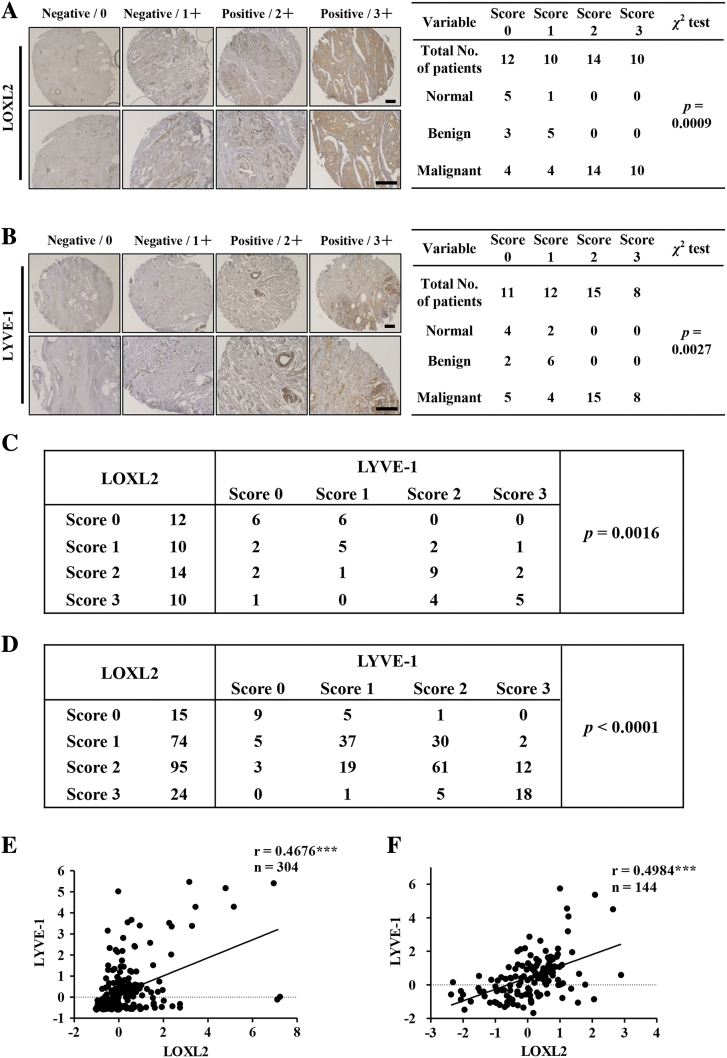
To investigate the clinical relevance of LOXL2 and lymphangiogenesis in human breast cancer patients, we directly detected LOXL2 and lymphatic vessel endothelial hyaluronan receptor 1 (LYVE-1, a LEC maker) expression levels in patients by immunohistochemistry (IHC) analysis of a breast tissue microarray (Table S2). The levels of LOXL2 and LYVE-1 in each specimen were divided into four categories according to the staining intensities. The IHC results showed that the positive staining rates of LOXL2 and LYVE-1 in malignant breast cancer tissues were 75% (24/32) and 72% (23/32), respectively. However, the staining intensities of LOXL2 and LYVE-1 in normal tissues and benign breast tumors were negative (P = .0009, Figure 1A; P = .0027, Figure 1B). These results suggested that both LOXL2 and lymphangiogenesis be crucial in malignant breast cancer. In addition, the correlation analysis demonstrated that LOXL2 expression was positively correlated with LYVE-1 level in breast cancer tissues (P = .0016, Figure 1C).
Figure 1.
The expression of LOXL2 positively correlated with lymphatic vessel density in malignant breast cancer tissues. (A, B) Representative LOXL2 and LYVE-1 staining in normal tissues, benign tumor and malignant breast cancer samples. Immunohistochemistry (IHC) analysis of LOXL2 and LYVE-1 on human breast samples (BR1006a, 46 of 50 were valid) respectively (left). The scores of staining intensity: 0 = negative, 1 = low, 2 = moderate and 3 = high. The correlation between protein levels and tumor malignancy was analyzed through chi-square test (right). (C) The correlation of LOXL2 with LYVE-1 protein levels in human breast tissues was analyzed through x2 test. (D) The x2 test was used to analyzed the correlation of LOXL2 with LYVE-1 protein levels in malignant breast tumor tissues (BR2161). (E) Pearson correlation analysis of LOXL2 and LYVE-1 in 304 subjects with malignant breast cancer (TCGA, PanCancer Atlas). (F) Pearson correlation analysis of LOXL2 and LYVE-1 in 144 subjects with malignant breast cancer (TCGA, Provisional). **P < .01; ***P < .001.
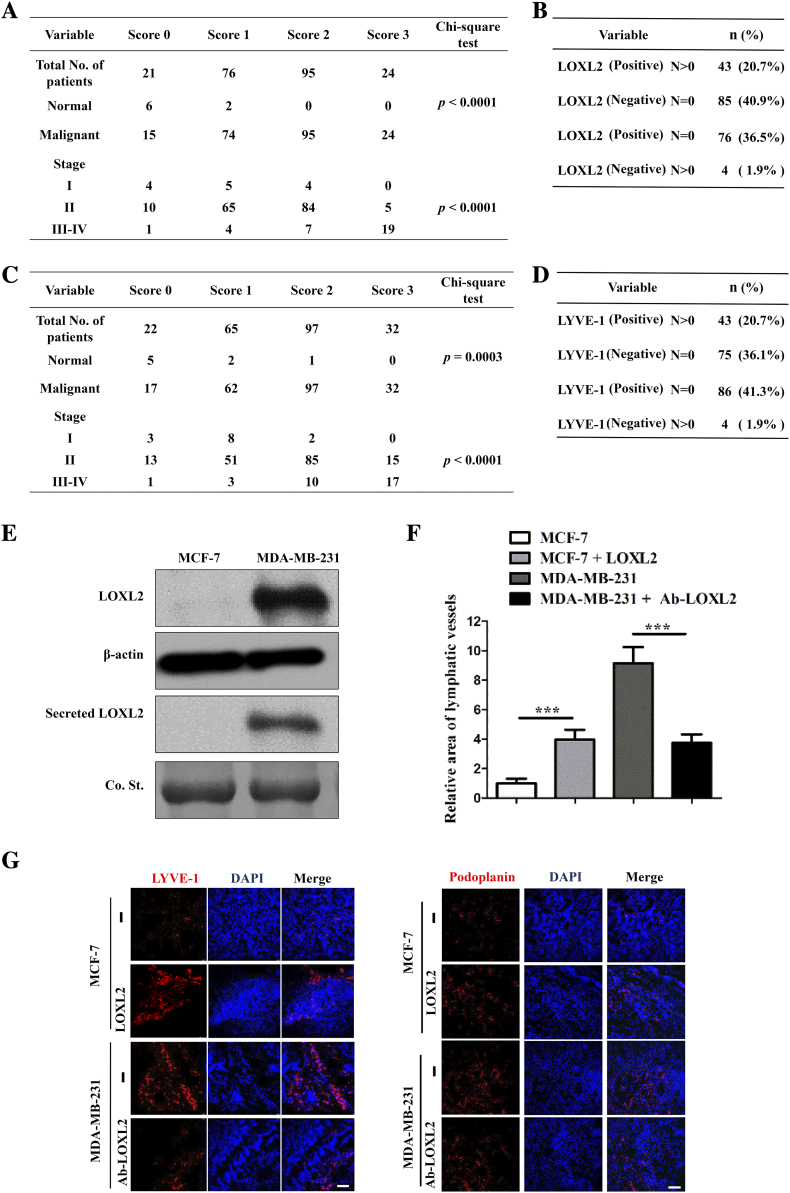
To further confirm these observations, we detected the levels of LOXL2 and LYVE-1 using another breast cancer tissue microarray (216 specimens with clinical records, Table S3), and found that LOXL2 expression was positively correlated with LYVE-1 level in malignant breast tissues (P < .0001, Figure 1D). Additionally, LOXL2 and LYVE-1 were positively correlated with tumor malignancy and lymph node metastasis (Figure S2, A-D). Furthermore, correlation analysis with the online TCGA datasets demonstrated a positive correlation between LOXL2 and LYVE-1 at mRNA level in malignant breast cancer samples (Figure 1, E and F).
Figure S2.
LOXL2 promotes lymphangiogenesis in clinical cancer specimens and breast cancer mice models. (A) Associations of LOXL2 IHC scores with tumor stage (I, II, III-IV) (B) Specimens were subsequently classified into 4 groups: positive expression level of LOXL2 with lymph node metastasis, negative expression level of LOXL2 with no lymph node metastasis, positive expression level of LOXL2 with no lymph node metastasis, negative expression level of LOXL2 with lymph node metastasis. (C) Associations of LYVE-1 IHC scores with tumor stage (I, II, III-IV). (D) Specimens were subsequently classified into 4 groups: positive expression level of LYVE-1 with lymph node metastasis, negative expression level of LYVE-1 with no lymph node metastasis, positive expression level of LYVE-1 with no lymph node metastasis, negative expression level of LYVE-1 with lymph node metastasis. (E) The protein levels of LOXL2 in the MCF-7 and MDA-MB-231 cells lysis and CM were measured by immunoblot, respectively. (F) Quantification results of IF staining area of LYVE-1 and podoplanin in panel G. (G) LYVE-1 and podoplanin expression detected by IF staining in primary tumors from xenograft-bearing mice injected with MCF-7 cells and MDA-MB-231 cells (n = 6). Columns: means ± SDs. **P < .01; ***P < .001. Scale bar = 100 μm.
To ascertain the role of LOXL2 in tumor lymphangiogenesis, we implanted MCF-7 cells with low level of LOXL2 expression and MDA-MB-231 cells with high level of LOXL2 expression (Figure S2E) into mice to establish an orthotopic breast cancer model, and detected lymphatic vessel density in tumor tissues by immunofluorescent (IF) staining. As expected, a higher density of lymphatic vessels was observed in MDA-MB-231 tumors compared with MCF-7 tumors. LOXL2 administration increased the lymphatic density in MCF-7 tumors by 4 folds, while blocking LOXL2 with a neutralizing antibody inhibited lymphangiogenesis in MDA-MB-231 tumors (Figure S2, F and G).
In short, these results clearly demonstrate that LOXL2 expression is necessary for lymphangiogenesis and positively correlated with breast cancer malignancy.
LOXL2 Promotes the Invasion and Tube Formation of LECs
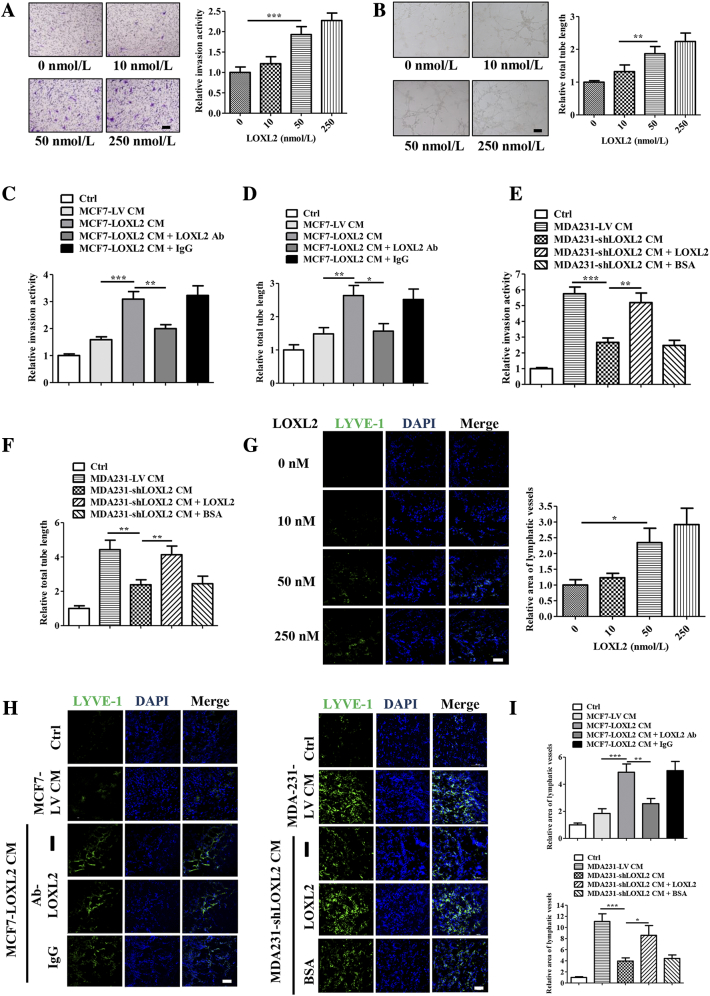
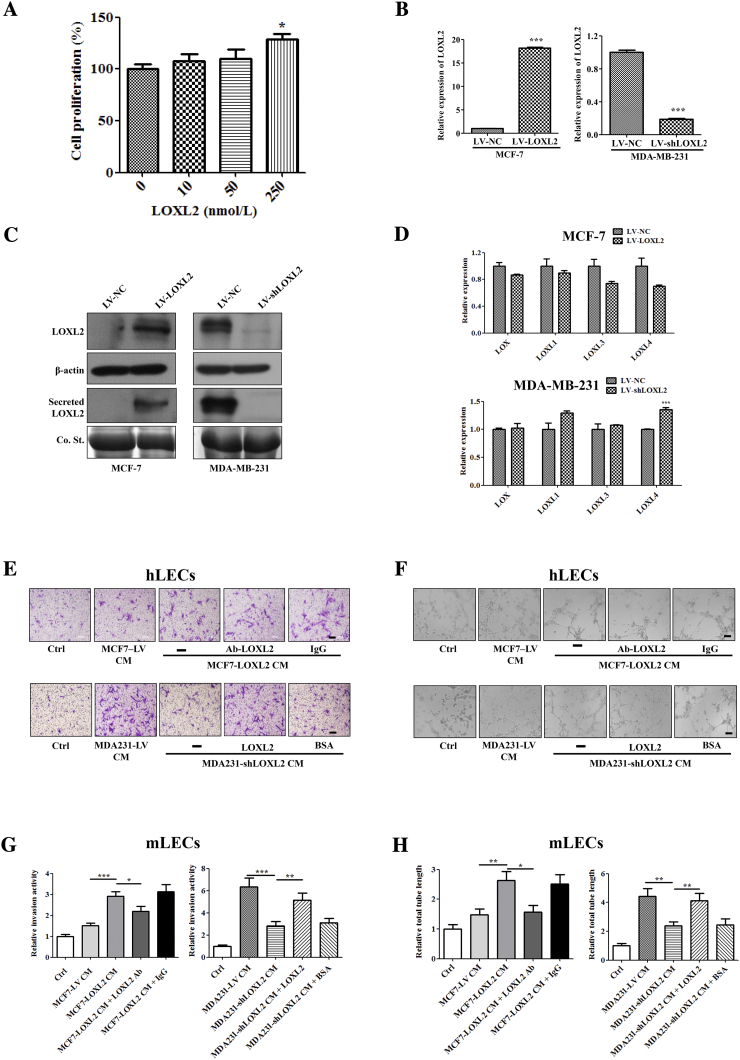
In order to study the mechanisms of LOXL2 in lymphangiogenesis modulation, we assessed LECs proliferation, invasion and tube formation abilities, all of which are essential steps for lymphangiogenesis in vivo [6]. It was shown that exogenous LOXL2 significantly promoted human LECs (hLECs) invasion and tube formation in a dose-dependent manner (Figure 2, A and B), while undetectable differences were observed in cell proliferation (Figure S3A).
Figure 2.
LOXL2 promotes the invasion and tube formation of LECs. (A) Representative and quantified results showing the relative invasion activity of the hLECs treated with the LOXL2 protein. (B) Representative and quantified results showing the relative tube formation activity of the hLECs treated with the LOXL2 protein. (C, D) Quantified results showing the relative invasion and tube formation activity of the hLECs treated with MCF7-LV CM or MCF7-LOXL2 CM in the presence of IgG or LOXL2-antibodies. (E, F) Quantified results showing the relative invasion and tube formation activity of the hLECs treated with MDA231-LV CM or MDA231-shLOXL2 CM in the presence of LOXL2 or BSA. Scale bar = 200 μm. (G) Representative photomicrographs showing LOXL2-induced lymphatic vessel formation in a Matrigel plug assay. Plugs containing the different dose of LOXL2 into the abdominal midline of BALB/c mice. After 8 days, IF staining of lymphatic vessels in dissected Matrigel plugs was performed. LYVE-1 (green) represents lymphatic vessels (left; Scale bar = 100 μm). Quantified results were shown (right; n = 6). (H) Density of lymphatic vessels in the Matrigel plug assay. Plugs containing indicated reagents were subcutaneously injected into BALB/c mice. After 8 days, plugs were dissected and applied to IF analysis for lymphatic vessel density. LYVE-1 (green) represents lymphatic vessels. (Scale bar = 100 μm). (I) Quantified results were shown (n = 6). The data are the means ± SD of three independent experiments.*P < .05; **P < .01; ***P < .001.
Figure S3.
LOXL2 promotes the invasion and tube formation of LECs. (A) Quantified results showing the relative proliferation of the hLECs treated with LOXL2 protein. (B) The mRNA levels of LOXL2 in the indicated cells were measured by qRT-PCR. (C) The protein levels of LOXL2 in the indicated cells and CM were measured by immunoblot using LOXL2 (ab96233) antibody. (D) The mRNA levels of LOX, LOXL1, LOXL3, LOXL4 in the MCF7-LOXL2, MDA231-shLOXL2 cells and their corresponding controls (MCF7-LV, MDA231-LV) were measured by RT-qPCR. (E, F) Representative results showing the invasion and tube formation activity of the hLECs treated with CM from the MCF7-LOXL2, MDA231-shLOXL2 and their corresponding controls (MCF7-LV, MDA231-LV). (G, H) Quantified results showing the relative invasion and tube formation activity of the mLECs treated with MCF7-LV CM or MCF7-LOXL2 CM in the presence of IgG or LOXL2-antibodies. Quantified results showing the relative invasion and tube formation activity of the hLECs treated with MDA231-LV CM or MDA231-shLOXL2 CM in the presence of LOXL2 or BSA. The data are the means ± SD of three independent experiments. *P < .05; **P < .01; ***P < .001.
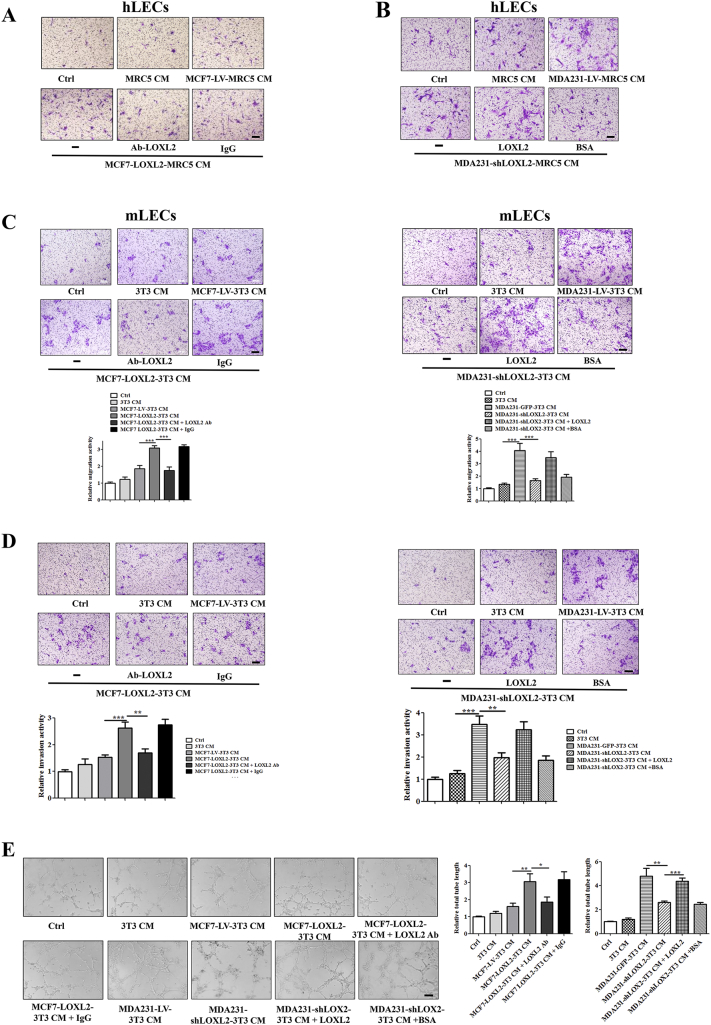
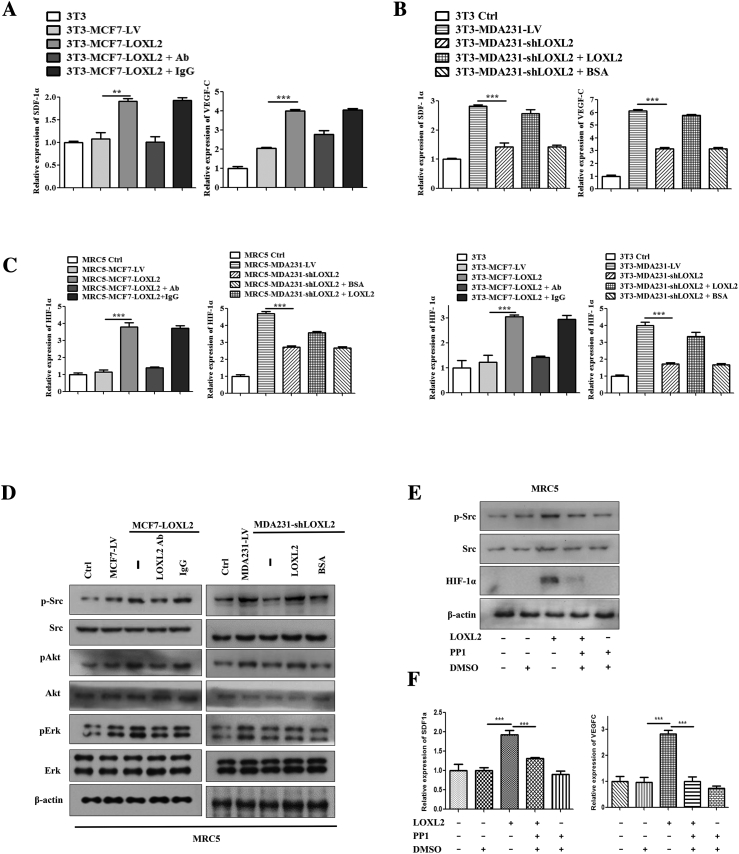
Furthermore, to evaluate the effects of tumor cell-derived LOXL2 on lymphangiogenesis, we stably knocked down LOXL2 in highly invasive MDA-MB-231 cells (MDA231-shLOXL2) and overexpressed LOXL2 in non-invasive MCF-7 cells (MCF7-LOXL2), then used their conditioned medium (CM) to treat LECs. The efficiencies of LOXL2 overexpression and knockdown were characterized by qRT-PCR and Western blotting (Figure S3, B and C). MCF7-LOXL2 CM increased the invasion and tube-forming activities of hLECs, which were disrupted by administration of LOXL2 antibody (Figures 2, C and D; S3, E and F). Consistently, hLECs treated with MDA231-shLOXL2 CM showed lower invasion and tube formation abilities than the control group (CM from MDA231-LV cells), while addition of LOXL2 protein could rescue the suppressed processes (Figures 2, E and F; S3, E and F). Similar results were also observed in mouse LECs (mLECs) models (Figure S3, G and H). These results demonstrate that the tumor-secreted LOXL2 promotes the invasion and tube formation activities of LECs.
To further confirm above results, in vivo Matrigel plug assay was conducted as previously described [12] and the results showed that LOXL2 increased the lymphatic vessel density in a dose-dependent manner (Figure 2G). Plugs containing MCF7-LOXL2 CM showed a higher lymphatic vessel density and a better tubule-like structure than those harboring MCF7-LV CM. However, addition of LOXL2 antibody impeded lymphatic vessel formation. On the contrary, compared with plugs containing MDA231-LV CM, those bearing MDA231-shLOXL2 CM had lower lymphatic vessel density, and a broken vessel structure, which were relieved by the administration of LOXL2 protein (Figure 2, H and I). Taken together, our data reveals that tumor cell-derived LOXL2 enhances lymphangiogenesis both in vitro and in vivo.
LOXL2 Promotes Lymphangiogenic Activity via Activation of the Akt-Snail and Erk Pathways
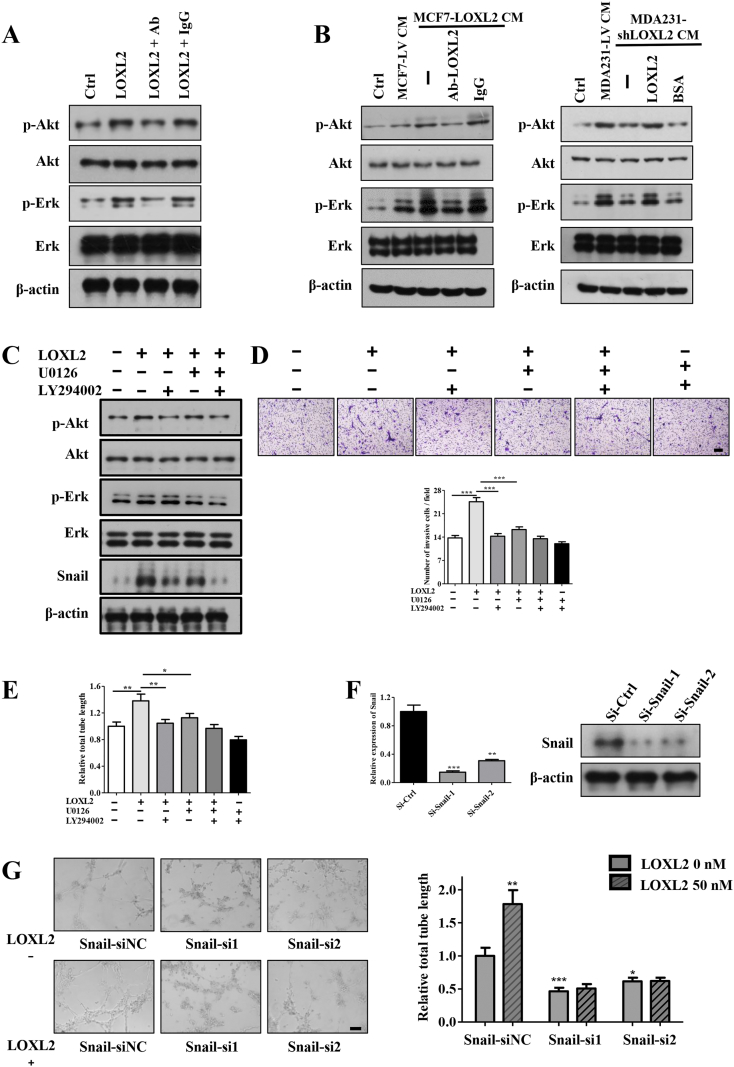
Previous studies have documented that activations of the Akt and Erk signaling pathways are important for LECs invasion and tube formation [35]. In order to investigate the molecular mechanism underlying LOXL2-dependent alterations in LEC s modulation, we examined the effects of purified LOXL2 and tumor CM on these signaling pathways by Western blotting analysis. As expected, upon LOXL2 stimulation, the phosphorylations of Akt and Erk were increased in LECs. When LOXL2 was blocked by the neutralizing antibody, the phosphorylations of Akt and Erk were significantly decreased (Figures 3A, S4A). Similarly, CM derived from LOXL2 knockdown or overexpression cells down- and upregulated Akt and Erk phosphorylation respectively (Figure 3B). Next, to define whether Akt or Erk was involved in LOXL2-facilitated LECs invasion and tube formation, the inhibitor of PI3k/Akt (LY294002) or Erk (U0126) was used to pretreat LECs. The phosphorylations of Akt and Erk were dramatically attenuated after the treatment (Figure 3C), accompanied by defects in LOXL2-induced LECs invasion and tube formation (Figure 3, D and E).
Figure 3.
LOXL2 promotes lymphangiogenic activity via activation of the Akt-Snail and Erk signaling pathways. (A, B) LOXL2 induces the phosphorylation of Akt and Erk. (A) The protein levels of p-Akt, p-Erk, Akt, Erk in hLECs treated with LOXL2 determined by Western blotting. (B) The protein levels of p-Akt, p-Erk, Akt, Erk in hLECs treated with tumor CM and indicated reagents determined by Western blotting. (C) Immunoblot analysis of p-Akt, p-Erk, Snail expression in the LOXL2, LY294002 (inhibitor of PI3K/Akt) or U0126 (inhibitor of Erk) treated hLECs. Cells were pretreated with LY294002 and U0126 for 30 min, and then stimulated with LOXL2 for 30 min. (D) The effect of LY294002 and U0126 on invasion of hLECs induced by LOXL2. (E) Quantified results showing the relative tube formation activity of the hLECs pretreated with LY294002 and U0126. (F) qRT-PCR and Immunoblot analysis of Snail expression in the LECs transfected with two specific siRNAs. (G) Representative and quantified data showing the relative tube formation activity of LECs transfected siCtrl and Snail-siRNAs. The data are the means ± SD of three independent experiments. *P < .05; **P < .01; ***P < .001. Scale bar = 200 μm.
Figure S4.
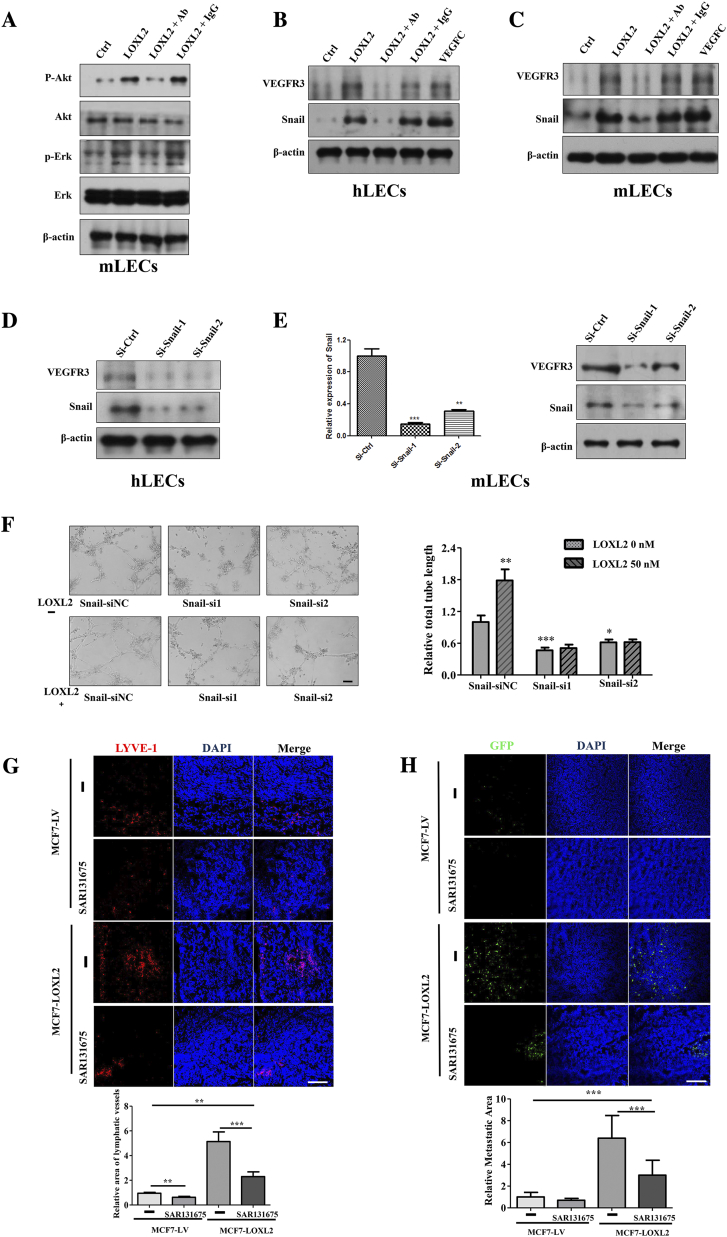
LOXL2 activates intracellular signaling pathways of LECs. (A) The protein levels of p-Akt, p-Erk, Akt, Erk in mLECs treated with LOXL2 determined by Western blotting. (B) The protein expression levels of Snail and VEGFR3 in the hLECs were detected by immunoblot. VEGF-C was used as a positive control. (C) The protein expression levels of Snail and VEGFR3 in the mLECs were detected by immunoblot. VEGF-C was used as a positive control. (D) Immunoblot analysis of Snail and VEGFR3 expression in the hLECs transfected with two specific Snail siRNAs. (E) qRT-PCR and Immunoblot analysis of Snail expression in the mLECs transfected with two specific siRNAs targeted to Snail. (F) Representative data showing the relative tube formation activity of mLECs transfected siNC and Snail-siRNAs. The data are the means ± SD of three independent experiments. Scale bar = 200 μm. (G) MCF7-LV and MCF7-LOXL2 cells were implanted into mice mammary fat pads (n = 6). Daily oral treatment with SAR131675 (30 mg/kg/d) or saline buffer started at day 8. Representative images of IF detection of lymphatic vessels (red, LYVE-1 staining) in MCF7-LV and MCF7-LOXL2 tumors (top). Quantified results of relative lymphatic vessels density were shown (bottom). (H) Representative images of immunofluorescence detection of GFP-tumor cells in lymph nodes (top). Quantified results of relative metastatic area were shown (bottom). *P < .05; **P < .01; ***P < .001. Scale bar = 100 μm.
Several studies have reported that the Akt-Snail pathway is activated in response to cell–cell and cell-ECM interactions during endothelial cells and LECs tube formation [36], [37]. Consistently, we also observed that LOXL2 increased the level of Snail in LECs (Figures 3C; S4, B and C), which could be decreased by LY294002 treatment (Figure 3C). To investigate whether Snail was essential for LOXL2-induced tube formation, we knocked down Snail by siRNAs (Figures 3F; S4, D and E) and observed a significantly reduction in LECs tube formation (Figures 3G, S4F). In summary, these results show that activations of the Akt-Snail and Erk pathways mediate LOXL2-induced lymphangiogenesis in vitro.
LOXL2 Stimulates Lymphangiogenic Activities Through Educating Fibroblasts
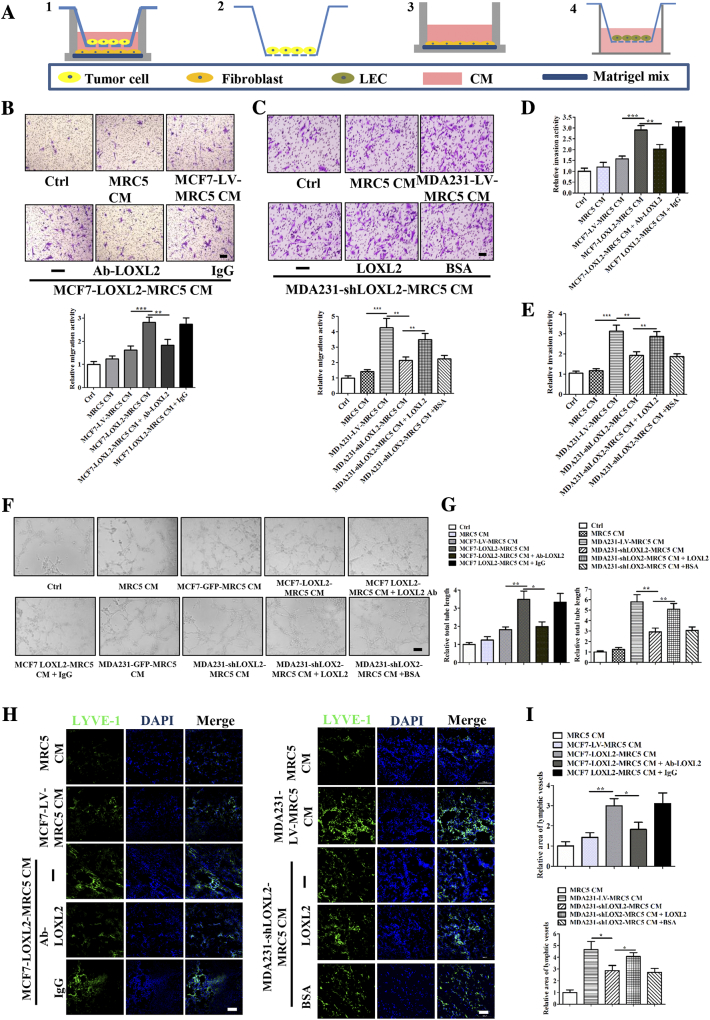
Based on the in vivo results (Figure S2, F and G), LOXL2 protein promoted tumor lymphangiogenesis to a more dramatic extent than that was observed in vitro. Therefore, we wondered whether other components in the tumor microenvironment assist tumor lymphangiogenesis under the influence of LOXL2. Previous studies have reported that cancer associated fibroblasts (CAFs) augment the malignant progression of cancer [38], [39], Given the fact that CAFs could be activated by tumor-secreted LOXL2 [31], we further analyzed whether in vivo lymphangiogenesis promoted by tumor-secreted LOXL2 was mediated by fibroblasts. We firstly co-cultured MRC5 fibroblasts and breast cancer cells, and collected the CM from the educated fibroblasts (Figure 4A). Then the collected CM was used to stimulate LECs in various assays. The results showed that CM from fibroblasts educated by MCF7-LOXL2 cells increased the migration activity of LECs. However, addition of LOXL2 antibody in co-cultured system attenuated the migration activity (Figure 4B). In consistence, LECs treated with CM from fibroblasts educated by MDA231-shLOXL2 showed weaker migration ability than the corresponding control group. The decreased activity of migration was restored by the treatment of LOXL2 protein in co-cultured system (Figure 4C). Similar results were obtained in invasion and tube formation assays in vitro (Figure 4, D–G; S5, A and B). We also used 3 T3 mouse fibroblasts and mLECs to confirm the effects of educated-fibroblasts on lymphangiogenesis, and observed similar results in migration, invasion and tube formation assays (Figure S5, C–E).
Figure 4.
Fibroblasts educated by tumor-derived LOXL2 stimulate migration, invasion and tube formation of LECs. (A) The schematic flowchart shows the co-culture system for fibroblasts and tumor cells:1. Co-culture fibroblasts with tumor cells, fibroblast cells cultured on the matrigel mix; 2. remove tumor cells; 3. culture fibroblasts in serum free medium; 4. collect CM from educated fibroblasts to treat LECs. (B, C) Representative images and quantification results of the cell migration abilities of LECs treated by CM and indicated reagents. (D, E) Quantification results of the cell invasion abilities of LECs treated by CM and indicated reagents. (F, G) Representative images and quantification results of the cell tube formation abilities of LECs treated by CM and indicated reagents. Scale bar = 200 μm. (H, I) Density of lymphatic vessels in the Matrigel plug assay. (H) Plugs containing CM and indicated reagents were subcutaneously injected into BALB/c mice. After 8 days, plugs were dissected and applied to IF analysis for lymphatic vessel density. Scale bar = 100 μm. (I) Quantified results were shown (n = 6). The data are the means ± SD of three independent experiments. *P < .05; **P < .01; ***P < .001.
Figure S5.
Fibroblasts educated by tumor-derived LOXL2 can stimulate migration, invasion and tube formation of LECs. (A, B) Representative images of the cell invasion abilities of hLECs treated by tumor CM. (C) Representative images and quantification results of the cell migration abilities of mLECs treated by tumor CM. (D) Representative images and quantification results of the cell invasion abilities of mLECs treated by CM. (E) Representative images and quantification results of the cell tube formation abilities of mLECs treated by CM. The data are the means ± SD of three independent experiments. **P < .01; ***P < .001.
To determine whether fibroblasts educated by tumor-derived LOXL2 inducing lymphangiogenesis in vivo, we conducted a Matrigel plug assay. IF staining of LYVE-1 revealed that addition of LOXL2 antibody in co-cultured system decreased the good tubule-like structure induced by CM from fibroblasts educated by MCF7-LOXL2 cells. However, adding LOXL2 in co-cultured system could alleviate the discontinuous lymphatic vessel structure induced by CM from MDA231-shLOXL2 group (Figure 4, H and I). Collectively, above results demonstrate that tumor-secreted LOXL2 contributes to lymphangiogenesis through educating fibroblasts.
LOXL2 Promotes the Expression and Secretion of Pro-Lymphangiogenic Factors in Fibroblasts Through HIF-1α
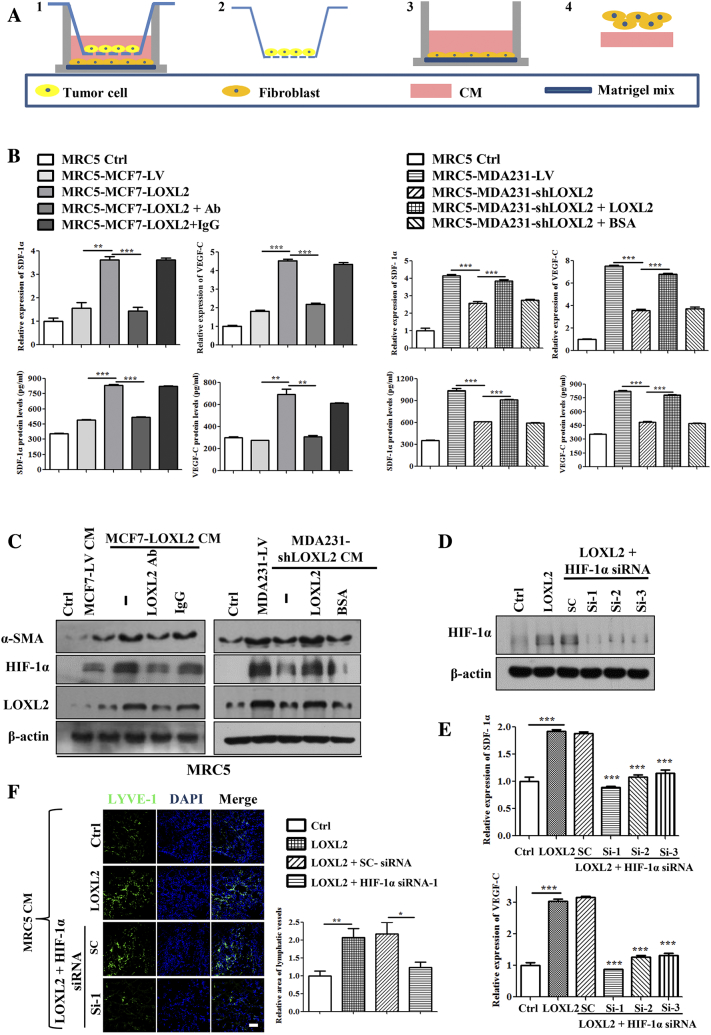
An important feature regulating on lymphangiogenesis by educated fibroblasts is the production of multiple pro-lymphangiogenic factors [39]. As such, gene expression and secretion of pro-lymphangiogenic factors of fibroblasts in the co-culture system were evaluated with qRT-PCR and ELISA assays, respectively (Figure 5A). As shown in Figure 5B, LOXL2 led to significant increases of SDF-1α and VEGF-C in fibroblasts and supernatants (Figure 5B; S6, A and B). Because HIF-1α was reported as the transcription factor to regulate pro-lymphangiogenic factors production [40], we speculated that LOXL2 upregulated SDF-1α and VEGF-C expression via HIF-1α. Immunoblot assays showed that HIF-1α expression was elevated in fibroblasts educated by MCF7-LOXL2 tumor cells, and LOXL2 antibody hindered the HIF-1α upregulation. In contrast, fibroblasts educated by MDA231-shLOXL2 tumor cells showed lower HIF-1α expression, which was reversed by the administration of LOXL2 protein (Figure 5C). The qRT-PCR data displayed similar results (Figure S6C). After knockdown of HIF-1α in fibroblasts by siRNAs (Figure 5D), LOXL2 failed to accelerate the expression of SDF-1α and VEGF-C (Figure 5E).
Figure 5.
LOXL2 promotes the expression and secretion of pro-lymphangiogenic factors in fibroblasts through HIF-1α. (A) The schematic flowchart shows the co-culture system for fibroblasts and tumor cells. Then detect the cell lysis and CM of the educated fibroblast cells. (B) The mRNA levels of SDF-1α and VEGF-C in fibroblast cells educated by tumor cells (top). The protein levels of SDF-1α and VEGF-C in fibroblasts CM educated by tumor cells were detected by ELLSA (bottom). (C) The protein levels of HIF-1α, LOXL2 and α-SMA in fibroblast cells educated by tumor cells were detected by Western blotting. (D) Immunoblot analysis of HIF-1α expression in the fibroblast cells transfected with specific siRNAs. (E) The effect of siRNAs on inhibiting VEGF-C, SDF-1α mRNA levels of fibroblast cells. (F) Density of lymphatic vessels in the Matrigel plug assay. Plugs containing CM from fibroblast cells and CM from fibroblasts transfected with specific siRNAs were subcutaneously injected into BALB/c mice. After 8 days, plugs were dissected and applied to IF analysis for lymphatic vessel density (left). Quantified results were shown (right; n = 6). The data are the means ± SD of three independent experiments. *P < .05; **P < .01; ***P < .001. Scale bar = 100 μm.
Figure S6.
LOXL2 promotes the expression and secretion of pro-lymphangiogenic factors in fibroblasts through HIF-1α. (A, B) The mRNA levels of VEGF-C and SDF-1α and in 3T3 cells educated by tumor cells were detected by qRT-PCR. (C) The mRNA levels of HIF-1α in MRC5 and 3T3 cells educated by tumor cells were detected by qRT-PCR. (D) The protein levels of Akt, Erk, pAkt, pErk, pSrc and Src in MRC5 cells educated by tumor cells were detected by Western blotting. (E) Immunoblot analysis of p-Src, Src and HIF-1α expression in the PP1 (inhibiting pSRC) pretreated fibroblast cells. Cells were pretreated with PP1, and then stimulated with LOXL2. (F) The mRNA levels of VEGF-C and SDF-1α in treated fibroblasts. The data are the means ± SD of three independent experiments. **P < .01; ***P < .001.
Furthermore, we determined whether HIF-1α mediated the promotion effect of LOXL2-educated fibroblasts on lymphangiogenesis in vivo. Consistent with in vitro results, plugs mixed with CM from HIF-1α depleted fibroblasts resulted in a significant reduction in lymphatic vessel density (Figure 5F). These results demonstrate that tumor-secreted LOXL2 facilitates lymphangiogenesis by enhancement of HIF-1α-regulated SDF-1α and VEGF-C expression in fibroblasts.
LOXL2 Promotes Breast Cancer Lymphangiogenesis and Lymph Node Metastasis In Vivo
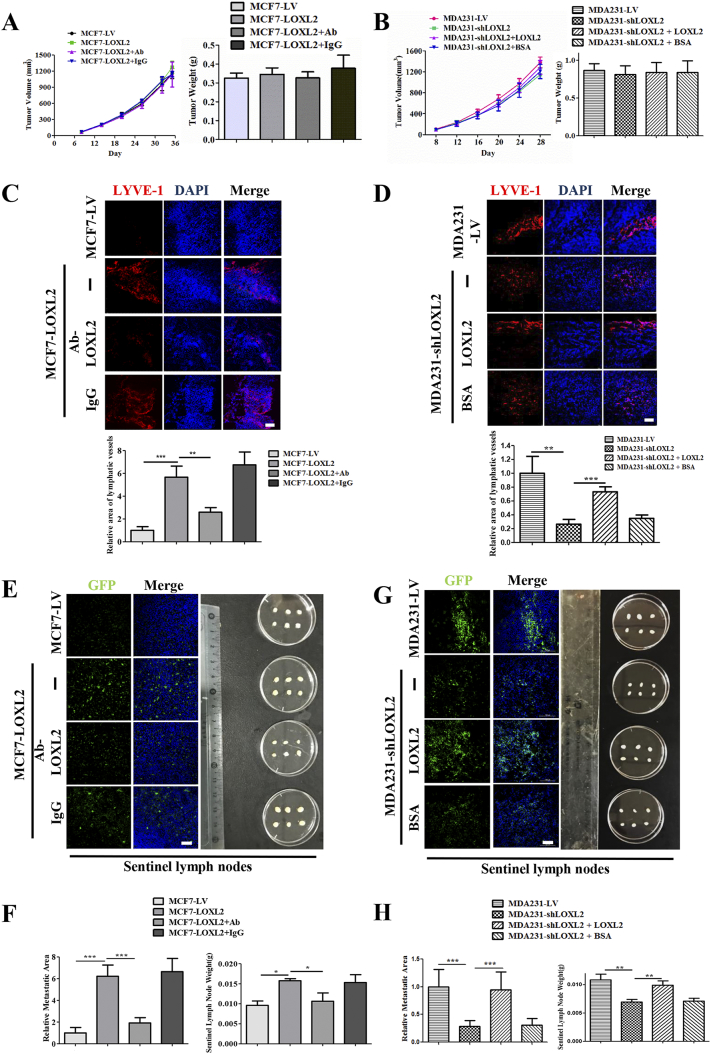
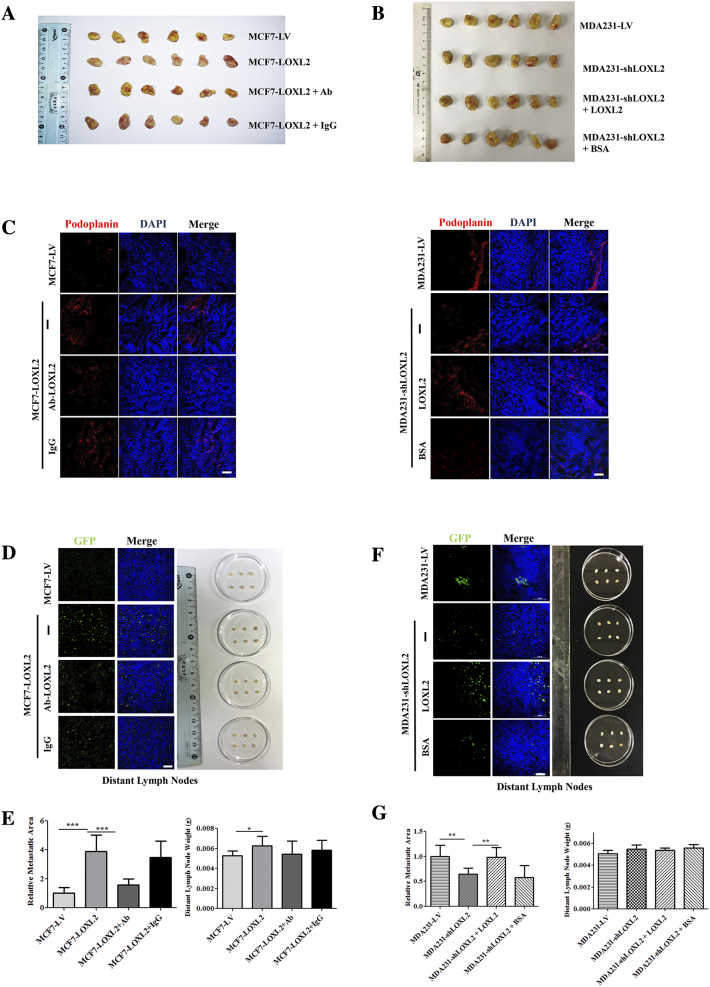
Finally, using a mouse xenograft model, we sought to further test the significance of LOXL2 in breast cancer lymphangiogenesis and metastasis, MCF7-LOXL2 cells, MDA231-shLOXL2 cells and their corresponding controls were implanted into mice mammary fat pads. 8 days after implantation, MCF7-LOXL2 tumor-bearing mice were intravenously injected with LOXL2-neutralizing antibody or IgG antibody, while MDA231-shLOXL2 tumor-bearing mice were injected with LOXL2 protein or BSA twice weekly. Tumor volumes were monitored regularly and tumor weights were measured when the mice were sacrificed. No prominent differences were observed among primary tumor growth and tumor weight (Figures 6, A and B; S7, A and B).
Figure 6.
LOXL2 promotes breast cancer lymphangiogenesis and lymph node metastasis in vivo. (A) Tumor volume of MCF7-LV and MCF7-LOXL2 mouse models (left). Tumor weight of MCF7-LV and MCF7-LOXL2 mouse models (right). (B) Tumor volume of MDA231-LV and MDA231-shLOXL2 mouse models (left). Tumor weight of MCF7-LV and MCF7-LOXL2 mouse models (right). (C) Representative images of immunofluorescence detection of lymphatic vessels (red, LYVE-1 staining) in MCF7-LV and MCF7-LOXL2 tumors (top). Quantified results of relative lymphatic vessels density were shown (bottom). (D) Representative images of immunofluorescence detection of lymphatic vessels (red, LYVE-1 staining) in MDA231-LV and MDA231-shLOXL2 tumors (top). Quantified results of relative lymphatic vessels density were shown (bottom). (E) Axillary lymph nodes were removed from MCF7-GFP and MCF7-LOXL2 tumor bearing mice. Dissected lymph nodes were photographed (right). Representative images of immunofluorescence detection of GFP-tumor cells in lymph nodes (left). (F) Quantified results of relative metastatic area were shown (left). Sentinel lymph nodes weight of MCF7-LV and MCF7-LOXL2 mouse models (right). (G) Axillary lymph nodes were removed from MDA231-LV and MDA231-shLOXL2 tumor bearing mice. Dissected lymph nodes were photographed (right). Representative images of immunofluorescence detection of GFP-tumor cells in lymph nodes (left). (H) Quantified results of relative metastatic area were shown (left). Sentinel lymph nodes weight of MDA231-LV and MDA231-shLOXL2 mouse models (right). The data are the means ± SD of three independent experiments. n = 6. *P < .05; **P < .01; ***P < .001. Scale bar = 100 μm.
Figure S7.
LOXL2 promotes breast cancer lymphangiogenesis and lymph node metastasis in vivo. (A) Primary tumors were removed from MCF7-LV and MCF7-LOXL2 tumor bearing mice. Dissected tumors were photographed. (B) Primary tumors were removed from MDA231-LV and MDA231-shLOXL2 tumor bearing mice. Dissected tumors were photographed. (C) Representative images of IF detection of lymphatic vessels (red, podoplanin staining) in MCF7-LV and MCF7-LOXL2 tumors (left). Representative images of IF detection of lymphatic vessels (red, podoplanin staining) in MDA231-LV and MDA231-shLOXL2 tumors (right). (D) Distant lymph nodes were removed from MCF7-LV and MCF7-LOXL2 tumor bearing mice. Dissected lymph nodes were photographed (right). Representative images of IF detection of GFP-tumor cells in lymph nodes (left). (E) Quantified results of relative metastatic area were shown (left). Distant lymph nodes weight of MCF7-LV and MCF7-LOXL2 mouse models (right). (F) Distant lymph nodes were removed from MDA231-LV and MDA231-shLOXL2 tumor bearing mice. Dissected lymph nodes were photographed (right). Representative images of IF detection of GFP-tumor cells in lymph nodes (right). (G) Quantified results of relative metastatic area were shown (left). Distant lymph nodes weight of MDA231-LV and MDA231-shLOXL2 mouse models (right). n = 6, error bars represent SD. P values, Student’s t test, *P < .05; **P < .01; ***P < .001. Scale bar = 100 μm.
To confirm the role of LOXL2 in promotion of lymphangiogenesis and lymph node metastasis, tumors and lymph nodes were dissected. Quantification of LYVE-1 and podoplanin-positive areas in tumor tissues demonstrated that lymphangiogenesis was increased in MCF7-LOXL2 tumors, whereas treatment with LOXL2 antibody abolished this effect. A striking decrease of lymphatic vessel density was observed in MDA231-shLOXL2 tumors compared with MDA231-LV control tumors, which could be reversed by treatment with LOXL2 protein (Figures 6, C and D; S7C). Strikingly, mice bearing MCF7-LOXL2 tumors developed 6-fold more lymph node metastasis and higher lymph node weight than those bearing MCF7-LV control tumors. Treatment with anti-LOXL2 antibody significantly decreased lymph node metastasis (Figures 6, E and F; S7, D and E). Moreover, mice bearing MDA231-shLOXL2 tumors developed significantly less lymph node metastasis and lower weight than mice bearing control MDA231-LV tumors. LOXL2 treatment recovered the decreased lymph node metastasis (Figures 6, G and H; S7, F and G). The IF analysis indicated that LOXL2 induces breast cancer lymphangiogenesis and lymph node metastasis in vivo.
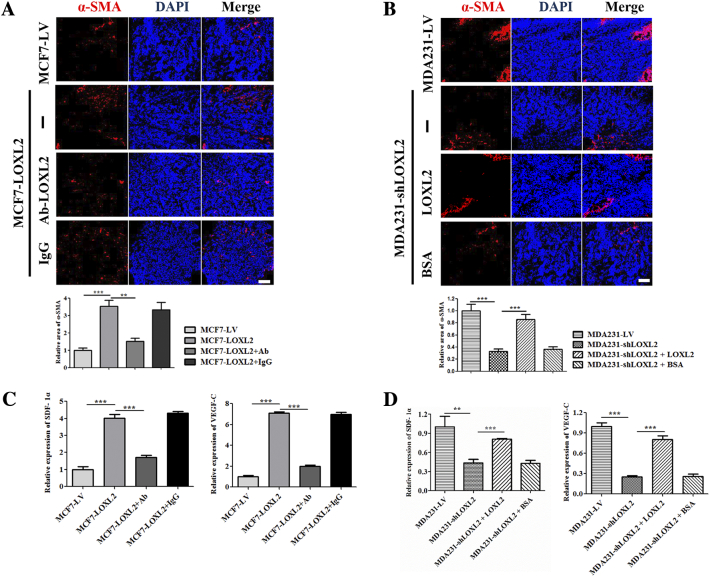
To further validate the role of fibroblasts in promoting lymphangiogenesis in vivo, α-SMA (CAFs maker) levels in tumor sections were analyzed by IF. Quantification results showed that compared with control group, α-SMA positive area in MCF7-LOXL2 tumors increased by about 3 folds, which was diminished by injecting LOXL2 antibody (Figure 7A). LOXL2 treatment resulted in relieving the decline of α-SMA positive regions in MDA231-shLOXL2 tumors (Figure 7B). In addition, fibroblasts isolated from tumor tissues were evaluated by qRT-PCR assays, and the results confirmed that LOXL2 enhanced the expression levels of SDF-1α and VEGF-C in fibroblasts from tumor tissues (Figure 7, C and D).
Figure 7.
LOXL2 activates CAFs in breast cancer in vivo. (A) Representative images of immunofluorescence detection of CAFs (red, α-SMA staining) in MCF7-LV and MCF7-LOXL2 tumors (top). Quantified results of relative α-SMA positive regions were shown (bottom). (B) Representative images of immunofluorescence detection of CAFs (red, α-SMA staining) in MDA231-LV and MDA231-shLOXL2 tumors (top). Quantified results of relative α-SMA positive regions were shown (bottom). (C, D) The mRNA levels of SDF-1α and VEGF-C in fibroblast cells isolated from tumor tissues. n = 6, error bars represent SD. P values, Student's t test, **P < .01; ***P < .001. Scale bar = 100 μm.
This in vivo evidence strongly demonstrates that LOXL2 significantly induces breast tumor lymphangiogenesis and lymph node metastasis.
Discussion
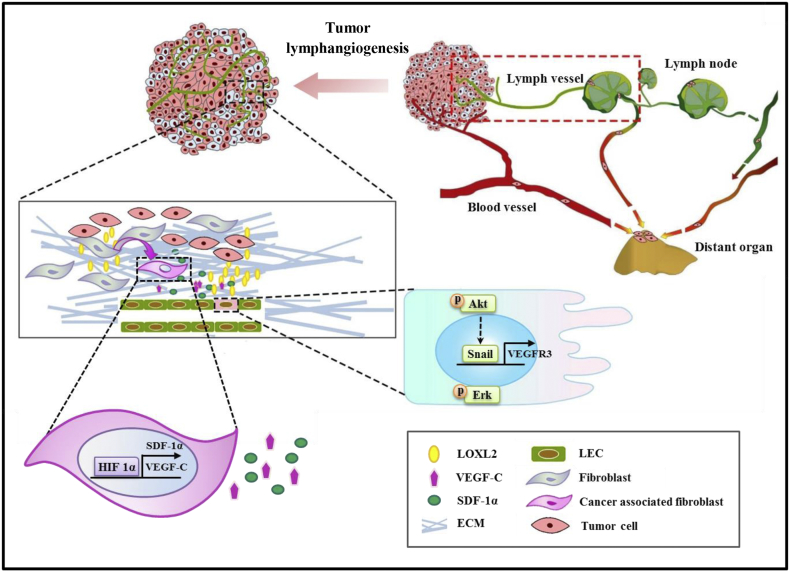
Metastasis is the main cause of cancer mortality and results in 90% of cancer patients' death [1]. Multiple studies demonstrated that lymphangiogenesis is positively correlated with lymphatic metastasis and decreased survival in breast cancer patients [41], [42]. Recently, two articles published in Science revealed that cancer cells from lymph node metastasis can be a source of distant metastases [43], [44]. Consistently, our results confirmed that lymphangiogenesis is positively correlated with the breast cancer malignancy and lymph node metastasis (Figure 1, Figure S2A–D). Therefore, studies on the mechanisms of lymphangiogenesis are essential for developing new drugs for treatment and improving the prognosis of patients. In the present study, we revealed a novel event that LOXL2 is a vital pro-lymphangiogenic molecule, which directly affects the function of LECs in vitro and in vivo. In addition, fibroblasts educated by cancer cell-derived LOXL2 promote the migration and tube formation of LECs, which assist lymphangiogenesis in primary tumors (Figure 8).
Figure 8.
Schematic model of LOXL2 promoting lymphangiogenesis and lymph node metastasis. In tumor microenvironment, LOXL2 induces lymphangiogenesis directly and indirectly. 1) Tumor-derived LOXL2 induces LECs invasion and tube formation directly via activation of the Akt and Erk signaling pathways; Further, LOXL2 increases VEGFR3 expression through enhancing the pAKT-Snail pathway. 2) In addition, tumor-derived LOXL2 actives tumor stroma fibroblasts to secrete VEGF-C and SDF-1α through enhancing HIF-1α expression, then these factors assist lymphangiogenesis in primary tumors. ECM: extracellular matrix. The design of this schematic model refers to several articles [42], [50].
Several previous studies demonstrated that the upregulation of LOXL2 is associated with poor prognosis in several cancers, including breast cancer [15]. Accumulating evidence suggests that LOXL2 promotes tumor progression and metastasis through multiple pathways and some LOXL2 small molecule inhibitors have shown potential promise in the treatment of breast cancer [14], [45]. In 2017, professor Cano’ group used conditional transgenic mouse models to establish that LOXL2 promoted metastasis of breast cancer independent of its conventional role in extracellular matrix remodeling [46]. Furthermore, many studies showed LOXL2 has been characterized as a pro-angiogenic factor [47], [48], [49]. Nevertheless, the effects of LOXL2 on tumor lymphangiogenesis, an important prognosis predictor in breast cancer patients, have not been reported yet. Herein, LOXL2 knocking-down and overexpressing tumor cells were used to evaluate the effects of tumor cell-derived LOXL2 on lymphangiogenesis. Considering the possible influence of other LOX family members, the expression levels of LOX, LOXL1, LOXL3 and LOXL4 were detected, and only LOXL4 mRNA level was slightly upregulated in MDA-shLOXL2 tumor cells (Figure S3D), but the protein level remained unchanged (data not shown). In addition, we demonstrated that tumor-derived LOXL2 could increase the expression level of a well-characterized pro-lymphangiogenic receptor VEGFR3 by upregulating Snail [50] (Figure S4, B and C), suggesting that LOXL2 can also enhance lymphangiogenesis via increasing the VEGF-C/VEGFR3 signaling. To further evaluate the role of the VEGF-C/VEGFR-3 pathway in LOXL2-induced lymphangiogenesis and metastasis progression, we performed animal experiments to investigate the impact of VEGF-C/VEGFR-3 pathway inhibitor (SAR131675). Quantification of LYVE-1 and GFP-positive areas in tumor tissues and lymph nodes demonstrated that after inhibition of the VEGF-C/VEGFR-3 pathway, partly deceased LOXL2-induced lymphangiogenesis and metastasis (Figure S4, G and H). Thus, LOXL2 promoted breast tumor lymphangiogenesis and metastasis is partly dependent on the VEGF-C/VEGFR-3 pathway.
Tumor microenvironment is heterogeneous, in which fibroblasts are the most abundant cellular constituents and are frequently regarded as the prominent modifiers in the malignant progression of cancer [38]. Emerging data has shown that CAFs differ from normal fibroblasts and promote tumor initiation and progression by secreting various growth factors [51]. Consistently, we discovered that the pro-lymphangiogenic factors SDF-1α and VEGF-C were highly expressed and secreted in fibroblasts educated by tumor-derived LOXL2 in vitro and in vivo (Figures 5B; 7, C and D). In addition, fibroblasts educated by tumor-secreted LOXL2 expressed more LOXL2, which may drive a feed-forward loop to further enhance cancer lymphangiogenesis (Figure 5C). Moreover, Barker et al. reported that tumor-derived LOXL2 directly activated fibroblasts in 4 T1 tumor tissues by modulating their signaling pathway [31]. Therefore, we examined the signaling pathways associated with fibroblasts activation, and showed that the Akt, Erk, Src signaling pathways were activated by LOXL2 (Figure S6D). Of particular note, the Src signaling pathway was involved in the HIF-1α-mediated SDF-1α and VEGF-C expression (Figure S6, E and F). Furthermore, we also demonstrated tumor-derived LOXL2 can upregulate CAFs in vitro and in vivo by detecting α-SMA expression (Figures 5C; 7, A and B). These results unraveled that fibroblasts activated by tumor-derived LOXL2 are undergoing many changes involving the activation of signaling pathways and increase of pro-lymphangiogenic factors secretion to facilitate lymphangiogenesis. Therefore, exploring the role of fibroblasts activated by tumor-derived LOXL2 on tumor progression, including angiogenesis, lymphangiogenesis and drug resistance will be of considerable interest in the immediate future.
Another interesting discovery in our current study is that we revealed LOXL2 remarkably induced lymphangiogenesis and lymph node metastasis, without affecting tumor growth in an orthotopic breast cancer model (Figure 6). Consistent results were reported by other groups, showing that LOXL2 promoted tumor metastasis, independent of primary tumor growth [30], [52]. However, contradictory findings were observed on functions of LOXL2 in breast cancer growth [15], [22]. This paradox is likely resulted from differences in the number of tumor cells injected in the mammary fat pads and the different experimental settings used among the diverse studies.
Conclusions
In conclusion, our data reveals a hitherto unknown role of LOXL2 on promoting breast cancer lymphangiogenesis and lymph node metastasis, which extends our understanding of the functions of LOXL2 on tumor procession. Secreted LOXL2 increases lymphangiogenesis by directly activating LECs and indirectly modulating fibroblasts to enhance LECs activities. Consequently, LOXL2 represents a diagnostic marker to indicate malignancy of breast cancer, and a promising novel therapeutic target for blocking lymphangiogenesis and lymphatic metastasis.
The following are the supplementary data related to this article.
siRNA sequences of the Snail and HIF-1α Gene.
The clinical features of patient samples in the tissue microarray (BR1006a).
The clinical features of patient samples in the tissue microarray (BR2161).
Declarations
Acknowledgements
We thank professor Gera Neufeld for the LOXL2-plasmid.
We thank insightful discussions and technical assistances from all members of the Luo laboratory.
Funding
This work was supported by the General Programs of the National Natural Science Foundation of China (Nos. 81672865, 81461148021, and 81272529) and the Major Scientific and Technological Special Project for “significant new drugs creation” (No. 2013ZX09509103).
Availability of Data and Materials
The data included in this study are available. Microarray data is presented in Table S2, Table S3 and TCGA data is available at the public database (http://www.cbioportal.org/).
Authors' Contributions
C. Wang, Y. Luo and Y. Fu conceived and designed all the experiments. C. Wang conducted most of the experiments for this work and evaluated the data. A. J, Y. Tian, S. Xu and Q. Hou contributed to the animal manipulations. C. Wang, Y. Luo and Y. Fu wrote the manuscript. J. Liu helped to review the manuscript. All authors read and approved the final manuscript.
Ethics Approval and Consent to Participate
All animal studies were approved by the Institutional Animal Care and Use Committee of Tsinghua University (F16–00228; A5061–01). The breast tissue specimens are anonymous, and detail information was demonstrated in Supplementary material.
Consent for Publication
Not applicable.
Competing Interests
The authors disclose no potential conflicts of interest.
Footnotes
Competing interests statement: No conflicts of interest were declared.
References
- 1.Mehlen P, Puisieux A. Metastasis: a question of life or death. Nat Rev Cancer. 2006;6:449–458. doi: 10.1038/nrc1886. [DOI] [PubMed] [Google Scholar]
- 2.Paduch R. The role of lymphangiogenesis and angiogenesis in tumor metastasis. Cell Oncol (Dordr) 2016;39:397–410. doi: 10.1007/s13402-016-0281-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karaman S, Detmar M. Mechanisms of lymphatic metastasis. J Clin Invest. 2014;124:922–928. doi: 10.1172/JCI71606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sleeman JP, Thiele W. Tumor metastasis and the lymphatic vasculature. Int J Cancer. 2009;125:2747–2756. doi: 10.1002/ijc.24702. [DOI] [PubMed] [Google Scholar]
- 5.Stacker SA, Williams SP, Karnezis T, Shayan R, Fox SB, Achen MG. Lymphangiogenesis and lymphatic vessel remodelling in cancer. Nat Rev Cancer. 2014;14:159–172. doi: 10.1038/nrc3677. [DOI] [PubMed] [Google Scholar]
- 6.Stacker SA, Baldwin ME, Achen MG. The role of tumor lymphangiogenesis in metastatic spread. FASEB J. 2002;16:922–934. doi: 10.1096/fj.01-0945rev. [DOI] [PubMed] [Google Scholar]
- 7.Skobe M, Hawighorst T, Jackson DG, Prevo R, Janes L, Velasco P, Riccardi L, Alitalo K, Claffey K, Detmar M. Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat Med. 2001;7:192–198. doi: 10.1038/84643. [DOI] [PubMed] [Google Scholar]
- 8.Joukov V, Pajusola K, Kaipainen A, Chilov D, Lahtinen I, Kukk E, Saksela O, Kalkkinen N, Alitalo K. A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. EMBO J. 1996;15:290–298. [PMC free article] [PubMed] [Google Scholar]
- 9.Achen MG, Jeltsch M, Kukk E, Makinen T, Vitali A, Wilks AF, Alitalo K, Stacker SA. Vascular endothelial growth factor D (VEGF-D) is a ligand for the tyrosine kinases VEGF receptor 2 (Flk1) and VEGF receptor 3 (Flt4) Proc Natl Acad Sci U S A. 1998;95:548–553. doi: 10.1073/pnas.95.2.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jeltsch M, Kaipainen A, Joukov V, Meng X, Lakso M, Rauvala H, Swartz M, Fukumura D, Jain RK, Alitalo K. Hyperplasia of lymphatic vessels in VEGF-C transgenic mice. Science. 1997;276:1423–1425. doi: 10.1126/science.276.5317.1423. [DOI] [PubMed] [Google Scholar]
- 11.Hirakawa S, Kodama S, Kunstfeld R, Kajiya K, Brown LF, Detmar M. VEGF-A induces tumor and sentinel lymph node lymphangiogenesis and promotes lymphatic metastasis. J Exp Med. 2005;201:1089–1099. doi: 10.1084/jem.20041896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhuo W, Jia L, Song N, Lu XA, Ding Y, Wang X, Song X, Fu Y, Luo Y. The CXCL12-CXCR4 chemokine pathway: a novel axis regulates lymphangiogenesis. Clin Cancer Res. 2012;18:5387–5398. doi: 10.1158/1078-0432.CCR-12-0708. [DOI] [PubMed] [Google Scholar]
- 13.Lucero HA, Kagan HM. Lysyl oxidase: an oxidative enzyme and effector of cell function. Cell Mol Life Sci. 2006;63:2304–2316. doi: 10.1007/s00018-006-6149-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moon HJ, Finney J, Ronnebaum T, Mure M. Human lysyl oxidase-like 2. Bioorg Chem. 2014;57:231–241. doi: 10.1016/j.bioorg.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akiri G, Sabo E, Dafni H, Vadasz Z, Kartvelishvily Y, Gan N, Kessler O, Cohen T, Resnick M, Neeman M. Lysyl oxidase-related protein-1 promotes tumor fibrosis and tumor progression in vivo. Cancer Res. 2003;63:1657–1666. [PubMed] [Google Scholar]
- 16.Peng L, Ran YL, Hu H, Yu L, Liu Q, Zhou Z, Sun YM, Sun LC, Pan J, Sun LX. Secreted LOXL2 is a novel therapeutic target that promotes gastric cancer metastasis via the Src/FAK pathway. Carcinogenesis. 2009;30:1660–1669. doi: 10.1093/carcin/bgp178. [DOI] [PubMed] [Google Scholar]
- 17.Fong SF, Dietzsch E, Fong KS, Hollosi P, Asuncion L, He Q, Parker MI, Csiszar K. Lysyl oxidase-like 2 expression is increased in colon and esophageal tumors and associated with less differentiated colon tumors. Genes Chromosomes Cancer. 2007;46:644–655. doi: 10.1002/gcc.20444. [DOI] [PubMed] [Google Scholar]
- 18.Peinado H, Moreno-Bueno G, Hardisson D, Perez-Gomez E, Santos V, Mendiola M, de Diego JI, Nistal M, Quintanilla M, Portillo F. Lysyl oxidase-like 2 as a new poor prognosis marker of squamous cell carcinomas. Cancer Res. 2008;68:4541–4550. doi: 10.1158/0008-5472.CAN-07-6345. [DOI] [PubMed] [Google Scholar]
- 19.Park PG, Jo SJ, Kim MJ, Kim HJ, Lee JH, Park CK, Kim H, Lee KY, Kim H, Park JH. Role of LOXL2 in the epithelial-mesenchymal transition and colorectal cancer metastasis. Oncotarget. 2017;8:80325–80335. doi: 10.18632/oncotarget.18170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peinado H, Del Carmen Iglesias-de la Cruz M, Olmeda D, Csiszar K, Fong KS, Vega S, Nieto MA, Cano A, Portillo F. A molecular role for lysyl oxidase-like 2 enzyme in snail regulation and tumor progression. EMBO J. 2005;24:3446–3458. doi: 10.1038/sj.emboj.7600781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moon HJ, Finney J, Xu L, Moore D, Welch DR, Mure M. MCF-7 cells expressing nuclear associated lysyl oxidase-like 2 (LOXL2) exhibit an epithelial-to-mesenchymal transition (EMT) phenotype and are highly invasive in vitro. J Biol Chem. 2013;288:30000–30008. doi: 10.1074/jbc.C113.502310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moreno-Bueno G, Salvador F, Martin A, Floristan A, Cuevas EP, Santos V, Montes A, Morales S, Castilla MA, Rojo-Sebastian A. Lysyl oxidase-like 2 (LOXL2), a new regulator of cell polarity required for metastatic dissemination of basal-like breast carcinomas. EMBO Mol Med. 2011;3:528–544. doi: 10.1002/emmm.201100156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wong CC, Tse AP, Huang YP, Zhu YT, Chiu DK, Lai RK, Au SL, Kai AK, Lee JM, Wei LL. Lysyl oxidase-like 2 is critical to tumor microenvironment and metastatic niche formation in hepatocellular carcinoma. Hepatology. 2014;60:1645–1658. doi: 10.1002/hep.27320. [DOI] [PubMed] [Google Scholar]
- 24.Weidenfeld K, Schif-Zuck S, Abu-Tayeh H, Kang K, Kessler O, Weissmann M, Neufeld G, Barkan D. Dormant tumor cells expressing LOXL2 acquire a stem-like phenotype mediating their transition to proliferative growth. Oncotarget. 2016;7:71362–71377. doi: 10.18632/oncotarget.12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Christiansen A, Detmar M. Lymphangiogenesis and cancer. Genes Cancer. 2011;2:1146–1158. doi: 10.1177/1947601911423028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jung ST, Kim MS, Seo JY, Kim HC, Kim Y. Purification of enzymatically active human lysyl oxidase and lysyl oxidase-like protein from Escherichia coli inclusion bodies. Protein Expr Purif. 2003;31:240–246. doi: 10.1016/s1046-5928(03)00217-1. [DOI] [PubMed] [Google Scholar]
- 27.Rodriguez HM, Vaysberg M, Mikels A, McCauley S, Velayo AC, Garcia C, Smith V. Modulation of lysyl oxidase-like 2 enzymatic activity by an allosteric antibody inhibitor. J Biol Chem. 2010;285:20964–20974. doi: 10.1074/jbc.M109.094136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang Y, Shi H, Zhou H, Song X, Yuan S, Luo Y. The angiogenic function of nucleolin is mediated by vascular endothelial growth factor and nonmuscle myosin. Blood. 2006;107:3564–3571. doi: 10.1182/blood-2005-07-2961. [DOI] [PubMed] [Google Scholar]
- 29.Wang QS, He R, Yang F, Kang LJ, Li XQ, Fu L, Sun B, Feng YM. FOXF2 deficiency permits basal-like breast cancer cells to form lymphangiogenic mimicry by enhancing the response of VEGF-C/VEGFR3 signaling pathway. Cancer Lett. 2018;420:116–126. doi: 10.1016/j.canlet.2018.01.069. [DOI] [PubMed] [Google Scholar]
- 30.Barker HE, Chang J, Cox TR, Lang G, Bird D, Nicolau M, Evans HR, Gartland A, Erler JT. LOXL2-mediated matrix remodeling in metastasis and mammary gland involution. Cancer Res. 2011;71:1561–1572. doi: 10.1158/0008-5472.CAN-10-2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barker HE, Bird D, Lang G, Erler JT. Tumor-secreted LOXL2 activates fibroblasts through FAK signaling. Mol Cancer Res. 2013;11:1425–1436. doi: 10.1158/1541-7786.MCR-13-0033-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mueller L, Goumas FA, Himpel S, Brilloff S, Rogiers X, Broering DC. Imatinib mesylate inhibits proliferation and modulates cytokine expression of human cancer-associated stromal fibroblasts from colorectal metastases. Cancer Lett. 2007;250:329–338. doi: 10.1016/j.canlet.2006.10.024. [DOI] [PubMed] [Google Scholar]
- 33.Nagasaki T, Hara M, Nakanishi H, Takahashi H, Sato M, Takeyama H. Interleukin-6 released by colon cancer-associated fibroblasts is critical for tumour angiogenesis: anti-interleukin-6 receptor antibody suppressed angiogenesis and inhibited tumour-stroma interaction. Br J Cancer. 2014;110:469–478. doi: 10.1038/bjc.2013.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL, Weinberg RA. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 35.Coso S, Zeng Y, Sooraj D, Williams ED. Conserved signaling through vascular endothelial growth (VEGF) receptor family members in murine lymphatic endothelial cells. Exp Cell Res. 2011;317:2397–2407. doi: 10.1016/j.yexcr.2011.07.023. [DOI] [PubMed] [Google Scholar]
- 36.Lee JG, Jung E, Heur M. Fibroblast growth factor 2 induces proliferation and fibrosis via SNAI1-mediated activation of CDK2 and ZEB1 in corneal endothelium. J Biol Chem. 2018;293:3758–3769. doi: 10.1074/jbc.RA117.000295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang SH, Chang JS, Hsiao JR, Yen YC, Jiang SS, Liu SH, Chen YL, Shen YY, Chang JY, Chen YW. Tumour cell-derived WNT5B modulates in vitro lymphangiogenesis via induction of partial endothelial-mesenchymal transition of lymphatic endothelial cells. Oncogene. 2017;36:1503–1515. doi: 10.1038/onc.2016.317. [DOI] [PubMed] [Google Scholar]
- 38.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 39.Buchsbaum RJ, Oh SY. Breast Cancer-Associated Fibroblasts: Where We Are and Where We Need to Go. Cancers (Basel) 2016;8 doi: 10.3390/cancers8020019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schindl M, Schoppmann SF, Samonigg H, Hausmaninger H, Kwasny W, Gnant M, Jakesz R, Kubista E, Birner P, Oberhuber G. Overexpression of hypoxia-inducible factor 1alpha is associated with an unfavorable prognosis in lymph node-positive breast cancer. Clin Cancer Res. 2002;8:1831–1837. [PubMed] [Google Scholar]
- 41.Ran S, Volk L, Hall K, Flister MJ. Lymphangiogenesis and lymphatic metastasis in breast cancer. Pathophysiology. 2010;17:229–251. doi: 10.1016/j.pathophys.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tobler NE, Detmar M. Tumor and lymph node lymphangiogenesis--impact on cancer metastasis. J Leukoc Biol. 2006;80:691–696. doi: 10.1189/jlb.1105653. [DOI] [PubMed] [Google Scholar]
- 43.Pereira ER, Kedrin D, Seano G, Gautier O, Meijer EFJ, Jones D, Chin SM, Kitahara S, Bouta EM, Chang J. Lymph node metastases can invade local blood vessels, exit the node, and colonize distant organs in mice. Science. 2018;359:1403–1407. doi: 10.1126/science.aal3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brown M, Assen FP, Leithner A, Abe J, Schachner H, Asfour G, Bago-Horvath Z, Stein JV, Uhrin P, Sixt M. Lymph node blood vessels provide exit routes for metastatic tumor cell dissemination in mice. Science. 2018;359:1408–1411. doi: 10.1126/science.aal3662. [DOI] [PubMed] [Google Scholar]
- 45.Chang J, Lucas MC, Leonte LE, Garcia-Montolio M, Singh LB, Findlay AD, Deodhar M, Foot JS, Jarolimek W, Timpson P. Pre-clinical evaluation of small molecule LOXL2 inhibitors in breast cancer. Oncotarget. 2017;8:26066–26078. doi: 10.18632/oncotarget.15257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Salvador F, Martin A, Lopez-Menendez C, Moreno-Bueno G, Santos V, Vazquez-Naharro A, Santamaria PG, Morales S, Dubus PR, Muinelo-Romay L. Lysyl Oxidase-like Protein LOXL2 Promotes Lung Metastasis of Breast Cancer. Cancer Res. 2017;77:5846–5859. doi: 10.1158/0008-5472.CAN-16-3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zaffryar-Eilot S, Marshall D, Voloshin T, Bar-Zion A, Spangler R, Kessler O, Ghermazien H, Brekhman V, Suss-Toby E, Adam D. Lysyl oxidase-like-2 promotes tumour angiogenesis and is a potential therapeutic target in angiogenic tumours. Carcinogenesis. 2013;34:2370–2379. doi: 10.1093/carcin/bgt241. [DOI] [PubMed] [Google Scholar]
- 48.de Jong OG, van der Waals LM, Kools FRW, Verhaar MC, van Balkom BWM. Lysyl oxidase-like 2 is a regulator of angiogenesis through modulation of endothelial-to-mesenchymal transition. J Cell Physiol. 2018 doi: 10.1002/jcp.27695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bignon M, Pichol-Thievend C, Hardouin J, Malbouyres M, Brechot N, Nasciutti L, Barret A, Teillon J, Guillon E, Etienne E. Lysyl oxidase-like protein-2 regulates sprouting angiogenesis and type IV collagen assembly in the endothelial basement membrane. Blood. 2011;118:3979–3989. doi: 10.1182/blood-2010-10-313296. [DOI] [PubMed] [Google Scholar]
- 50.Park JA, Kim DY, Kim YM, Lee IK, Kwon YG. Endothelial Snail Regulates Capillary Branching Morphogenesis via Vascular Endothelial Growth Factor Receptor 3 Expression. PLoS Genet. 2015;11 doi: 10.1371/journal.pgen.1005324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Luo H, Tu G, Liu Z, Liu M. Cancer-associated fibroblasts: a multifaceted driver of breast cancer progression. Cancer Lett. 2015;361:155–163. doi: 10.1016/j.canlet.2015.02.018. [DOI] [PubMed] [Google Scholar]
- 52.Wong CC, Gilkes DM, Zhang H, Chen J, Wei H, Chaturvedi P, Fraley SI, Wong CM, Khoo US, Ng IO. Hypoxia-inducible factor 1 is a master regulator of breast cancer metastatic niche formation. Proc Natl Acad Sci U S A. 2011;108:16369–16374. doi: 10.1073/pnas.1113483108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
siRNA sequences of the Snail and HIF-1α Gene.
The clinical features of patient samples in the tissue microarray (BR1006a).
The clinical features of patient samples in the tissue microarray (BR2161).
Data Availability Statement
The data included in this study are available. Microarray data is presented in Table S2, Table S3 and TCGA data is available at the public database (http://www.cbioportal.org/).