Abstract
Background
A 50-year-old mother of four children was newly diagnosed with arterial hypertension and bilateral neck pulsations.
Case summary
Her current blood pressure was 170/100 mmHg in the right arm and 122 mmHg systolic in the right ankle. There was a radio-femoral delay palpable. The electrocardiogram showed signs of left ventricular hypertrophy. On the chest X-ray, a figure of 3-sign was found at the aortic knuckle and notching of the inferior ribs was present. An echocardiogram showed concentric left ventricular hypertrophy, a mildly stenotic bicuspid aortic valve, and a low peak-gradient across the descending aorta. Magnetic resonance imaging demonstrated severe focal coarctation with complete interruption of the descending aorta. Large collaterals vessels were present, effectively bridging the aortic interruption.
Discussion
In light of the extensive collateral vessels and the bleeding risk, an extra-anatomic aortic bypass was considered the least risky procedure. The patient agreed to the intervention and had an uncomplicated surgical course and recovery. At the 12-month follow-up, she was doing well and normotensive on Lisinopril 5 mg OD.
Keywords: Coarctation of the aorta, Hypertension, Extra-anatomic aortic bypass, Case report
Learning points
Aortic coarctation (CoA) is sometimes diagnosed in adult life.
Clinical findings like an arm–leg blood pressure difference or typical chest X-ray signs may suggest an underlying diagnosis of CoA.
A low peak gradient on echocardiography in the aortic isthmus does not exclude severe CoA.
Introduction
Coarctation of the aorta (CoA) is a common heart defect (5–8% of all congenital cardiac defects). In historical cohorts, 25% of children with severe CoA survived to age 46 years1 and 10% to age 58 years without intervention.2 Death in patients with un-repaired CoA was due to aortic rupture or dissection, heart failure, coronary artery disease, infective endocarditis, or cerebral haemorrhage.3 Despite being a potentially life-threating cardiac defect, CoA is sometimes diagnosed only in adult life.4,5 This case describes the late presentation of severe CoA in an apparently asymptomatic adult woman.
Timeline
| Childhood |
|
| 1994, 1997, 2000, 2003 |
|
| January 2016 |
|
| June 2016 |
|
| December 2016 |
|
| December 2017 |
|
Case presentation
Patient information
A 50-year-old female presented with a history of newly diagnosed arterial hypertension and prominent neck pulsations. Hypertensive blood pressures were incidentally diagnosed by the family physician following a flu episode. Forty years ago, an incidental heart murmur was noted. It was considered to be an innocent childhood flow murmer and no further follow-up was scheduled. The patient reported a medically uneventful childhood and adolescence. Between 1997 and 2003, she had four uncomplicated pregnancies. During the last pregnancy, mild arterial hypertension was noted for the first time. On magnetic resonance imaging (MRI) of her head for evaluation of the neck pulsations, enlarged bilateral carotid arteries were noted.
The patient was referred to our institution for further evaluation. She had office blood pressure recordings of 170/100 mmHg on her right arm. On clinical inspection, pronounced bilateral neck pulsations were noted. On palpitation, a palpable sustained left apical impulse was felt. On auscultation, she had a normal first heart sound, the second heart sound was normally split with respiratory variability, but the aortic component was prominent. She had a Grade 2/6 aortic ejection systolic murmur with radiation to the carotids and weakly palpable pulses in both lower limbs with a radio-femoral delay. There was systolic blood pressure difference of 50 mmHg between her right arm and the left leg.
Diagnostic assessment
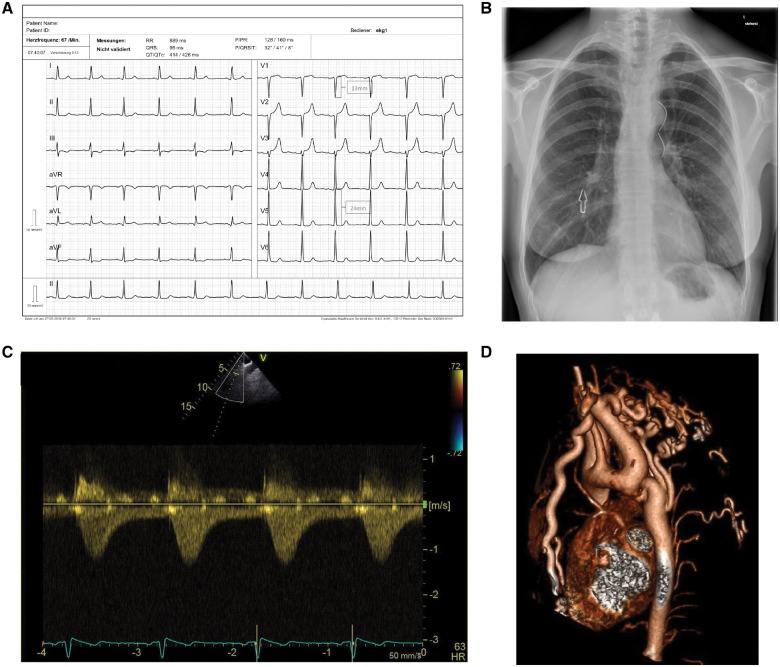
The electrocardiogram showed sinus rhythm and signs of left ventricular hypertrophy (Figure 1A). On chest X-ray, there was a figure of 3-sign at the aortic knuckle and notching of the inferior ribs (Figure 1B). A transthoracic echocardiogram showed concentric left ventricular hypertrophy, a mildly stenotic bicuspid aortic valve (BAV) with a mean/peak gradient of 12/22 mmHg, a normally sized ascending aorta and aortic arch, and a peak gradient of 6 mmHg with a serrated flow pattern in the continuous wave Doppler (CW-Doppler) across the region of the aortic isthmus (Figure 1C). The aortic flow pattern in the abdominal aorta demonstrated continuous diastolic flow. The flow pattern across the aortic isthmus region and in the abdominal aorta was highly suggestive of hemodynamically important CoA, despite the low peak gradient. On a cardiac MRI severe focal CoA with probable complete interruption after the aortic arch was noted. On cardiac catherization with aortic angiography (access via right femoral and right radial artery), no patency between the aortic arch and the thoracic aorta was detected, indicating complete aortic interruption. There was no coronary artery disease. Abdominal blood flow was maintained by numerous large collateral vessels (Figure 1D) contributing to the enlargement of both carotid arteries. The origin of the low peak gradient in the CW-Doppler was uncertain. It may be a velocity measured across one of the collateral vessels.
Figure 1.
(A) Resting 12-lead electrocardiogram with signs of left ventricular hypertrophy (positive Sokolow-Lyon index). (B) Chest X-ray with a figure of 3-sign produced by the dilated left subclavian artery, the narrowing at the aortic isthmus, and the post-stenotic dilatation of the descending aorta. There is also notching of the ribs (arrow). (C) Continuous wave Doppler across the region of the aortic isthmus shows a serrated flow pattern and low transcoarctation velocities of 1.3 m/s. (D) On a cardiac magnetic resonance imaging severe focal coarctation of the aorta with probable complete interruption after the aortic arch was present. Abdominal blood flow was maintained by numerous large collateral vessels.
Interventions
The risks of a percutaneous approach with needle puncture and wire crossing of the interrupted aorta vs. a surgical repair strategy were discussed. A resection of the stenotic aorta with end-to-end anastomosis was not possible, as the gap between the two parts of the aorta was too large. An interposition graft or the placement of an ascending to descending aortic bypass were evaluated. In light of the extensive collateral vessels and the bleeding risk, an extra-anatomic aortic bypass was considered the least risky procedure. In situ anatomic repair is associated with paraplegia risk of 3–5%. An extra-anatomic bypass is thought to reduce the risk of spinal ischaemia because blood supply is preserved during the procedure.6 The patient agreed to the intervention and had an uncomplicated surgical course and recovery.
Follow-up and outcomes
During 3- and 12-month follow-up, she was doing well and was normotensive. The antihypertensive therapy prior to surgery consisted of Irbesartan 150 mg OD. At discharge from the surgical ward, the antihypertensive therapy had been switched to Lisinopril 5 mg OD and Metoprolol 25 mg OD. At the last visit 1 year after the intervention, she had normotensive blood pressures on Lisinopril 5 mg OD only. A cardiac MRI with contrast angiography 6 months after surgery showed no complications (stenosis, pseudoaneurysm formation) at the anastomosis sites.
Discussion
Coarctation of the aorta is a congenital defect with variable clinical presentation, depending on the location and severity of the aortic narrowing. In clinical practice, CoA is classified as pre-ductal or post-ductal. In the pre-ductal CoA type, the flow to the distal aorta depends in utero on the ductus arteriosus. Once the duct closes after birth, the infant may become severely symptomatic in a short time. Collateral vessel formation is usually absent, because the flow to the abdominal aorta is maintained in utero by the ductus arteriosus.7 In post-ductal CoA, the narrowing is distal to insertion of the ductus arteriosus. In this type of CoA, collateral vessel formation may already occur in utero. These patients may be asymptomatic after ductal closure in the presence of sufficient collateral circulation. Coarctation of the aorta is associated with a BAV,8–10 subaortic stenosis, mitral valve abnormalities such as parachute mitral valve, a ventricular septal defect, and circle of Willis cerebral artery aneurysms.1,11
In native CoA or after re-coarctation, indications for treatment include a non-invasive peak-to-peak pressure difference >20 mmHg between upper and lower limbs in combination with proximal hypertension, or a pathological blood pressure response during exercise, or significant left ventricular hypertrophy. Independent of the pressure gradient, hypertensive patients with ≥50% aortic narrowing relative to the aortic diameter at the diaphragm level (on cardiac MRI, computed tomography, or invasive angiography) should also be considered for intervention.12
Common late cardiovascular complications in repaired patients are systemic hypertension,13 premature coronary artery disease due to longstanding hypertension,14 re-coarctation,15 aortic arch hypoplasia,16,17 and major aortic wall complications, such as true or false aortic aneurysm,18 rupture,19 dissection,20 endarteritis,21 and fistula.22
Early repair does not preclude the development of late arterial hypertension, but is associated with a lower likelihood in adult life.23 In patients with late repair (i.e. in late adolescence or adult life) arterial hypertension may persist even after complete relief of the aortic obstruction and long-term antihypertensive drugs may be necessary, albeit in usually lower doses than prior to the intervention. In adults with native CoA and appropriate anatomy, stenting has become the treatment of first choice in many centres. For adults with recurring or residual CoA, angioplasty with or without stent implantation has been shown to be safe and effective. In our case with an interrupted aorta and extensive collateral vessels, surgery with an extra-anatomic bypass grafting was considered to be less risky than a transluminal intervention.
Echocardiography is the first line diagnostic imaging test for diagnosis and follow-up. The blood velocity proximal to the CoA site should be measured with pulsed wave Doppler. If this velocity is >1 m/s, it should be subtracted from the peak velocity at the CoA site obtained with CW-Doppler to avoid overestimation of the aortic gradient: transcoarcation gradient = 4 (v2peak − v2PW Doppler) mmHg. In the presence of significant collateral vessels, the transcoarctation velocities may be low since the pressure distal to the CoA is maintained by collateral flow. Hence, a low transcoarctation peak gradient on CW-Doppler echocardiography does not exclude a severe stenosis. A ‘serrated’ CW-Doppler flow tracing in the isthmus region with rapid acceleration followed by gradual deceleration throughout diastole is a characteristic echocardiography sign of CoA. Pulsed Doppler evaluation of the abdominal aorta typically shows low-velocity systolic-diastolic flow with little phasic variations in this setting.
Besides echocardiography, conventional X-ray images can point to the correct diagnosis. Notching of the ribs is a classic sign caused by collateral flow through dilated pulsatile intercostal arteries. It appears seldom before 6 years of age and is rare above the third or below the ninth rib.24 The combination of a dilated left subclavian artery, the aortic narrowing at the isthmic side and post-stenotic aortic dilatation create the figure of 3-sign, also present in our case.
Patient perspective
The patient needs life-long specialized cardiac care because of the potential long-term complications after successful repair mentioned above. She continues to take antihypertensive medication; however, her blood pressure is now well controlled with a small dose of Lisinopril.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The author/s confirm that written consent for submission and publication of this case report including image(s) and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: none declared.
Supplementary Material
References
- 1. Warnes CA, Williams RG, Bashore TM, Child JS, Connolly HM, Dearani JA, del Nido P, Fasules JW, Graham TP, Jr., Hijazi ZM, Hunt SA, King ME, Landzberg MJ, Miner PD, Radford MJ, Walsh EP, Webb GD. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to develop guidelines on the management of adults with congenital heart disease). Circulation 2008;118:e714–e833. [DOI] [PubMed] [Google Scholar]
- 2. Campbell M. Natural history of coarctation of the aorta. Br Heart J 1970;32:633–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cohen M, Fuster V, Steele PM, Driscoll D, McGoon DC.. Coarctation of the aorta: long-term follow-up and prediction of outcome after surgical correction. Circulation 1989;80:840–845. [DOI] [PubMed] [Google Scholar]
- 4. Zivelonghi C, Pighi M, Perandini S, Vassanelli C, Ribichini F.. Asymptomatic severe aortic coarctation at old age. Int J Cardiol 2014;173:e56–e57. [DOI] [PubMed] [Google Scholar]
- 5. Ouali S, Kortas C, Brockmeier K, Boughzela E.. Adult aortic coarctation discovered incidentally after the rupture of sinus of Valsalva aneurysm: combined surgical and interventional approach. Interact Cardiovasc Thorac Surg 2011;13:688–690. [DOI] [PubMed] [Google Scholar]
- 6. Schoenhoff FS, Berdat PA, Pavlovic M, Kadner A, Schwerzmann M, Pfammatter J-P, Carrel TP.. Off-pump extraanatomic aortic bypass for the treatment of complex aortic coarctation and hypoplastic aortic arch. Ann Thorac Surg 2008;85:460–464. [DOI] [PubMed] [Google Scholar]
- 7. Hoffman JIE. The challenge in diagnosing coarctation of the aorta. Cardiovasc J Afr 2018;29:252–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bondy C, Bakalov VK, Cheng C, Olivieri L, Rosing DR, Arai AE.. Bicuspid aortic valve and aortic coarctation are linked to deletion of the X chromosome short arm in Turner syndrome. J Med Genet 2013;50:662–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brickner ME, Hillis LD, Lange RA.. Congenital heart disease in adults. N Engl J Med 2000;342:256–263. [DOI] [PubMed] [Google Scholar]
- 10. Roos-Hesselink JW, Schölzel BE, Heijdra RJ, Spitaels SE, Meijboom FJ, Boersma E, Bogers AJ, Simoons ML. Aortic valve and aortic arch pathology after coarctation repair. Heart 2003;89:1074–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Connolly HM, Huston J 3rd, Brown RD Jr, Warnes CA, Ammash NM, Tajik AJ.. Intracranial aneurysms in patients with coarctation of the aorta: a prospective magnetic resonance angiographic study of 100 patients. Mayo Clin Proc 2003;78:1491–1499. [DOI] [PubMed] [Google Scholar]
- 12. Baumgartner H, Bonhoeffer P, De Groot NM, de Haan F, Deanfield JE, Galie N, Gatzoulis MA, Gohlke-Baerwolf C, Kaemmerer H, Kilner P, Meijboom F, Mulder BJ, Oechslin E, Oliver JM, Serraf A, Szatmari A, Thaulow E, Vouhe PR, Walma E, Vahanian A, Auricchio A, Bax J, Ceconi C, Dean V, Filippatos G, Funck-Brentano C, Hobbs R, Kearney P, McDonagh T, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Vardas P, Widimsky P, Swan L, Andreotti F, Beghetti M, Borggrefe M, Bozio A, Brecker S, Budts W, Hess J, Hirsch R, Jondeau G, Kokkonen J, Kozelj M, Kucukoglu S, Laan M, Lionis C, Metreveli I, Moons P, Pieper PG, Pilossoff V, Popelova J, Price S, Roos-Hesselink J, Uva MS, Tornos P, Trindade PT, Ukkonen H, Walker H, Webb GD, Westby J. ESC guidelines for the management of grown-up congenital heart disease (new version 2010). Eur Heart J 2010;31:2915–2957. [DOI] [PubMed] [Google Scholar]
- 13. Daniels SR. Repair of coarctation of the aorta and hypertension: does age matter? Lancet 2001;358:89.. [DOI] [PubMed] [Google Scholar]
- 14. Stewart AB, Ahmed R, Travill CM, Newman CG.. Coarctation of the aorta life and health 20–44 years after surgical repair. Br Heart J 1993;69:65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dodge-Khatami A, Backer CL, Mavroudis C.. Risk factors for recoarctation and results of reoperation: a 40-year review. J Card Surg 2000;15:369–377. [DOI] [PubMed] [Google Scholar]
- 16. Jost CHA, Schaff HV, Connolly HM, Danielson GK, Dearani JA, Puga FJ, Warnes CA.. Spectrum of reoperations after repair of aortic coarctation: importance of an individualized approach because of coexistent cardiovascular disease. Mayo Clin Proc 2002;77:646–653. [DOI] [PubMed] [Google Scholar]
- 17. Ma ZL, Yan J, Li SJ, Hua ZD, Yan FX, Wang X, Wang Q. Coarctation of the aorta with aortic arch hypoplasia: midterm outcomes of aortic arch reconstruction with autologous pulmonary artery patch. Chin Med J 2017;130:2802–2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kirsh MM, Perry B, Spooner E.. Management of pseudoaneurysm following patch grafting for coarctation of the aorta. J Thorac Cardiovasc Surg 1977;74:636–639. [PubMed] [Google Scholar]
- 19. Parks WJ, Ngo TD, Plauth WH, Bank ER, Sheppard SK, Pettigrew RI, Williams WH.. Incidence of aneurysm formation after Dacron patch aortoplasty repair for coarctation of the aorta: long-term results and assessment utilizing magnetic resonance angiography with three-dimensional surface rendering. J Am Coll Cardiol 1995;26:266–271. [DOI] [PubMed] [Google Scholar]
- 20. Moodie DS. Aortic dissection and coarctation. Curr Opin Cardiol 1990;5:649–654. [DOI] [PubMed] [Google Scholar]
- 21. Perez Day CM, Angela MP, Mangione SA, Furque JC.. Coarctation of the aorta complicated by infectious endarteritis, mycotic aneurysm and rupture of the spleen. Rev Esp Cardiol 1986;39:68–71. [PubMed] [Google Scholar]
- 22. Favre JP, Gournier JP, Adham M, Rosset E, Barral X.. Aortobronchial fistula: report of three cases and review of the literature. Surgery 1994;115:264–270. [PubMed] [Google Scholar]
- 23. Van Son JA, Mohr FW, Hess H, Hambsch J, Haas GS.. Early repair of coarctation of the aorta. Ann Thorac Cardiovasc Surg 1999;5:237–244. [PubMed] [Google Scholar]
- 24. Boone ML, Swenson BE, Felson B.. Rib notching: its many causes. Am J Roentgenol Radium Ther Nucl Med 1964;91:1075.. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.