Abstract
Background
Ectatic coronary segments are nidi for thrombus formation due to altered flow dynamics and stasis—an important component of Virchow’s triad. Ectasia accompanied by an adjacent coronary stenosis with or without a plaque event can lead to acute myocardial infarctions complicated by huge thrombus burden.
Case summary
Here, we present a case of a young male with acute inferior wall myocardial infarction complicated by Type IV coronary artery ectasia of the right coronary artery and huge thrombus burden that was refractory to conventional methods of thrombus management like thrombo-suction and intracoronary cocktails including tenecteplase. After a stormy course of transient complete heart block and recurrent ventricular tachycardia, the thrombus was successfully extracted using a stent retriever to achieve thrombolysis in myocardial infarction (TIMI) II plus flow distally. The lesion just distal to the ectasia was stented 24 h later to achieve TIMI III flow and the patient had an uneventful recovery subsequently.
Discussion
Angiographically visible thrombus that is refractory to intracoronary medications and aspiration thrombectomy can be successfully managed using a stent retriever.
Keywords: Coronary artery ectasia, Thrombectomy, Solitaire AB, Thrombus retrieval, Case report
Learning points
Coronary ectasia can complicate acute myocardial infarction with huge thrombus burden that is resistant to conventional thrombus management strategy.
Thrombus retrieval devices like Solitaire AB system can work in such situations where aspiration thrombectomy has failed.
Introduction
Focal or diffuse dilatation of coronary artery up to 1.5 times the adjacent normal vessel with a width to length ratio of the dilated segment <1 differentiates a coronary artery ectasia (CAE) from a coronary artery aneurysm (CAA: >1.5 times and width: length >1). Based on the number and extent of vessels involved it’s further classified into four types by Markis et al.1 Localized CAE involving a single vessel is classified as Type IV and is one of the most frequent types, most commonly involving the proximal or mid-right coronary artery (RCA).1 Coronary artery ectasia can present as acute myocardial infarction with or without an underlying plaque event and can complicate primary percutaneous intervention (PPCI) because of the associated high thrombus burden that can cause distal embolization and no reflow. The success of PPCI in the presence of CAE is suboptimal due to slow flow during the procedure and floating stent struts in the ectatic segments leading to recurrent events in the long term.2,3
Timeline
| Time | Events |
|---|---|
| Prior to presentation |
|
| Day 1 |
|
| Day 2 |
|
| Day 3 |
|
| Day 4 |
|
| 1- and 6-month follow-up |
|
Case presentation
A 30-year-old male smoker presented with a 2 h history of compressive type of central chest pain, associated with sweating and radiation to left shoulder. It was not associated with nausea, vomiting, breathlessness, or syncope and there were no other conventional risk factors of coronary artery disease (CAD). His prior functional capacity was good without any angina or dyspnoea.
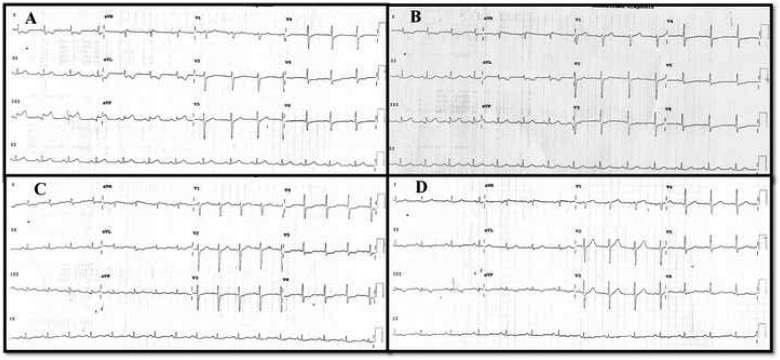
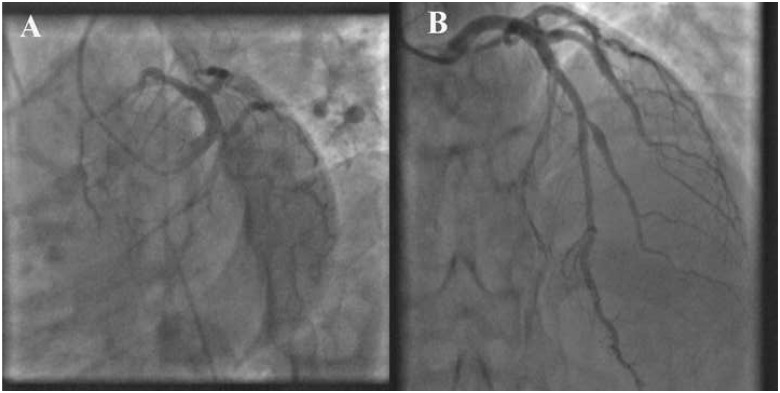
In the emergency room, he was anxious with a pulse rate of 90 b.p.m. and a blood pressure of 130/80 mmHg. The neck veins were not distended. His room air oxygen saturation was 99%, with normal heart sounds and there were no murmurs or rales on auscultation. An electrocardiogram (ECG) showed sinus rhythm with ST-segment elevation inferior wall myocardial infarction (Figure1A). Transthoracic echocardiogram showed hypokinesia of the basal and mid-inferior wall with mild mitral regurgitation and adequate left ventricular ejection fraction. He was promptly transferred to the catheterization laboratory after loading with 325 mg of aspirin, 600 mg of clopidogrel, and 80 mg of atorvastatin. Coronary angiogram via right radial access showed an occluded proximal RCA (Figure2A) and minimal disease of the left coronary artery (Figure3A and B,Supplementary material online, Video S1) and its branches.
Figure 1.
(A) Electrocardiogram at presentation showing ST elevation inferior wall myocardial infarction. (B) Electrocardiogram after thrombus retrieval showed near complete resolution of ST segment in inferior leads. (C) Twenty-four hours later the electrocardiogram showed a small Q wave with complete ST-segment resolution. (D) The Q wave become deep and T wave got inverted after stenting.
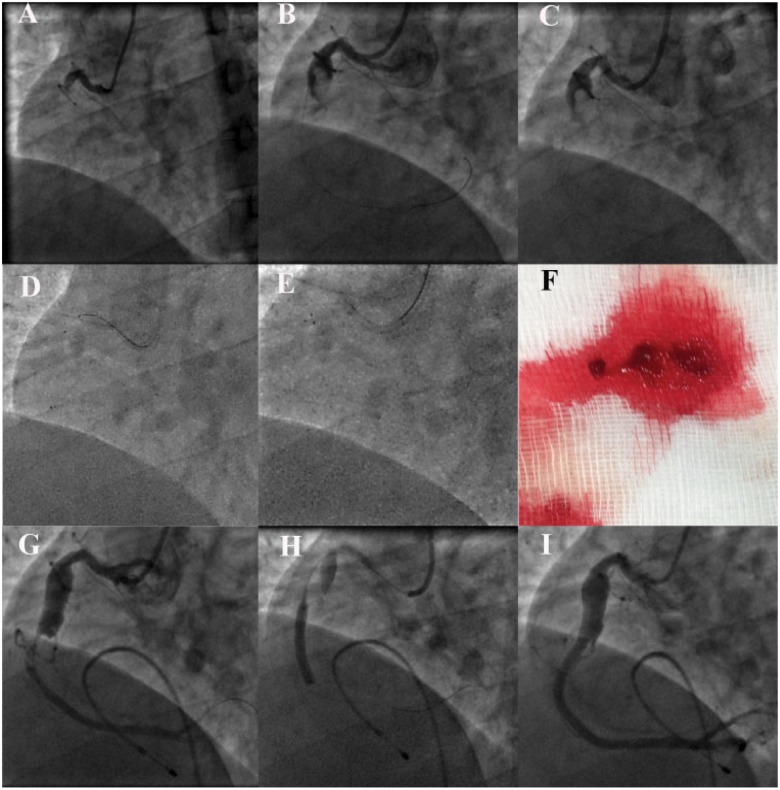
Figure 2.
(A) Occluded proximal right coronary artery. (B) Proximal right coronary artery coronary artery ectasia with a large thrombus. (C) Rebar micro-catheter tip at mid-right coronary artery and the wire removed. (D) Solitaire AB distal tip positioned in the mid-right coronary artery and partially unsheathed by pulling the micro-catheter out. (E) Pulling back the retriever into the guide catheter in the deployed state. (F) Retrieved thrombus. (G) Thrombolysis in myocardial infarction II flow in distal right coronary artery with a tight lesion just distal to the coronary artery ectasia. (H) Lesion being stented. (I) Final result after post-dilatation.
Figure 3.
(A, B) Left coronary artery in caudal and cranial view showed minimal disease involving its branches.
Primary percutaneous intervention was planned and the RCA was engaged using a 6-Fr JR 3.5 guiding catheter. Repeat acquisition after crossing the RCA lesion with a 0.014″ Floppy wire showed a huge ectatic segment (Figure2B andSupplementary material online, Video S1) involving the proximal to mid-RCA segment with a large thrombus within the ectatic segment. Repeated manual aspiration thrombectomy using a 6-Fr Export catheter failed to clear the thrombus. Intracoronary (i.c.) thrombolysis using 10 mg of tenecteplase induced recurrent ventricular tachycardia that was successfully cardioverted, but the thrombus burden was persistent. Our previous experience with Solitaire AB for thrombus retrieval from cerebral arteries in stroke patients prompted us to use it in this desperate situation.
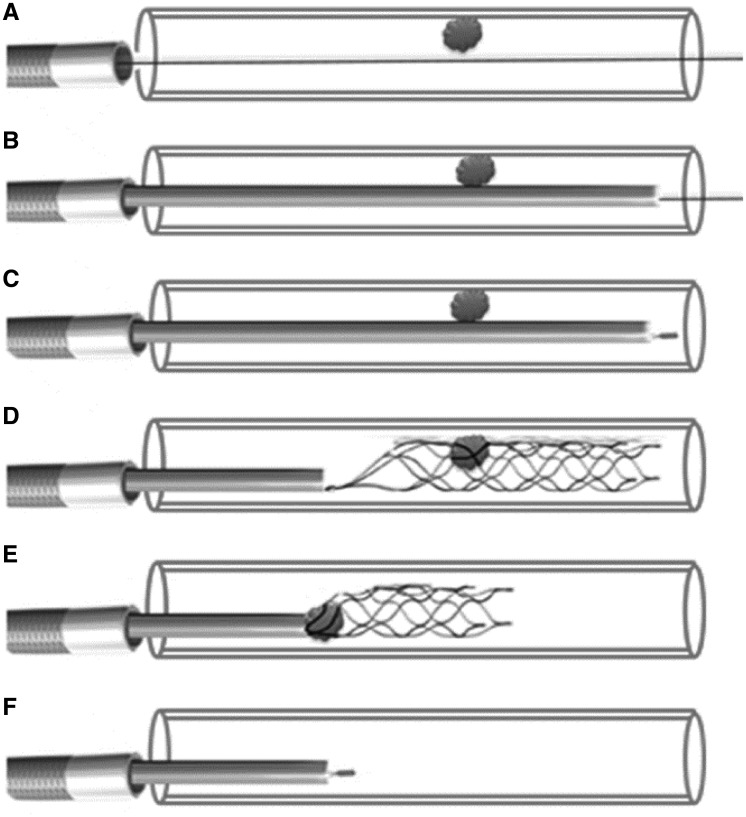
The Solitaire device is a self-expanding a-traumatic nitinol stent attached to a pusher and it requires a 2.4-Fr micro-catheter (Rebar) for its delivery and re-capture. The first step is to advance the micro-catheter over the wire and to position its tip just distal to the thrombotic segment. After removing the wire, the solitaire is back loaded into the micro-catheter and pushed up to its tip that is already positioned in the coronary just distal to the thrombus. Now the micro-catheter is pulled back using one hand, simultaneously maintaining the position of the solitaire device by holding the pusher in place using the other hand. This manoeuvre will un-sheath the device across the thrombus and entangles it. Next the device is pulled back into micro-catheter along with the entangled thrombus. Finally the micro-catheter and the recaptured solitaire device (with the retrieved thrombus) are pulled out of the guide catheter with simultaneous aspiration from the proximal hub of the guide catheter. Technically this leads to loss of the wire after each retrieval, and some operators prefer complete removal of the guide catheter; this also allows the operator to flush out any thrombus that might be retained within it. A line diagram (Figure4) is presented here to illustrate the procedural technique.
Figure 4.
Line diagram to illustrate the technique of using the thrombus retrieval device. (A) The vessel with thrombus is crossed with a workhorse wire that was pre-loaded with a Rebar micro-catheter. (B) Rebar micro-catheter was advanced beyond the thrombus. (C) The wire was removed; an appropriate sized Solitaire AB device was back loaded and advanced to the tip of the micro-catheter positioned beyond the thrombus. (D) The micro-catheter was pulled back maintaining the tip of the device at the same position. This makes the stent expand and trap the thrombus between its struts. (E) The stent device is then pulled back into the micro-catheter along with the trapped thrombus. (F) Once the device is completely within the micro-catheter the whole system is pulled out.
The 153 cm, 2.4-Fr Rebar micro-catheter was pre-loaded with a 0.014″ Floppy wire and its tip was advanced distal to the thrombus in the ectatic segment over the wire. Then a 4 × 20 mm Solitaire AB stent was back loaded into the micro-catheter (after removing the wire) and delivered to its tip (Figure2D). The solitaire stent was unsheathed and deployed across the ectatic segment by pulling back the micro-catheter. The position of the deployed stent was confirmed by the radio opaque markers (distally four and proximally one) and its relation to thrombus was confirmed by a cine angiogram. The device was retrieved back into the micro-catheter and then into the guide catheter as an assembly to retrieve a large thrombus on the 3rd attempt (Figure2E and F). Simultaneously, the guide catheter was aspirated to prevent escape of the thrombus while the stent collapses and enters the guide catheter. Repeat acquisition showed a tight lesion just after the ectatic segment with mild residual thrombus (Figure2G). The stenotic segment was dilated with a 2.5 × 15 mm non-compliant balloon and then i.c. nicorandil and tirofiban were given selectively into the distal RCA, through the aspiration port of the thrombo-suction catheter to achieve thrombolysis in myocardial infarction (TIMI) II plus flow. Post-thrombus retrieval ECG (Figure1B) showed significant ST-segment resolution. The patient was monitored on the coronary care unit for 24 h whilst receiving an intravenous tirofiban infusion and back-up temporary pacing. Stenting was not considered at this stage fearing intramural haematoma with i.c. thrombolytics and worsening of slow flow. Twenty-four hours later ECG (Figure 1C) showed complete ST-segment resolution with a small Q wave in lead III. Repeat angiogram showed near total resolution of thrombus within the ectatic segment and the post-ectatic critical stenosis was further pre-dilated and stented using a 3 × 28 mm everolimus eluting stent to restore TIMI III flow in the distal RCA and its branches (Figure2H and I, Supplementary material online, Video S1). Care was taken to position the proximal edge of the stent exactly at the distal margin of the CAE and the proximal stent edge was flared by high-pressure post-dilation. The pacing lead was removed 24 h later and the lead III on ECG showed Q wave with T inversions (Figure1D). The patient had an uneventful recovery subsequently and he is asymptomatic at 6-month follow-up. There was no history suggestive for Kawasaki’s disease in childhood or any other autoimmune disorders and the stenotic lesion distal to the ectatic segment points to an atherosclerotic aetiology for the ectasia also.
Discussion
Late presentation after acute ST elevation myocardial infarction is the most important predictor for huge thrombus burden and no reflow phenomenon.4 In addition, degenerated vein grafts and CAE2 with acute coronary syndromes (ACS) are important causes as in this case. Independent of the thrombus burden CAE is known to cause slow flow by releasing inflammatory mediators.5 Managing thrombus with i.c. medications and thrombo-suction using manual aspiration catheters can restore flow in situations of slow flow without large angiographically visible thrombus. Angiographically large fixed and partially organized thrombi as seen in this case with CAE are seldom affected by the above thrombus management strategies. Special devices are required to retrieve such thrombus. Arnous et al.6 has reported a series of cases with large i.c. thrombus refractory to aspiration thrombectomy that were successfully retrieved using the ev3 Spider filter device. This is a distal embolic protection device conventionally used during degenerated vein graft interventions. Theoretically, this technique appears to have more control over the basketed thrombus and it is an over the wire technique throughout, hence there is no loss of wire access. Uribe et al.7 has reported a case of left main thrombus in a patient without any conventional CAD risk factors that were successfully retrieved using a Solitaire AB stent system. In this case out of the many thrombus management strategy used, only the Solitaire AB device helped to retrieve the thrombus and establish an antegrade flow on the 3rd attempt. Retrospectively it appears a larger retriever system would have succeeded with fewer attempts but it involves the risk of scraping the endothelium of the proximal normal RCA segment. Similarly, the role of larger aspiration catheters is also debateable as we have used only a 6-Fr system in this case. Regarding the composition of the clot Silvain et al.8 has demonstrated that the fibrin composition of a clot increases with increasing ischaemic time and vice versa for the platelet composition. In our case, whilst we did not study the extracted clot for its composition, we presume the clot to be more fibrin rich in spite of the relatively shorter ischaemic time because of its organized nature. The stasis aggravated by the CAE could have contributed for the fibrin rich nature of the clot by a mechanism that is somewhat similar to the stasis-related fibrin-rich clots that form with deep vein thrombosis. Moreover, the majority of the thrombus that was successfully extracted from cerebral arteries using these devices in stroke patients was red and fibrin rich, hence we presume that this system works better for organized and fibrin-rich clots than for friable and platelet-rich clots. Also refractoriness to tenecteplase argues against a fresh fibrin-rich clot.
Thrombus retrieval has emerged as a first line treatment to restore cerebral flow in patients with acute ischaemic stroke9 but the same is not true with CAD and cannot be extrapolated to ACS due to the following pathophysiologic differences between these two entities. The majority of ischaemic strokes are embolic in nature (cardio-embolic or athero-embolic from the carotids) as in situ atherosclerosis leading to a plaque event of the intracranial vessels is rare.10,11 Hence the thrombus causing ischaemic stroke is often organized whereas in ACS there is a plaque event that is complicated by onsite friable thrombus. Hence the distal embolization and slow flow during coarse manipulation of the thrombus by these devices in ACS is many fold compared with the retrieval of organized thrombus in stroke. Pulling these devices in the deployed state across the coronaries involves the risk of eroding a non-culprit plaque on its way back to the guide catheter. This is not a problem with the intracranial part of the cerebral vessels which are usually not diseased. However, in situations where the thrombus appears organized and refractory to the conventional methods such thrombus retrieval devices are useful as demonstrated in this case.
Conclusion
Angiographically visible thrombus that is refractory to i.c. medications and aspiration thrombectomy can be successfully managed using a stent retriever. To our knowledge, this is the first case report of the successful use of Solitaire AB stent retriever for thrombus retrieval from an ectatic coronary artery.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The author/s confirm that written consent for submission and publication of this case report including image(s) and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: none declared.
Supplementary Material
References
- 1. Markis JE, Joffe CD, Cohn PF, Feen DJ, Herman MV, Gorlin R.. Clinical significance of coronary arterial ectasia. Am J Cardiol 1976;37:217–222. [DOI] [PubMed] [Google Scholar]
- 2. Doi T, Kataoka Y, Noguchi T, Shibata T, Nakashima T, Kawakami S, Nakao K, Fujino M, Nagai T, Kanaya T, Tahara Y, Asaumi Y, Tsuda E, Nakai M, Nishimura K, Anzai T, Kusano K, Shimokawa H, Goto Y, Yasuda S.. Coronary artery ectasia predicts future cardiac events in patients with acute myocardial infarction. Arterioscler Thromb Vasc Biol 2017;37:2350–2355. [DOI] [PubMed] [Google Scholar]
- 3. Campanile A, Sozzi FB, Consonni D, Piscione F, Sganzerla P, Indolfi C, Stabile A, Migliorini A, Antoniucci D, Ferraresi R, Boccuzzi G, Danzi GB.. Primary PCI for the treatment of ectatic infarct-related coronary artery. Minerva Cardioangiol 2014;62:327–333. [PubMed] [Google Scholar]
- 4. Mazhar J, Mashicharan M, Farshid A.. Predictors and outcome of no-reflow post primary percutaneous coronary intervention for ST elevation myocardial infarction. Int J Cardiol Heart Vasc 2015;10:8–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brunetti ND, Salvemini G, Cuculo A, Ruggiero A, De Gennaro L, Gaglione A, Di Biase M.. Coronary artery ectasia is related to coronary slow flow and inflammatory activation. Atherosclerosis 2014;233:636–640. [DOI] [PubMed] [Google Scholar]
- 6. Arnous S, Agelaki M, Shakhshir N, Kelly D, Ordoubadi FF, Mamas MA, Fraser D.. Thrombus capture by withdrawal of an open filter device: a useful treatment for large non-occlusive coronary thrombus. EuroIntervention 2014;10:689–693. [DOI] [PubMed] [Google Scholar]
- 7. Uribe CE, Zuñiga M, Madrid C.. Mechanical thrombectomy using the Solitaire stent in a left main coronary artery: a novel approach to coronary thrombus retrieval. Catheter Cardiovasc Interv 2017;89:71–77. [DOI] [PubMed] [Google Scholar]
- 8. Silvain J, Collet J-P, Nagaswami C, Beygui F, Edmondson KE, Bellemain-Appaix A, Cayla G, Pena A, Brugier D, Barthelemy O, Montalescot G, Weisel JW.. Composition of coronary thrombus in acute myocardial infarction. J Am Coll Cardiol 2011;57:1359–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Brown M, Demaerschalk BM, Hoh B, Jauch EC, Kidwell CS, Leslie-Mazwi TM, Ovbiagele B, Scott PA, Sheth KN, Southerland AM, Summers DV, Tirschwell DL; on behalf of the American Heart Association Stroke Council. 2018 Guidelines for the early management of patients with acute ischaemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2018;49:e46–e110. [DOI] [PubMed] [Google Scholar]
- 10. Denswil NP, van der Wal AC, Ritz K, de Boer OJ, Aronica E, Troost D, Daemen MJAP.. Atherosclerosis in the circle of Willis: spatial differences in composition and in distribution of plaques. Atherosclerosis 2016;251:78–84. [DOI] [PubMed] [Google Scholar]
- 11. Mathur KS, Kashyap SK, Kumar V.. Correlation of the extent and severity of atherosclerosis in the coronary and cerebral arteries. Circulation 1963;27:929–934. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.