Abstract
Background
Updated urticaria guidelines recommend that patients should be assessed for disease activity, severity, control, and quality of life at baseline and follow up. Regarding treatment, guidelines consider second generation antihistamines as the cornerstone in therapy for chronic urticaria (CU), while other drugs, such as omalizumab, are conceived as second-line alternatives. In regards to omalizumab, despite advances in the management of CU, there are still open questions about timing, dosing, and objective measures for clinical response. This study was designed to portray the use of patient-reported outcomes (PROs) in chronic urticaria management, as well as the effectiveness and treatment patterns of omalizumab in CU, as seen in a real-life setting in Latin America.
Methods
This is a retrospective observational study, involving 72 Latin American patients with chronic urticaria treated with omalizumab. Patient reported outcomes and treatment patterns, response, quality of life improvement and discontinuation were analyzed.
Results
From the 72 patients, 91.7% (n = 66) were assessed through PROs, where urticaria control test (UCT) was the most used (79.2%; n = 57). Overall, 80.0% (n = 44) responded to omalizumab at some point of the treatment. Omalizumab 300 mg was associated with earlier response compared to lower doses. Regardless of dosage, most patients assessed with CU-Q2oL improved quality of life (80.8%; n = 21). With respect to omalizumab discontinuation, 20.8% (n = 15) patients interrupted omalizumab before the 3rd month of treatment (p = .000).
Conclusions
The present study highlights how the use of PROs and omalizumab in Latin America differ from guidelines’ recommendations and clinical trials. Even though most patients were initiated under omalizumab 300 mg, most of them finished with lower doses. Regardless of dosage, most patients responded to omalizumab and improved quality of life at some point during treatment. However, such features were seen earlier with omalizumab 300 mg. Regarding treatment discontinuation, one-fifth of patients interrupted omalizumab before the third month.
Keywords: Chronic spontaneous urticaria, Patient-reported outcomes, Omalizumab, Quality of life, Latin America
Abbreviations: CU, chronic urticaria; LA, Latin America; PRO, patient-reported outcomes; UAS7, urticaria activity score 7; EAACI/GA2LEN/EDF/WAO, European Academy of Allergology and Clinical Immunology; Global Allergy and Asthma European Network, European Dermatology Forum and World Allergy Organization; MCID, minimal clinical important difference; UCT, urticaria control test; CU-Q2oL, chronic urticaria quality of life questionnaire; SD, standard deviation
Background
Chronic urticaria (CU) is a common skin disorder characterized by spontaneous recurrent wheals, that may present with associated angioedema and occur for at least 6 weeks.1 Often, urticaria needs to be differentiated from other medical conditions such as anaphylaxis (in the case of acute urticaria), autoinflammatory syndromes, or urticarial vasculitis, where hives with or without angioedema can also occur.1 Updated guidelines recommend omalizumab, an anti-IgE monoclonal antibody, in cases of antihistamine refractoriness. It was first reported to be effective in patients with chronic autoimmune urticaria who were symptomatic despite antihistamine therapy, with subsequent clinical trials supporting its efficacy and safety for the treatment of CU.2, 3 Furthermore, treatment with omalizumab has been shown to improve quality of life, effectively treat relapses after treatment discontinuation, and is safe for long-term treatment.4, 5 Despite the current knowledge of omalizumab for CU, studies of this subject in Latin America (LA) are largely missing; therefore, little is known about the use and effects of omalizumab in Latin America (LA).
As evidenced by a recent real-world systematic review of omalizumab for CU, most studies originate from developed countries in Europe and the United States, where omalizumab is readily available for physicians to use, and it is usually reimbursed by health insurance.6 In contrast, in Latin America, economic and health insurance limitations appear to influence the prescription of omalizumab to a greater extent. For instance, in a publication addressing omalizumab discontinuation in LA, it was found that most patients could not achieve a 3-month regimen since health insurance programs did not support this therapy, and patients could not afford the costs.7 These circumstances provide a unique setting and challenge for the use of omalizumab in the treatment of patients with CU, and they may apply to other developing countries outside of LA.
In view of the previous findings, our study aims to objectively assess the effectiveness and describe the treatment patterns of omalizumab for CU, particularly regarding dose, and the use of patient reported outcomes (PROs), as seen in a real-life setting in LA. We believe the knowledge and learnings obtained from real-life experiences of treating CU patients with omalizumab might help to establish a framework for physicians working in places with similar health care systems and socioeconomic conditions.
Methods
This is a retrospective observational study, involving 72 Latin American (Ecuador, n = 23; Peru, n = 26, Brazil, n = 9; Colombia, n = 6; Argentina, n = 4; Mexico, n = 2 and Dominican Republic, n = 2) patients with chronic urticaria (CU) treated with omalizumab from January to December 2017. Patient demographics and clinical characteristics were reported. PRO use and treatment patterns, treatment regimens and response, quality of life improvement and medication discontinuation were analyzed. To be included, patients were required to have CU diagnosis according to the EAACI/GA2LEN/EDF/WAO guideline and at least one application of omalizumab, either 150 mg or 300 mg, within the previous designated period.1 Data were collected by physicians from clinical records from either private and/or public practice, under the supervision of an ethics committee.
The primary variable analyzed was treatment response/urticaria control, defined as an Urticaria Activity Score summed over 7 days (UAS7) ≤6 and/or Urticaria Control Test (UCT) ≥12 at any point during the treatment.8, 9 In addition, patients were assessed for how long they took to achieve response and stratified into early responders (response during the first month), intermediate responders (response between the first and third month) and late responders (response after the sixth month).8, 10, 11 Finally, the degree of response was classified into complete responders (UAS7 = 0) and partial responders (UAS ≤6 but not 0).8, 9, 10 The degree of response could manifest at any point of the treatment and was exclusively analyzed in patients with two UAS7 measurements, including their baseline status.
For quality of life improvement response, a change in the minimal clinical important difference (MCID) of 15 points in the total CU-Q2oL score from the baseline measurement was considered meaningful.12 Time to achieve quality of life improvement was categorized applying the same criteria as for treatment response. The improvement could be objectively measured at any point during treatment.
Ethical considerations
This study was approved by the ethics committee: Comité de ética e Investigación en Seres Humanos (CEISH).
Statistical analyses
Descriptive statistics were performed for demographic and a number of clinical variables including age, gender, years with disease, medications, PRO patterns/preferences, effective omalizumab dose and frequency. Chi-square tests for association were performed between the independent variable omalizumab dose and the dependent variables, type of responder (according to time to achieve response), degree of response and quality of life improvement. A Cramer's V test was use further to assess the strength of association if present.
In addition, we performed separate analyses concerning individuals completing 3- and 6-months regimens as previously investigated in clinical trials.13 A two-way mixed analysis of variance (ANOVA) was performed to understand the effect of omalizumab dose and time on UAS7, UCT and each of the CU-Q2oL domains scores. Chi-square tests for association were performed between the independent variable omalizumab dose and the dependent variable responses (present/absent), and quality of life improvement.
In addition, omalizumab discontinuation was evaluated at 3 and 6 months. Adjusted binomial logistic regressions were performed to ascertain the effects of age, gender, angioedema, years with urticaria, Omalizumab dose, treatment response and quality of life improvement on the likelihood that participants discontinued omalizumab before the 3 and 6 months of treatment.
Results
Descriptive statistics
A total of 72 patients were included in this analysis. The average age was 43.3 years (SD 14.3), and 55 (76.4%) were female. The average duration of urticaria before diagnosis was 3.8 years, and the mean treatment duration with omalizumab was 7.7 months (SD 7.7). The most common primary diagnosis of urticaria was chronic spontaneous urticaria (91.7%), wheras only 6 (8.3%) patients had a primary diagnosis of chronic inducible urticaria (Table 1).
Table 1.
Demographic characteristics of the studied population.
| Characteristics (n = 72) | n (%) |
|---|---|
| Average age (SD) | 43.3 ± 14.3 |
| Average time since diagnosis (in years) | 3.8 |
| Mean treatment duration in month (SD) | 7.7 (7.7) |
| Sex | |
| Female | 55 (76.4%) |
| Male | 17 (23.6%) |
| Type of urticaria | |
| Chronic spontaneous urticaria | 66 (91.7%) |
| Chronic inducible urticaria | 6 (8.3%) |
| Angioedema | 17 (23.6%) |
Notes: SD, standard deviation.
Baseline assessment
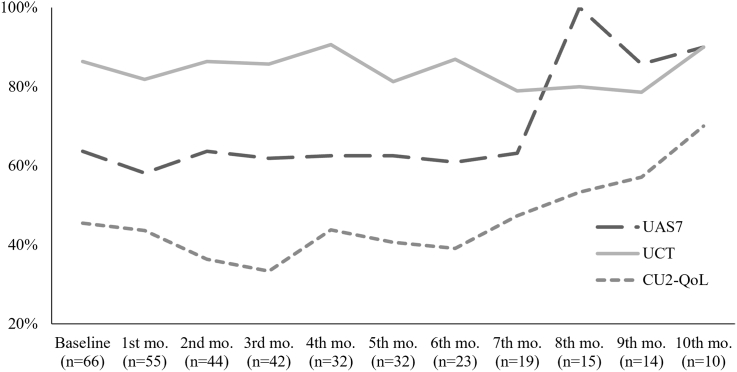
Among the 72 patients, one-third of the patients used all 3 PRO tools, the UAS7, the UCT and the Cu-Q2oL, one-third used 2 tools, and one-third used 1. Moreover, 91.7% (n = 66) were assessed objectively using questionnaires, while 8.3% (n = 6) were assessed by recall and physical exam only (p = .000). Overall, UCT was the most used questionnaire (79.2%; n = 57; p = .000), followed by UAS7 (58.3%; n = 42; p = .157) and CU-Q2oL (41.7%; n = 30; p = .157). When two questionnaires were used, the combination of UAS7 plus UCT was most often used (71.4; n = 15; p = .050). Patterns and combination of questionnaires are described in Table S1. Questionnaire use over time is depicted in Figure 1
Fig. 1.
Usage rate of patient-reported outcomes over time. Frequencies of each questionnaire used are shown as percentages at each month of follow-up. N, patients assessed by at least 1 questionnaire; Mo, month; UAS7, Urticaria Activity Score 7; UCT, Urticaria Control Test. CU-Q2oL, Chronic Urticaria Quality of Life.
From the 42 patients assessed through UAS7, most patients had severe disease (47.6%; n = 20; p = .000). Based on the use of the UCT, CU in 96.5% (n = 55; p = .000) of patients was not controlled when omalizumab was initiated (Table S2). The mean for UAS7, UCT and each CU-Q2oL domain are summarized in Table S3.
Treatment with omalizumab
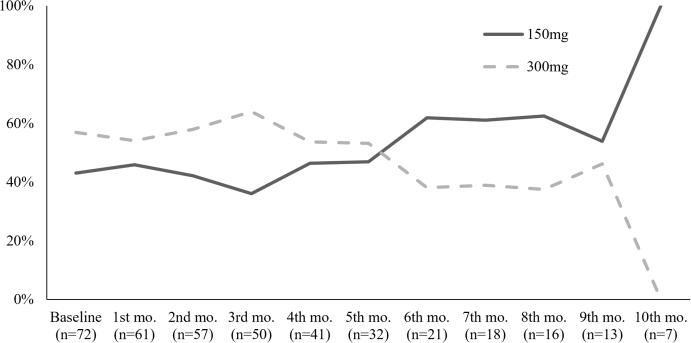
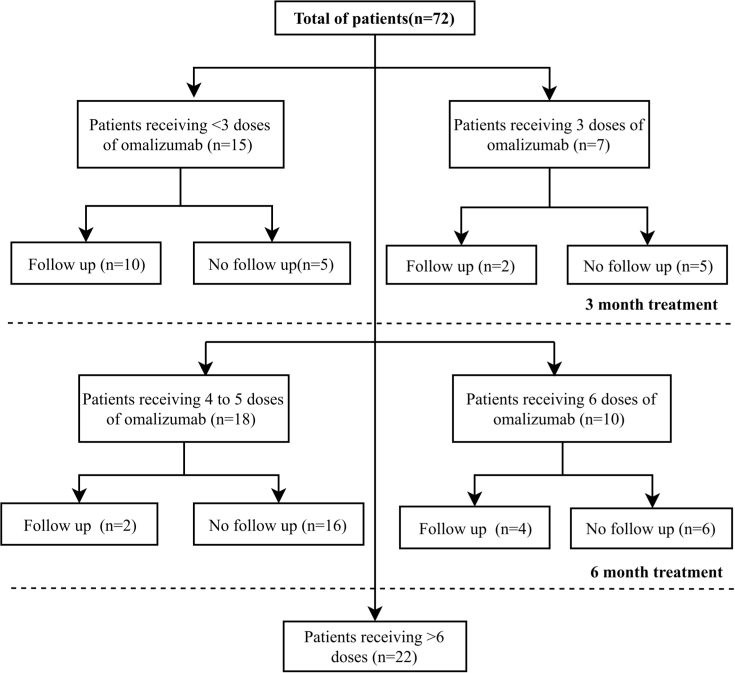
The mean number of omalizumab doses applied during the time period of the study was 5.45 (SD, 3.15). The mean treatment duration was 7.67 months (SD, 7.67). 56.9% (n = 41) and 43.1% (n = 31) of patients started on omalizumab 150 mg and 300 mg monthly, respectively. Of the 72 patients, 7 (9.9%) completed a 3-month treatment course, and 10 (13.8%) patients received omalizumab for 6 months. Omalizumab dosing over time is illustrated in Figure 2. The remaining patients received different treatment schemes other than those used in clinical trials (Figure 3). Concerning other medications, all patients used antihistamines and 29.2% (n = 21) used steroids. Additional use of medication is summarized in Table S4.
Fig. 2.
Omalizumab dosage over time. Frequency of omalizumab dose used (150mg and 300mg) is shown as a percentage at each month. N, number patients receiving omalizumab at each month; Mo, month.
Fig. 3.
Treatment duration and follow-up after last dose received. Frequencies of patients according to number of doses received: <3, 3, 4-5, 6, and more than 6 doses, classified according to follow-up. Follow-up indicates at least 1 questionnaire was performed after last dose received.
Four of five patients treated with omalizumab benefit from the treatment and most respond fast.
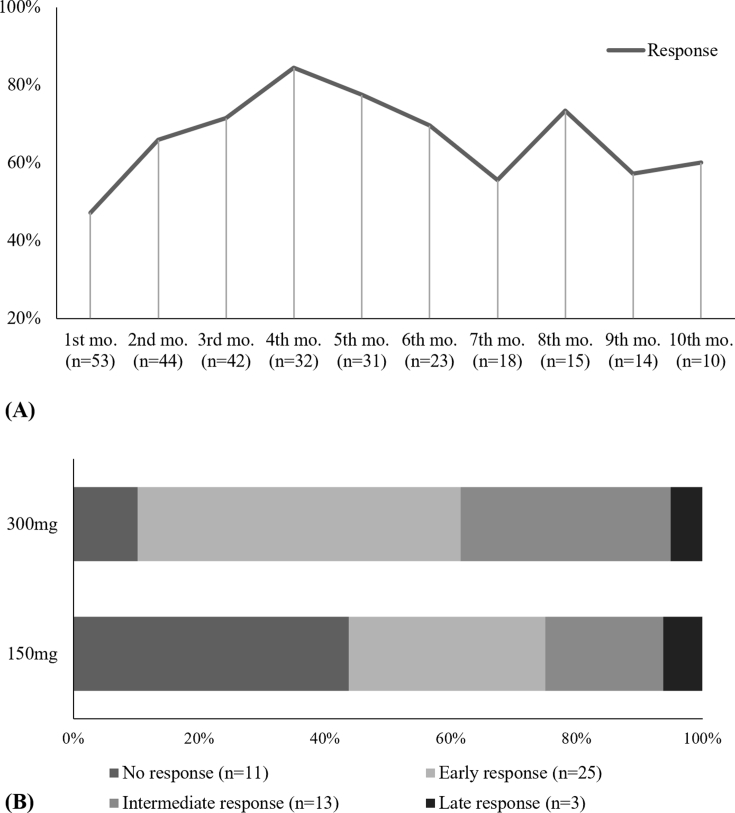
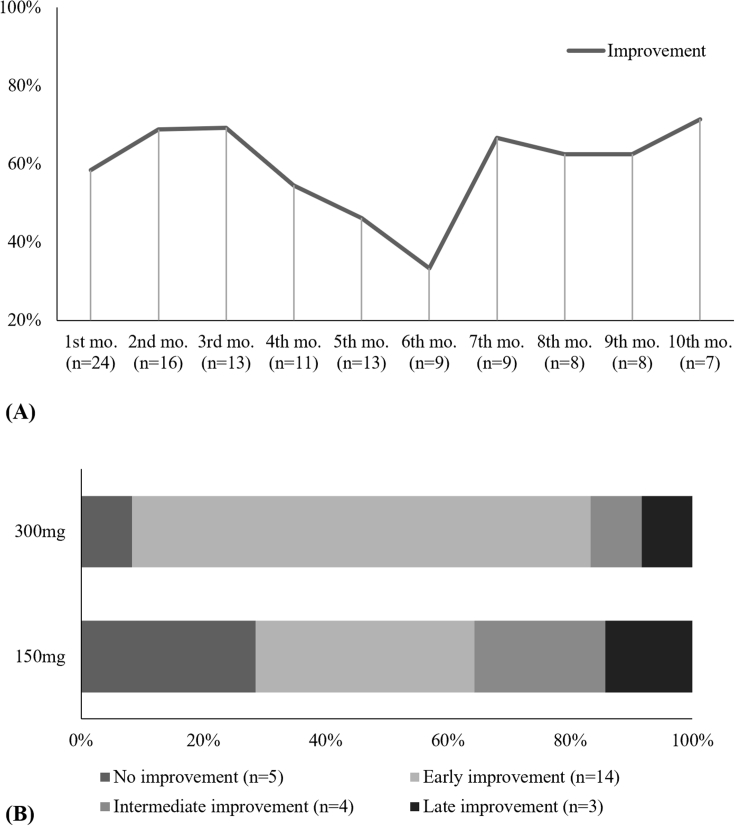
Among the 72 patients evaluated, 55 had at least one follow-up office visit to assess the degree of response to omalizumab using the UAS7 and/or UCT. Overall, 80.0% (n = 44) had some degree of benefit to omalizumab, while 20.0% (n = 11) had no response at all. The mean time to response was 1.75 months (SD, 1.28). Furthermore, 45.5% (n = 25) of patients were considered early responders, 29.1% (n = 16) intermediate responders and 5.5% (n = 3) late responders (p = .000). There was a weak but statistically significant association between omalizumab dosing and the speed of onset of the response (χ2(3) = 7.577, p = .048; φC = 0.387, p = .042). The overall treatment response and proportions of each type of responder according to omalizumab dose are illustrated in Figures 4A and B, respectively.
Fig. 4.
Treatment and type of response according to UAS and/or UCT. (A) Treatment response over time shown as a percentage of patients that achieved response at each month. Treatment response was defined as an UAS7 ≤6 and/or UCT ≥12. (B) Type of responders according to how long they took to achieve response with each omalizumab dose. Mo, month; Early responders, response at first month; Intermediate responders, response between the first and third month; Late responders, response after the sixth month; UAS7, Urticaria Activity Score 7; UCT, Urticaria Control Test. CU-Q2oL, Chronic Urticaria Quality of Life.
Degree of response according to UAS7
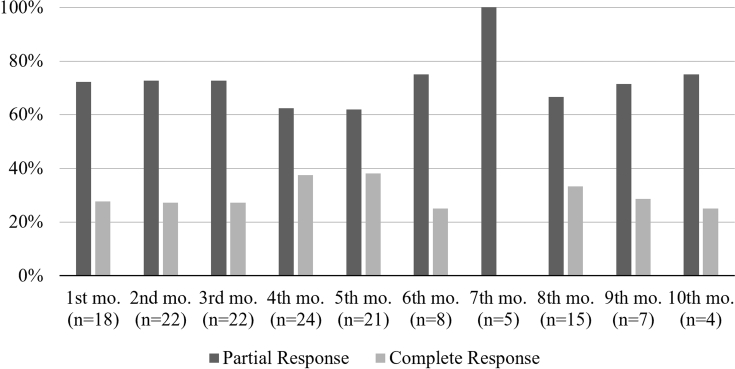
Among the 37 patients assessed using the UAS7 at least twice during treatment, 54.1% (n = 20) of patients presented with complete response at some point during treatment (p = .622). Among the complete responders, 13 were treated with Omalizumab 300 mg every 4 weeks.14, 15 There was not a statistically significant association between Omalizumab dose and complete response (χ2(1) = 1.205; p = .272). The mean time to achieve complete response was 3.3 months (SD, 2.34), with the highest complete response rate at the 4th month of follow-up. The degree of response according to UAS7 is depicted in Figure 5.
Fig. 5.
Degree of response over time according to UAS7. Frequencies of patients with partial and complete responses shown as percentages at each month. Partial response was defined as an UAS ≤6; Complete response was defined as an UAS7 = 0; N=number of patients achieving at least one type of response as defined by their UAS7 score; Mo, month; UAS7, Urticaria Activity Score 7.
Quality of life improvement
Among the 26 patients assessed with CU-Q2oL who presented with at least one follow-up, 80.8% (n = 21) of patients experienced some degree of quality of life improvement at some point during their treatment (p = .002).
Among these patients, 53.8% (n = 14) were early improvers, 15.4% (n = 4) intermediate improvers, and 11.5% (n = 3) late improvers (p = .008) (Figure 6). There was not a statistically significant association between Omalizumab dose and type of improvement (χ2(2) = 3.962; p = .330).
Fig. 6.
Treatment and type of quality of life improvement according to CU-Q2oL. (A) Percentage of patients that achieved quality of life improvement at each month. Quality of life improvement was defined by the minimal clinical important difference of 15 points in the total CU-Q2oL score with respect to the baseline measurement. (B) Type of quality of life improvement according to how long it took to achieve improvement with each omalizumab dose. Mo, month; Early improvement, quality of life improvement at first month; Intermediate improvement, quality of life improvement between the first and third month; Late improvement, quality of life improvement after the sixth month; CU-Q2oL, Chronic Urticaria Quality of Life.
Response and quality of life improvement in patients completing the 3- and 6-month treatment courses.
Using the UAS7, UCT and CU-Q2oL score values as continuous variables, a two-way mixed ANOVA showed that there were no statistically significant interactions between the omalizumab dose and time for UAS7 and CU-Q2oL. The main effect time showed a statistically significant difference for all mean scores. However, the main effect of omalizumab dose showed no statistically significant difference for any mean score. Due to an assumption violation concerning homogeneity of variances and covariances, mean difference analyses could not be performed for UCT and sleep domain of CU-Q2oL.
Of the 42 patients assessed by either UAS7 or UCT at 3 months, 71.4% (n = 30) responded to treatment (p = .005). From these responders, 61.9% (n = 26) received with Omalizumab 300 mg and 9.5% reveived 150 mg every 4 weeks. The association between Omalizumab dose and the presence/absence of response at month 3 was statistically significant, χ2(1) = 6.353, p = .020.
Of the 23 patients assessed by either UAS7 or UCT at 6 months, 69.6% (n = 16) had a beneficial response to treatment (p = .061), and all of them received 300 mg of omalizumab. The association between omalizumab dose and the presence/absence of response at month 6 was statistically significant (χ2(1) = 14.603; p = .001). Proportions of patients with manifesting treatment response and quality of life improvement at 3 and 6 months are summarized in Tables S5 and S6, respectively.
Discontinuation of omalizumab and follow up
The omalizumab treatment was interrupted before the third treatment month in 20.8% (n = 15) of patients and in 55.6% (n = 40) before the sixth month. Those patients who discontinued treatment showed a higher proportion of uncontrolled urticaria with worse quality of life than those who continued treatment (supplemental appendix Tables S7 and S8).
Discussion
To our knowledge, this is the first study to analyze the use of omalizumab in Latin America and its role in the management of CU. In addition, this study highlights how the experiences with omalizumab by Latin American physicians contrast with evidence-based recommendations provided by international guidelines.
Our study provides real-life data on the timing and dosing of omalizumab in the treatment of CU patients as well as on the use of PRO measures for assessing their clinical response.16, 17, 18 Currently, there are no reliable biomarkers to identify and measure disease activity in CU.19 Consequently, the use of PRO instruments remains crucial to evaluate and monitor various aspects of CU including activity, severity, control, and quality of life.19 Among the available PRO instruments, UAS7 has been considered the gold standard and has been extensively used in clinical trials.20 Despite this, the reported use of PRO instruments is low, as evidenced by a recent “real world” systematic review by Bernstein and colleagues. According to this review, PRO instruments were used to assess the response of CU patients to omalizumab in only 41.7% of studies, with UAS7 being used in 28.6% of studies and UCT only in 3.6% of studies.6 In our study, 58.3% of patients were evaluated with UAS7, while almost 80% of patients were assessed using the UCT at baseline.
The higher reported use of UCT in our patients, compared to UAS7 and CU-Q2oL, might be the result of the former being simpler and faster to use among physicians and patients.19 On the other hand, CU-Q2oL was the least used PRO at baseline (approximately 37.5%), with a similar low overall use throughout the patients’ follow up. The CU-Q2oL provides detailed information on the effects of CU on different QoL domains, and it can be useful to compare changes over time including those that occur in response to different treatments. On the other hand, it is generally considered to be more time consuming than other PRO measures, both for patients as well as physicians.19 Our findings are consistent with a European study that reported low use (approximately 50% of patients) of the CU-Q2oL at baseline evaluation.21
The current version of the international urticaria guideline recommends second generation H1-antihistamines as the first line treatment, and it suggests updosing this medication up to 4-fold when CU control cannot be achieved with standard doses.1 Of the studied patients, we found that nearly half of them were treated following this recommendation at the start of omalizumab treatment, a finding comparable to that of a recent global report.22 Nevertheless, the level of awareness of CU guidelines among Latin American physicians is still low, and the fear of side effects when updosing 2nd generation H1-antihistamines is still high.23, 24
For antihistamine-refractory patients, omalizumab has been shown to be very effective and safe in the treatment of CU.15 In fact, several studies point to high discontinuation rates for other medications, e.g. antihistamines, over time when omalizumab is used for the treatment of patients with CU.6 Our study confirms this and shows a significant decrease in the use of pharmacologic agents by the end of omalizumab treatment.
Clinical trials have shown that 300 mg of omalizumab monthly is the most effective starting dose in CU. In real life clinical practice, however, approximately 25% of patients are still started on 150 mg of omalizumab.6, 13 In the present study, 43.1% of our patients were initiated on this latter regimen. The most likely explanation for this is that, in Latin America, the economic burden, insurance restrictions, and omalizumab availability limit the use of 300 mg as the starting dose.7 While it is clear that 300 mg is the best starting dose, there may be cases where the use of 150 mg may be preferred for treatment continuation, for example in patients with partial response to 300 mg every 4 weeks, who may experience a better response when dosed with 150 mg every two weeks.25
Even though most of our patients were initiated at a dose of 300 mg every 4 weeks, by the 6th month of follow-up, 150 mg every 4 weeks was far more common. This finding relates to a recent systematic review, in which 300 mg had the highest initiation rates, but was able to be stepped down to a lower dose by the end of treatment.6 Changes in dosage appear to be common in clinical practice. It is still unclear, however, if these changes occur secondary to treatment success and achieving urticaria control or due to limitations in access to omalizumab as previously described. Regardless of dosage, we found an overall response rate at any point in treatment of 80%, with 20% of patients not responding at all. These findings compare to previous real real-life studies.26 In contrast, complete responder rates appear to be more heterogeneous. An earlier real-life study by Sussman et al. found a complete response rate of 79% at any point during treatment, while a recent retrospective observational study by Nettis and colleagues observed a complete response of 67%.26, 27 In our study, the complete response rate was lower, achieved only in 54.1% of cases, and presenting with the highest rate at 4th and 5th months as depicted in Fig. 5. In the POLARIS trial, the proportion of complete response versus placebo for omalizumab 300 mg was even lower than that, 36%, and omalizumab 150 mg resulted in only 19% complete responders.28 In the present study we did not find any statistically significant association between omalizumab dose and complete response, although most complete responders were treated with omalizumab 300 mg. Clearly, complete responder rates are highly variable, and the patient population treated, the tools and definitions used to assess complete response, and when this is done after the initiation of omalizumab treatment are important variables.
When considering the time to response to omalizumab treatment, our analyses show that omalizumab 300 mg was associated with higher rates of earlier responders. In our study, 45.5% of individuals were early responders, as assessed by UAS7 and UCT, of which 36.4% were treated with Omalizumab 300 mg (p = .042). This finding compares very well with the ASTERIA I and II clinical trials, in which the rate of early responders for omalizumab 300 mg was 37% and 51%, respectively.10
Regarding omalizumab discontinuation, 20.8% of patients interrupted their treatment before 3 months. This contrasts with ASTERIA II and ASTERIA I, where overall treatment discontinuation rates at 3 months of treatment were 6% and 15%, respectively.10 The discrepancies between our study and clinical trials in treatment discontinuation highlight how much omalizumab regimens vary in the real-life setting and that they are frequently subject to factors that may interrupt the course of treatment such as poor response to treatment, socioeconomical problems, lack of time for treatment, insurance changes, symptom resolution or, less commonly, adverse effects.26, 29 For instance, in a publication addressing omalizumab discontinuation in LA patients, 65.4% patients could not complete a 3-month treatment course since health insurance programs did not support this therapy and patients could not afford the costs.7 Discontinuing omalizumab has been associated with disease relapse and significant increase in symptoms, as suggested by other studies.14, 15, 30 In a real-life study, 61% of patients who discontinued treatment with omalizumab reported worsening of symptoms, however, some of these patients were retreated successfully with omalizumab.31
It is well known that improvement in disease activity does not always translate to better quality of life. CSU disease activity assessed by UAS7 is a significant predictor for overall CU-Q2oL score, but patients with a low UAS7 score may still have markedly impaired quality of life.19, 32 This highlights the importance of assessing CU patients with multiple tools in order to achieve a more complete status of disease impact. In our study, omalizumab improved mean quality of life scores over time, a finding that has also been reported in previous clinical trials and other real-life studies.10, 33 Overall, we found that 80.8% of patients experienced improved quality of life at some point during treatment. This finding is comparable to a recent systematic review, in which quality of life outcomes measured by CU-Q2oL improved 71.1%6
Limitations
There are several limitations in our study that require mentioning. First, the 72 patients included did not achieve enough power to generate statistically significant results for all comparisons. For instance, chi square tests with 3° of freedom as well as two-way-mixed ANOVA procedures presented difficulties either because of type II errors or assumptions violations. Moreover, even though we describe and portray the variability in patterns and preferences in PRO and omalizumab use in the real-life clinical practice in LA, the heterogeneity of data limits the extrapolation and generalization of some observations/conclusions. The results reported in this study might have been influenced by other factors that have not been considered in the analyzes, and as our conclusions concerning the potential benefits of omalizumab are mostly descriptive, they are subject to bias. Despite including few patients with chronic inducible urticaria, the PRO evaluated in this study have not been validated for this disease. Another potential limitation is that it was not possible to consider in detail the clinical background of each patient, such as the type of medications prescribed and their effectiveness prior to the start of omalizumab treatment, or the reason why patients could not achieve follow-up after the last omalizumab dose received. Despite these limitations, many of the reported observations from this real-life study correlated fairly well with the pivotal omalizumab clinical trial results and other real-life studies from other regions of the world.
Conclusions
Our results suggest that patient-reported outcomes measurements, in addition to clinical judgement, are useful to record improvements or lack thereof, in patients with chronic urticaria treated with omalizumab. Even though most patients are initiated on omalizumab 300 mg, many can be maintained well with lower doses. Regardless of the dose selected, most patients experienced improved quality of life at some point during treatment. Good responses were observed earlier with omalizumab 300 mg. Treatment discontinuation remains a problem: 20% of patients stopped omalizumab before the third month for various reasons not related to the medication's effectiveness. In summary, there are still many unanswered questions regarding the use of omalizumab for CU in clinical practice. We believe that these questions can ultimately only be answered by additional studies, using registries, real life settings and controlled trials.
Ethics approval and consent to participate
This study was approved by the ethics committee Comité de ética e Investigación en Seres Humanos (CEISH), Guayaquil-Ecuador, in accordance to the principles established by the declaration of Helsinki.
Consent for publication
Not applicable.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of interest
The following authors have relevant competing interests to disclose:
Dr. Marcus Maurer: is or recently was a speaker and/or advisor for and/or has received research funding from Allakos, Aralez, Genentech, GSK, Menarini, Merckle Recordati, Moxie, Novartis, Sanofi, MSD, and Uriach.
Dr. Jonathan A. Bernstein: PI – Novartis and Genentech; PI, speaker, consultant - Shire, CSL Behring, Pharming; PI, consultant – Biocryst, Consultant – Kalvista
Dr. Luis Felipe Ensina: Speaker for Novartis, Sanofi and Takeda; Clinical Research for Novartis; Advisory Board for Novartis.
Dr. José Ignacio Larco Sousa: Speaker for Novartis, Sanofi, Faes Farma.
Dr. Ricardo Cardona Villa: Clinical Research for Inmunotek.
Dr. Patricia Latour Staffeld: Speaker for: Astra Zeneca, Faes, Novartis, Sanofi, Advisory Board Novartis.
Dr. Blanca María Morfin-Maciel: Speaker for Astra-Zeneca.
The rest of authors have no relevant conflicts of interest to disclose related to this work.
Author's contributions
Authors have made substantial contributions to conception and design, acquisition, analysis and interpretation of data, have been involved in drafting the manuscript or revising it critically for important intellectual content, and given final approval of the version to be published. ICO, EV, MF, VLM, AC, GDR, LFE, JILS, EEMB, RCV, PLS, BMMM, JM, PWC participated in the recollection of data. EV performed the statistical analyses. ICO, EV, MV, MM, JAB participated in the interpretation of analyses, figures, tables, and drafting of the manuscript. MM, and JAB collaborated in revising and improving the final version of the manuscript. All authors read and approved the final version.
Acknowledgements
The authors acknowledge the guidance and knowledge imparted by the MECOR Program for this study, especially from Sonia Buist MD, Ana Menezes MD, and Juliana Ferreira M.D. Special thanks to all members of Respiralab Research Group. Finally, we want to express our gratitude to Universidad Espiritu Santo for their continuous support.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.waojou.2019.100011.
Contributor Information
Ivan Cherrez-Ojeda, Email: ivancherrez@gmail.com.
Marcus Maurer, Email: marcus.maurer@charite.de.
Jonathan A. Bernstein, Email: jonathan.bernstein@uc.edu.
Emanuel Vanegas, Email: emnlv@hotmail.com.
Miguel Felix, Email: miguel.felixromero@gmail.com.
German D. Ramon, Email: clinicalresearch@iais.com.ar.
Luis Felipe Ensina, Email: 100alergia@gmail.com.
José Ignacio Larco Sousa, Email: jilarco@gmail.com.
Edgar Emilio Matos Benavides, Email: ematben@hotmail.com.
R. Cardona Villa, Email: rcv2016udea@gmail.com.
P. Latour Staffeld, Email: latour_patricia@hotmail.com.
Blanca María Morfin-Maciel, Email: blancamorfin@hotmail.com.
Jose Mori, Email: josemori23@yahoo.es.
Paul Wilches C, Email: pwilches2@gmail.com.
Valeria L. Mata, Email: valeria.matac@gmail.com.
Annia Cherrez, Email: anniacherrez@hotmail.com.
Funding
This study was partially supported by an unrestricted grant from Universidad Espíritu Santo. The sponsor had no role in the design of the study or in the collection, analysis, and interpretation of data.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Zuberbier T., Aberer W., Asero R. The EAACI/GA(2)LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73(7):1393–1414. doi: 10.1111/all.13397. [DOI] [PubMed] [Google Scholar]
- 2.Kaplan A.P., Joseph K., Maykut R.J., Geba G.P., Zeldin R.K. Treatment of chronic autoimmune urticaria with omalizumab. J Allergy Clin Immunol. 2008;122(3):569–573. doi: 10.1016/j.jaci.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 3.Maurer M., Altrichter S., Bieber T. Efficacy and safety of omalizumab in patients with chronic urticaria who exhibit IgE against thyroperoxidase. J Allergy Clin Immunol. 2011;128(1):202–209. doi: 10.1016/j.jaci.2011.04.038. e205. [DOI] [PubMed] [Google Scholar]
- 4.Maurer M., Sofen H., Ortiz B., Kianifard F., Gabriel S., Bernstein J.A. Positive impact of omalizumab on angioedema and quality of life in patients with refractory chronic idiopathic/spontaneous urticaria: analyses according to the presence or absence of angioedema. J Eur Acad Dermatol Venereol : JEADV. 2017;31(6):1056–1063. doi: 10.1111/jdv.14075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maurer M., Kaplan A., Rosen K. The XTEND-CIU study: long-term use of omalizumab in chronic idiopathic urticaria. J Allergy Clin Immunol. 2018;141(3):1138–1139. doi: 10.1016/j.jaci.2017.10.018. e1137. [DOI] [PubMed] [Google Scholar]
- 6.Bernstein J.A., Kavati A., Tharp M.D. Effectiveness of omalizumab in adolescent and adult patients with chronic idiopathic/spontaneous urticaria: a systematic review of 'real-world' evidence. Expert Opin Biol Ther. 2018;18(4):425–448. doi: 10.1080/14712598.2018.1438406. [DOI] [PubMed] [Google Scholar]
- 7.Wilches P., Wilches P., Calderon J.C., Cherrez A., Cherrez Ojeda I. Omalizumab for chronic urticaria in Latin America. World Allergy Org j. 2016;9(1) doi: 10.1186/s40413-016-0127-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferrer M., Boccon-Gibod I., Goncalo M. Expert opinion: defining response to omalizumab in patients with chronic spontaneous urticaria. Eur J Dermatol : EJD. 2017;27(5):455–463. doi: 10.1684/ejd.2017.3085. [DOI] [PubMed] [Google Scholar]
- 9.Donald Stull D.M., Gimenez-Arnau Ana, Grattan Clive, Khalil Sam, Balp Maria-Magdalena. Validation of chronic spontaneous/idiopathic urticaria (CSU/CIU) health states using weekly urticaria activity score (UAS7) and Dermatology Life Quality Index (DLQI) J Am Acad Dermatol. 2015;72(5):AB154. [Google Scholar]
- 10.Kaplan A., Ferrer M., Bernstein J.A. Timing and duration of omalizumab response in patients with chronic idiopathic/spontaneous urticaria. J Allergy Clin Immunol. 2016;137(2):474–481. doi: 10.1016/j.jaci.2015.08.023. [DOI] [PubMed] [Google Scholar]
- 11.Syrigos N., Grapsa D., Zande M., Tziotou M., Syrigou E. Treatment response to omalizumab in patients with refractory chronic spontaneous urticaria. Int J Dermatol. 2018;57(4):417–422. doi: 10.1111/ijd.13935. [DOI] [PubMed] [Google Scholar]
- 12.Kulthanan K., Chularojanamontri L., Tuchinda P., Rujitharanawong C., Baiardini I., Braido F. Minimal clinical important difference (MCID) of the Thai chronic urticaria quality of life questionnaire (CU-Q2oL) Asian Pac J Allergy Immunol. 2016;34(2):137–145. doi: 10.12932/AP0674.34.2.2016. [DOI] [PubMed] [Google Scholar]
- 13.Larenas-Linnemann D.E.S., Parisi C.A.S., Ritchie C. Update on omalizumab for urticaria: what's new in the literature from mechanisms to clinic. Curr Allergy Asthma Rep. 2018;18(5):33. doi: 10.1007/s11882-018-0787-5. [DOI] [PubMed] [Google Scholar]
- 14.Maurer M., Rosén K., Hsieh H.-J. Omalizumab for the treatment of chronic idiopathic or spontaneous urticaria. N Engl J Med. 2013;368(10):924–935. doi: 10.1056/NEJMoa1215372. [DOI] [PubMed] [Google Scholar]
- 15.Kaplan A., Ledford D., Ashby M. Omalizumab in patients with symptomatic chronic idiopathic/spontaneous urticaria despite standard combination therapy. J Allergy Clin Immunol. 2013;132(1):101–109. doi: 10.1016/j.jaci.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 16.Maurer M., Church M.K., Marsland A.M. Questions and answers in chronic urticaria: where do we stand and where do we go? J Eur Acad Dermatol Venereol : JEADV. 2016;30(Suppl 5):7–15. doi: 10.1111/jdv.13695. [DOI] [PubMed] [Google Scholar]
- 17.Asero R., Canonica G.W., Cristaudo A. Critical appraisal of the unmet needs in the treatment of chronic spontaneous urticaria with omalizumab: an Italian perspective. Curr Opin Allergy Clin Immunol. 2017;17(6):453–459. doi: 10.1097/ACI.0000000000000404. [DOI] [PubMed] [Google Scholar]
- 18.Ensina L.F., Valle S.O., Juliani A.P. Omalizumab in chronic spontaneous urticaria: a Brazilian real-life experience. Int Arch Allergy Immunol. 2016;169(2):121–124. doi: 10.1159/000444985. [DOI] [PubMed] [Google Scholar]
- 19.Moestrup K., Ghazanfar M.N., Thomsen S.F. Patient-reported outcomes (PROs) in chronic urticaria. Int J Dermatol. 2017;56(12):1342–1348. doi: 10.1111/ijd.13668. [DOI] [PubMed] [Google Scholar]
- 20.Mlynek A., Zalewska-Janowska A., Martus P., Staubach P., Zuberbier T., Maurer M. How to assess disease activity in patients with chronic urticaria? Allergy. 2008;63(6):777–780. doi: 10.1111/j.1398-9995.2008.01726.x. [DOI] [PubMed] [Google Scholar]
- 21.Thomsen S.F., Pritzier E.C., Anderson C.D. Chronic urticaria in the real-life clinical practice setting in Sweden, Norway and Denmark: baseline results from the non-interventional multicentre AWARE study. J Eur Acad Dermatol Venereol : JEADV. 2017;31(6):1048–1055. doi: 10.1111/jdv.14210. [DOI] [PubMed] [Google Scholar]
- 22.Kolkhir P., Pogorelov D., Darlenski R. Management of chronic spontaneous urticaria: a worldwide perspective. World Allergy Org J. 2018;11(1):14. doi: 10.1186/s40413-018-0193-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cherrez A., Maurer M., Weller K., Calderon J.C., Simancas-Racines D., Cherrez Ojeda I. Knowledge and management of chronic spontaneous urticaria in Latin America: a cross-sectional study in Ecuador. World Allergy Org j. 2017;10(1):21. doi: 10.1186/s40413-017-0150-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guillen-Aguinaga S., Jauregui Presa I., Aguinaga-Ontoso E., Guillen-Grima F., Ferrer M. Updosing nonsedating antihistamines in patients with chronic spontaneous urticaria: a systematic review and meta-analysis. Br J Dermatol. 2016;175(6):1153–1165. doi: 10.1111/bjd.14768. [DOI] [PubMed] [Google Scholar]
- 25.Turk M., Kocaturk E., Cure K., Yilmaz I. Two-week intervals during omalizumab treatment may provide better symptom control in selected patients with chronic urticaria. Journal Allergy Clin Immunol Practice. 2018;6(4):1389–1390. doi: 10.1016/j.jaip.2018.01.027. [DOI] [PubMed] [Google Scholar]
- 26.Sussman G., Hebert J., Barron C. Real-life experiences with omalizumab for the treatment of chronic urticaria. Ann Allergy Asthma Immunol : Off Pub Am College Allergy Asthma Immunol. 2014;112(2):170–174. doi: 10.1016/j.anai.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 27.Nettis E., Cegolon L., Di Leo E., Lodi Rizzini F., Detoraki A., Canonica G.W. Omalizumab chronic spontaneous urticaria. Ann Allergy Asthma Immunol. 2018;121(4):474–478. doi: 10.1016/j.anai.2018.06.014. [DOI] [PubMed] [Google Scholar]
- 28.Hide M., Park H.S., Igarashi A. Efficacy and safety of omalizumab in Japanese and Korean patients with refractory chronic spontaneous urticaria. J Dermatol Sci. 2017;87(1):70–78. doi: 10.1016/j.jdermsci.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 29.Wang L., Ke X., Kavati A. Real-world treatment patterns and outcomes of omalizumab use in patients with chronic idiopathic urticaria. Curr Med Res Opin. 2018;34(1):35–39. doi: 10.1080/03007995.2017.1395732. [DOI] [PubMed] [Google Scholar]
- 30.Bongiorno M.R., Crimi N., Corrao S. Omalizumab for the treatment of chronic spontaneous urticaria in clinical practice. Ann Allergy Asthma Immunol : Off Pub Am College Allergy Asthma Immunol. 2016;117(6):703–707. doi: 10.1016/j.anai.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 31.Turk M., Yilmaz I., Bahcecioglu S.N. Treatment and retreatment with omalizumab in chronic spontaneous urticaria: real life experience with twenty-five patients. Allergol Int : Off J Japan Soc Allergol. 2018;67(1):85–89. doi: 10.1016/j.alit.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 32.Koti I., Weller K., Makris M. Disease activity only moderately correlates with quality of life impairment in patients with chronic spontaneous urticaria. Dermatology (Basel, Switzerland) 2013;226(4):371–379. doi: 10.1159/000351711. [DOI] [PubMed] [Google Scholar]
- 33.Larrea-Baca I., Gurpegui-Resano M. Improvement in the quality of life of patients with chronic spontaneous urticaria treated with omalizumab in real life. Enfermeria clinica. 2017;27(6):361–368. doi: 10.1016/j.enfcli.2017.03.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.