Abstract
Background
Allergic rhinitis is the main symptom of pollinosis, relieved by non-specific treatment universally. This study aimed to find the changes of serum metabolites between the seizure and remission periods of pollinosis and provide assistance in the diagnosis and/or therapy.
Methods
Metabonomics based on 1H nuclear magnetic resonance (NMR) was used to study the 37 serum samples of pollinosis patients.
Results
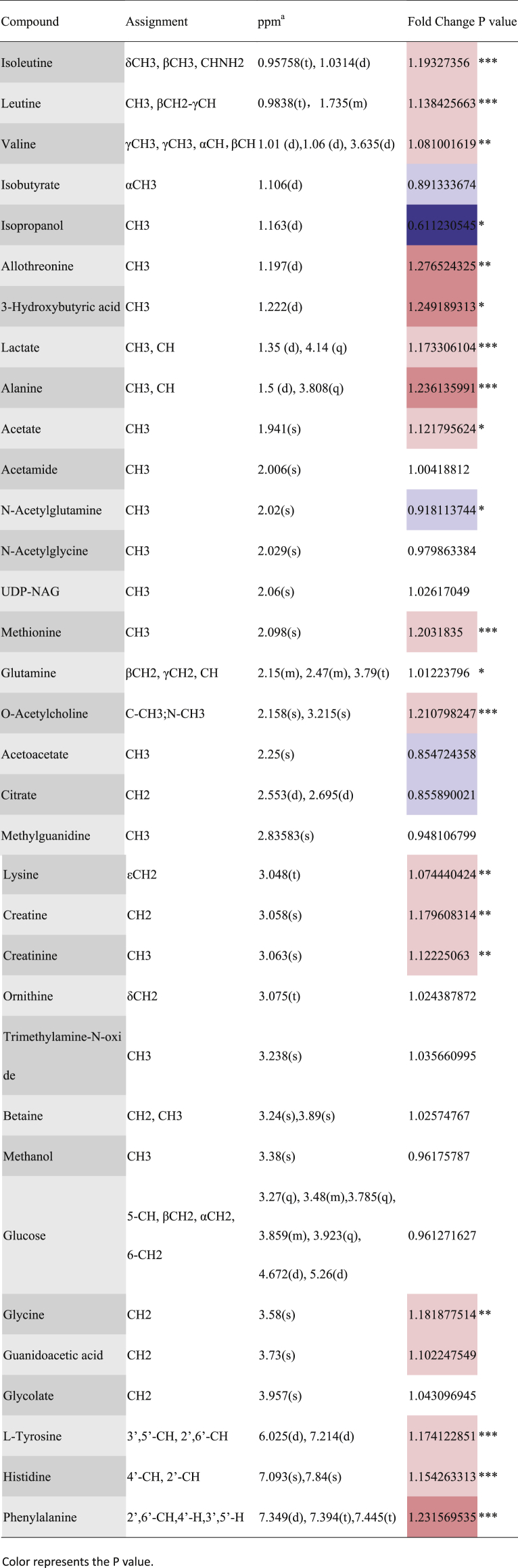
We believed that the decreased levels of isoleutine, leutine, valine, 3-hydroxybutyric acid, allo-threonine, alanine, methionine, glutamine, lysine, glycine, l-tyrosine, histidine, phenylalanine, lactate, acetate, O-acetylcholine, creatine and creatinine and the increased level of N-acetylglutamine at the seizure stage were statistically significant.
Conclusions
Pollinosis could change the metabolic profiles of energy, amino acid and lipid in patients, which might be the diagnosis and/or prognosis markers for hay fever patients.
Keywords: Pollinosis, Metabonomics, Energy, Amino acid, Lipid metabolism
Abbreviations: NMR, nuclear magnetic resonance; SIT, allergen-specific immunotherapy; PBS, phosphate buffer solution; TSP, 3-trimethylsilyl-propionic acid; PCA, principle component analysis; OPLS-DA, orthogonal partial least squares discriminant analysis; OSC-PLS-DA, orthogonal signal correction-partial least squares discriminant analysis; FIDs, free induction decay; SD, standard deviation; TCA, tricarboxylic acid cycle; SLE, systemic lupus erythematosus
Introduction
Pollinosis belongs to type I allergic reaction. Its pathogenesis is relatively clear. After re-exposure to allergens, mast cells are stimulated and degranulated, releasing the allergic medium such as histamine, leukotriene, serotonin, and peptides. These allergic mediums will cause mucosal edema, fluid exudation, increased secretion, local stimulation and smooth muscle contraction.1 The main clinical symptoms of pollinosis are nasal itching, sneezing, rhinobyon and rhinorrhea. Some patients also get allergic conjunctivitis, allergic asthma, allergic dermatitis, etc.2 Pollinosis has become a global health problem.3
Diagnosis of pollinosis is based on anamnesis, skin tests and determination of specific IgE (sIgE) in the serum. Treatment of pollinosis includes allergen avoidance, pharmacotherapy and allergen-specific immunotherapy (SIT).4, 5 SIT can fundamentally solve the problem. But many patients do not want to try because of the long course and high failure rate. Additionally, pharmacotherapy is more universal which includes antihistamines, mast cell stabilizers, H1 receptor antagonists, anticholinergic drugs, glucocorticoids, etc.6, 7, 8
Pollen allergy patients differ in clinical symptoms during and after the pollen season. It is worth considering whether there is a significant difference of serum metabolites between the seizure and remission periods. So far no research has been done in this field, so we collected serum from patients with pollen allergy to study the changes of metabolites.
In recent decades, metabonomics becomes a new tool to study complex diseases, including allergic disease.9, 10 Metabonomics based on 1H nuclear magnetic resonance (NMR) can simultaneously detect hundreds of low molecular weight metabolites in biological matrices and the change of endogenous metabolic profile in typical external stimulation reactions.11 NMR-based metabonomics has been widely used in disease diagnosis,12 toxicity13 and efficacy evaluation.14
The purpose of this study was to find out the changes of serum metabolites between the seizure and remission periods of pollinosis, and to provide assistance in the diagnosis and/or therapy.
Materials and methods
Sample collection and preparation
Each subject had a history of spring pollen allergy for at least one year and the skin test of pollen allergens was positive. Blood samples were collected on an empty stomach at the seizure (the Pre group) and remission (the Pro group) stages respectively. The samples were stored at −80 °C until used.
All serum samples were thawed and 10000 g centrifuged 10 min at 4 °C before the 1H NMR spectra. The 300 μL upper serum was added to the 5 mm NMR test tube, and then 200 μL, 0.2 mol/L phosphate buffer solution (PBS, pH = 7.4) and 50 μL thick water was added to the test tube to oscillate and mix.
NMR experiments
1H NMR spectra of samples were recorded on a Bruker AVANCE III 500 MHz NMR spectrometer at 298 K D2O was used for field frequency locking and sodium 3-trimethylsilyl-propionic acid (TSP) was used as a chemical shift reference (1H, 0.00 ppm). A transverse relaxation edited Carr–Purcell–Meiboom–Gill (CPMG) sequence (90(τ–180–τ) n-acquisition) with a total spin-echo delay (2 nτ) of 40 ms was used to suppress the signals of proteins. 1H NMR spectra were recorded with 128 scans into 32 K data points over a spectral width of 10 000 Hz. The spectra were Fourier transformed after multiplying the FIDs (free induction decay) by an exponential weighting function corresponding to a line broadening of 0.5 Hz.
Data processing and statistical analysis
Based on least squares minimization with shifts corrected by the TSP signal, the spectra of 1H NMR were aligned and binned into integrated segments with widths of 0.005 ppm after removing residual water signals (4.22–6.7 ppm in plasma spectra). Then, principle component analysis (PCA) and orthogonal partial least squares discriminant analysis (OPLS-DA) were applied to distinguish differences in serum metabolites of two groups and determine the differential metabolic components. In addition, corresponding loading plots were used to provide variables which may influence clustering of the samples. Taking component indices of subjects in both groups as study factors, SPSS 13.0 was used to process general data (SPSS Inc., Chicago, IL, USA). The measured data were presented as mean ± standard deviation (SD), and t-test of two groups was conducted for inter-group comparison. P < 0.05 was considered as statistically significant.
Results
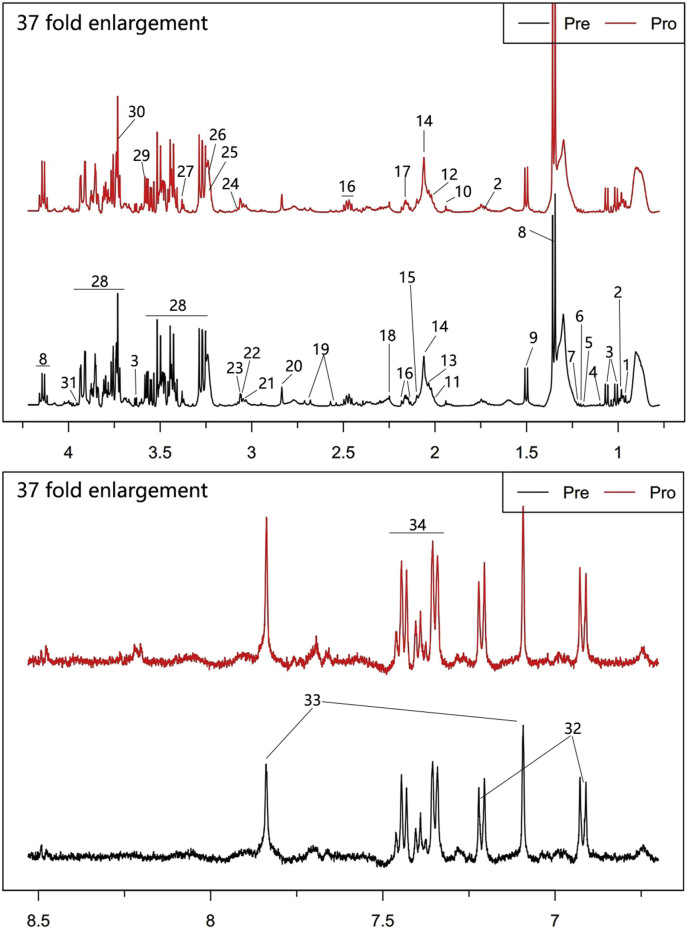
The representative 1H NMR spectra that were obtained from serum samples were shown with peak assignments in Fig. 1. Detailed information, including peak assignments and multiplicity, for the analyzed metabolites are listed in Table 1(*p < 0.05, **p < 0.01, ***p < 0.001).
Fig. 1.
Typical 500 MHz CPMG 1H NMR spectra of plasma with the metabolites labeled. 1. Isoleutine, 2. Leutine, 3. Valine, 4. Isobutyrate, 5. Isopropanol, 6. Allothreonine, 7.3-Hydroxybutyric acid, 8. Lactate, 9. Alanine, 10. Acetate, 11. Acetamide, 12. N-Acetylglutamine, 13. N-Acetylglycine, 14. UDP-NAG, 15. Methionine, 16. Glutamine, 17. O-Acetylcholine, 18. Acetoacetate, 19. Citrate, 20. Methylguanidine, 21. Lysine, 22. Creatine, 23. Creatinine, 24. Ornithine, 25. Trimethylamine N-oxide, 26. Betaine, 27. Methanol, 28. Glucose, 29. Glycine, 30. Guanidoacetic acid, 31. Glycolate, 32. l-Tyrosine, 33. Histidine, 34. Phenylalanine.
Table 1.
Assignments of NMR signals for endogenous metabolites in serum samples, their changes and associated p-values.
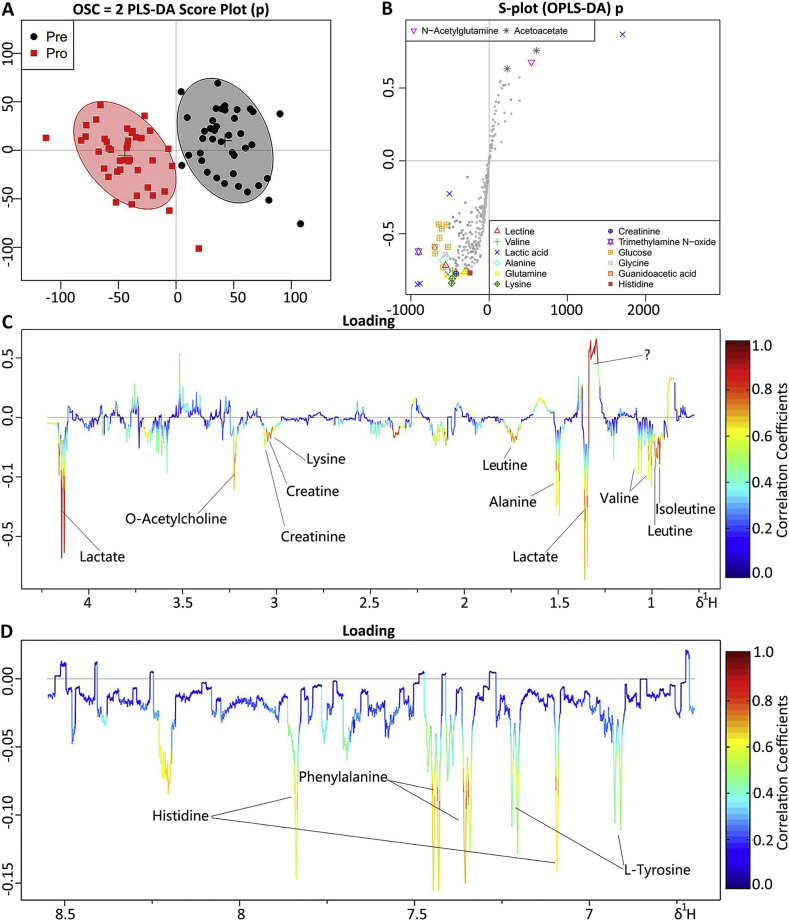
A supervised OSC-PLS-DA was performed on the data to filter out variations that were unrelated to class discrimination and to obtain a clear separation. A scores plot (Fig. 2A) was used to demonstrate that the results in the Pro group were completely separated with those in the Pre group after removed a few discrete values. The loading plot (Fig. 2C&D) was shown color-coded according to the absolute values of the correlation coefficients (r2) and constructed using a covariance-based pseudo-spectrum. The weight of a variable in the discrimination model was determined by calculating the square of its r2 and ranged from zero (blue areas) to high values (red areas).The S-plot (Fig. 2B) was shown as a scatter plot that demonstrates both the covariance (X axis) and correlation (Y axis) structures of the loading profiles. This plot was generated to identify differences in markers (e.g., those far away from the point of origin, which are located in the upper right and lower left quadrants) between classes. This visualization of our data was helpful for identifying interesting metabolites in our projection and for lowering the risk of false positives during metabolite selection.
Fig. 2.
OPLS-DA analyses of metabonomics profiles between the Pro group and the Pre group. Score plots (A) and color-coded coefficient loadings plot (C and D) for 1H NMR spectra of serum. Significantly changed metabolites were assigned in the loadings plot. Positive signals correspond to the metabolites present at increased concentrations in patients' serum. Conversely, negative signals correspond to the metabolites present at decreased concentrations in patients' serum. Symbols of ● (black filled circles) and  (red filled squares) represented the Pre and Pro group respectively. S-plot (B) from the OSC–PLS–DA analysis of the Pre and Pro groups.
(red filled squares) represented the Pre and Pro group respectively. S-plot (B) from the OSC–PLS–DA analysis of the Pre and Pro groups.
Combined with the coefficient loading plot and S-plot from OPLS-DA analysis, we identified several metabolites that were significantly different between the seizure and remission periods. Because of the fasting samples, the effect of food can be excluded. The level of serum metabolites at the remission stage was close to normal, so we believed that the decreased levels of isoleutine, leutine, valine, 3-hydroxybutyric acid, allo-threonine, alanine, methionine, glutamine, lysine, glycine, l-tyrosine, histidine, phenylalanine, lactate, acetate, O-acetylcholine, creatine and creatinine and the increased level of N-acetylglutamine at the seizure stage were statistically significant.
Discussion
In this study, a 1H NMR-based metabonomics approach was used to identify the important metabolites with significant differences in serum levels during and after the pollen season (seizure and remission). The changes of metabolites in this study will help us to understand more about the mechanism.
Patients with pollen allergy need a lot of energy during the disease. At the seizure stage, clinical manifestations are sneezing, runny nose, some are nasal congestion. They could not sleep at night and were in the state of excitement and exhaustion alternate. In immune stress reactions, amino acids are redistributed and mainly used to synthesize proteins involved in inflammation and immune response, as well as important compounds involved in immune cell proliferation and other immune responses.15 A large amount of histidine catalyzes histamine production by histidine decarboxylase, and the serum histidine content at the seizure stage is significantly lower than normal. This should be the most obvious and intuitive change in pollen allergy (type 1 hypersensitivity).16 Glutamine can promote the synthesis of protein and improve the level of immunoglobulin.17 Although its change is not obvious, it is still of great significance. Methionine is high in immune response protein. Methionine can be converted into homocysteine, and the increase of its concentration can up-regulate the adhesion of T cells, monocytes and endothelial cells.18 Branched chain amino acids including valine, leucine and isoleucine are very important nutritional supplement. Branched chain amino acids can stimulate the proliferation of monocytes, regulate the secretion of cytokines, and promote the development of ThI (helper T cell) type immune response. Imbalance of branched chain amino acids leads to immune damage.19 Deficiency of valine can lead to differentiation and maturation of dendritic cells.20 d-Valine is an important organic chiral source and its derivatives have shown great activity in clinical use, such as penicillamine for the treatment of immune-deficiency diseases.21 Allo-threonine was decreased at the seizure stage and can also be converted into glycine. LX519290, a derivative of L-allo threonine has the potential to ameliorate asthmatic symptoms by treating inflammatory factors in the lung.22
The level of glycine was decreased at the seizure stage. Glycine is an essential substrate for the synthesis of several biologically important biomolecules and compounds. It participates in the synthesis of proteins, of the tripeptide glutathione and in detoxification reactions. It has a broad spectrum of anti-inflammatory, cyto-protective and immune modulatory properties.23 When the body function is normal, the level of creatine and creatinine remains normal. However, some diseases or lifestyle lead to low creatinine levels. Creatine and creatine analogs such as cyclocreatine were found to have antitumor, antiviral, and antidiabetic effects and to protect tissues from hypoxic, ischemic, neurodegenerative, or muscle damage.24 The alteration in serum metabolites might reflect an increased energy demand relative to the decreased energy supply under inflammatory conditions.25 The level of lactate significantly decreased at the seizure stage, which is the intermediate product produced by metabolism of glucose in vivo.26 The intermediate product of TCA (tricarboxylic acid cycle) can be used as a precursor to other metabolic pathways, such as oxaloacetic acid to aspartic acid; alpha ketoglutaric acid to glutamic acid; oxaloacetate to alanine.27 The level of alanine decreased evidently at the seizure stage which may also reflect the up-regulated glycolysis. This result is identical to that of SLE(systemic lupus erythematosus) and rheumatoid arthritis patients.28 This may be the common feature of type I and III hypersensitivity. In the pathogenesis of type I and III hypersensitivity, immune complexes deposit locally and activate the complement and trigger a series of chain reactions in platelets, neutrophils and other cells, resulting in tissue damage and hypoxia.29
Phenylalanine is mostly oxidized to tyrosine by phenylalanine hydroxylase catalysis in vivo, and together with tyrosine to synthesize important neurotransmitters and hormones, which participate in carbohydrate metabolism and lipid metabolism.30, 31 In Fig. 2C, according to some other studies on serum metabonomics,28, 32, 33 the unknown metabolites may be high-density lipoprotein which indicated that patients may have up-regulated lipid metabolism at the seizure stage. The energy consumption of patients at the seizure stage is huge, and the carbohydrate supply is insufficient. The ketone body instead of glucose becomes the main energy source of brain tissue and muscle.34 The ketone body is a product of fat decomposition, not a product of hyperglycemia. So the changes of 3-hydroxybutyric acid in this study, a kind of ketone body, also indicated the abnormality of lipid metabolism.
In summary, pollinosis mainly influenced energy, amino acid and lipid metabolism, which might be the diagnosis and/or prognosis markers for hay fever patients. A variety of amino acids were consumed to synthesize immunoglobulins and related substances at the seizure stage. Due to the serum samples representing a cross-section of metabolic events and involving multiple organs, it was impossible to assign alterations to specific pathways based on the methods of this study. Future studies are needed to replicate these findings, and functional experiments are also needed to assess in individual patients. It may be possible to conduct in-depth analysis by collecting samples from specific locations.
Declarations
Ethics approval and consent to participate
The study protocol was in accordance with the ethical standards of the Declarations of Helsinki and Istanbul. The protocol of this study was approved by the local Ethics Committee of Peking Union Medical College Hospital, and written informed consent was obtained from all subjects.
Consent for publication
All authors agreed to publish the manuscript.
Availability of data and materials
We certify that the submission is original work and this paper has not been published elsewhere in whole or in part. All authors have read and approved the content, and agree to submit it for consideration for publication in your journal. There is no ethical/legal conflicts involved in the article.
Conflicts of interest
The authors declare that they have no competing interests.
Authors' contribution
Li-Sha Li completed the work of sample collection and patient information collection. Yan-jun Zhou completed the experiment and data analysis and wrote this thesis. Jin-Lu Sun, Kai Guan and Ji-Fu Wei conceived of the study and participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
Authors' information
Professor Jin-Lu Sun is the director of the Department of Allergy of Peking Union Medical College Hospital and has very rich experience in clinical diagnosis and treatment of allergic diseases. Professor Ji-Fu Wei is the director of the Research Division of Clinical Pharmacology of the First Affiliated Hospital of Nanjing Medical University who has studied allergic diseases for many years.
Funding
This project was sponsored by the grants from the National Natural Science Foundation of China (81571568, 81771725 and 81871265); CAMS Innovation Fund for Medical Sciences (CIFMS:2016-I2M-1003); the Program The Beijing Municipal Science And Technology project (Z161100000516006); Innovation team of Jiangsu Provincial Commission of Health and Family Planning (CXTDA2017049); the Priority Academic Program Development (PAPD) of Jiangsu Higher Education Institutions.
Acknowledgments
Thanks for the support of all the above funds.
Contributor Information
Yan-jun Zhou, Email: 244045736@qq.com.
Li-Sha Li, Email: 732842714@qq.com.
Jin-Lu Sun, Email: sunjl5@yahoo.com.
Kai Guan, Email: dr_guankai@126.com.
Ji-Fu Wei, Email: weijifu@hotmail.com.
References
- 1.Enerback L., Karlsson G., Pipkorn U. Nasal mast cell response to natural allergen exposure. Int Arch Allergy Appl Immunol. 1989;88:209–211. doi: 10.1159/000234788. [DOI] [PubMed] [Google Scholar]
- 2.Leonardi A., Castegnaro A., Valerio A.L. Epidemiology of allergic conjunctivitis: clinical appearance and treatment patterns in a population-based study. Curr Opin Allergy Clin Immunol. 2015;15:482–488. doi: 10.1097/ACI.0000000000000204. [DOI] [PubMed] [Google Scholar]
- 3.Smith M., Berger U., Behrendt H. Pollen and pollinosis. Chem Immunol Allergy. 2014;100:228–233. doi: 10.1159/000358743. [DOI] [PubMed] [Google Scholar]
- 4.Mailhol C., Didier A. Specific immunotherapy in grass pollen allergy. Hum Vaccines Immunother. 2012;8:1544–1547. doi: 10.4161/hv.22357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wallner M., Briza P., Thalhamer J. Specific immunotherapy in pollen allergy. Curr Opin Mol Ther. 2007;9:160–167. [PubMed] [Google Scholar]
- 6.Ben-Eli H., Solomon A. Topical antihistamines, mast cell stabilizers, and dual-action agents in ocular allergy: current trends. Curr Opin Allergy Clin Immunol. 2018;18:411–416. doi: 10.1097/ACI.0000000000000473. [DOI] [PubMed] [Google Scholar]
- 7.Sharma A.K., Arya A., Sahoo P.K. Overview of biopolymers as carriers of antiphlogistic agents for treatment of diverse ocular inflammations. Mater Sci Eng C Mater Biol Appl. 2016;67:779–791. doi: 10.1016/j.msec.2016.05.060. [DOI] [PubMed] [Google Scholar]
- 8.Platt M. Pharmacotherapy for allergic rhinitis. Int Forum Allergy Rhinol. 2014;4(Suppl 2):S35–S40. doi: 10.1002/alr.21381. [DOI] [PubMed] [Google Scholar]
- 9.Sitole L.J., Williams A.A., Meyer D. Metabonomic analysis of HIV-infected biofluids. Mol Biosyst. 2013;9:18–28. doi: 10.1039/c2mb25318f. [DOI] [PubMed] [Google Scholar]
- 10.Chai Y., Wang J., Liu Z. Metabonomics-a useful tool for individualized cancer therapy. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2013;42:705–710. doi: 10.3785/j.issn.1008-9292.2013.06.020. [DOI] [PubMed] [Google Scholar]
- 11.Pauli G.F., Jaki B.U., Lankin D.C. Quantitative 1H NMR: development and potential of a method for natural products analysis. J Nat Prod. 2005;68:133–149. doi: 10.1021/np0497301. [DOI] [PubMed] [Google Scholar]
- 12.Tritten L., Keiser J., Godejohann M. Metabolic profiling framework for discovery of candidate diagnostic markers of malaria. Sci Rep. 2013;3:2769. doi: 10.1038/srep02769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fukuhara K., Ohno A., Ando Y. A 1H NMR-based metabonomics approach for mechanistic insight into acetaminophen-induced hepatotoxicity. Drug Metab Pharmacokinet. 2011;26:399–406. doi: 10.2133/dmpk.dmpk-11-rg-005. [DOI] [PubMed] [Google Scholar]
- 14.Li A.P., Li Z.Y., Sun H.F. Comparison of two different Astragali Radix by a (1)H NMR-based metabonomic approach. J Proteome Res. 2015;14:2005–2016. doi: 10.1021/pr501167u. [DOI] [PubMed] [Google Scholar]
- 15.Grohmann U., Bronte V. Control of immune response by amino acid metabolism. Immunol Rev. 2010;236:243–264. doi: 10.1111/j.1600-065X.2010.00915.x. [DOI] [PubMed] [Google Scholar]
- 16.Uzzaman A., Cho S.H. Chapter 28: classification of hypersensitivity reactions. Allergy Asthma Proc. 2012;33(Suppl 1):96–99. doi: 10.2500/aap.2012.33.3561. [DOI] [PubMed] [Google Scholar]
- 17.Windle E.M. Glutamine supplementation in critical illness: evidence, recommendations, and implications for clinical practice in burn care. J Burn Care Res. 2006;27:764–772. doi: 10.1097/01.BCR.0000245417.47510.9C. [DOI] [PubMed] [Google Scholar]
- 18.Grimble R.F. The effects of sulfur amino acid intake on immune function in humans. J Nutr. 2006;136:1660S–1665S. doi: 10.1093/jn/136.6.1660S. [DOI] [PubMed] [Google Scholar]
- 19.Negro M., Giardina S., Marzani B. Branched-chain amino acid supplementation does not enhance athletic performance but affects muscle recovery and the immune system. J Sports Med Phys Fitness. 2008;48:347–351. [PubMed] [Google Scholar]
- 20.Kakazu E., Kanno N., Ueno Y. Extracellular branched-chain amino acids, especially valine, regulate maturation and function of monocyte-derived dendritic cells. J Immunol. 2007;179:7137–7146. doi: 10.4049/jimmunol.179.10.7137. [DOI] [PubMed] [Google Scholar]
- 21.Chen M., Shi C., Zhao J. Application and microbial preparation of D-valine. World J Microbiol Biotechnol. 2016;32:171. doi: 10.1007/s11274-016-2119-z. [DOI] [PubMed] [Google Scholar]
- 22.Heo J.C., Lee S.H. Alleviation of asthma-related symptoms by a derivative of L-allo threonine. Int J Mol Med. 2013;31:881–887. doi: 10.3892/ijmm.2013.1265. [DOI] [PubMed] [Google Scholar]
- 23.Grotz M.R., Pape H.C., van Griensven M. Glycine reduces the inflammatory response and organ damage in a two-hit sepsis model in rats. Shock. 2001;16:116–121. doi: 10.1097/00024382-200116020-00006. [DOI] [PubMed] [Google Scholar]
- 24.Wyss M., Kaddurah-Daouk R. Creatine and creatinine metabolism. Physiol Rev. 2000;80:1107–1213. doi: 10.1152/physrev.2000.80.3.1107. [DOI] [PubMed] [Google Scholar]
- 25.Martin F.P., Rezzi S., Philippe D. Metabolic assessment of gradual development of moderate experimental colitis in IL-10 deficient mice. J Proteome Res. 2009;8:2376–2387. doi: 10.1021/pr801006e. [DOI] [PubMed] [Google Scholar]
- 26.Adeva-Andany M., Lopez-Ojen M., Funcasta-Calderon R. Comprehensive review on lactate metabolism in human health. Mitochondrion. 2014;17:76–100. doi: 10.1016/j.mito.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 27.Akram M. Citric acid cycle and role of its intermediates in metabolism. Cell Biochem Biophys. 2014;68:475–478. doi: 10.1007/s12013-013-9750-1. [DOI] [PubMed] [Google Scholar]
- 28.Ouyang X., Dai Y., Wen J.L. 1)H NMR-based metabonomic study of metabolic profiling for systemic lupus erythematosus. Lupus. 2011;20:1411–1420. doi: 10.1177/0961203311418707. [DOI] [PubMed] [Google Scholar]
- 29.Weljie A.M., Dowlatabadi R., Miller B.J. An inflammatory arthritis-associated metabolite biomarker pattern revealed by 1H NMR spectroscopy. J Proteome Res. 2007;6:3456–3464. doi: 10.1021/pr070123j. [DOI] [PubMed] [Google Scholar]
- 30.Tessari P., Vettore M., Millioni R. Effect of liver cirrhosis on phenylalanine and tyrosine metabolism. Curr Opin Clin Nutr Metab Care. 2010;13:81–86. doi: 10.1097/MCO.0b013e32833383af. [DOI] [PubMed] [Google Scholar]
- 31.Holm G., Bjorntorp P., Jagenburg R. Carbohydrate, lipid and amino acid metabolism following physical exercise in man. J Appl Physiol Respir Environ Exerc Physiol. 1978;45:128–131. doi: 10.1152/jappl.1978.45.1.128. [DOI] [PubMed] [Google Scholar]
- 32.Li J., Zhao X.L., Liu Y.X. 1HNMR-based metabonomic profile of rats with experimental acute pancreatitis. BMC Gastroenterol. 2014;14:115. doi: 10.1186/1471-230X-14-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu J., Wang D., Chen Y. 1H NMR-based metabonomic analysis of serum and urine in a nonhuman primate model of diabetic nephropathy. Mol Biosyst. 2013;9:2645–2652. doi: 10.1039/c3mb70212j. [DOI] [PubMed] [Google Scholar]
- 34.Cotter D.G., Schugar R.C., Crawford P.A. Ketone body metabolism and cardiovascular disease. Am J Physiol Heart Circ Physiol. 2013;304:H1060–H1076. doi: 10.1152/ajpheart.00646.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
We certify that the submission is original work and this paper has not been published elsewhere in whole or in part. All authors have read and approved the content, and agree to submit it for consideration for publication in your journal. There is no ethical/legal conflicts involved in the article.