Abstract
Background
Insects have become increasingly interesting as alternative nutrient sources for feeding humans and animals, most reasonably in processed form. Initially, some safety aspects — among them allergenicity — need to be addressed.
Objective
To reveal the cross-reactivity of shrimp-, mite- and flies-allergic patients to different edible insects, and further to assess the efficacy of food processing in reducing the recognition of insect proteins by patients' IgE and in skin prick testing of shrimp-allergic patients.
Methods
IgE from patients allergic to crustaceans, house dust mite or flies was evaluated for cross-recognition of proteins in house cricket Acheta domesticus (AD), desert locust Schistocerca gregaria (SG) and Yellow mealworm Tenebrio molitor (TM). Changes in IgE-binding and SPT-reactivity to processed insect extracts were determined for migratory locust (Locusta migratoria, LM), after different extraction methods, enzymatic hydrolysis, and thermal processing were applied.
Results
IgE from patients with crustacean-allergy shows cross-recognition of AD, SG and stable flies; house dust mite allergics' IgE binds to AD and SG; and the flies-allergic patient recognized cricket, desert locust and migratory locust. Cross-reactivity and allergenicity in SPT to LM can be deleted by conventional processing steps, such as hydrolysis with different enzymes or heat treatment, during the preparation of protein concentrates.
Conclusion
The results show that crustacean-, HDM- and stable flies-allergic patients cross-recognize desert locust and house cricket proteins, and crustacean-allergic patients also flies proteins. Furthermore, this study shows that appropriate food processing methods can reduce the risk of cross-reactivity and allergenicity of edible insects.
Keywords: Allergenicity, Cross-recognition, Edible insects, Enzymatic hydrolysis, Food allergy, Immunoreactivity, Thermal processing
Abbreviations:
- AD
Acheta domesticus (house cricket)
- db
dry basis
- dm
dry matter
- EDTA
ethylenediaminetetraacetic acid
- HDM
house dust mite
- IUS
ISAC Standardized Units
- LM
Locusta migratoria (migratory locust)
- LMPC
Locusta migratoria (migratory locust) processed
- NaN3
sodium azide
- SG
Schistocerca gregaria (desert locus)
- SPT
skin prick test
- TM
Tenebrio molitor (Yellow mealworm)
Introduction
Edible insects are gaining increasing attention as trendy foods and are inter alia discussed as novel alternative protein sources for human food and animal feed.1 So far, edible insects are mainly consumed as snack products and are included in feed for fish and poultry. The further development of insect-based products for human consumption as well as the use of insects or insect-derived fractions for other livestock like cattle is progressing.1 The development of industrial-scale mass rearing systems and efficient processes for the recovery of functional insect-derived fractions such as protein, fat or chitin will be a prerequisite to promote the commercial use of edible insects in Western countries and explore a wide range of potential food and non-food applications.2 Migratory locust (Locusta migratoria L.) is among the most promising candidates for the integration of edible insect in western food and feed industry due to auspicious protein content of 65% db, well-balanced amino acid profile and already existing rearing know-how on a commercial scale for pet food and even human nutrition.3, 4, 5, 6
As insects have not been widely used for consumption by humans in the Western countries, they have to be declared as novel foods and their safety needs to be assessed.2 As part of this assessment, edible insects need to be characterized with focus on the nutritional value as well as on other safety-related aspects such as microorganisms, toxins, and heavy metals.7 In this context, the risk of allergenicity has to be taken into consideration as well.8 Few studies exist on this topic and mainly case reports on primary sensitization against insects are provided. They often describe respiratory allergies in breeders and –as a modern trend- also in reptile pet keepers, who use insects as feed for their pets.9
Even scarcer are reports about true primary food allergies against edible insects, which include symptoms upon ingestion of grasshopper Mecopoda elongata10 and stink bugs.11 Anaphylactic reactions occurred due to consumption of different insect species, e.g. fried grasshoppers and crickets,12 lentis weevil Bruchus lentis,13 Mopane worm from the Emporer moth Gonimbrasia belina,14 mealworm and superworm.15 A review of the Chinese literature from 1980 to 200716 revealed that ingested insects where often the cause for anaphylactic events: locust (27 cases), grasshopper (27), silkworm pupae (5), cicada pupae (1), bee pupae (1), bee larvae (1) or moth Clanis bilineata (1).
More is known about the cross-reactivity of patients allergic to shrimps (and other crustacean) or house dust mite, who also react to grasshoppers, field cricket and mealworm.17, 18, 19 However, studies on cross-reactivity of crustacean- and house dust mite-allergic patients against house cricket (Acheta domesticus) as well as desert locust (Schistocerca gregaria) have not been performed.
In addition, flies (Brachycera), e.g. larvae from black soldier fly Hermetia illucens and house fly (Musca domestica) have already been produced as alternative to soybean meal in animal feed20, 21 and are on their way into human diet. However, apart from one case report about sensitization to common house fly,22 no further investigations of flies allergy, cross-reactivity or allergens were performed. In the present study, a patient with inhalative allergies against stable flies could be included.
In Western countries, insects are not very likely to be consumed in raw state. First, in order to ensure microbiological safety, the application of at least one decontamination step during post-harvest processing is essential, and thermal processes such as blanching, pasteurization, sterilization, cooking or autoclaving are suitable unit operations for this purpose. Second, the most likely form of future use and consumption will be the processed state, either as a whole, as food component (e.g. in cereal bars or pastes), or as food ingredient (flour, extracted proteins).21 In this respect, enzymatic hydrolysis is an important means to increase functionality and functional properties of proteins.23
Food processing operations, in particular enzymatic hydrolysis and heat treatment, have already been demonstrated to affect the allergenicity of plant- and animal-based allergens, e.g. pasteurization of milk increases allergenicity of the proteins.24 In the case of edible insects, studies investigating the impact of processing on the allergenicity of insect proteins are scarce. Broekman et al evaluated the effect of thermal processing such as blanching, boiling, baking, and frying on Yellow mealworm (Tenebrio molitor) larvae on protein allergenicity.25 IgE binding in blot and basophil activation test occurred in both, processed and native extracts, and furthermore skin prick test reaction was not affected by the applied thermal treatments. In another study, van Broekhoven and colleagues reported diminished house dust mite and tropomyosin IgE cross-reactivity of Yellow mealworm, Lesser mealworm (Alphitobius diaperinus) and super mealworm (Zophobas atratus) larvae after heat treatment (frying, boiling) or in vitro digestion.26 Also alterations in IgE-binding intensity of allergens after frying Bombay locust (Patanga succincta) towards (i) a higher allergenicity in the case of pyruvate kinase and GADPH and (ii) a reduced allergenicity for arginine kinase, hexamerin, and enolase was reported.19 Due to this limited knowledge, it is crucial to further investigate how production and processing technologies, e.g. thermal treatment and enzymatic hydrolysis, might influence i) protein integrity, and ii) the immunoreactivity and allergenicity of processed insect species.
Therefore, the present study provides i) additional information on cross-reactions for patients allergic to crustacean, house dust mite and stable flies, ii) as well as pioneering work regarding the influence of technological processing (protein fractionation, conventional heat treatment and enzymatic hydrolysis) on immunoreactivity and allergenicity of insect proteins from Locusta migratoria.
Material and methods
Protein extract preparation from shrimp, house dust mite, Schistocerca gregaria, Acheta domesticus, stable flies
Shrimp (from supermarket), Schistocerca gregaria and Acheta domesticus (from pet store) and stable flies (from cow's stable obtained from the University of Veterinary Medicine, Vienna, Austria) were frozen in liquid nitrogen, grinded and extracted 1:10 (w/v) in PBS supplemented with 0.5 M EDTA (2 mL/L) and 0.19503 g/L NaN3 at 4 °C o.n. under continuous agitation. After multiple centrifugation steps at 3000 g (30 min, 30 min, 80 min), the final supernatant was filtered sterile and stored at −20 °C.
Production of Tenebrio molitor larvae (TM) extract
Frozen larvae were crushed using a blender (Braun 4162 MQ300, Braun, Poland) and mixed with deionized water (1:1.7 w/v). The slurry was stirred for 2 h to solubilize the proteins. Afterwards, the slurry was centrifuged (Sigma Laborzentrifugen, Type 4–15, Osterode, Germany) for separation of the protein extract. The insoluble fractions (solid chitin pellet, fat layer) were removed after centrifugation. The liquid protein extract was finally frozen at −30 °C, freeze-dried (FreeZone 6, Labconco, Kansas City, USA), ground using a mortar grinder and stored at 3 °C until further use.
Raw materials for migratory locust, reagents and chemicals
Frozen adult migratory locusts (Locusta migratoria, LM) were purchased from NGN BV (Helvoirt, Netherlands) and stored at −30 °C until further use.
The enzyme preparations Alcalase® 2.4 L FG (2.4 AU-A/g, endoprotease from Bacillus licheniformis), Neutrase® 0.8 L (endoprotease from Bacillus amyloliquefaciens), and Flavourzyme® 1000 L (1000 LAPU/g, endo- and exoprotease from Aspergillus oryzae) were kindly provided by Novozymes A/S (Bagsvaerd, Denmark). Papain, a cysteine-protease from papaya latex (≥10 units/mg, E.C. 3.4.22.2, Sigma no P4762), was purchased from Sigma-Aldrich Inc. (St. Louis, U.S.A). For fat extraction of LM, n-hexane with a purity of ≥95.0% was used (AnalaR, VWR Chemicals, Fontenay-sous-Bois, France).
Production of different LM protein concentrates (LMPC)
The different production and process steps for LMPC are summarized in Table 1. Established extraction and processing conditions comparable to the production schemes of plant-based proteins were chosen. These steps include inter alia basic and acidic extraction as well as a basic extraction step followed by acidic protein precipitation.
Table 1.
Parameters of differently processed protein concentrates from LM.
| No. | Sample ID | Processing | Dry matter (dm) (% ± SD) |
Protein content (% dm ± SD) |
|---|---|---|---|---|
| 1 | LM | – | 96.35 ± 0.01 | 65.87 ± 0.42 |
| 2 | LMPC 1 | Basic extraction | 94.90 ± 0.13 | 82.26 ± 0.62 |
| 3 | LMPC 2 | Acid extraction | 95.18 ± 0.08 | 61.32 ± 0.22 |
| 4 | LMPC 3 | IE precipitation | 92.89 ± 0.37 | 59.18 ± 0.39 |
| 5 | LMPC Flavourzyme | One-step hydrolysis (E/S: 0.5%) | 94.90 ± 0.13 | 82.26 ± 0.62 |
| 6 | LMPC Alcalase | One-step hydrolysis (E/S: 0.5%) | 94.90 ± 0.13 | 82.26 ± 0.62 |
| 7 | LMPC Papain | One-step hydrolysis (E/S: 0.05%) | 94.90 ± 0.13 | 82.26 ± 0.62 |
| 8 | LMPC Neutrase | One-step hydrolysis (E/S: 0.5%) | 94.90 ± 0.13 | 82.26 ± 0.62 |
| 9 | LMPC Flavourzyme & Papain |
One-step hydrolysis (E/S: 1.0% and 0.05%) | 94.90 ± 0.13 | 82.26 ± 0.62 |
| 10 | LMPC Flavourzyme & Papain |
Two-step hydrolysis (E/S: 1.0% and 0.05%) | 94.90 ± 0.13 | 82.26 ± 0.62 |
| 11 | LMPC thermal 1 | Cooking (80 °C, 10 min) | 94.90 ± 0.13 | 82.26 ± 0.62 |
| 12 | LMPC thermal 2 | Autoclaving (121 °C, 20 min) | 94.90 ± 0.13 | 82.26 ± 0.62 |
| 13 | LMPC thermal 3 | Cooking (100 °C, 10 min) | 94.90 ± 0.13 | 82.26 ± 0.62 |
| 14 | LMPC thermal 4 | Autoclaving (138 °C, 20 min) | 94.90 ± 0.13 | 82.26 ± 0.62 |
Basic protein extraction - LMPC 1
Initially, frozen LM were freeze-dried (FreeZone 6, Labconco, Kansas City, USA) at 0.2 mbar for 72 h. Afterwards, wings and legs were manually removed and LM were ground into coarse meal using a blender (C-Series 5200, Vitamix, Cleveland, USA). For fat extraction, n-hexane was added at a ratio of 1:5 w/v and continuously stirred for 27 h at room temperature. After 1 h of settling, the fat-containing hexane supernatant was separated via decantation and the solid residue was dried in a vacuum dryer (VD 23, Binder, Tuttlingen, Germany) at 35 °C and 200 mbar for 72 h. For protein extraction, the dried and defatted LM coarse meal was mixed with deionized water (1:2 w/v) and the pH was set to 9.0 by adding 4 M or 1 M NaOH for rough and fine adjustment, respectively. After 2 h of stirring, the LM slurry was filtered through a cheese-filtering cloth (pore size 0.2–0.5 mm, 100% cotton) for chitin separation. Finally, the filtrate was freeze-dried, ground in a mortar grinder and stored at 3 °C until further use.
Acidic protein pre-extraction – LMPC 2
Wings and legs of frozen LM were removed manually. Subsequently, the remaining bodies were mixed with Millipore water (1:2 w/w) and ground in a kitchen blender. In order to minimize enzymatic decolorisation processes, the pH value was lowered to 3.8 using citric acid at a concentration of 0.36 g/100 g LM. The slurry was filtered through a flexible filter (pore size 1 mm) to separate the chitin. Filter and filter cake were rinsed once with Millipore water (10% w/w of the initial slurry mass) to remove the remaining protein from the chitin fraction. Afterwards, the filtrate was centrifuged (Centaur 2, MSE Ltd., London, UK) at 3130 g for 20 min at ambient temperature for fat separation. After centrifugation, the fat layer was removed manually and the residual slurry was frozen at −30 °C and freeze-dried at 200 mbar for 72 h. Finally, the freeze-dried protein extract was ground using a mortar grinder and stored at 3 °C until analysis.
Basic protein extraction and subsequent acidic protein precipitation – LMPC 3
LMPC 1 was suspended in deionized water at a ratio of 1:10 w/v by stirring. Afterwards, pH was steadily reduced to 4.3 using 1 M HCl to induce protein precipitation. The precipitated protein was separated from the serum by centrifugation at 3220g for 20 min at room temperature. The supernatant was removed by decantation and the protein pellet was frozen, freeze-dried, manually ground using a mortar grinder and stored at 3 °C until further use.
Modification of LMPC 1 by processing
Due to its high protein content (82% db), LMPC 1 (see 2.4.1) was used as raw material for the following modification, including enzymatic hydrolysis and thermal treatment. All experiments were performed in duplicate. For the hydrolysis experiments, four different commercially available food-grade enzymes were used. These protease preparations are commonly used in the food industry for the production of protein hydrolysates. Reaction conditions (50 °C and pH 7.0) were chosen according to producers' application sheet. The dosage of 0.05%, 0.5% and 1% for endo- and exoproteases has been applied according to unpublished preliminary experiments (data not shown). Further, the processing conditions and combinations were chosen according to the experiments performed by Meinlschmidt et al.27, 28 Thermal treatments were performed in order to study the effect of conventional food preservation (e.g. autoclaving) on the resulting allergenicity of insects.
Enzymatic hydrolysis of LMPC 1
Four different commercially available food-grade enzyme preparations (Alcalase, Neutrase, Flavourzyme, and papain) were used. Reaction conditions (50 °C and pH 7.0) were chosen according to producers' application sheet in order to ensure maximum enzyme activity. The dosage of 0.05%, 0.5% and 1% for endo- and exoproteases has been applied according to own unpublished experiments. Enzymatic hydrolysis of LMPC 1 was performed by applying both single enzyme preparations and combinations thereof as described earlier.27, 28 Briefly, a 5% (w/v) LMPC 1 dispersion using deionized water was prepared and the pH value was adjusted from initially pH 6.6 to 7.0 with 1 M NaOH. The dispersions were heated up to 50 °C in a shaking water bath.
Single enzymes Alcalase, Flavourzyme and Neutrase were added to the heated dispersions with an enzyme-to-substrate (E/S) ratio of 1:20 (w/v), whereas papain was added with an E/S ratio of 1:200 (w/w) based on own unpublished data. Enzymatic hydrolysis was conducted for 2 h at 50 °C without pH adjustment in a shaking water bath. Enzymes were inactivated by heat treatment at 90 °C for 10 min and samples were stored at – 20 °C until further use.
The combined application of papain and Flavourzyme (enzyme combinations) has been shown to provide synergetic effects (own unpublished data). It was performed as one- and two-step process, applying the enzymes concomitantly or subsequently. For the one-step process, 0.05% (w/w) papain and 1% (w/v) Flavourzyme were added simultaneously and LMPC 1 was digested for 2 h. For the two-step process, 0.05% (w/w) papain was added to the pre-heated (50 °C) LMPC 1 dispersion. After 1 h of pre-digestion, 1% (w/v) Flavourzyme was added, and the hydrolysis was continued for another 60 min without pH adjustment. Enzyme activity was terminated by heat treatment (90 °C, 10 min) and samples were stored at −20 °C.
Thermal treatment of LMPC 1
For heat treatment experiments, a 5% (w/v) LMPC 1-dispersion was prepared. An aliquot of 80 mL was heated at 80 and 100 °C for 10 min in a water bath. In addition, samples were also subjected to autoclaving (Varioklav 400, Ehret, Tulln, Austria) at 121 and 138 °C for a dwell time of 20 min (Fo = 20 and 980 min respectively based on Tref = 121.1 °C and z = 10 °C and C = 100 and 370 min respectively based on Tref = 100 °C and z = 30 °C, only the dwell time was considered for calculation). Samples were stored at −20 °C prior to analysis.
Chemical composition of LM
The chemical composition of LM samples in terms of dry matter and crude protein content was analyzed. The dry matter content was determined by oven drying method at 105 °C based on AOAC 950.46.29 The total nitrogen content was analysed using the Kjeldahl method according to AOAC 928.08.29 The crude protein content was calculated using the nitrogen conversion factor (N) of 6.25 recommended by Finke MD.30
Protein determination in SDS-PAGE by silver staining
The protein content of all extracts was determined by bicinchoninic acid assay (Pierce BCA Protein Assay Kit, Thermo Scientific, 23225), according to manufacturer's instructions. Protein extracts (concentration equally adjusted according to BCA to 2.5 μg/slot or 140 μg/gel or were separated via SDS-PAGE (15%) under reducing conditions, and visualized by silver staining.
Patient selection
Sera were collected from patients (with written informed consent) during routine allergy testing in an allergy-outpatient clinic (Vienna): 3 sera from patients diagnosed with crustacean allergy (based on suggestive history and/or specific IgE acc. to CAP-FEIA), 8 patients with house dust mite allergy (based on suggestive history and sensitization) and 1 patient with stable flies allergy (based on suggestive history) were included to test cross-reactivity as well as the effect of processing on protein immunoreactivity using immunoblot. Details about patients' serum characteristics can be found in Table 2. Ethical approval was granted by the local ethics committee of the Medical University of Vienna (No 2002/2012).
Table 2.
Patients' characteristics.
| Patient No, Sex, Age | Used in pool for | Reported allergies | IgE in FEIA (class) | Total IgE in ELISA (ng/ml) | Specific IgE on ImmunoCAP ISAC chip |
Allergen source | |
|---|---|---|---|---|---|---|---|
| Allergen | ISAC Standardized Units (ISU) | ||||||
| 1, f, 32 | Crustacean | Lobster Prawn Latex Fish Orange |
Lobster (6) Prawn (4) Latex (2) Fish-Mix (2) Orange (2) |
1920.4 | Che a 1 | 21.02 | Goosefoot |
| Cyn d 1 | 26.93 | Bermuda grass | |||||
| Der p 10 | 22.01 | House dust mite | |||||
| Phl p 1 | 133.68 | Timothy grass | |||||
| Phl p 4 | 27.42 | ||||||
| Phl p 5 | 87.05 | ||||||
| Phl p 6 | 39.12 | ||||||
| Phl p 11 | 32.45 | ||||||
| Pla a 2 | 2.27 | Plane tree | |||||
| nApi g 5 | 1.49 | Celery | |||||
| Jug r 2 | 1.37 | Walnut | |||||
| Pen m 1 | 33.46 | Shrimp | |||||
| Pen m 4 | 42.91 | ||||||
| Ana c 2 | 2.78 | Pineapple | |||||
| Ves v 5 | 5.19 | Yellow jacket wasp | |||||
| Ani s 3 | 24.01 | Herring worm | |||||
| Bla g 7 | 27.55 | German cockroach | |||||
| 2, f, 30 | Crustacean | Fish | Lobster (2) Prawn (2) Different fish and mussel (2) |
1146.6 | Bet v 1 | 1.85 | Birch tree |
| Bet v 4 | 0.82 | ||||||
| Cry j 1 | 5.92 | Cypress tree | |||||
| Cup a 1 | 8.06 | Cypress tree | |||||
| Cyn d 1 | 11.90 | Bermuda grass | |||||
| Der f 1 | 33.07 | House dust mite | |||||
| Der f 2 | 68.85 | ||||||
| Der p 1 | 19.43 | House dust mite | |||||
| Der p 2 | 55.24 | ||||||
| Fel d 1 | 2.11 | Cat | |||||
| Phl p 1 | 3.04 | Timothy grass | |||||
| Phl p 4 | 12.06 | ||||||
| Phl p 5 | 1.66 | ||||||
| Pla a 2 | 7.90 | Plane tree | |||||
| nApi g 5 | 3.76 | Celery | |||||
| Jug r 2 | 7.20 | Walnut | |||||
| Ana c 2 | 5.99 | Pineapple | |||||
| Pol d 5 | 1.77 | Paper wasp | |||||
| Ves v 5 | 15.70 | Yellow jacket | |||||
| Gad c 1 | 1.63 | Baltic cod | |||||
| 3, m, 30 | Crustacean | Crustacean Egg Milk; Grass Birch |
n.d. | 139.4 | Alt a 1 | 5.29 | Alternaria plant |
| Cyn d 1 | 1.05 | Bermuda grass | |||||
| Der p 10 | 7.62 | House dust mite | |||||
| Phl p 1 | 4.87 | Timothy grass | |||||
| Rat Albumin | 0.86 | Rat | |||||
| Act d 2 | 1.19 | Kiwi | |||||
| Bos d 6 | 1.16 | Cow | |||||
| Pen m 1 | 15.15 | Shrimp | |||||
| Pen m 4 | 9.27 | ||||||
| Rabbit albumin | 1.25 | Rabbit | |||||
| Ani s 3 | 8.46 | Herring worm | |||||
| Bla g 7 | 11.71 | Cockroach | |||||
| Gal d 5 | 3.30 | Chicken | |||||
| Gal d 3 | 1.90 | ||||||
| 4, m, 23 | HDM | Pollen, grass; dog, cat; fungi; HDM |
971.9 | Bet v 2 | 7.34 | Birch tree | |
| Cyn d 1 | 44.05 | Bermuda grass | |||||
| Der p 2 | 1.79 | House dust mite | |||||
| Fel d 1 | 1.21 | Cat | |||||
| Lep d 2 | 0.78 | Storage mite | |||||
| Mer a 1 | 6.37 | Herb | |||||
| Ole e 1 | 6.35 | Olive | |||||
| Phl p 1 | 75.25 | Timothy grass | |||||
| Phl p 2 | 32.33 | ||||||
| Phl p 4 | 12.59 | ||||||
| Phl p 5 | 19.94 | ||||||
| Phl p 6 | 0.90 | ||||||
| Phl p 12 | 4.03 | ||||||
| Pla a 2 | 2.35 | Plane tree | |||||
| nApi g 5 | 2.33 | Celery | |||||
| Jug r 2 | 4.06 | Walnut | |||||
| Hev b 8 | 9.69 | Latex | |||||
| Ana c 2 | 2.36 | Pineapple | |||||
| Pol d 5 | 1.46 | Paper wasp | |||||
| Ves v 5 | 1.05 | Yellow jacket | |||||
| 5, m, 44 | HDM | HDM; Grass; Cat, horse |
160.1 | Bet v 2 | 1.81 | Birch tree | |
| Cyn d 1 | 1.50 | Bermuda grass | |||||
| Der f 1 | 1.27 | House dust mite | |||||
| Der f 2 | 6.35 | ||||||
| Der p 2 | 4.73 | House dust mite | |||||
| Equ c 1 | 1.39 | Horse | |||||
| Mer a 1 | 1.77 | Herb | |||||
| Phl p 1 | 9.93 | Timothy grass | |||||
| Phl p 5 | 7.92 | ||||||
| Hev b 8 | 2.05 | Latex | |||||
| 6, f, 20 | HDM | HDM; Pollen; Horse, rabbit |
713.0 | Aln g 1 | 4.64 | Alder tree | |
| Art v 1 | 12.53 | Mugwort | |||||
| Bet v 1 | 10.05 | Birch tree | |||||
| Bet v 2 | 2.13 | ||||||
| Cor a 1.0101 | 3.16 | Hazelnut | |||||
| Cyn d 1 | 12.50 | Bermuda grass | |||||
| Der f 2 | 17.96 | House dust mite | |||||
| Der p 2 | 16.93 | House dust mite | |||||
| Fel d 1 | 1.43 | Cat | |||||
| Mer a 1 | 3.04 | Herb | |||||
| Ole e 1 | 4.39 | Olive | |||||
| Phl p 1 | 45.66 | Timothy grass | |||||
| Phl p 4 | 19.42 | ||||||
| Phl p 5 | 62.12 | ||||||
| Phl p 6 | 33.20 | ||||||
| Phl p 11 | 11.60 | ||||||
| Phl p 12 | 1.15 | ||||||
| Ara h 8 | 2.08 | Peanut | |||||
| Cor a 1.0401 | 4.65 | Hazelnut | |||||
| Mal d 1 | 1.90 | Apple | |||||
| Pru p 1 | 3.01 | Peach | |||||
| Hev b 8 | 2.66 | Latex | |||||
| 7, m, 26 | HDM | HDM; Apple, kiwi, carrot, soy; Grass, birch; Dog, cat, mouse |
655.2 | Aln g 1 | 12.30 | Alder tree | |
| Bet v 1 | 26.90 | Birch tree | |||||
| Can f 1 | 9.51 | Dog | |||||
| Can f 5 | 2.19 | ||||||
| Che a 1 | 1.20 | Lambs quarter | |||||
| Cor a 1.0101 | 7.93 | Hazelnut | |||||
| Cyn d 1 | 18.50 | Bermuda grass | |||||
| Der f 1 | 23.55 | House dust mite | |||||
| Der f 2 | 53.73 | ||||||
| Der p 1 | 5.58 | House dust mite | |||||
| Der p 2 | 15.39 | ||||||
| Equ c 1 | 6.74 | Horse | |||||
| Fel d 1 | 35.60 | Cat | |||||
| Mus m 1 | 2.56 | Mouse | |||||
| Phl p 1 | 24.52 | Timothy grass | |||||
| Phl p 2 | 2.66 | ||||||
| Phl p 4 | 4.61 | ||||||
| Phl p 5 | 5.77 | ||||||
| Phl p 6 | 6.31 | ||||||
| Phl p 11 | 3.45 | ||||||
| Pla l 1 | 2.01 | Plane tree | |||||
| Act d 8 | 0.96 | Kiwi | |||||
| Api g 1 | 2.78 | Celery | |||||
| Ara h 8 | 1.90 | Peanut | |||||
| Cor a 1.0401 | 7.37 | Hazelnut | |||||
| Gly m 4 | 2.29 | Soybean | |||||
| Mal d 1 | 5.62 | Apple | |||||
| Pru p 1 | 8.62 | Peach | |||||
| Fel d 4 | 5.51 | Cat | |||||
| 8 | HDM | n.d. | 1607.8 | Art v 1 | 19.09 | Mugwort | |
| Art v 3 | 0.80 | ||||||
| Api m 1 | 11.85 | Honey bee | |||||
| Ves v 5 | 1.11 | Yellow jacket | |||||
| 9 | HDM | n.d. | 1774.6 | Aln g 1 | 15.34 | Alder tree | |
| Alt a 1 | 19.19 | Alternaria plant | |||||
| Bet v 1 | 63.14 | Birch tree | |||||
| Cor a 1.0101 | 14.33 | Hazelnut | |||||
| Der f 2 | 25.05 | House dust mite | |||||
| Der p 2 | 24.59 | ||||||
| Fel d 1 | 1.83 | Cat | |||||
| Ole e 1 | 3.47 | Olive | |||||
| Phl p 4 | 3.43 | Timothy grass | |||||
| Api g 1 | 1.91 | Celery | |||||
| Ara h 8 | 10.34 | Peanut | |||||
| Cor a 1.0401 | 27.75 | Hazelnut | |||||
| Gly m 4 | 6.15 | Soybean | |||||
| Mal d 1 | 15.66 | Apple | |||||
| Ole e 9 | 0.81 | Olive | |||||
| Pen m 2 | 9.87 | Shrimp | |||||
| Pru p 1 | 9.25 | Peach | |||||
| 10, m, 56 | Flies | Stable flies; Wasp, hornet |
492.1 | Cyn d 1 | 5.86 | Bermuda grass | |
| Fel d 1 | 1.15 | Cat | |||||
| Phl p 4 | 1.67 | Timothy grass | |||||
| Pol d 5 | 0.87 | Paper wasp | |||||
| Ves v 5 | 1.28 | Yellow jacket | |||||
| 11, f, 34 | NHS | 0 | n.d. | 89.2 | 0 | 0 | 0 |
0: none reported/negative result; n.d. = not determined.
Shrimp-allergic patients (n = 5 with written informed consent) were tested with extracts 1–14 (Table 1) and unprocessed mealworm (TM) in addition to routine allergy testing in an allergy clinic (Mexico). Ethical approval was granted by the Committee of Ethics in Investigation of Médica Sur, S.A.B. De C.V. (No 2018-EXT-339). Their ImmunoCap reactivity to crustaceans is listed in Table 3.
Table 3.
Skin prick test results of crustacean-allergic patients.
| Patient A Female, 60 years |
Patient B Female, 25 years |
Patient C Male, 22 years |
Patients D Male, 43 years |
Patient E Female, 46 years |
|
|---|---|---|---|---|---|
| ImmunoCap IgE f24 (Shrimp) (kUA/L) | 0.01 | 0.74 | 99.9 | 0.18 | n.d. |
| Symptoms upon shrimp consumption | Local reactions and moderate-to-severe GI symptoms | Local reactions and moderate-to-severe GI symptoms | Anaphylaxis, = Generalized hives, glossodynia, odynophagia, erythema conjunctiva | Anaphylaxis = Moderate-to-severe GI symptoms and generalized hives and malaise | Local reactions, moderate-to-severe GI symptoms |
| Sample (1 mg/ml total protein) | W/E (mm) | W/E | W/E | W/E | W/E |
| 1 | 6/28 × 34 | 5/14 | 8/20 | 5/21 | 5/21 × 14 |
| 2 | 5/20 | 4/13 | 5 | 0 | 6/18 |
| 3 | 6/22 | 9/21 | 5/22 | 9 × 8/30 × 32 | 0 |
| 4 | 7/25 | 0 | 0 | 7/26 × 19 | 0 |
| 5 | 0 | 0 | 0 | 0 | 0 |
| 6 | 0 | 0 | 0 | 0 | 0 |
| 7 | 0 | 0 | 0 | 0 | 0 |
| 8 | 0 | 0 | 0 | 0 | 0 |
| 9 | 0 | 0 | 0 | 0 | 0 |
| 10 | 0 | 0 | 0 | 0 | 0 |
| 11 | 0 | 0 | 0 | 0 | 0 |
| 12 | 0 | 0 | 0 | 0 | 0 |
| 13 | 0 | 0 | 6/18 | 0 | 0 |
| 14 | 0 | 0 | 0 | 0 | 0 |
| Control unprocessed mealworm (TM) | 7/28 | 9/27 | 11/34 | 8/31 × 22 | 3–4 |
| Negative control (50/50 glycerine/saline) | 0 | 0 | 5 | 0 | 0 |
| Other sensitizations (apart from shrimp/prawn) | Tuna, Dermatophagoides spp. | Dermatophagoides spp. | Dermatophagoides spp. | Dermatophagoides spp. | Fish, Dermatophagoides spp. |
For all patients histamine positive controls were included and gave results as expected between 7–10/22–28 mm (data not shown). A wheal diameter 3 mm larger than the negative control was considered positive. W/E: wheal/erythema; n.d. = not determined; GI = gastrointestinal.
Determination of total and specific IgE in patients' sera
The individual sera (30 μl) were tested on a custom-designed allergen-chip (ImmunoCAP™ ISAC, Thermo Fisher Scientific, Austria) with 127 spotted allergens from 60 different allergen sources to determine molecule-specific IgE-reactivities, according to manufacturer's instructions. Individual sera were incubated undiluted for 2 h, after washing, fluorescence-labeled anti-human IgE (30 μL) was applied for 30 min. Fluorescence was measured with an ISAC chip-reader (LUX-Scan-10K/A; CapitalBio Corporation, Beijing, China) and evaluated with MIA Software (Phadia Microarray Image Analysis Software, Version 1.2, Thermo Fisher Scientific, Uppsala, Sweden).
For detection of total IgE, an ELISA (Thermo Fisher Scientific, Waltham, Massachusetts, USA) was performed according to manufacturer's instructions. Sera were diluted 1:10 and measured as single reads.
Immunoblotting with patients' sera
Extracts of mealworm, shrimp, cricket legs, cricket body, desert locust legs, body and wings, stable flies or the processed extracts of migratory locust were blotted individually onto a nitrocellulose membrane (140 μg/blot with accompanying protein weight marker) and blocked with TBST/3% BSA for 30 min, thereafter washed 4x with TBST. Sera from patients with allergy to crustacean (n = 3, pooled), house dust mite (n = 8 for Fig. 2, n = 6 for Fig. 3B, pooled) or flies (n = 1), or non-hypersensitivity serum as negative control (n = 1) were used at a final dilution of 1:20 in TBST/0.1% BSA. Blots were incubated with sera o.n. at 4 °C, then washed 4x with TBST. For detection of IgE-binding, goat anti-human IgE-HRP (Invitrogen, REF A18793) was diluted 1:3000 and incubated for 2 h at RT. Development of reaction was performed with ECL (Bio-Rad, Clarity Western ECL, 1705061) according to manufacturer's instructions. All experiments were performed twice.
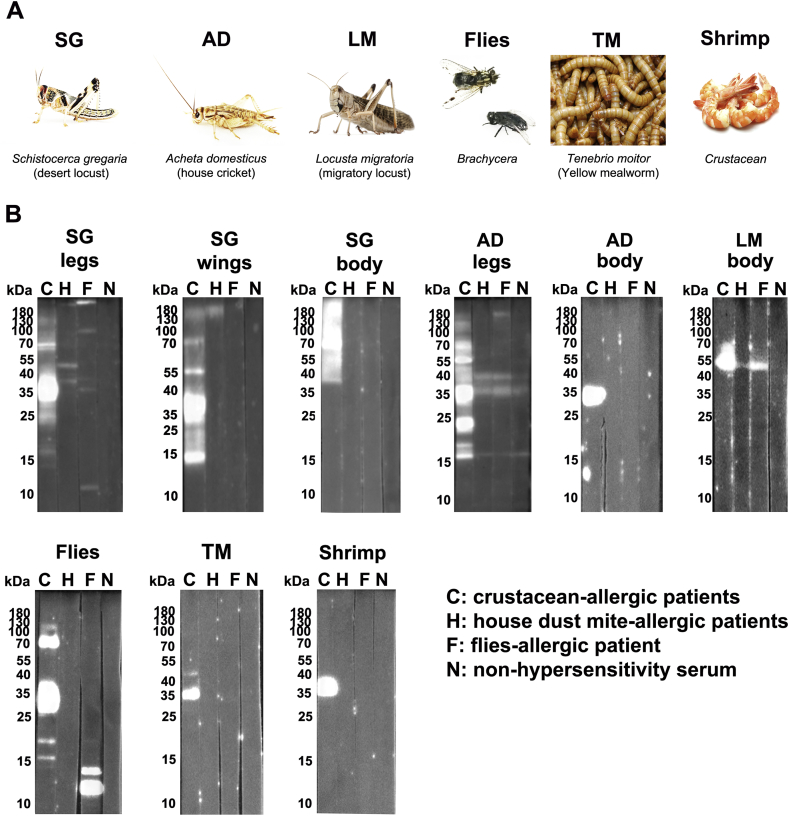
Fig. 2.
Immunoblot of different insect extracts with sera of patients allergic to crustaceans, house dust mite or stable flies. Individual insect extracts (A) of desert locust (Schistocerca gregaria, SG) legs, body and wings, house cricket (Acheta domesticus, AD) legs and body, migratory locust body (Locusta migratoria, LM), as well as a mix of stable flies, mealworm larvae (Tenebrio molitor, TM) and shrimp were (B) incubated with pooled sera from patients allergic to crustaceans (C), house dust mite (H) or flies (F), or with non-hypersensitivity serum (N) as negative control, and human IgE was detected. Photo credits: SG and AD = Fotolia.com©Daniel Nimmervoll; LM = Fotolia.com©EricIsselée; TM = Fotolia.com©shenk1; Flies: courtesy of authors; Shrimp = Fotolia.com©NarongJongsirikul.
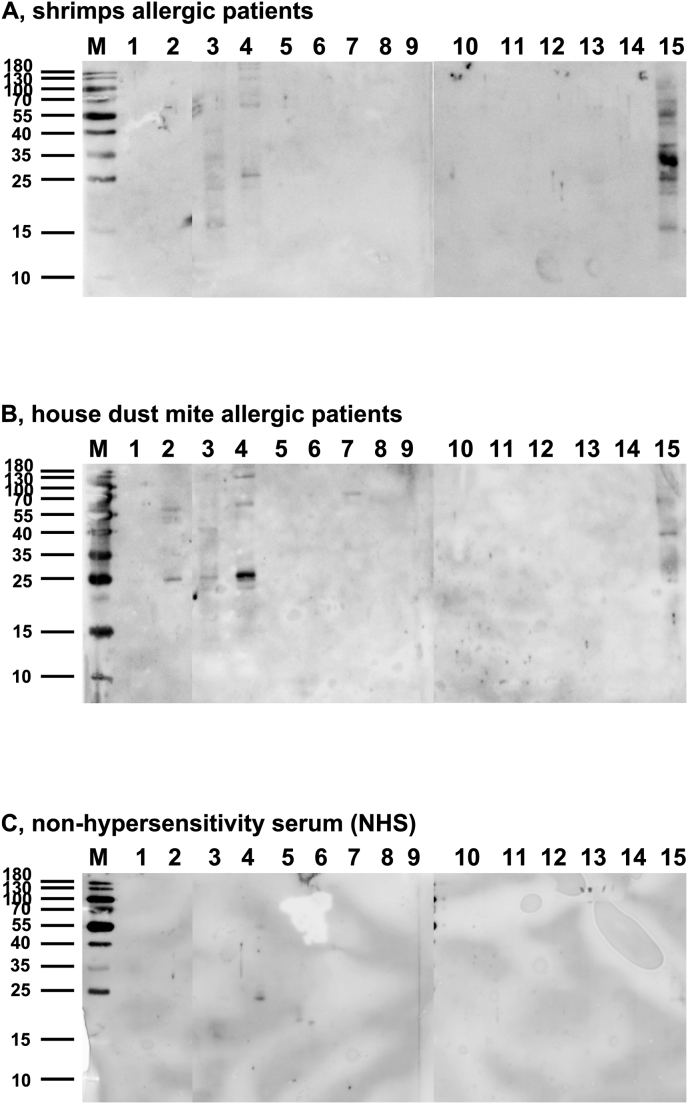
Fig. 3.
Influence of processing of LM extract on IgE-binging from crustacean-allergic patients. Processed extracts of migratory locust (Locusta migratoria, LM, lanes 1–14) and unprocessed mealworm (Tenebrio molitor, lane 15) were separated individually on 15% SDS-gels with accompanying protein weight marker (M). Process parameters for lanes 1–14: see Table 1 (A) Blotted extracts were incubated with a serum pool from crustacean-allergic patients. (B) Blotted extracts were incubated with a serum pool from house dust mite-allergic patients. (C) Blotted extracts were incubated with non-hypersensitivity serum as negative control.
Skin prick testing
Shrimp-allergic patients (n = 5, specific IgE to crustaceans tested in ImmunoCap, see Table 3) were tested with LM extracts 1–14 (Table 1) and unprocessed mealworm (TM) in 1 mg/ml on either the inner side of the forearms or on their back. Positive control (histamine) and negative control (glycerol/NaCl) were included. Reactions were read out after 20 min and diameter of the wheal-and-flare reaction (erythema) were taken. Ethical approval was granted by the Committee of Ethics in Investigations of Médica Sur, S.A.B. De C.V. (No 2018-EXT-339).
Results
Chemical composition
The compositional analysis of locust protein concentrates revealed crude protein contents of 82.3%, 61.3%, and 59.2% db for LMPC 1, LMPC 2, and LMPC 3 (Table 1). The chemical composition of LM, having a proximate crude protein content of 65.9% db, is comparable to data reported in literature for adult migratory locusts reared in captivity, e.g. crude protein contents of whole L. migratoria between 55.5 and 64.9% db were reported.5, 6
Total and specific IgE in patients' sera
Individual testing of patients revealed IgE-binding to a number of specific allergen molecules (Table 2). Interestingly, although patient No.2 was shown to have specific IgE against lobster and prawn by CAP-FEIA, no reaction to Pen m 1 (tropomyosin), Pen m 2 (arginine kinase) or Pen m 4 (sarcoplasmic calcium binding protein) from shrimp was observed on the ImmunoCAP ISAC-chip, indicating that other molecules may play a role in the crustacean-allergy of this patient. Furthermore, patient 8 did not show positive reactions to any of the spotted allergens from house dust mite, despite self-reporting HDM-allergy, also here other allergens may play a role.
The cross-reactivity of crustacean-IgE to HDM-allergens and vice versa was also observed in ImmunoCAP ISAC, as some patients crosswise detected the respective allergens. NHS remained negative on all tested allergens.
Total IgE values reached from 139.4 to 1920.4 ng/ml in the individual allergic patients sera, and was 89.2 ng/ml in NHS (Table 2).
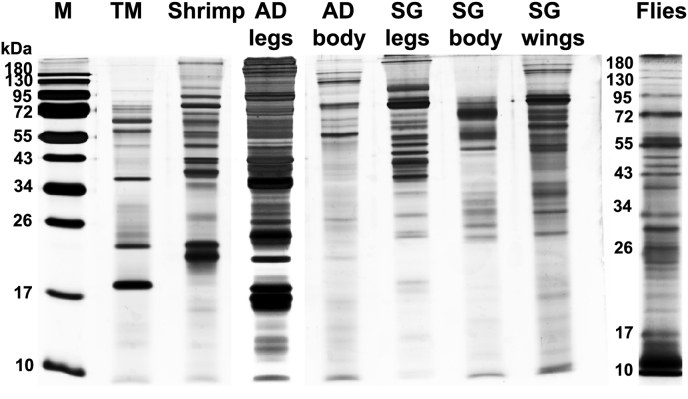
Determination of proteins in extracts by silver staining
Protein content of extracts from mealworm, shrimp, house cricket, desert locust and stable flies was visualized by silver staining and proved that in all extracts proteins from 10 to at least 180 kDa size were present (Fig. 1). The mealworm extract showed the most prominent bands at 18, 23, 37 and 41 kDa. In the shrimp extract, proteins with 21, 23 and around 37 kDa dominated among proteins in the range of 10 and > 180 kDa. For the flies mix with proteins between <10 and >180 kDa, the most intensive bands appeared at 11, 55 and 72 kDa.
Fig. 1.
Silver staining of different insect extracts. Extracts of mealworm (Tenebrio molitor, TM), shrimp, house cricket (Acheta domesticus, AD) legs and body, desert locust (Schistocerca gregaria, SG) legs, body and wings, as well as a stable flies mix were separated on a 15% SDS-gel and silver stained. M = protein weight marker.
As expected, different body parts of house cricket and desert locust contain different proteins.
IgE cross-recognition of crustacean-, HDM- and flies-allergic patients to insect extracts
Immunoblots of insect extracts (Fig. 2A) were incubated with sera of allergic patients (Fig. 2B) and confirmed the known cross-reactivity for crustacean-allergic patients to mealworm (IgE binds a protein around 35–38 kDa, most presumably tropomyosin26) and migratory locust LM (IgE binds to protein around 52 kDa, probably α-amylase, which was described as cross-reactive protein for HDM-patients in mealworm earlier26). Importantly, IgE from sera of crustacean-allergic patients very intensively detected proteins in extracts of legs, wings and body of the desert locus Schistocerca gregaria, where IgE bound to different proteins in different body parts. Unexpectedly, the protein around 35–38 kDa (tropomyosin) was detected in the extremities of the insect rather than the body. In comparison, in house cricket Acheta domesticus, IgE-binding was seen to the 35–38 kDa protein and also to a protein around 23 kDa. For the first time, it was shown that crustacean-allergic patients intensively recognize proteins at 35–38 kDa as well as around 72 kDa in stable flies extract. For HDM-allergic patients, reactions to SG legs and wings of desert locust as well as legs of house cricket were detected, and could point towards a possible cross-reaction at least via the inhalative route. For the flies-allergic patient, IgE-binding to proteins around 12 and 14 kDa in flies extract was revealed, and furthermore to proteins around 12, 35, 100 and > 180 kDa in desert locust legs, at >180 kDa in desert locust wings, and at 17, 35, 40 and 180 kDa in house cricket legs. The reaction of the flies-allergic patient to migratory locust was directed against the same protein that was recognized by crustacean- and HDM-patients, although completely different proteins where important in the flies extract.
Influence of food processing on IgE-binding of crustacean- and HDM allergic patients' sera
Extracts from edible migratory locust were used for processing, as they are among the most frequently consumed insect species in Western Countries and their cross-reactivity for shrimp- and house dust mite-allergic patients is well described.
Immunoblots of processed LM extracts were tested with sera of crustacean- (Fig. 3A) or HDM-allergic patients (Fig. 3B) or non-hypersensitivity serum (negative control, Fig. 3C). It became obvious that different extraction methods resulted in different IgE-binding in the extracts. Most important, IgE-binding, which was seen in untreated, differently extracted or precipitated LM extracts, was lost for crustacean- and HDM-allergic patients when enzymatic hydrolysis or heat treatment was applied (Fig. 3A and B).
Influence of food processing on skin prick test reaction of crustacean- allergic patients' sera
Processed LM extracts (Samples 1–14, Table 1) and unprocessed mealworm were tested in skin prick testing of 5 crustacean-allergic patients. These patients reacted to most or all of the non-hydrolyzed samples 1–4 with positive skin test reactions (Table 3). In contrast, no reactions where observed to all the enzymatically or heat-treated extracts (samples 5–14, Table 3). As control, untreated mealworm extract (TM, sample 15) was also applied and all 5 patients showed intensive, positive reactions (Table 3). All 5 patients were also positive in SPT for HDM.
Discussion
The present work shows for the first time that crustacean- and house dust mite-allergic patients are at risk for cross-reactions to desert locust and house cricket. Crustacean-allergic patients also intensively reacted to stable flies, necessitating a warning if crustacean-allergic patients would consume flies as edible insects. Vice versa, a flies-allergic patient also showed IgE-binding to legs of cricket, desert locust and migratory locust legs and therefore is probably at risk for cross-reactions to these insects, not only via the inhalative route but also when ingesting insects like migratory locust. Furthermore, house dust mite allergic patients' IgE binds to proteins in legs of desert locust and wings as well as legs of house cricket, also suggesting the risk at least for inhalative allergies to these insect species. Therefore, HDM-allergic patients should be alerted when keeping a reptile pet, which is fed with living insects like grasshopper, house cricket and locusts. Importantly, as there was also a reaction to LM to the same protein that crustacean- and flies-allergic patients reacted to, we suggest that a realistic risk for cross-reactivity of HDM to migratory locust exists also by ingestion of these as well as closely related insects (e.g. Brachycera). In summary, these reactions strongly point towards cross-reactivity for crustacean-allergic patients to desert locust, house cricket and stable flies, and patients should be warned before consuming these insects, as these patients were especially prone to react to a broad number of insect species.31
For the first time, also a flies-allergic patient, reacting with rhinoconjunctivitis symptoms to stable flies, was investigated on different extracts and individual allergen molecules for IgE-cross-recognition. The results strongly point towards a realistic risk for the flies-allergic patient for allergic cross-reactivity when eating LM.
Interestingly, although crustacean-IgE intensively binds to several proteins in the flies extract, vice versa no reaction of the flies-allergic patient to shrimp could be seen, indicating that IgE-binding and/or cross-reactivity is more insect-restricted for flies-allergic patients.
Finally, the results reveal that certain food processing methods, like enzymatic hydrolysis or thermal treatment, are able to reduce the IgE-binding from crustacean- and HDM-allergic patients to migratory locust. Most important, the in vivo relevance of this observation was proven in skin prick testing of crustacean-allergic patients, where reactions were only seen to the untreated extracts, but not to the enzymatically or heated samples. The appropriate food processing method therefore most likely can reduce cross-reactivity and allergenicity in edible insects.
The risk of food allergy after insect ingestions urgently needs further investigations and greater attention is required as edible insects emerge as alternative protein source for human as well as veterinary diet. For example, the true prevalence of food allergy to edible insects is completely unknown. It is anticipated that cases will increase in total numbers due to increased consumption also in Westernized countries.
Furthermore, the full cross-reactivity potential needs to be revealed. Obviously, there are many other members of the insect family, which are used for human nutrition. For instance, honeybee or honeybee nest are very common as insect-produced food. Future studies therefore need to investigate the cross-reactivity and allergenicity, and also the applicability of the food processing methods for honeybee and alike.
In this respect it is equally important that veterinary patients, like dogs, allergic to house dust mite or crustacean could show cross-reactions, and this should be kept in mind when including edible insects in animal feed, although the confirmation of this assumption is out of scope of this paper.
In addition, the effect of processing technologies on insect protein allergenicity requires further studies at the molecular level. Then, the development of targeted concepts for the mitigation or even elimination of insect allergens needs to take into account the resulting effects on food quality such as techno-functionality and sensory perception.
Ethics approval and consent to participate
Ethical approval was granted by the local ethics committee of the Medical University of Vienna (No 2002/2012) and the Committee of Ethics in Investigation of Médica Sur, S.A.B. De C.V. (No 2018-EXT-339).
Consent for publication
All authors have seen and approved the last version.
Availability of data and material
All data are available upon request from the corresponding author.
Conflicts of interest
The authors declare that they have no competing interests.
Authors' contributions
IPS and HJ designed the study, supervised experiments and wrote and edited the manuscript; PM and BP carried out the work for extract preparation and technological treatment of mealworm and LM and wrote the respective parts for the manuscript; GH was responsible for the experiments with other insects and in vitro immunological work; DLL and FARM selected shrimp-allergic patients by clinical history and/or positive serum tests and performed SPT with extracts of edible insects and derivates; NML was responsible for the ethical approval and the handling of the human samples for in vitro testing; LE carried out the allergy chip experiments. All authors read and approved the final version of the manuscript.
Acknowledgments
The authors would like to thank Helene Tanzmeister and Yhosemar Méndez Sanchez for the production of different LM and TML concentrates, and Stefanie Schönbauer as well as Maja Prvulovic for excellent technical assistance with SDS-PAGE and immunoblot experiments. Furthermore, we would like to thank the team of Prof. Thomas Wittek at the University of Veterinary Medicine, Vienna, for opportunity and help with collection of stable flies and finally we would like to thank Ely Figueroa from the Laboratorio de Alergia Molecular (LAM) Mexicocity, for running the InmunoCAP IgE against shrimp of the SPT patients.
Funding
Supported by grants of the Austrian Science Fund FWF (project SFB F4606-B28 and MCCA DK W 1248-B13 to EJJ) and by the Austrian Research Promotion Agency FFG (“Talente entdecken/Discover talents” 7121459-3+4 to IPS).
References
- 1.van Huis A., Van Itterbeeck J., Klunder H. FAO Forestry Paper; 2013. Edible Insects: Future Prospects for Food and Feed Security; p. 171. [Google Scholar]
- 2.Schluter O., Rumpold B., Holzhauser T. Safety aspects of the production of foods and food ingredients from insects. Mol Nutr Food Res. 2017;61(6):1–14. doi: 10.1002/mnfr.201600520. [DOI] [PubMed] [Google Scholar]
- 3.van Huis A. Edible insects contributing to food security? Agric Food Secur. 2015;4:1–9. [Google Scholar]
- 4.EFSA Risk profile related to production and consumption of insects as food and feed. EFSA J. 2015;13 [Google Scholar]
- 5.Oonincx D.G.B.A., van der Poel A.F.B. Effects of diet on the chemical composition of migratory locusts (Locusta migratoria) Zoo Biol. 2011;30:9–16. doi: 10.1002/zoo.20308. [DOI] [PubMed] [Google Scholar]
- 6.Barroso F.G., de Haro C., Sánchez-Muros M.-J., Venegas E., Martínez-Sánchez A., Pérez-Bañón C. The potential of various insect species for use as food for fish. Aquaculture. 2014;422:193–201. [Google Scholar]
- 7.Regulation (EU) 2015/2283 2015/2283 of the European Parliament and of the Council of 25 November 2015 on novel foods, amending Regulation (EU) No 1169/2011 of the European Parliament and of the Council and repealing Regulation (EC) No 258/97 of the European Parliament and of the Council and Commission Regulation (EC) No 1852/2001. Offic J Eur Union. 2015;58:L327. [Google Scholar]
- 8.Verhoeckx K., Broekman H., Knulst A., Houben G. Allergenicity assessment strategy for novel food proteins and protein sources. Regul Toxicol Pharmacol. 2016;79:118–124. doi: 10.1016/j.yrtph.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 9.Jensen-Jarolim E., Pali-Scholl I., Jensen S.A., Robibaro B., Kinaciyan T. Caution: reptile pets shuttle grasshopper allergy and asthma into homes. World Allergy Organ J. 2015;8:24. doi: 10.1186/s40413-015-0072-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pener M.P. Allergy to locusts and acridid grasshoppers: a review. J Orthoptera Res. 2014;23:59–67. [Google Scholar]
- 11.Barennes H., Phimmasane M., Rajaonarivo C. Insect Consumption to Address Undernutrition, a National Survey on the Prevalence of Insect Consumption among Adults and Vendors in Laos. PLoS One. 2015;10:e0136458. doi: 10.1371/journal.pone.0136458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Piromrat K., Chinratanapisit S., Trathong S. Anaphylaxis in an emergency department: a 2-year study in a tertiary-care hospital. Asian Pac J Allergy Immunol. 2008;26:121–128. [PubMed] [Google Scholar]
- 13.Armentia A., Lombardero M., Blanco C., Fernandez S., Fernandez A., Sanchez-Monge R. Allergic hypersensitivity to the lentil pest Bruchus lentis. Allergy. 2006;61:1112–1116. doi: 10.1111/j.1398-9995.2006.01123.x. [DOI] [PubMed] [Google Scholar]
- 14.Okezie O.A., Kgomotso K.K., Letswiti M.M. Mopane worm allergy in a 36-year-old woman: a case report. J Med Case Rep. 2010;4:42. doi: 10.1186/1752-1947-4-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freye H.B., Esch R.E., Litwin C.M., Sorkin L. Anaphylaxis to the ingestion and inhalation of Tenebrio molitor (mealworm) and Zophobas morio (superworm) Allergy Asthma Proc. 1996;17:215–219. doi: 10.2500/108854196778996903. [DOI] [PubMed] [Google Scholar]
- 16.Ji K., Chen J., Li M. Anaphylactic shocks and lethal anaphylaxis caused by food consumption in China. Trends Food Sci Technol. 2009;20:227–231. doi: 10.1016/j.tifs.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Broekman H., Verhoeckx K.C., den Hartog Jager C.F. Majority of shrimp-allergic patients are allergic to mealworm. J Allergy Clin Immunol. 2016;137:1261–1263. doi: 10.1016/j.jaci.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 18.Verhoeckx K.C., van Broekhoven S., den Hartog-Jager C.F. House dust mite (Der p 10) and crustacean allergic patients may react to food containing Yellow mealworm proteins. Food Chem Toxicol. 2014;65:364–373. doi: 10.1016/j.fct.2013.12.049. [DOI] [PubMed] [Google Scholar]
- 19.Srinroch C., Srisomsap C., Chokchaichamnankit D., Punyarit P., Phiriyangkul P. Identification of novel allergen in edible insect, Gryllus bimaculatus and its cross-reactivity with Macrobrachium spp. allergens. Food Chem. 2015;184:160–166. doi: 10.1016/j.foodchem.2015.03.094. [DOI] [PubMed] [Google Scholar]
- 20.Ravindran V., Blair R. Feed resources for poultry production in Asia and the Pacific. III. Animal protein sources. Worlds Poultr Sci J. 1993;49:219–235. [Google Scholar]
- 21.Bussler S., Rumpold B.A., Jander E., Rawel H.M., Schluter O.K. Recovery and techno-functionality of flours and proteins from two edible insect species: meal worm (Tenebrio molitor) and black soldier fly (Hermetia illucens) larvae. Heliyon. 2016;2:e00218. doi: 10.1016/j.heliyon.2016.e00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Focke M., Hemmer W., Wohrl S., Gotz M., Jarisch R., Kofler H. Specific sensitization to the common housefly (Musca domestica) not related to insect panallergy. Allergy. 2003;58:448–451. doi: 10.1034/j.1398-9995.2003.00126.x. [DOI] [PubMed] [Google Scholar]
- 23.Wouters A.G.B., Rombouts I., Fierens E., Brijs K., Delcour J.A. Relevance of the functional properties of enzymatic plant protein Hydrolysates in food systems. Compr Rev Food Sci Food Saf. 2016;15:786–800. doi: 10.1111/1541-4337.12209. [DOI] [PubMed] [Google Scholar]
- 24.Roth-Walter F., Berin M.C., Arnaboldi P. Pasteurization of milk proteins promotes allergic sensitization by enhancing uptake through Peyer's patches. Allergy. 2008;63:882–890. doi: 10.1111/j.1398-9995.2008.01673.x. [DOI] [PubMed] [Google Scholar]
- 25.Broekman H., Knulst A., den Hartog Jager S. Effect of thermal processing on mealworm allergenicity. Mol Nutr Food Res. 2015;59:1855–1864. doi: 10.1002/mnfr.201500138. [DOI] [PubMed] [Google Scholar]
- 26.van Broekhoven S., Bastiaan-Net S., de Jong N.W., Wichers H.J. Influence of processing and in vitro digestion on the allergic cross-reactivity of three mealworm species. Food Chem. 2016;196:1075–1083. doi: 10.1016/j.foodchem.2015.10.033. [DOI] [PubMed] [Google Scholar]
- 27.Meinlschmidt P., Sussmann D., Schweiggert-Weisz U., Eisner P. Enzymatic treatment of soy protein isolates: effects on the potential allergenicity, technofunctionality, and sensory properties. Food Sci Nutr. 2016;4:11–23. doi: 10.1002/fsn3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meinlschmidt P., Schweiggert-Weisz U., Brode V., Eisner P. Enzyme assisted degradation of potential soy protein allergens with special emphasis on the technofunctionality and the avoidance of a bitter taste formation. LWT - Food Sci Technol. 2016;68:707–716. [Google Scholar]
- 29.AOAC . Association of Official Analytical Chemists; Gaithersburg: 2002. Official Methods of Analysis. [Google Scholar]
- 30.Finke M.D. Estimate of chitin in raw whole insects. Zoo Biol. 2007;26:105–115. doi: 10.1002/zoo.20123. [DOI] [PubMed] [Google Scholar]
- 31.Broekman H., Knulst A.C., de Jong G. Is mealworm or shrimp allergy indicative for food allergy to insects? Mol Nutr Food Res. 2017;61:1–9. doi: 10.1002/mnfr.201601061. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are available upon request from the corresponding author.