Abstract
Background
The urticaria control test (UCT) is a questionnaire designed to determine if chronic urticaria (CU) is controlled or not and to aid therapeutic decision-making. It collects retrospective information about the symptoms and quality of life impairment over the last 4 weeks. The current study aimed to investigate the validity, reliability and sensitivity to change of the Turkish version of the UCT. We also evaluated its correlation with other tools and compared the UCT results of patients with chronic spontaneous urticaria (CSU) and patients with chronic inducible urticaria (CINDU).
Methods
Following forward/backward translation and cognitive debriefing, the Turkish version of the UCT was used in 81 CSU and 78 CINDU patients. Dermatology life quality index (DLQI), Chronic urticaria quality of life questionnaire (CU-Q2oL), urticaria activity score (UAS), patients' and physicians’ global assessment visual analog scores and Likert scales were used at baseline and after four weeks to assess quality of life impairment, disease activity and disease control. Statistical analysis to determine the validity and reliability of the Turkish version of the UCT as well as comparison between CINDU and CSU patients were performed.
Results
Duration of disease was longer, disease control was poorer and severe complaints were more frequent in CINDU patients (duration of disease: 36.3 (24) ± 49.1 vs 31.5 (9) ± 67.9, p = .007, UCT baseline: 8.4 (8) ± 3.4 vs 10.4 (11) ± 3.9, p = .001 and patient's global assessment Likert scale severe complaints: 6 vs 15, p < .001, respectively). The UCT showed excellent internal consistency for CSU and a minimally acceptable consistency for CINDU (Cronbach's α 0.89 for CSU versus 0.68 for CINDU). It showed strong correlation with CU-Q2oL but a moderate correlation with DLQI (r = −0.649, P < .001 and r = −0.545, P < .001, respectively). It was able to discriminate between patients with different disease control and was sensitive to detect changes in the disease control in both groups. The minimally important difference of the UCT was found to be 3.
Conclusions
The Turkish version of the UCT is a valid and reliable tool for the management of CU patients and can be used both in CSU and CINDU patients to determine if the treatment is sufficient and if disease activity and quality of life impairment are under control or not.
Keywords: Chronic urticaria, Inducible urticaria, Urticaria control test (UCT), Minimal clinically important difference (MCID), Reliability, Validity, Quality of life, Patient reported outcomes
Introduction
Chronic urticaria (CU) is a common skin disorder characterized by recurrent pruritic hives (wheals) and/or angioedema that occur for more than 6 weeks affecting up to 1% of the total population. It is classified as chronic inducible urticarias (CINDUs) and chronic spontaneous urticaria (CSU) depending on whether a specific trigger can be identified or not.1
The clinical signs and symptoms of CU patients are unpredictable and show waxing and waning. Thus, the clinical picture of a patient at the time of presentation is only rarely representative of the actual current disease status. This makes it difficult for physicians to estimate patients’ disease activity and disease burden, and to adjust treatment accordingly.
To overcome this obstacle, several patient-reported outcome (PRO) measures have been developed for CU patients in the recent years, including the Urticaria Activity Score (UAS),2, 3, 4 the Angioedema Activity Score (AAS),5 Chronic Urticaria Quality of Life questionnaire (CU-Q2oL),6, 7, 8, 9, 10 the Angioedema Quality of Life Questionnaire,11, 12 the Urticaria Activity and Impact Measure,13 and the Urticaria Control Test (UCT).14, 15, 16, 17, 18, 19, 20, 21 Among these tools, UCT is the easiest and least time-consuming. It was specifically designed to retrospectively assess the level of disease control in CU patients and thereby aid to evaluate the control of various types of urticaria and decision of treatment change.14 In addition, it overcomes some of the limitations of the UAS and AAS. First, it is a retrospective instrument that works in all types of physician consultations (largely independently of patient compliance). Second, it is a tool for all types of chronic urticaria (not only for CSU), and third, it can also be used in urticaria patients with recurrent angioedema.
The UCT includes four simple questions that assess the control of signs and symptoms of the disease, quality of life (QoL) impairment, efficacy of treatment and overall disease control, over the prior 4 weeks. It is completed by the patient and can be evaluated by both the patient and the physician. The questions are rated from 0 to 4, and the total score is calculated by summing the four individual question scores. The lowest UCT score possible is 0 (no control) and the highest score possible is 16 (complete control). A score of ≥12 indicates well-controlled urticaria, while a score of ≤11 indicates poor disease control.14
The current study aimed to investigate the validity, reliability and sensitivity to change of the Turkish version of the UCT in both CSU and CINDU. We also compared the UCT results of patients with CSU and patients with CINDU.
Methods
Patients
Turkish patients with CU (N = 159, 18–75 years, 67.9% females) attending the Urticaria Clinic of the Urticaria Centre of Reference and Excellence22 at the Department of Dermatology Okmeydanı Training and Research Hospital participated in this study. A total of 81 CSU and 78 CINDU patients were included and the subtypes of CINDU were symptomatic dermographism (56), cholinergic urticaria,7 cold urticaria,6 aquagenic urticaria1 and mixed CINDU (eg; symptomatic dermographism plus cold urticaria in a patient).8
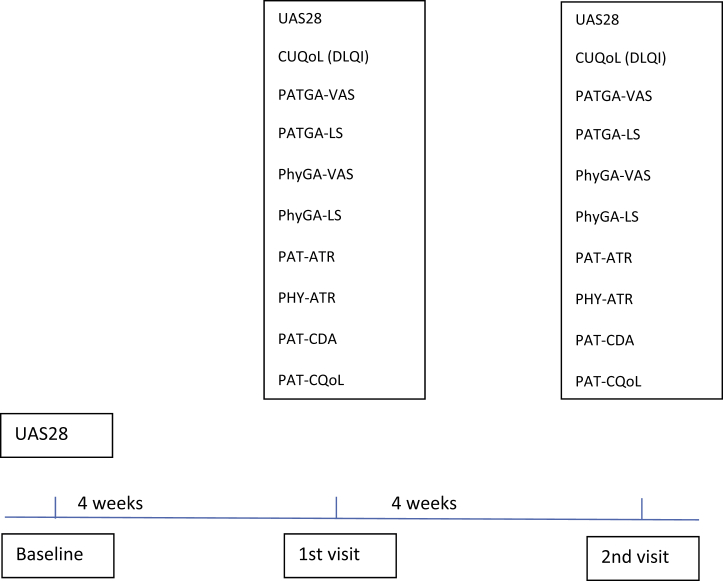
All patients were assessed at three visits in 4-week intervals; baseline, 1st and 2nd visit. The flow of the study and the questionnaires given are shown in Fig. 1. During each visit, the participants received appropriate treatment according to their disease activity and control as recommended by the EAACI/ GA2LEN/EDF/WAO guideline.1 The study was approved by the ethics committee of Okmeydanı Training and Research Hospital and it was performed in line with institutional guidelines and regulations. Informed consent to be included in the study and consent to publish pseudonymized data were taken from the participants.
Fig. 1.
Study flow. Urticaria Activity Score 28 (UAS28), Chronic Urticaria Quality of Life Questionnaire (CU-Q2oL), Physician's global assessment-visual analog scale (PhyGA-VAS), Patient's global assessment of disease severity-visual analog scale (PatGA-VAS), Patient's global assessment of disease severity - Likert scale (PatGA-LS), Physicians global assessment of disease control Likert-scale (PhyGA-LS), Patient's assessment of treatment Response (Pat-ATR), Physician's assessment of treatment response (Phy-ATR), Patient's assessment of change in disease activity (Pat-CDA), Patient's assessment of change in quality of life (Pat- CQoL).
Translation and linguistic validation of the Turkish UCT version
To obtain a linguistically validated Turkish version of the UCT, a structured forward-backward translation was performed. The original English version of the UCT was independently translated into Turkish by two native Turkish speakers with a command of English language. Then, these two Turkish versions were reviewed for comprehensibility by dermatologists specialized in treating urticaria patients (Kocatürk, Kızıltaç, Can). After these dermatologists reached consensus, the final Turkish version was back-translated into German by two independent bilingual translators. The back-translated versions were then reviewed against the original by the original authors. Potential misconceptions or misinterpretations introduced in the translation process were discussed between the Turkish research team and the original authors. After consensus on the final Turkish version was achieved, the Turkish version of UCT was tested in 10 CU patients (cognitive debriefing interviews). Here, it was recognized that the patients were incapable of understanding the third question of the UCT “How often was the treatment for your urticaria in the last 4 weeks not enough to control your urticaria symptoms?” which was answered as “very often”, “often”, “sometimes”, “seldom” and “not at all”. This was attributed to the reverse nature of the question and then it was changed as “How successful was the treatment for your urticaria in controlling your urticaria symptoms in the last 4 weeks?” with the answers “not at all”, “a little”, “somewhat”, “well” and “very well”. Subsequently, the final Turkish version of UCT was used for this study.
Clinical measures
The weekly Urticaria Activity Score (UAS7): The UAS7 is a prospective diary-type tool to assess disease activity of CSU patients for one week.2, 3, 4 It is based on the once-daily assessment of itch intensity/severity and numbers of hives over a period of 7 days. It sums up the number of wheals and the intensity of pruritus on a four-point scale (0–3) with a minimum and maximum score of 0 and 6 points per day, respectively. The UAS7 ranges from 0 to 42. The UAS28 is the sum of the UAS7 scores of 4 consecutive weeks (range from 0 to 168).23
The validated Turkish version of the Chronic Urticaria Quality of Life Questionnaire (CU-Q2oL): The CU-Q2oL was specifically designed for the assessment of health-related quality of life impairment in patients with CU, including the physical, psychosocial and practical aspects of this condition.6, 7 A total of 23 questions were found to cover six key CU-specific domains: itch, swelling, impact on life activities, sleep problems, looks and limits. For each question, patients were asked to choose between five response options (scored 0–4) indicating the intensity of each item in the last two weeks. A total score across all questions was calculated and transformed into scores ranging from 0 to 100, with a score of 100 indicating the worst possible health related quality of life (HRQoL) impairment. Dermatology Life Quality Index (DLQI) was used for the CINDU patients since the CU-Q2oL was specifically designed for CSU patients.
The Physician's global assessment of disease control - visual analog scale (PhyGA-VAS) is a physician evaluation instrument for assessing urticaria control during the last four weeks.14 It is a 10-cm unmarked line which ranges between 0 cm (not at all under control) and 10 cm (completely under control).
The Physician's global assessment of disease control - Likert-scale (PhyGA-LS) was used to assess disease control on a 5-point scale (1 complete control, 2 good control, 3 moderate control, 4 little control, 5 no control).14
The Patient's global assessment of disease severity-visual analog scale (PatGA-VAS) was used to assess disease severity during the previous four weeks.14 The PatGA-VAS is an unmarked line anchored at the two ends with “no complaints” (0 cm) and “maximal complaints” (10 cm).
The Patient's global assessment of disease severity - Likert scale (PatGA-LS) was used to assess disease severity during the previous four weeks.14 The PatGA-LS is a 5-point scale for assessing disease control (1 no complaints, 2 mild complaints, 3 moderate complaints 4 severe complaints)
The Physician's assessment of treatment response (Phy-ATR) is a physician evaluation instrument for assessing a patient's response to treatment during the last four weeks.14 The physician evaluates the adequacy of treatment as sufficient or not sufficient (treatment sufficient = 0, treatment not sufficient = 1).
The Patient's assessment of treatment response – Likert scale (Pat-ATR) is a patient evaluation instrument for assessing treatment during the last four weeks.14 The patient evaluates the adequacy of treatment as sufficient or not sufficient (treatment sufficient = 0, treatment not sufficient = 1).
The Patient's assessment of change in disease activity – Likert scale (Pat-CDA) is a patient evaluation instrument for assessing change in disease activity during the last four weeks (Significant improvement = 0, mild improvement = 1, no change = 2, mild worsening = 3, significant worsening = 4).14
The Patient's assessment of change in quality of life – Likert scale (Pat-CQoL) is a patient evaluation instrument for assessing change in quality of life during the last four weeks (significant improvement = 0, mild improvement = 1, no change = 2, mild worsening = 3, significant worsening = 4).14
Assessment of the Turkish UCT consistency, validity, sensitivity to change, reliability, and screening accuracy
Internal consistency: Cronbach's α coefficient was used to test the internal consistency of the Turkish UCT. We used the suggested guidelines for interpretation of Cronbach's α coefficient: < 0.60 unacceptable, 0.60–0.65 undesirable, 0.65–0.70 minimally acceptable, 0.70–0.80 respectable, 0.80–0.90 excellent and >0.90 excessive consistency.24
Convergent validity assesses whether measures that should be related are related. The correlation between the Turkish version of UCT and CU-Q2oL, DLQI, UAS28, PhyGA-VAS, PatGA-VAS, and PatGA-LS were evaluated by Pearson's correlation coefficient. Weak, moderate and strong correlations were defined as correlation coefficient values of <0.3, 0.3–0.6, and >0.6 respectively.14
Known-groups validity measures the ability of the tool to discriminate between patients with different disease severity and disease control. The known-group validity of the Turkish UCT was explored by using the Kruskal Wallis test. The UAS28 scores of <6, 7–24, 25–64, >65 were used to classify the disease activity of the patients into none, mild, moderate and severe, respectively.14 To determine patients with different levels of disease control PatGA-LS and PhyGA-LS were used and patients with different levels of treatment responses were determined by using Pat-ATR and Phy-ATR.
Sensitivity to change: All of the patients returned for a second visit. The changes in the UCT scores and UAS28, CU-Q2oL, DLQI, PatGA-VAS and PhyGA-VAS change scores between the two visits were calculated and the correlation between the changes in these variables was analysed (computing of rank correlation coefficient Spearman's rho).
Test-retest reliability (intraclass correlation coefficient - ICC): To determine the UCT's test-retest reliability patients who had no change in the PatGA-LS scores (stable patients) during the four-week interval between the 1st and the 2nd visit were included in the analysis for test-retest reliability. UCT scores of patients with CINDU and CSU and total were compared between two visits by using Wilcoxon signed rank test. An ICC of 0.5–0.7 indicated moderate-to-good reproducibility while an ICC of greater than 0.7 was considered to demonstrate excellent reproducibility.14
Screening accuracy (categorization): Screening accuracy (categorization) is the ability of UCT to categorize patients into suffering from poorly-controlled and well-controlled disease. ROC curve analysis and area under the curve (AUC) were used to determine screening accuracy of the UCT. PhyGA-LS was categorized into well-controlled and poorly controlled urticaria for ROC curve analysis. Good control or complete control were accepted as well-controlled urticaria whereas no control, little control, or moderate control were considered as poorly controlled urticaria. The cut off values for well-controlled urticaria versus poor controlled urticaria were determined for both visits. AUCs of 1, 0.9, 0.8, 0.7, and 0.5 were defined as perfect, excellent, good, fair, and no better than chance, respectively.15
Minimal important difference (MID), minimal clinically important difference and smallest detectable change (SDC) of the Turkish UCT
Anchor based, and distributional-criterion approaches were applied to calculate the MID which is the change in a score that can be considered clinically relevant.16 Anchor based methods include the mean change method and ROC curve analysis.2, 16, 25 A ROC curve analysis was applied and the Phy-GA-LS was used as an external anchor for the anchor-based methods. To use Phy-GA-LS as an anchor, the correlation between UCT score changes and Phy-GA-LS changes were determined (Spearman's correlation). At least good correlation (r ∼ 0.5) should be determined in order to use the Phy-GA-LS as an anchor. Patients were classified into “improved” disease (≥1st step improvement in Phy-GA-LS) and “not improved” disease (unchanged or worsened Phy-GA-LS) in order to determine the cut-off value of UCT changes. The ROC cut-off point was chosen by getting smallest sum of percentages of false positive and false negative classifications ([1-sensitivity] + [1-specificity]).25 The mean change approach was applied, and the mean UCT score improvement was determined in patients with a 1st step improvement in their Phy-GA-LS from 1st visit to 2nd.2, 16 Another method to calculate MID indirectly is the distributional-criterion approach which can be calculated by dividing the standard deviation (SD) of the baseline UCT scores was by 2.12, 16, 25 Besides the MID, the smallest detectable change of the UCT score was also calculated.2, 16 The SDC was analysed as 1.96 times the SD of the UCT's score changes between two visits in patients whose Phy-GA-LS was stable.
Results
Duration of disease, disease control and patients’ complaints
Duration of disease was longer, disease control was poorer, severe complaints were more frequent in CINDU patients. The demographic characteristics of the patients and the scores of the instruments used through the study are shown in Table 1. The table shows significant differences between the two groups; CSU and CINDU. See also Table 4 for details of PatGA-LS, PhyGA-LS, Pat-ATR, Phy-ATR distributions between CSU and CINDU.
Table 1.
Demographic characteristics and comparison between CSU and CINDU.
| Total | CSU | CINDU | P | |
|---|---|---|---|---|
| Mean (median)±SD | Mean (median)±SD | Mean (median)±SD | ||
| Gender | ||||
| Female n (%) | 108 (67.9%) | 57 (70.4%) | 51 (65.4%) | 0.501 |
| Age (years) | 39.1 (39) ± 13.3 | 40.5 (38) ± 14 | 37.7 (39) ± 12,5 | 0.328 |
| Min-max | 14–75 | 14–75 | 17–72 | |
| Disease duration (mo) | 33.8 (12) ± 59.3 | 31.5 (9) ± 67.9 | 36.324 ± 49.1 | 0.007 |
| Min-max | 2–480 | 2–480 | 2–240 | |
| UAS28-Baseline | 42.728 ± 36.4 | – | ||
| Min--max | 0–127 | |||
| UAS28-1st | 34.3 (23) ± 37.6 | – | ||
| Min-max | 0–165 | |||
| UCT-1st | 9.5 (9) ± 3.8 | 10.4 (11) ± 3.9 | 8.48 ± 3.4 | 0.001 |
| Min-max | 1–16 | 1–16 | 1–16 | |
| UCT-2nd | 11.6 (12) ± 3.3 | 11.7 (12) ± 3.4 | 11.412 ± 3.2 | 0.379 |
| Min-max | 2–16 | 2–16 | 2–16 | |
| CU-Q2oL-1st | 22.7 (17.3) ± 19.5 | – | ||
| Min-max | 0–79.34 | |||
| CU-Q2oL-2nd | 18.5 (14.1) ± 18.0 | – | ||
| Min-max | 0–73.90 | |||
| PatGA-VAS-1st | 4.0 (4.5) ± 2.8 | 3.1 (2) ± 2.7 | 4.95 ± 2.5 | <0.001 |
| Min-max | 0–10 | 0–10 | 0–10 | |
| PatGA-VAS-2nd | 3.1 (2) ± 2.7 | 2.5 (1.5) ± 2.7 | 3.83 ± 2.7 | 0.001 |
| Min-max | 0–10 | 0–9 | 0–10 | |
| PhyGA-VAS-1st | 6.8 (6) ± 5.6 | 8.1 (8) ± 7.4 | 5.55 ± 2.2 | <0.001 |
| Min-max | 1–7 | 2–7 | 1–10 | |
| PhyGA-VAS-2nd | 7.2 (8) ±2.6 | 7.7 (9) ± 2.4 | 6.6 (7.5) ± 2.6 | 0.003 |
| Min-max | 1–10 | 1–10 | 1–10 | |
| Pat ATR-1st n,% - treatment sufficient | 82 (51.6%) | 53 (65.4%) | 29 (37.2%) | <0.001 |
| Pat ATR-2nd n,%- treatment sufficient | 105 (66%) | 55 (67.9%) | 50 (64.1%) | 0.613 |
| PHY ATR-1st n,%- treatment sufficient | 71 (44.7%) | 52 (64,2%) | 19 (24,4%) | <0.001 |
| PHY ATR-2nd n,%-treatment sufficient | 106 (66.7%) | 56 (69.1%) | 50 (64.1%) | 0.501 |
| DLQI-1st m (md±SD) | – | 8.57 ± 6.3 | ||
| Min-max | 0–24 | |||
| DLQI-2nd m (md±SD) | 5.74 (4) ±6.26 | |||
| Min-max | 0–27 |
Pearson Chi-Square, Mann-Whitney U.
UAS28-Baseline: the 4-weekly UAS score before the 1st visit, UAS28, UAS28-1st: the 4-weekly UAS score between the 1st and 2nd visit, UCT-1st: Urticaria control test score at the 1st visit, UCT-2nd: Urticaria control test score at the 2nd visit, CU-Q2oL-1st: Chronic urticaria quality of life questionnaire score at the 1st visit, CU-Q2oL-2nd: Chronic urticaria quality of life questionnaire score at the 2nd visit, PatGA-VAS-1st: Patient's global assessment of disease severity-visual analog scale at the 1st visit, PatGA-VAS-2nd: Patient's global assessment of disease severity-visual analog scale at the 2nd visit, PhyGA-VAS-1st: Physician's global assessment of disease control - visual analog scale at the 1st visit, PhyGA-VAS-2nd: Physician's global assessment of disease control - visual analog scale at the 2nd visit, DLQI-1st: Dermatology Life Quality Index Score at the 1st visit, DLQI-2nd: Dermatology Life Quality Index Score at the 2nd visit.
Table 4.
Known groups validity of the UCT (CSU and CINDU).
| N | Mean | SD | Median | Kruskal-Vallis Test | p | |
|---|---|---|---|---|---|---|
| PatGA-LS-1st CSU | ||||||
| No complaints | 16 | 15.13 | 2.062 | 16.00 | 47.178 | <0.001 |
| Mild complaints | 34 | 11.09 | 2.690 | 12.00 | ||
| Moderate complaints | 25 | 8.04 | 2.336 | 8.00 | ||
| Severe complaints | 6 | 4.33 | 3.204 | 3.50 | ||
| PatGA-LS-1st CINDU | ||||||
| No complaints | 3 | 13.67 | 1.155 | 13.00 | 34.226 | <0.001 |
| Mild complaints | 20 | 11.25 | 2.826 | 12.00 | ||
| Moderate complaints | 40 | 7.80 | 2.662 | 8.00 | ||
| Severe complaints | 15 | 5.27 | 2.086 | 6.00 | ||
| PHYGA-LS-1st CSU | ||||||
| Complete control | 19 | 14.16 | 2.651 | 15.00 | 43.629 | <0.001 |
| Good control | 34 | 11.24 | 2.742 | 12.00 | ||
| Moderate control | 23 | 7.65 | 2.367 | 7.00 | ||
| Little control | 2 | 7.00 | 1.414 | 7.00 | ||
| No control | 3 | 1.67 | 0.577 | 2.00 | ||
| PHY-LS-1st CINDU | ||||||
| Complete control | 1 | 16.00 | 16.00 | 49.100 | <0.001 | |
| Good control | 18 | 12.78 | 1.396 | 12.50 | ||
| Moderate control | 36 | 7.89 | 1.997 | 8.00 | ||
| Little control | 20 | 5.85 | 2.277 | 6 | ||
| No control | 3 | 3.33 | 2.082 | 4 | ||
| UAS28-1st-R | ||||||
| <6 | 14 | 14.79 | 2.517 | 16.00 | 34.281 | 0.000 |
| 7-24 | 23 | 11.39 | 3.394 | 12.00 | ||
| 25-64 | 19 | 9.63 | 3.804 | 10.00 | ||
| >65 | 25 | 7.76 | 2.454 | 8.00 | ||
| PAT-ATR-1st CSU | ||||||
| Treatment sufficient | 53 | 12.19 | 3.051 | 12.00 | 196.000 | 0.000 |
| Treatment not sufficient | 28 | 7.14 | 3.147 | 7.00 | ||
| PAT-ATR-1st CINDU | ||||||
| Treatment not sufficient | 49 | 6.53 | 2.399 | 6.00 | 87.500 | 0.000 |
| Treatment sufficient | 29 | 11.62 | 2.321 | 12.00 | ||
| PHY-ATR-1st CSU | ||||||
| Treatment sufficient | 52 | 12.31 | 3.052 | 12.00 | 170.000 | 0.000 |
| Treatment not sufficient | 29 | 7.10 | 2.920 | 7.00 | ||
| PHY-ATR-1st CINDU | ||||||
| Treatment not sufficient | 59 | 6.92 | 2.336 | 7.00 | 49.555 | 0.000 |
| Treatment sufficient | 19 | 13.11 | 1.329 | 13.00 | ||
Kruskal-Vallis.
The internal consistency for CSU and CINDU
The UCT showed excellent internal consistency for CSU and a minimally acceptable internal consistency for CINDU. When calculated for the total patient population (both CSU and CINDU), the Cronbach's α was found 0.81. But when analysed separately, the Cronbach's α was found 0.89 versus 0.68 for CSU and CINDU patients respectively (Table 2).
Table 2.
Internal consistency of the Turkish UCT.
| Item | Mean | S.D | N | Cronbach's Alpha | |
|---|---|---|---|---|---|
| CSU | UCT-1 | 2.33 | 1.19 | 81 | 0.891 |
| UCT-2 | 2.73 | 1.17 | 81 | ||
| UCT-3 | 2.81 | 1.09 | 81 | ||
| UCT-4 | 2.70 | 0.98 | 81 | ||
| CINDU | UCT-1 | 1.94 | 1.17 | 78 | 0.684 |
| UCT-2 | 2.10 | 1.21 | 78 | ||
| UCT-3 | 2.36 | 1.23 | 78 | ||
| UCT-4 | 2.03 | 1.16 | 78 | ||
| TOTAL | UCT-1 | 2.14 | 1.19 | 159 | 0.817 |
| UCT-2 | 2.42 | 1.23 | 159 | ||
| UCT-3 | 2.59 | 1.18 | 159 | ||
| UCT-4 | 2.37 | 1.12 | 159 |
Cronbach's α coefficient: < 0.60 unacceptable, 0.60–0.65 undesirable, 0.65–0.70 minimally acceptable, 0.70–0.80 respectable, 0.80–0.90 excellent and >0.90 excessive consistency.
Correlations of UCT with other tools
The UCT showed strong correlation with CU-Q2oL but a moderate correlation with DLQI. The UCT scores of the total patient population (CSU and CINDU) showed strong correlations with disease severity (PatGA-VAS, PatGA-LS) and disease control (PhyGA-VAS, PhyGA-LS). UCT scores of CSU patients showed a strong negative correlation with disease activity (UAS28) and with CU-Q2oL but UCT scores of CINDU patients showed a moderate negative correlation with DLQI (r = −0.649, P < .001 and r = −0.545, P < .001, respectively) (Table 3).
Table 3.
Convergent validity of UCT.
| UCT Score | Correlation Coefficienta | |
|---|---|---|
| CSU | UAS28-Baseline | −0.662, P < .001 |
| CUQ2oL-1st | −0.649, P < .001 | |
| PatGA-VAS-1st | −0.756, P < .001 | |
| PhyGA-VAS-1st | 0.708, P < .001 | |
| PatGA-LS-1st | −0.763, P < .001 | |
| PhyGA-LS-1st | −0.733, P < .001 | |
| CINDU | DLQI-1st | −0.545, P < .001 |
| PatGA-VAS-1st | −0.678, P < .001 | |
| PhyGA-VAS-1st | 0.624, P < .001 | |
| PatGA-LS-1st | −0.664, P < .001 | |
| PhyGA-LS-1st | −0.774, P < .001 | |
| TOTAL | PatGA-VAS-1st | −0.741, P < .001 |
| PhyGA-VAS-1st | 0.698, P < .001 | |
| PatGA-LS-1st | −0.737, P < .001 | |
| PhyGA-LS-1st | −0.784, P < .001 |
UAS28-Baseline: the 4-weekly UAS score before the 1st visit, UAS28, UAS28-1st: the 4-weekly UAS score between the 1st and 2nd visit, UCT-1st: Urticaria control test score at the 1st visit, UCT-2nd: Urticaria control test score at the 2nd visit, CU-Q2oL-1st: Chronic urticaria quality of life questionnaire score at the 1st visit, CU-Q2oL-2nd: Chronic urticaria quality of life questionnaire score at the 2nd visit, PatGA-VAS-1st: Patient's global assessment of disease severity-visual analog scale at the 1st visit, PatGA-VAS-2nd: Patient's global assessment of disease severity-visual analog scale at the 2nd visit, PhyGA-VAS-1st: Physician's global assessment of disease control - visual analog scale at the 1st visit, PhyGA-VAS-2nd: Physician's global assessment of disease control - visual analog scale at the 2nd visit, DLQI-1st: Dermatology Life Quality Index Score at the 1st visit, DLQI-2nd: Dermatology Life Quality Index Score at the 2nd visit.
Pearson's correlation coefficient. Weak, moderate and strong correlations were defined as correlation coefficient values of <0.3, 0.3–0.6, and >0.6 respectively.
The known group validity for UCT
The UCT showed similar known groups validity for CSU and CINDU patients. The UCT was able to discriminate between groups with different disease severity (demonstrated by PatGA-LS) and different levels of disease control (demonstrated by PhyGA-LS) (p = 0,000). Patients with different disease severity had different UCT scores. Even though there were slight differences between CSU and CINDU patients, the overall known groups validity was similar (Table 4). A higher number of CINDU patients rated their symptoms as severe complaints (PatGA-LS-1st 15 vs 6 in CINDU and CSU, respectively).
The sensitivity to change of the UCT
The UCT was sensitive to detect changes in disease activity and quality of life. The correlations between change in UCT scores and UAS28, CU-Q2oL, DLQI, PATGA-VAS and PhyGA-VAS change scores were statistically significant for both CSU and CINDU (p < .01).
Change in UCT scores have a moderate correlation with PhyGA-VAS-change (r = 0.573), strong correlations with PatGA-LS change (r = −0.632), PhyGA-LS change (r = −0.713) and PatGA-VAS change (−0.623) in total. Change in UCT scores have moderate correlations with UAS28 change (r = −0.598), CU-Q2oL change (r = −0.543) and PhyGA-VAS change (r = 0.570), strong correlations with PatGA-VAS change (r = −0.633), PatGA-LS change (r = −0.689), PhyGA-LS change (r = −0.612) in the CSU group, while change in UCT scores have moderate correlations with DLQI change (r = −0.543), PatGA-LS change (r = −0.511), PatGA-VAS change (r = −0.556), PhyGA-VAS change (r = 0.523) and a strong correlation with PhyGA-LS change (r = −0.752) in CINDU patients.
The reproducibility of the Turkish UCT
The Turkish UCT showed excellent reproducibility. Seventy patients (34 CSU patients and 36 CINDU patients) who had stable PatGA-LS scores during the four-week interval between the 1st and the 2nd visits were included into analysis of test-retest reliability. ICC of UCT in CSU and CINDU were analysed separately and also totally. The ICCs of UCT in patients with CSU and CINDU were 0.89 (95% confidence interval = 0.79–0.95) and 0.74 (95% confidence interval = 0.50–0.87), respectively, that demonstrated excellent reproducibility of both UCTs. ICC was 0.83 (95% confidence interval = 0.72–0.89) showing excellent reproducibility of Turkish UCT when it is determined totally.
Screening accuracy (categorization)
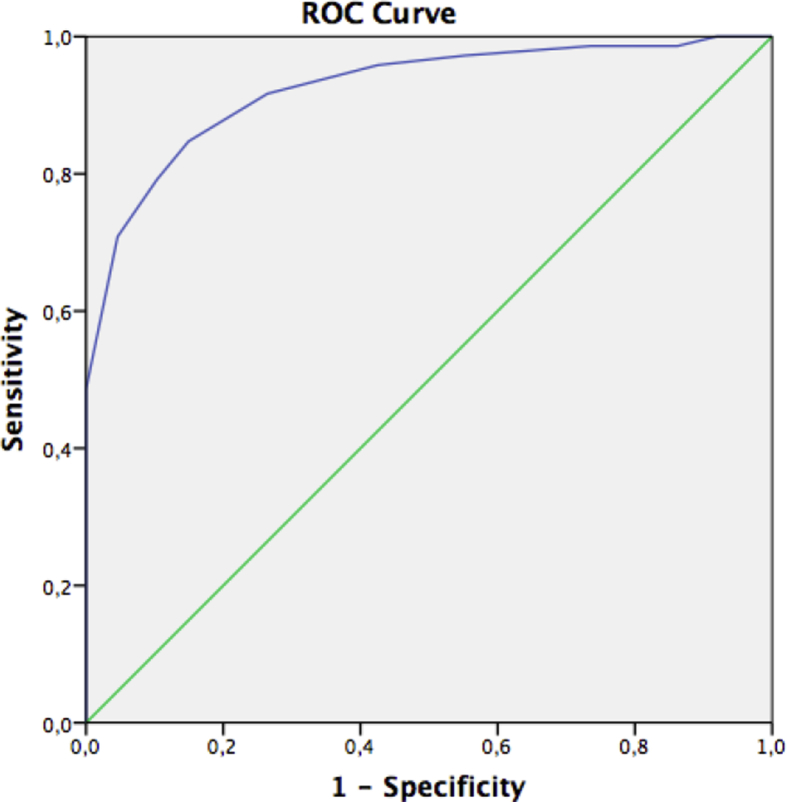
Seventy-two and 101 patients had well-controlled urticaria by using PhyGA-LS at 1st and 2nd visits, respectively. The AUCs on ROC analyses showed excellent accuracy of both visits of Turkish UCT (Table 5) (Fig. 2). UCT scores of ≥9,5 (sensitivity 84.7%, specificity 85.1%) and ≥11.5 (sensitivity 93.1%, specificity 91.4%) were found to be suitable cut off values to define well-controlled disease for the 1st and the 2nd visits, respectively. When the CSU and CINDU groups were analysed separately, UCT scores of ≥10.5 and ≥ 11.5 were defined for CINDU patients, ≥8.5 and ≥ 10.5 were defined for CSU patients for the 1st and 2nd visits, respectively.
Table 5.
Cut-off values for the UCT for screening patients for well controlled disease.
|
Total |
PhyGA-LS |
Total |
PhyGA-LS |
||
|---|---|---|---|---|---|
| UCT score | 1st visit |
UCT score |
2nd visit |
||
|
Well-controlled urticaria (n:72) |
|
Well-controlled urticaria (n:101) |
|||
| Cut-off value | Sensitivity (%) | Specificity (%) | Cut-off value | Sensitivity (%) | Specificity (%) |
| 8.5–9 | 91.7 | 73.6 | 8.5–9 | 98 | 55.2 |
| 9.5–10 | 84.7 | 85.1 | 9.5–10 | 98 | 69 |
| 10.5–11 | 79.2 | 89.7 | 10.5–11 | 96 | 81 |
| 11.5–12 | 70.8 | 95.4 | 11.5–12 | 93.1 | 91.4 |
| 12.5–13 | 48.6 | 100 | 12.5–13 | 69.3 | 100 |
| 13.5–14 | 33.3 | 100 | 13.5–14 | 47.5 | 100 |
| AUC (95% CI) | 0.925 (0.883–0.966) | 0.967 (0.943–0.992) | |||
Abbreviations: AUC (95% CI): area under the curve (95% confidence interval).
Fig. 2.
ROC curve of first visit UCT. Area under the ROC curve for UCT score = 0.925 (95% CI, (0,883–0966).
Minimal important difference (MID) and smallest detectable change (SDC) of the UCT
The mean UCT score change in cases of minimal improvement (1 step improvement in Phy-GA-LS) was found to be 4.2 ± 3.0 points, while mean UCT score change in cases of an unchanged Phy-GA-LS was only 0.6 ± 2.2 points. This mean change method indicated that MID is 4, while the distributional-criterion approach determined MID as 1.9 (the SD of the baseline UCT scores (3.8) was divided by 2). The Phy-GA-LS change and UCT score change showed strong correlation (r = −0.713, p < .01). On the ROC curve analysis, UCT score change was determined by improved disease versus not improved disease. AUC was 0.896 (0.841–950) (95% CI) and UCT change score that identifies improved disease was 2.5 (sensitivity 82.5%, specificity 87.5%). Sixty-seven patients had stable Phy-GA-LS between two visits and the SD of the UCT's score changes in patients with stable Phy-GA-LS was 2.2 and SDC was 4.3.
Discussion
The UCT is a simple and easy to use patient-reported outcome (PRO) tool which was originally developed to determine disease control in CU patients.14 The linguistic adaptation and validation studies have been performed in Thai, Arabic and Spanish.15, 17, 18 We performed the recommended steps for validation of PRO-tools rigorously for the Turkish UCT and we determined the reliability, validity, sensitivity to change and minimally important difference (MID) of the tool.26
We found that UCT scores were lower in the CINDU group at referral (8.4 in CINDU vs 10.4 in CSU, p = .001) and PhyGA-VAS was also lower in the CINDU group compared to CSU group (5.5 vs 8.1, p < .001). PatGA-VAS were higher (4.9 vs 3.1; p < .001) and Pat-ATR and Phy-ATR were lower in CINDU compared to CSU (29 vs 53, p < .001 and 19 vs 52, p < .001, respectively). Most of the CSU patients rated their disease activity as mild (34 patients) while most of the CINDU patients rated as moderate (40 patients), severe activity was rated by 6 of the CSU patients while 15 of CINDU patients rated themselves as severe. Nineteen patients with CSU reported complete control of urticaria while only one patient with CINDU reported complete control. These findings suggest a higher disease impact and poorer control of urticaria in CINDU patients which might be associated with the longer disease duration in these patients (36.3 vs 31.5 months in CINDU and CSU patients, respectively). Since we had to use different QoL measures to assess QoL impairment in CSU and CINDU patients we are not able to make a direct comparison between the QoL impairment of CSU and CINDU patients but we could get some information from the second question of UCT which provides information on how the disease impacted the quality of life of the patient. Twenty-four patients with CINDU while 13 patients with CSU reported to have their quality of life impairment as “much and very much effected” by urticaria which are in parallel to the findings of O'Donnell et al. and Poon et al. who described poorer quality of life in patients with delayed pressure urticaria and cholinergic urticaria and with Schoepke et al. who reported that quality of life significantly impaired in 44% and normal life not possible in 7% of symptomatic dermographism patients.27, 28, 29
The Turkish version of UCT showed excellent consistency for CSU which was higher than the original tool (Cronbach's α original versus Turkish 0.84 vs 0.89) and a minimally acceptable consistency for CINDU (Cronbach's α 0.89 vs 0.68). The reason for this might be the nature of CINDU that shows exaggeration with exposure to the specific trigger and moderation with avoidance which makes it hard to assess the disease activity without making the critical trigger threshold (CTT) measurement.19 It is unfortunate that by employing the short version of UCT, we missed the chance to ask the question of “How much, in the last 4 weeks, did you have to avoid physical exercise or stimuli such as heat, cold, pressure light or friction because of your urticaria?” to CINDU patients which is included in the long version of the UCT.14 By asking this question, maybe we could get a thorough assessment of the activity of the disease.
The evaluation for the correlation between other tools revealed that the UCT showed strong negative correlations with the UAS28, CU-Q2oL, Pat-GA-VAS, Phy-GA-VAS and Pat-LS and a strong positive correlation with Phy-GA-LS which is because higher scores of the former tools are linked to higher disease activity and poor control. The DLQI showed a moderate negative correlation with the UCT. We believe this is again due to the difficulty to assess disease impact in CINDU patients and could also be linked to the differences in the period of assessment times which assesses the last 4 weeks in UCT and the last week for DLQI. Although a study from Japan showed good correlation with UCT and DLQI in CU,30 since DLQI is not a disease specific tool which is designed specifically for CINDU, it might not be able to show the real impact on QoL impairment of CINDU patients. As the CU-Q2oL has been reported to be more sensitive in comparison with DLQI to reflect QoL impairment in CSU, certain disease specific questionnaires are needed to assess QoL in patients with CINDU31.
UCT also showed to be sensitive to changes in disease activity in both CSU and CINDU patients. The test-retest reliability showed excellent reproducibility for both CSU and CINDU patients but there was a clear superiority for CSU patients (first visit UCT 12.4 vs 12.2 for CSU, 8.9 vs 10.9 for CINDU, respectively). At baseline, the mean UCT scores of CINDU patients were lower than the CSU patients (8.4 vs 10.4, respectively) but after treatment, the total scores clearly increased at the follow up visit (11.7 for CSU and 11.4 for CINDU, respectively). The lower reproducibility of UCT for CINDU could be attributed to its changeability according to avoidance behaviours.
Here we report that the UCT is also suited to determine changes in disease control over time and to assess treatment effects (that it is responsive to change). Our results demonstrate that changes in the UCT score strongly correlate with disease-specific assessments of changes in urticaria activity (UAS7) and HR-QoL (CU-Q2oL), while the correlation with changes in the less-specific DLQI are less strong (but still good). Importantly, the change of UCT ratings from ‘‘poorly controlled’’ to ‘‘well-controlled’’ was well in accordance with the change of the patients' self-assessment of treatment efficacy, with the physicians' global assessment of the treatment response, and with the UAS7-based assessment of treatment response. This supports the appropriateness of the current UCT cut-off value of >12 points to detect patients with well-controlled urticaria.
MID is the smallest change in a score that can be considered clinically relevant and the knowledge of the MID of an outcome measure is important for the interpretation of changes in its score by time or after treatment.25 By using the mean change method we found an MID of 4 and with the ROC curve analysis UCT change score that identifies improved disease was found 2.5. Like the other investigators14, 15 we regarded an MID 3 points as the most appropriate for UCT score changes.
We unfortunately have some limitations in this study; even though the test-retest reliability analysis should be done in patients without change in disease activity, since we could not keep patients without treatment for one month, we had to give treatment and the disease activities decreased. For this reason, test-retest reliability could only be performed in 34 CSU and 42 CINDU patients. And by using the long version of UCT we could have evaluated the avoidance behaviours of the CINDU patients and thoroughly assess the impact and activity of CINDU. Another point is using SF-36 instead of DLQI could be more effective in showing the QoL impairment in patients with CINDU because it has a physical functioning domain which measures the repercussion of possible physical limitations on daily activities such as taking a bath, dressing, walking moderate distances, running, climbing stairs, carrying bags, bending over and kneeling.32
As a conclusion, the UCT is a valuable tool which aids in treatment decisions, allowing to assess whether the disease is controlled by two aspects; namely both in severity and quality of life impairment and the Turkish version of UCT is shown to be valid and reliable for both CSU and CINDU patients.
Authors' contributions*
Conceived and designed the study: Emek Kocatürk and Karsten Weller.
Data collection, patient inclusion and follow up: Emek Kocatürk, Utkan Kızıltaç, Kübra Kızıltaç, Pelin Can, Nagihan Sahillioğlu, Rabia Öztaş Kara, Teoman Erdem, Aslı Gelincik.
Wrote the manuscript: Emek Kocatürk and Utkan Kızıltaç.
Statistical analysis: Pelin Can.
Critically reviewed and revised the manuscript: Marcus Maurer.
Final language editing: Marcus Maurer, Emek Kocatürk.
Agreement with manuscript and conclusions: all.
Designed the figures and tables: Pelin Can, Emek Kocatürk.
All authors read and approved the final manuscript.
Conflicts of interest
None of the authors have conflicts of interest regarding the content of this manuscript.
Ethics approval and consent to participate
Letter of ethical clearance was secured from ethical review board of Okmeydanı Training and Research Hospital. Privacy and confidentiality of medical information was ensured. Informed consent of patients was obtained prior to inclusion.
Funding
There was no external source of funding obtained. All expenses related to this research work were covered by the authors.
Availability of data and materials
The datasets generated during the current study are available from the corresponding author on reasonable request.
Acknowledgements
Not applicable.
Contributor Information
Emek Kocatürk, Email: dremekozgur@gmail.com.
Utkan Kızıltaç, Email: utkancyp@hotmail.com.
Pelin Can, Email: pulkumen@gmail.com.
Rabia Öztaş Kara, Email: r.oztas.kara@gmail.com.
Teoman Erdem, Email: teomanerdem@gmail.com.
Kübra Kızıltaç, Email: kubracure@gmail.com.
Nagihan Sahillioğlu, Email: nagehantarikci@hotmail.com.
Aslı Gelincik, Email: gelincikasli@hotmail.com.
Marcus Maurer, Email: marcus.maurer@charite.de.
Karsten Weller, Email: karsten.weller@charite.de.
List of abbreviations
- AAS
Angioedema Activity Score
- AUC
Area under the curve
- CINDU
Chronic inducible urticaria
- CSU
Chronic spontaneous urticaria
- CU
Chronic urticaria
- CU-Q2oL
Chronic Urticaria Quality of Life questionnaire
- DLQI
Dermatology Life Quality Index
- HRQoL
Health related quality of life
- ICC
Intraclass correlation coefficient
- MID
Minimal important difference
- Pat-ATR
Patient's assessment of treatment response – Likert scale
- Pat-CDA
Patient's assessment of change in disease activity – Likert scale
- Pat-CQoL
Patient's assessment of change in quality of life – Likert scale
- PatGA-VAS
Patient's global assessment of disease severity-visual analog scale
- PatGA-LS
Patient's global assessment of disease severity - Likert scale
- PhyGA-VAS
Physician's global assessment of disease control - visual analog scale
- PhyGA-LS
Physician's global assessment of disease control - Likert-scale
- Phy-ATR
Physician's assessment of treatment response
- PRO
Patient-reported outcome
- QoL
Quality of life
- SDC
Smallest detectable change
- SD
Standard deviation
- UAS
Urticaria Activity Score
- UCT
Urticaria Control Test
References
- 1.Zuberbier T., Aberer W., Asero R. The EAACI/GA2LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. The 2017 revision and update. Allergy. 2018 May;73(5):1145–1146. doi: 10.1111/all.13414. [DOI] [PubMed] [Google Scholar]
- 2.Hawro T., Ohanyan T., Schoepke N. The urticaria activity score-validity, reliability, and responsiveness. J Allergy Clin Immunol Pract. 2018 Jul–Aug;6(4):1185–1190. doi: 10.1016/j.jaip.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Hollis K., Proctor C., McBride D. Comparison of urticaria activity score over 7 days (UAS7) values obtained from once-daily and twice-daily versions: results from the ASSURE-CSU study. Am J Clin Dermatol. 2018;19:267–274. doi: 10.1007/s40257-017-0331-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hawro T., Ohanyan T., Schoepke N. Comparison and interpretability of the available urticaria activity scores. Allergy. 2018;73:251–255. doi: 10.1111/all.13271. [DOI] [PubMed] [Google Scholar]
- 5.Weller K., Groffik A., Magerl M. validation, and initial results of the angioedema activity score. Allergy. 2013;68:1185–1192. doi: 10.1111/all.12209. [DOI] [PubMed] [Google Scholar]
- 6.Baiardini I., Pasquali M., Braido F. A new tool to evaluate the impact of chronic urticaria on quality of life: chronic urticaria quality of life questionnaire (CU-Q2oL) Allergy. 2005;60(8):1073–1078. doi: 10.1111/j.1398-9995.2005.00833.x. [DOI] [PubMed] [Google Scholar]
- 7.Kocaturk E., Weller K., Martus P. Turkish version of the chronic urticaria quality of life questionnaire: cultural adaptation, assessment of reliability and validity. Acta Derm Venereol. 2012;92(4):419–425. doi: 10.2340/00015555-1199. [DOI] [PubMed] [Google Scholar]
- 8.Kessel A., Graif Y., Vadasz Z. Adaption and validation of the Israeli version of the chronic urticaria quality of life questionnaire (CU-Q2oL) Isr Med Assoc J. 2016;18:461–465. [PubMed] [Google Scholar]
- 9.Brzoza Z., Badura-Brzoza K., Mlynek A. Adaptation and initial results of the polish version of the GA2LEN chronic urticaria quality of life questionnaire (CU-Q2oL) J Dermatol Sci. 2011;62:36–41. doi: 10.1016/j.jdermsci.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 10.Mlynek A., Magerl M., Hanna M. The German version of the chronic urticaria quality-of-life questionnaire (CU-Q2oL): factor analysis, validation and initial clinical findings. Allergy. 2009;64:927–936. doi: 10.1111/j.1398-9995.2008.01920.x. [DOI] [PubMed] [Google Scholar]
- 11.Weller K., Groffik A., Magerl M. Development and construct validation of the angioedema quality of life questionnaire (AE-QoL) Allergy. 2012;67:1289–1298. doi: 10.1111/all.12007. [DOI] [PubMed] [Google Scholar]
- 12.Weller K., Magerl M., Peveling-Oberhag A., Martus P., Staubach P., Maurer M. The Angioedema Quality of Life Questionnaire (AE-QoL) – assessment of sensitivity to change and minimal clinically important difference. Allergy. 2016;71:1203–1209. doi: 10.1111/all.12900. [DOI] [PubMed] [Google Scholar]
- 13.Maurer M., Mathias S.D., Crosby R.D., Rajput Y., Zazzali J.L. Validity and responsiveness of the urticaria activity and impact measure (U-AIM), a new patient-reported tool. Allergy. 2018 Jun;120(6):641–647. doi: 10.1016/j.anai.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 14.Weller K., Groffik A., Church M.K. Development and validation of the Urticaria Control Test: a patient-reported outcome instrument for assessing urticaria control. J Allergy Clin Immunol. 2014 May;133(5):1365–1372. doi: 10.1016/j.jaci.2013.12.1076. 1372.e1- [DOI] [PubMed] [Google Scholar]
- 15.Kulthanan K., Chularojanamontri L., Tuchinda P., Rujitharanawong C., Maurer M., Weller K. Validity, reliability and interpretability of the Thai version of the urticaria control test (UCT) Health Qual Life Outcomes. 2016;14:61. doi: 10.1186/s12955-016-0466-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ohanyan T., Schoepke N., Bolukbasi B. Responsiveness and minimal important difference of the urticaria control test. J Allergy Clin Immunol. 2017;140:1710–1713. doi: 10.1016/j.jaci.2017.04.050. e11. [DOI] [PubMed] [Google Scholar]
- 17.Irani C., Hallit S., Weller K., Maurer M., El Haber C., Salameh P. Chronic urticaria in most patients is poorly controlled. Results of the development, validation, and real life application of the Arabic urticaria control test. Saudi Med J. 2017 Dec;38(12):1230–1236. doi: 10.15537/smj.2017.12.21206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ortiz de Frutos F.J. Spanish version of the urticaria control test now available: good news for all. Actas Dermosifiliogr. 2015 Nov;106(9):697–698. doi: 10.1016/j.ad.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 19.Weller K., Siebenhaar F., Hawro T., Altrichter S., Schoepke N., Maurer M. Clinical measures of chronic urticaria. Immunol Allergy Clin. 2017 Feb;37(1):35–49. doi: 10.1016/j.iac.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 20.Weller K., Zuberbier T., Maurer M. Clinically relevant outcome measures for assessing disease activity, disease control and quality of life impairment in patients with chronic spontaneous urticaria and recurrent angioedema. Curr Opin Allergy Clin Immunol. 2015;15:220–226. doi: 10.1097/ACI.0000000000000163. [DOI] [PubMed] [Google Scholar]
- 21.Weller K., Zuberbier T., Maurer M. Chronic urticaria: tools to aid the diagnosis and assessment of disease status in daily practice. J Eur Acad Dermatol Venereol. 2015;S3:38–44. doi: 10.1111/jdv.13200. [DOI] [PubMed] [Google Scholar]
- 22.Maurer M., Metz M., Bindslev-Jensen C. Definition, aims, and implementation of GA2LEN urticaria centers of reference and excellence. Allergy. 2016;71:1210–1218. doi: 10.1111/all.12901. [DOI] [PubMed] [Google Scholar]
- 23.Mlynek A., Zalewska-Janowska A., Martus P., Staubach P., Zuberbier T., Maurer M. How to assess disease activity in patients with chronic urticaria? Allergy. 2008;63:777–780. doi: 10.1111/j.1398-9995.2008.01726.x. [DOI] [PubMed] [Google Scholar]
- 24.Streiner D., Norman G. second ed. Oxford University Press; Oxford: 1995. Health Measurement Scales; pp. 232–235. [Google Scholar]
- 25.de Vet H.C., Ostelo R.W.J.G., Terwee C.B. Minimally important change determined by a visual method integrating an anchor-based and a distribution-based approach. Qual Life Res. 2007;16:131–142. doi: 10.1007/s11136-006-9109-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wild D., Grove A., Martin M. ISPOR task force for translation and cultural adaptation. Principles of good practice for the translation and cultural adaptation process for patient-reported outcomes (PRO) measures: report of the ISPOR task force for translation and cultural adaptation. Value Health. 2005 Mar-Apr;8(2):94–104. doi: 10.1111/j.1524-4733.2005.04054.x. [DOI] [PubMed] [Google Scholar]
- 27.O'Donnell B.F., Lawlor F., Simpson J., Morgan M., Greaves M.W. The impact of chronic urticaria on the quality of life. Br J Dermatol. 1997;136:197–201. [PubMed] [Google Scholar]
- 28.Poon E., Seed P.T., Greaves M.W., Black A.K. The extent and nature of disability in different urticarial conditions. Br J Dermatol. 1999;140:667–671. doi: 10.1046/j.1365-2133.1999.02767.x. [DOI] [PubMed] [Google Scholar]
- 29.Schoepke N., Młynek A., Weller K., Church M.K., Maurer M. Symptomatic dermographism: an inadequately described disease. J Eur Acad Dermatol Venereol. 2015 Apr;29(4):708–712. doi: 10.1111/jdv.12661. [DOI] [PubMed] [Google Scholar]
- 30.Itakura A., Tani Y., Kaneko N., Hide M. Impact of chronic urticaria on quality of life and work in Japan: results of a real-world study. J Dermatol. 2018 Jun 13 doi: 10.1111/1346-8138.14502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weller K., Church M.K., Kalogeromitros D. Chronic spontaneous urticaria: how to assess quality of life in patients receiving treatment. Arch Dermatol. 2011;147(10):1221–1223. doi: 10.1001/archdermatol.2011.310. [DOI] [PubMed] [Google Scholar]
- 32.Ue A.P., Souza P.K., Rotta O., Furlani Wde J., Lima A.R., Sabbag D.S. Quality of life assessment in patients with chronic urticaria. An Bras Dermatol. 2011 Sep-Oct;86(5):897–904. doi: 10.1590/s0365-05962011000500006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during the current study are available from the corresponding author on reasonable request.