Abstract
Background
Asthma is one of the most common non-communicable respiratory diseases, affecting about 6% of the general population. Severe asthma, even if afflicts a minority of asthmatics, drives the majority of costs of the disease. The aim of this study is to create a pharmacoeconomic model to predict the costs of corticosteroid-related adverse events in severe asthmatics and applying it to the first published epidemiologic data from the Severe Asthma Network in Italy (SANI) registry.
Methods
The analysis was conducted from the perspective of the Italian National Healthcare System (INHS). Model inputs, derived from literature, included: asthma epidemiology data, frequency of adverse events, percentage of severe asthma treated with OCS and adverse event cost (Diagnosis-Related Group (DRG) national tariffs). We estimated costs per different patient groups: non-asthma controls, mild/moderate and severe asthmatics. Final results report estimated direct cost per patient and total direct cost for overall target population, showing economic impact related to corticosteroid complication.
Results
Based on epidemiological data input, in Italy, asthmatic subjects resulted about 3,999,600, of which 199,980 with severe asthma. The number of patients with severe asthma OCS-treated was estimated at 123,988. Compared to the non-asthma control cohort and to that with moderate asthma annual cost per severe asthmatic patient resulted respectively about €892 and €606 higher, showing a corticosteroids shadow cost ranging from 45% to 30%.
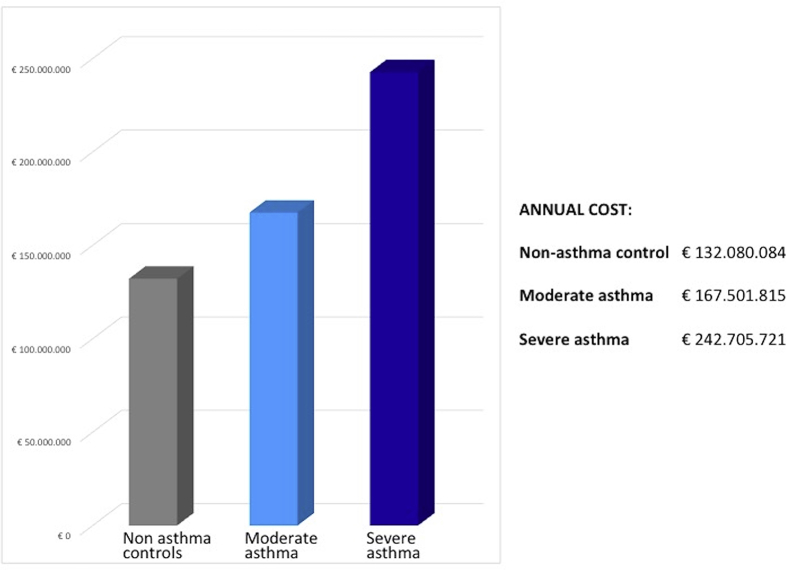
Applying the cost per patient to the target population identified for Italy, the budget impact model estimated a total annual cost related to OCS-related adverse events of €242.7 million for severe asthmatics. In respect with non-asthmatic and moderate population, an incremental expenditure of about € 110.6 million and €75.2, respectively, were shown.
Conclusions
Our study provides the first estimates of additional healthcare costs related to corticosteroid induced adverse events in severe asthma patient. Budget impact model results highlighted the relevant economic impact of OCS-related adverse events in severe asthma patients. The future extrapolation of additional data from SANI registry will support the development of a model to investigate the role of corticosteroids sparing drugs.
Keywords: Severe asthma, Oral corticosteroids, Adverse events, Pharmacoeconomy, Costs, Diabetes, Glaucoma, Obesity, Bone fracture, Chronic kidney disease
Introduction
Asthma is one of the most common non-communicable respiratory disease, affecting about 6% of general population, that is currently more than 300 millions of people in the world1, 2; the trend of asthma prevalence seems to be at least stable,1 if not even increasing in the last few years.2, 3 This epidemiological situation accounts for high and increasing health-related costs (direct and indirect),4, 5, 6, 7, 8, 9 particularly in those patients with suboptimal control of asthma.10, 11
Most of the patients with asthma achieve a good control of the disease using regularly low-to-medium doses of inhaled corticosteroids±other controllers, but a small proportion (accounting about 3.5–10% of all asthmatics12, 13, 14, 15) requires high dose of these drugs (and/or systemic corticosteroids) to be controlled or remains uncontrolled despite this therapy: these patients are defined as “severe asthmatics” according to the latest European Respiratory Society (ERS)/American Thoracic Society (ATS) guidelines.16 Severe asthma, although afflicting a minority of these patients, drives most costs of the disease.17
Moreover, it is precisely the patients with more severe asthma (particularly if they have associated comorbidities and high peripheral eosinophils levels) who use systemic corticosteroids more frequently and at high dosages, with an increased risk of corticosteroid-related adverse events.18, 19, 20 Indeed, it is known that systemic corticosteroids are not without side effects, and that these are more frequent and severe in patients who use them continuously or frequently.21, 22 The major adverse events related to the use of systemic corticosteroids (that are common to patients using high doses of inhaled corticosteroids for prolonged time23) are: hypertension, osteoporosis and bone fracture, cataract and glaucoma, diabetes, respiratory infections, reduced growth velocity in children, and hypothalamic-pituitary-adrenal axis suppression.
Few studies analysed the impact, in terms of costs, of corticosteroid-related adverse events in several diseases, including asthma19, 20, 22, 24, 25, 26, 27: the estimated annual cost in asthmatics ranges from about 600 Euros26 to about 5000 Euros in more severe patients.27
The aim of this study is to create a pharmacoeconomic model to predict the costs of corticosteroid-related adverse events in severe asthmatics and applying it to the first published epidemiologic data from the Severe Asthma Network in Italy (SANI) registry.28, 29
Materials and methods
Study design
A budget impact model (BIM) was developed to evaluate the pharmacoeconomic consequences of oral corticosteroid adverse events (OCS-AE) in adult patients with severe asthma. The analysis was conducted through a dynamic simulation model in Microsoft Excel® and carried out from the perspective of the Italian National Healthcare System (INHS). Our study initially provided a literature review aimed to identify data on:
-
•
asthma epidemiology
-
•
asthmatic comorbidities related to corticosteroids use
-
•
frequency of adverse events in severe asthma patients
-
•
adverse events cost
Model inputs included asthma epidemiology data, frequency of adverse events, percentage of severe asthma patients treated with OCS and adverse events cost. After identifying the study population and main OCS-related adverse events in adult's asthma patients, the economic impact of adverse events was estimated based on adverse events rate and events cost. Economic evaluation was conducted considering a main scenario, including all adverse events considered in a Sweeney's et al study that investigated the prevalence of systemic corticosteroid-induced morbidity in severe asthma using data from Optimum Patient Care Research Database (OPCRD) and British Thoracic Society (BTS) Difficult Asthma Registry.19 Specifically, we adopted prevalence data obtained from OPCRD database that included 7195 subjects in three age and gender matched groups: severe asthma (808), mild/moderate asthma (3975) and non-asthma controls (2412): patients with SA requiring regular OCS (GINA step 5) were compared with patients with mild/moderate asthma and non-asthmatic controls.
Adverse events evaluation was developed adopting Diagnosis-Related Group (DRG)-based national tariffs (diagnosis-related group tariffs system). In addition, an analysis subgroup, including some of most common OCS-related adverse events reported in available studies was considered: we researched cost of illness studies available in literature for Italy for diseases in analysis in order to use an alternative evaluation method, not related only to the acute event as it happens with the DRG system, and to observe their impact on the total. Based on different prevalence rates of morbidities associated with systemic steroid exposure provided by literature,19 we estimated costs per different patient groups: non-asthma controls, mild/moderate and severe asthmatics. Combining epidemiology data with frequency and OCS-related adverse events cost, we obtained budget impact analysis results. Final results report estimated direct cost per patient and total direct cost for overall target population, showing economic impact related to corticosteroid complications, potentially avoidable if these drugs were no longer administered.
Lastly, in order to assess the robustness of results, a deterministic sensibility analysis was developed changing main parameters used for the calculation (cost of adverse events and prevalence rates of OCS-related adverse events) by ±20%. In addition, for event costs we considered DRG costs related to Lombardy Region, as an alternative to national tariffs given the variability on the territory.
Study population
In order to obtain the target population, we started from national epidemiological data derived from Italian National Statistical Institute (ISTAT).30 As shown in Table 1, a 6.6% of asthma prevalence was applied to the total resident population in Italy, and 5% of asthmatics were considered to be affected by severe asthma (based on literature and expert opinion).3, 28
Table 1.
Study population: demographic data input.
| Demographic data | |
|---|---|
| Italian resident population (2017) | 60,589,445 |
| Asthma prevalence in Italy | 6.60% |
| SA patients of total asthmatics | 5% |
| SA patients treated with OCS | 62% |
Finally, it was considered that 62% of patients with severe asthma were chronically treated with OCS, as emerged from the first extractions from SANI database (Severe Asthma Network in Italy).29
Adverse events rate
Rates of OCS-related adverse events were provided from an observational study published by Sweeney et al., Table 2. The study was based on Optimum Patient Care Research Database (UK setting) and compared patients with SA requiring regular OCS with patients with mild/moderate asthma and non-asthmatic controls (rhinitis diagnosis with no asthma diagnosis/asthma drugs and no exposure to OCS). The aim of Sweeney's study was to determine the prevalence of systemic corticosteroid-induced morbidity in severe asthma over 2-year analysis. Subjects (7195) had at least 2 years of continuous medical records and were aged >12 years.
Table 2.
Prevalence rates of potential systemic corticosteroid-induced comorbidity.
| Comorbidity | Prevalence ratesa |
||
|---|---|---|---|
| Non-astma controls |
Mild/moderate Asthma |
Severe Asthma |
|
| % | % | % | |
| Type II diabetes | 6% | 7% | 10% |
| Obesity (BMI >30) | 23% | 35% | 42% |
| Osteopenia | 2% | 2% | 10% |
| Osteoporosis | 3% | 4% | 16% |
| Fracture | 4% | 3% | 5% |
| Dyspeptic disorders | 24% | 34% | 65% |
| Glaucoma | 3% | 3% | 4% |
| Cataract | 4% | 5% | 9% |
| Cardiovascular disease | 7% | 7% | 10% |
| Hypertension | 25% | 29% | 34% |
| Psychiatric disorders | 25% | 31% | 38% |
| Hypercholesterolaemia | 11% | 14% | 15% |
| Sleep disorder | 2% | 3% | 4% |
| Chronic kidney disease | 7% | 9% | 14% |
Sweeney J et al. Thorax 2016; 71:339–346.
2-year study period.
Adverse events cost
Economic evaluation of OCS-related adverse events was conducted by DRG Tariffs System, Table 3. This system classifies all patients in homogeneous groups according to resources absorption, allowing to economically quantify the use of resources and to provide an estimate of the cost per acute event from the INHS perspective.
Table 3.
Asthma comorbidities: cost per event.
| Comorbidity | Event cost |
|---|---|
| Type II diabetes | €1391.49 |
| Obesity (BMI >30) | €1757.84 |
| Osteopenia | €940.33 |
| Osteoporosis | €1037.82 |
| Fracture | €1984.96 |
| Dyspeptic disorders | €958.51 |
| Glaucoma | €1051.07 |
| Cataract | €1051.07 |
| Cardiovascular disease | €2096.82 |
| Hypertension | €963.36 |
| Psychiatric disorders | €1941.81 |
| Hypercholesterolaemia | €428.43 |
| Sleep disorder | €1408.88 |
| Chronic kidney disease | €3734.46 |
Moreover, in order to extrapolate data cost from literature, we adopted results from cost of illness studies available for Italy to implement model subgroup analysis. We considered cost of the following illness: type 2 diabetes mellitus (T2DM), obesity, osteoporosis, glaucoma and chronic kidney disease, Table 4.31, 32, 33, 34, 35
Table 4.
Asthma comorbidities: cost of illness study results.
| Comorbidity | Cost of Illness (mean annual cost) |
|---|---|
| Type II diabetes | € 2792 |
| Obesity | € 1166 |
| Osteoporosis | € 1325 |
| Glaucoma | € 734 |
| Chronic kidney disease | € 4508 |
Pagano et al. Nutrition, Metabolism & Cardiovascular Diseases (2016).
Colao A et al. BMJ Open 2017;7:e013899.
Degli Esposti L, et al. Farmeconomia e percorsi terapeutici 2011; 12(3).
Koleva D et al. Ophthalmologica 2007; 221: 340-347.
Turchetti G et al.Eur J Health Econ (2017) 18:847–858.
Results
Based on epidemiological data input, in Italy asthmatic subjects turned out to be about 3,999,600, of whom 199,980 with severe asthma. The number of patients with severe asthma OCS-treated was estimated to be 123,988 which represents the proportion of subjects potentially at risk of developing morbidities associated with systemic steroid exposure.
The cost of OCS-related adverse events in the main scenario was derived by combining the cost of each event with the relative rate. Annual costs per patient related to OCS-related adverse events in non-asthma control, mild/moderate, and severe asthma groups resulted equal to €1065,27, €1350,96 and €1957,50 respectively, Table 4.
Compared to the non-asthma control cohort, the annual per patient cost related to OCS-related adverse events resulted about €892 and €285 higher in severe asthma and moderate/mild cohort respectively. Annual per patient cost difference between asthmatic groups was about €606 (see Table 5).
Table 5.
Cost per patient related to comorbidities.
| Comorbidity | Non-asthma control | Moderate asthma | Severe asthma |
|---|---|---|---|
| Type II diabetes | € 83,49 | € 97,40 | € 139,15 |
| Obesity (BMI >30) | € 404,30 | € 615,24 | € 738,29 |
| Osteopenia | € 18,81 | € 18,81 | € 94,03 |
| Osteoporosis | € 31,13 | € 41,51 | € 166,05 |
| Fracture | € 79,40 | € 59,55 | € 99,25 |
| Dyspeptic disorders | € 230,04 | € 325,89 | € 623,03 |
| Glaucoma | € 31,53 | € 31,53 | € 42,04 |
| Cataract | € 42,04 | € 52,55 | € 94,60 |
| Cardiovascular disease | € 146,78 | € 146,78 | € 209,68 |
| Hypertension | € 240,84 | € 279,38 | € 327,54 |
| Psychiatric disorders | € 485,45 | € 601,96 | € 737,89 |
| Hypercholesterolaemia | € 47,13 | € 59,98 | € 64,26 |
| Sleep disorder | € 28,18 | € 35,22 | € 56,36 |
| Chronic kidney disease | € 261,41 | € 336,10 | € 522,83 |
| Total for 2-year analysis | € 2130.54 | € 2701.91 | € 3915.00 |
| Annual total cost | 1065.27 € | 1350.96 € | 1957.50 € |
We extended the total annual cost for patient to the estimated severe asthma Italian population in treatment with OCS (n = 123,988) in order to obtain budget impact results: (Fig. 1).
Fig. 1.
Annual cost related to oral corticosteroids-related adverse events in target population.
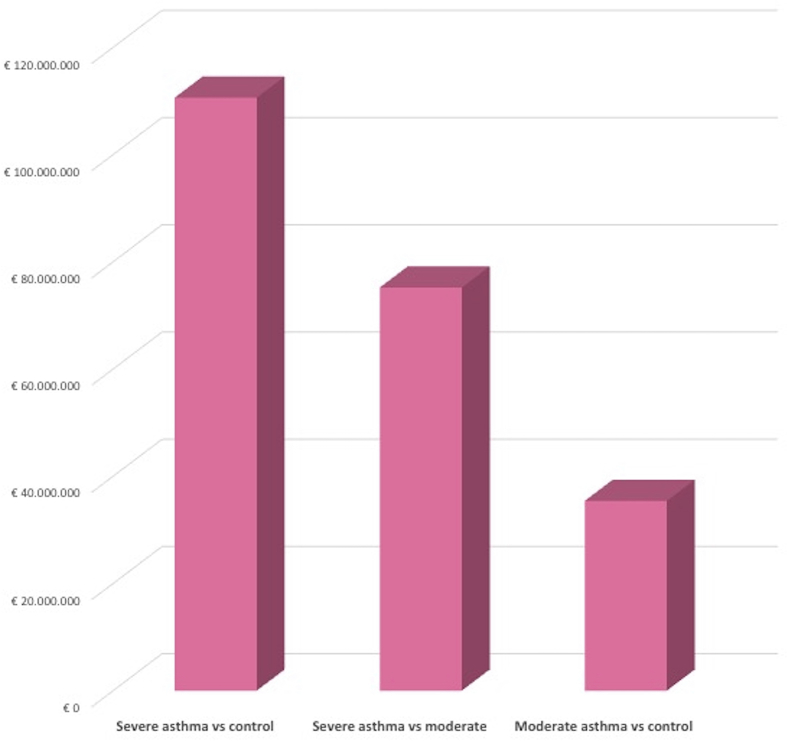
Comparing severe asthma annual costs with those of non-asthmatic population, an incremental expenditure of about € 110.6 million was shown, which was reduced to about € 75.2 compared to the moderate asthma. The difference between the population with moderate asthma compared to the non-asthmatic control was lower, reaching approximately €35.4 million (Fig. 2).
Fig. 2.
Incremental cost scenario: cost related to oral corticosteroids-related adverse events in main analysis.
Subgroup analysis
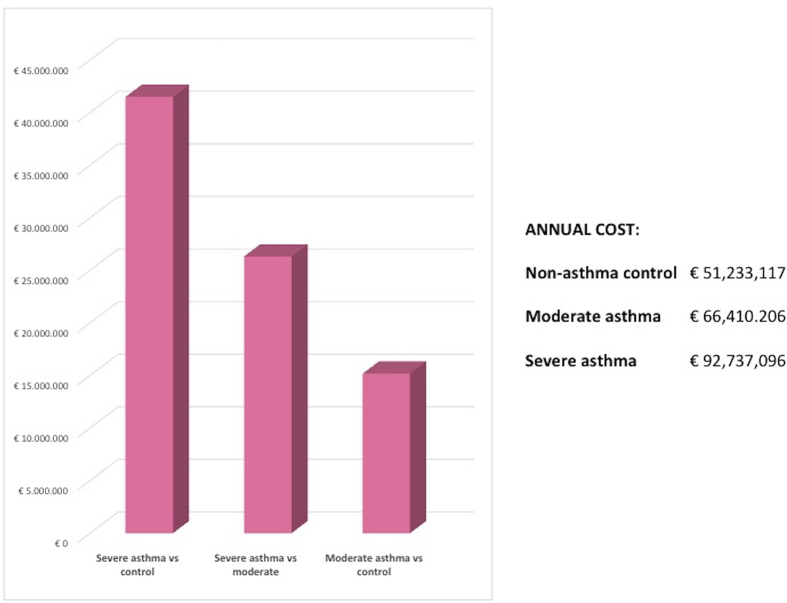
We evaluated the economic impact of the five most common OCS-adverse event, considering cost of illness studies available, in order to adopt an alternative method to DRG tariffs. The results are shown in Fig. 3. Specifically, the subgroup analysis allowed to highlight the economic impact exercised by type 2 diabetes, obesity, fractures, glaucoma and chronic kidney disease according to literature data. These five diseases alone were able to result in a total annual cost equal to €92.7 million in the population with severe asthma, involving an additional expenditure of approximately €41.5 million compared to control and €26.3 million compared to populations with moderate asthma (Fig. 3).
Fig. 3.
Incremental cost scenario: cost related to oral corticosteroids-related adverse events in sub-analysis scenario.
Sensibility analysis
In Table 6 sensibility analysis results are shown.
Table 6.
Sensibility analysis results.
| Main scenario | Annual cost | Incremental cost | |
|---|---|---|---|
| Non-asthma control | € 2131 | Severe asthma vs control | € 1784 |
| Moderate asthma | € 2702 | Severe asthma vs moderate | € 1213 |
| Severe asthma | € 3915 | Moderate asthma vs control | € 571 |
| Lombardia DRG cost |
Annual cost |
Incremental cost |
|
| Non-asthma control | € 2441 | Severe asthma vs control | € 2263 |
| Moderate asthma | € 3099 | Severe asthma vs moderate | € 1605 |
| Severe asthma | € 4704 | Moderate asthma vs control | € 658 |
| DRG National tarriffs -20% |
Annual cost |
Incremental cost |
|
| Non-asthma control | € 1704 | Severe asthma vs control | € 1428 |
| Moderate asthma | € 2162 | Severe asthma vs moderate | € 970 |
| Severe asthma | € 3132 | Moderate asthma vs control | € 457 |
| DRG National tarriffs +20% |
Annual cost |
Incremental cost |
|
| Non-asthma control | € 2557 | Severe asthma vs control | € 2141 |
| Moderate asthma | € 3242 | Severe asthma vs moderate | € 1456 |
| Severe asthma | € 4698 | Moderate asthma vs control | € 686 |
| Prevalence rates -20% |
Annual cost |
Incremental cost |
|
| Non-asthma control | € 1704 | Severe asthma vs control | € 1428 |
| Moderate asthma | € 2162 | Severe asthma vs moderate | € 970 |
| Severe asthma | € 3132 | Moderate asthma vs control | € 457 |
| Prevalence rates +20% |
Annual cost |
Incremental cost |
|
| Non-asthma control | € 2557 | Severe asthma vs control | € 2141 |
| Moderate asthma | € 3242 | Severe asthma vs moderate | € 1456 |
| Severe asthma | € 4698 | Moderate asthma vs control | € 686 |
Using a deterministic sensitivity analysis (DSA), we investigated the sensitivity of the results from the model-based analysis to variations in cost of adverse events and prevalence rates of OCS-related adverse events, which are among the main input parameters used for the cost analysis. Specifically, varying these values by ±20%, no significant changes in the final results were reported, highlighting the robustness of the budget impact analysis result.
Discussion
This budget impact analysis highlighted the significant economic burden of morbidity related to systemic corticosteroid exposure in severe asthma population from the Italian NHS perspective. Our analysis, starting from national demographic data, asthma epidemiological data and adopting first published extractions from the Severe Asthma Network in Italy (SANI) registry, identified the proportion of subjects potentially at risk of developing comorbidities associated with systemic steroid exposure and showed that co-morbid costs were greater in the severe asthma patients, subjected to treatment with high-medium oral corticosteroids, compared to moderate and non-asthmatic cohort.
Total annual cost per patient associated with comorbid conditions due to OCS use was estimated at €1957.50 for the severe asthma cohort, almost twice that non-asthmatic population, and more than 30% compared to the population with moderate asthma, (€1350.96). Annual cost per patient difference between severe and moderate groups was about €606. The cost was determined by the higher prevalence of OCS-related adverse events in severe asthma cohort, considering the correlation between regular intensive use of systemic steroid therapy and disease onset and consequent impact on cost. For the analysis we considered the co-morbidities most frequently associated with the use of corticosteroids taken from the Sweeney's study,19 which provided events prevalence data for the three cohorts in analysis.
Applying the cost per patient to the target population identified for Italy, the budget impact model estimated a total annual cost related to OCS-related adverse events of €242.7 million for severe asthmatics. In respect with non-asthmatic and moderate asthma population, an incremental expenditure of about € 110.6 million and €75.2, respectively, was shown. The micro-costing analysis conducted based on cost of illness studies available for Italy, allowed to evaluate the impact of a subset of diseases, offering an alternative economic evaluation method. Five OCS-related adverse events alone, type 2 diabetes, obesity, fractures, glaucoma and chronic kidney disease, resulted in a total annual cost equal to €92.7 million in the population with severe asthma. The robustness of main analysis was confirmed by sensitivity analysis. Moreover, given regional tariffs variability in Italy for acute care hospital services, in order to provide an example of regional valorisation, we adopted DRG tariffs used in Lombardy region, recognized higher than the national average.
According to international guidelines, oral corticosteroid (OCS) treatment in asthma should be reserved to acute exacerbations (with short course of therapy) or to those severe patients remaining clinically uncontrolled or at high risk for future loss of control, despite high dose of inhaled corticosteroids (ICS) plus another controller, such as long-term beta2-agonists (better if combined, in a single inhaler, with ICS), leukotriene-receptor antagonists (LTRA), tiotropium or theophylline.36 Anyway, even in case of severe asthma, the suggestion is to use low-dose of OCS as a second-choice treatment after having considered the possibility to use biologic agents such as anti-IgE or anti-IL5 monoclonal antibodies.36
However, the first published real-life data raised by the SANI registry29 put in evidence a dramatic situation in which more than 60% of severe asthmatics are chronically taking OCS, being therefore at high risk of OCS-related adverse events. An even more dramatic picture emerged from the updated SANI registry data recently published and showing a prevalence of OCS users higher than 64% of all severe asthmatics.37 A similar picture has been described by Zeiger et al. in a recent retrospective observational cohort study of adults with persistent asthma: they found an overall prevalence of OCS-treated patients of about 8.2% which corresponded to the great majority of those with severe asthma stigmata.18
Putting these real-life data together with the evidence that severe asthmatics are often affected by comorbidities requiring chronic use of topical (i.e.: intranasal) or systemic corticosteroids,19 it is easily predictable that these patients have a high probability to develop corticosteroid adverse events. This risk is further increased considering that also chronic use of high (often higher than suggested) dose of ICS has been associated with significant adverse events.23, 38
The high prevalence of OCS use and the well-known impact of these drugs on the onset of complications, from minor events to potentially life-threating conditions, in any case impacting patient's quality of life, have led to develop studies aimed at considering even their economic impact. Behind the low acquisition cost of these drugs, the cost associated with OCS-induced morbidity hides, representing a shadow cost not to be missed. The significance of this topic, also considering current OCS-sparing effect therapies availability, is demonstrated by various published studies that, in line with ours, paid attention to the economic burden of adverse events associated with drugs exposure.
Some reviews analysed the corticosteroids effect in term of adverse events and cost considering the effects on more pathologies or focusing on the asthma only.25, 39 Findings from these studies showed systemic corticosteroids are a common cause of comorbidities and the costs of managing these events can be substantial. Therefore, clinical and economic burden of systemic related adverse events highlights the need for OCS sparing therapies to be adopted.
Focusing on asthma, some studies have been carried out to evaluate the risk of systemic corticosteroids complications by steroid exposure and quantify the associated health care costs and resource use in patients with severe asthma requiring a regular therapy.
Lefebvre et al. performed a longitudinal, open-cohort, observational study using health insurance claims data; the adjusted risk of systemic corticosteroids related complications for patients with medium and high exposure compared with patients with low exposure and quantify the resulting health care resource use and costs were estimated: patients with medium and high systemic corticosteroids exposure had significantly higher risks of steroids related complications versus those with low exposure.40
Luskin et al with their cross-sectional, matched-cohort, retrospective study, using a commercial claims database, estimated the prevalence of possible oral corticosteroid (OCS)-related side effects and health care resource use and costs in patients with asthma.41 Adults with asthma diagnosis codes and evidence of asthma medication use were studied. Patients with high OCS use (≥30 days of OCS annually) with possible OCS-related adverse events were more likely to have office visits and hospitalizations than those without possible side effects. High OCS users with possible side effects had higher adjusted total annual mean health care costs ($25,168) than those without such side effects ($21,882).41
Our study is consistent with the data reported in literature, highlighting the correlation between the severity of asthmatic disease, and the increase in costs related to systemic corticosteroid-induced morbidities: differences in costs were significant between patients with asthma differentiated by steroid exposure. Moreover, this article is the first data from Italian Registry of Severe Asthma (SANI) that shows economic impact of OCS-overuse. This result is in line with that emerged from Barry's et al study,27 that have prevalence rates of corticosteroid-induced morbidity in common with our, corresponding to the economic evaluation of the results from the Sweeney study.19 According to UK NHS, Barry's study aimed to estimate the additional healthcare costs associated with steroid induced morbidity by comparing three patients' groups: those with severe asthma, moderate asthma and no asthma. Average healthcare costs per person per year range from £2603 - £4533 for the severe asthma cohort, to £978 - £2072 for the mild/moderate asthma cohort, to £560 - £1324 for the non-asthma control cohort, depending on different costing scenario considered.27
The excess risk of complications associated with long-term maintenance OCS use and related cost was also estimated by Tilden et al for the Australian population;26 this study, in addiction, considered quality-adjusted life year (QALY) losses due to corticosteroids exposure, focusing on eight disease outcomes significantly impacting costs QALY burden: type II diabetes, myocardial infarction, glaucoma, cataract, ulcer, osteoporosis, infection, and stroke. Expected annual cost of maintenance OCS-related disease outcomes resulted $598.32 per patient per year. Each patient treated with maintenance OCS also reported a QALY loss of 0.0367 per year of treatment. These effects are considered reversible once patients stop taking maintenance OCS.26
Different strategies have been developed to reduce the use of the corticosteroid load in asthma,42 ranging from allergy immunotherapy (not suitable for uncontrolled asthmatics),43 to biologic agents used in severe patients, as omalizumab,44 mepolizumab,45 benralizumab46 and dupilumab.47 The biologic treatment of severe asthma, into the context of a more personalized approach to the patients,48 seems to be a safe and effective steroid-sparing therapy; biologic agents are generally quite expensive and this, with payer policies, is currently one of the main limitation of their use in clinical practice, even if real-life studies demonstrated that their correct use is globally cost-effectiveness.49, 50, 51 To this purpose, the big data coming from registries like SANI will provide the opportunity to monitor OCS sparing effect of biologic agents in a real-life setting. However, even considering the more long-lived biologic agent for severe asthma (omalizumab), there is the real-life evidence that still a large group of individuals who may benefit from this drug are not receiving it.52 In the very selected population of patients followed by tertiary reference centers for severe asthma in Italy, composed in 95% of cases of patients classified as GINA V step severity, the underuse of biologic agents is confirmed: only about 60% of those eligible to at least one biologic agent are receiving this kind of therapy.29
Further studies are needed to investigate if a wider use biologic agent in severe asthmatics may be cost-effective also in terms of reduction of OCS-related adverse events costs.
Our study provides estimates of additional healthcare costs related to corticosteroid induced adverse events in severe asthma patients from the Italian NHS perspective, not analysed previously. Budget impact model results highlighted the relevant economic impact of OCS-related adverse events in severe asthma patients, subjected to regular exposure to corticosteroids, compared to not exposed. The availability of the first epidemiologic data from SANI registry allowed the definition of target population based on national real word data. The future extrapolation of additional data from SANI registry will support the development of a model populated with national data, providing an important tool for further analyses and greater robustness. Indeed, use of adverse events prevalence rates derived from a UK respiratory database (OPCRD)19 could be seen as a limit of our study. However, it was necessary, because there were no data available for Italy. To overcome the uncertainty of this data, together with that associated with the cost of adverse events, we conducted a sensitivity analysis. Economic evaluation was carried out adopting direct cost associated with corticosteroids related adverse events management, according to Italian NHS. Indirect costs were not considered despite their impact could be relevant due to effects of OCS-related adverse events on productivity and quality of life loss. Overall, the analysis conducted can be considered conservative, indeed the number of evaluable adverse events can be expanded and for economic evaluation we considered unique national tariffs, to achieve greater homogeneity, in a context of national variability.
Declarations
Ethics approval
The study has been approved by the Central Ethics Committee for the SANI Network (Comitato Etico Area Vasta Nord-Ovest Toscana; protocol number: study number 1245/2016, protocol number: 73714).
Consent for publication
Not applicable.
Availability of data
The data that support the findings of this study are available from the Severe Asthma Network in Italy (SANI) but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of SANI.
Competing interests
The authors declare that they have no competing interests.
Authors contribution
GWC, GLC, GMB, SDM, CM, FB, PP and EH: contributed in study conception, study design, literature review, data analysis, data interpretation, writing the manuscript and critical revision of the final draft. GLC,GMB, SDM and CM also created the pharmacoeconomic model here presented.
CB, NC, GPe, GPa and GS contributed in critical revision of the study concept and design, data interpretation, writing the manuscript and critical revion of the final draft.
The SANI Network members contributed in collecting data for the SANI registry and in critical revision of the final draft.
All the Authors read the final version of the manuscript and approved it.
Funding
The SANI Project was supported by GINA Italy/Federasma/SIAAIC/SIP-IRS through Unrestricted support from: AstraZeneca, Italy; Glaxo-Smith & Kline, Italy; Novartis, Italy; Sanofi, Italy; Teva, Italy.
Acknowledgements
We sincerely thank Dr. Concetta Sirena (SANI Project Manager).
Contributor Information
Giorgio Walter Canonica, Email: giorgio_walter.canonica@hunimed.eu.
Giorgio Lorenzo Colombo, Email: giorgio.colombo@savestudi.it.
Giacomo Matteo Bruno, Email: giacomo.bruno@savestudi.it.
Sergio Di Matteo, Email: sergio.dimatteo@savestudi.it.
Chiara Martinotti, Email: chiara.martinotti@savestudi.it.
Francesco Blasi, Email: francesco.blasi@unimi.it.
Caterina Bucca, Email: caterina.bucca@unito.it.
Nunzio Crimi, Email: crimi@unict.it.
Pierluigi Paggiaro, Email: pierluigi.paggiaro@unipi.it.
Girolamo Pelaia, Email: pelaia@unicz.it.
Giovanni Passalaqua, Email: passalacqua@unige.it.
Gianenrico Senna, Email: gianenrico.senna@aovr.veneto.it.
Enrico Heffler, Email: enrico.heffler@hunimed.eu.
SANI Network:
Aliberti Stefano, Bagnasco Diego, Barbuto Sarah, Camiciottoli Gianna, Caminati Marco, Colombo Giselda, Costantino Maria Teresa, Crimi Claudia, Crivellaro Mariangiola, D'Amato Mariella, Favero Elisabetta, Foschino Maria Pia, Guarnieri Gabriella, Latorre Manuela, Lombardi Carlo, Francesco Menzella, Patella Vincenzo, Puggioni Francesca, Ridolo Erminia, Rolla Giovanni, Savi Eleonora, Scichilone Nicola, Solidoro Paolo, Spadaro Giuseppe, and Triggiani Massimo
List of abbreviations
- ATS
American Thoracic Society
- BIM
Budget Impact Model
- BTS
British Thoracic Society
- DRG
Diagnosis-Related Group
- ERS
European Respiratory Society
- ICS
Inhaled Corticosteroids
- INHS
Italian National Healthcare System
- ISTAT
Italian National Statistical Institute
- LTRA
Leukotriene-Receptor Antagonists
- OCS
Oral Corticosteroids
- OCS-AE
Oral Corticosteroid Adverse Events
- OPCRD
Optimum Patient Care Research Database
- QALY
Quality-Adjusted Life Year
- SANI
Severe Asthma Network in Italy
- T2DM
Type 2 Diabetes Mellitus
References
- 1.Institute for Health Metrics and Evaluation (IHME) Global Burden of Diseases (GBD) Studies. http://www.healthdata.org/gbd
- 2.Loftus P.A., Wise S.K. Epidemiology and economic burden of asthma. Int Forum Allergy Rhinol. 2015;5(Suppl 1):S7–S10. doi: 10.1002/alr.21547. [DOI] [PubMed] [Google Scholar]
- 3.de Marco R., Cappa V., Accordini S., GEIRD Study Group Trends in the prevalence of asthma and allergic rhinitis in Italy between 1991 and 2010. Eur Respir J. 2012;39(4):883–892. doi: 10.1183/09031936.00061611. [DOI] [PubMed] [Google Scholar]
- 4.Costa E., Caetano R., Werneck G.L., Bregman M., Araújo D.V., Rufino R. Estimated cost of asthma in outpatient treatment: a real-world study. Rev Saude Publica. 2018 Apr 9;52:27. doi: 10.11606/S1518-8787.2018052000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chapel J.M., Ritchey M.D., Zhang D., Wang G. Prevalence and medical costs of chronic diseases among adult medicaid beneficiaries. Am J Prev Med. 2017 Dec;53(6S2):S143–S154. doi: 10.1016/j.amepre.2017.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dal Negro R.W., Distante C., Bonadiman L., Turco P., Iannazzo S. Cost of persistent asthma in Italy. Multidiscip Respir Med. 2016;11:44. doi: 10.1186/s40248-016-0080-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Accordini S., Corsico A.G., Braggion M. The cost of persistent asthma in Europe: an international population-based study in adults. Int Arch Allergy Immunol. 2013;160(1):93–101. doi: 10.1159/000338998. [DOI] [PubMed] [Google Scholar]
- 8.Nurmagambetov T., Kuwahara R., Garbe P. The economic burden of asthma in the United States, 2008-2013. Ann Am Thorac Soc. 2018;15(3):348–356. doi: 10.1513/AnnalsATS.201703-259OC. [DOI] [PubMed] [Google Scholar]
- 9.Fletcher M., Jha A., Dunlop W. Patient reported burden of asthma on resource use and productivity across 11 countries in Europe. Adv Ther. 2015;32(4):370–380. doi: 10.1007/s12325-015-0204-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zafari Z., Sadatsafavi M., Chen W., FitzGerald J.M. The projected economic and health burden of sub-optimal asthma control in Canada. Respir Med. 2018;138:7–12. doi: 10.1016/j.rmed.2018.03.018. [DOI] [PubMed] [Google Scholar]
- 11.Braido F., Brusselle G., Guastalla D., LIAISON Study Group Determinants and impact of suboptimal asthma control in Europe: the International Cross-Sectional and Longitudinal Assessment on Asthma Control (LIAISON) study. Respir Res. 2016;17(1):51. doi: 10.1186/s12931-016-0374-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lang D.M. Severe asthma: epidemiology, burden of illness, and heterogeneity. Allergy Asthma Proc. 2015;36(6):418–424. doi: 10.2500/aap.2015.36.3908. [DOI] [PubMed] [Google Scholar]
- 13.Hekking P.P., Wener R.R., Amelink M., Zwinderman A.H., Bouvy M.L., Bel E.H. The prevalence of severe refractory asthma. J Allergy Clin Immunol. 2015;135(4):896–902. doi: 10.1016/j.jaci.2014.08.042. [DOI] [PubMed] [Google Scholar]
- 14.Larsson K., Ställberg B., Lisspers K. Prevalence and management of severe asthma in primary care: an observational cohort study in Sweden (PACEHR) Respir Res. 2018;19(1):12. doi: 10.1186/s12931-018-0719-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.von Bülow A., Kriegbaum M., Backer V., Porsbjerg C. The prevalence of severe asthma and low asthma control among Danish adults. Allergy Clin Immunol Pract. 2014;2(6):759–767. doi: 10.1016/j.jaip.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 16.Chung K.F., Wenzel S.E., Brozek J.L. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43(2):343–373. doi: 10.1183/09031936.00202013. [DOI] [PubMed] [Google Scholar]
- 17.Antonicelli L., Bucca C., Neri M. Asthma severity and medical resource utilisation. Eur Respir J. 2004;23(5):723–729. doi: 10.1183/09031936.04.00004904. [DOI] [PubMed] [Google Scholar]
- 18.Zeiger R.S., Schatz M., Li Q., Chen W., Khatry D.B., Tran T.N. Burden of chronic oral corticosteroid use by adults with persistent asthma. J Allergy Clin Immunol Pract. 2017;5(4):1050–1060. doi: 10.1016/j.jaip.2016.12.023. [DOI] [PubMed] [Google Scholar]
- 19.Sweeney J., Patterson C.C., Menzies-Gow A., British Thoracic Society Difficult Asthma Network Comorbidity in severe asthma requiring systemic corticosteroid therapy: cross-sectional data from the Optimum Patient Care Research Database and the British Thoracic Difficult Asthma Registry. Thorax. 2016;71(4):339–346. doi: 10.1136/thoraxjnl-2015-207630. [DOI] [PubMed] [Google Scholar]
- 20.Lefebvre P., Duh M.S., Lafeuille M.H. Burden of systemic glucocorticoid-related complications in severe asthma. Curr Med Res Opin. 2017;33(1):57–65. doi: 10.1080/03007995.2016.1233101. [DOI] [PubMed] [Google Scholar]
- 21.Poetker D.M., Reh D.D. A comprehensive review of the adverse effects of systemic corticosteroids. Otolaryngol Clin. 2010;43(4):753–768. doi: 10.1016/j.otc.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 22.Rice J.B., White A.G., Scarpati L.M., Wan G., Nelson W.W. Long-term systemic corticosteroid exposure: a systematic literature review. Clin Therapeut. 2017;39(11):2216–2229. doi: 10.1016/j.clinthera.2017.09.011. [DOI] [PubMed] [Google Scholar]
- 23.Heffler E., Madeira L.N.G., Ferrando M. Inhaled corticosteroids safety and adverse effects in patients with asthma. J Allergy Clin Immunol Pract. 2018;6(3):776–781. doi: 10.1016/j.jaip.2018.01.025. [DOI] [PubMed] [Google Scholar]
- 24.Pisu M., James N., Sampsel S., Saag K.G. The cost of glucocorticoid-associated adverse events in rheumatoid arthritis. Rheumatology (Oxford) 2005;44(6):781–788. doi: 10.1093/rheumatology/keh594. [DOI] [PubMed] [Google Scholar]
- 25.Manson S.C., Brown R.E., Cerulli A., Vidaurre C.F. The cumulative burden of oral corticosteroid side effects and the economic implications of steroid use. Respir Med. 2009;103(7):975–994. doi: 10.1016/j.rmed.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 26.Tilden D., Makino K., Cottrell S., Needham M. Quantifying the cost and quality of life implications of adverse events associated with long-term oral corticosteroid use. Value Health. 2015;18(7):A688. [Google Scholar]
- 27.Barry L.E., Sweeney J., O'Neill C., Price D., Heaney L.G. The cost of systemic corticosteroid-induced morbidity in severe asthma: a health economic analysis. Respir Res. 2017;18(1):129. doi: 10.1186/s12931-017-0614-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Senna G., Guerriero M., Paggiaro P.L., SANI SANI-Severe Asthma Network in Italy: a way forward to monitor severe asthma. Clin Mol Allergy. 2017;15:9. doi: 10.1186/s12948-017-0065-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferrando M., Heffler E., Sirena C. European Academy of Allergy and Clinical Immunology (EAACI) Congress 2018, Munich. 26–30 May 2018. Severe Asthma Network Italy (SANI): first results from a national severe asthma registry. Abstract n. 376. [Google Scholar]
- 30.Geodemo Istat Tavola Popolazione Totale Italia. [Geodemo Istat Table Total Population Italy] http://demo.istat.it/pop2017/index.html
- 31.Pagano E., De Rosa M., Rossi E. The relative burden of diabetes complications on healthcare costs: the population-based CINECA-SID ARNO Diabetes Observatory. Nutr Metabol Cardiovasc Dis. 2016;26(10):944–950. doi: 10.1016/j.numecd.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 32.Colao A., Lucchese M., D'Adamo M. Healthcare usage and economic impact of non-treated obesity in Italy: findings from a retrospective administrative and clinical database analysis. BMJ Open. 2017;7(2):e013899. doi: 10.1136/bmjopen-2016-013899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Degli Esposti L., Adami S., Iolascon G. Il costo delle fratture da osteoporosi in Italia. Risultati dello studio BLO CK (Bone Loss and Osteoporosis: cost-of-illness Knowledge) Farmecon Percorsi Ter. 2011;12(3) [Google Scholar]
- 34.Koleva D., Motterlini N., Schiavone M., Garattini L., Study Group GLAUCO Ophthalmologica. 2007;221(5):340–347. doi: 10.1159/000104765. [DOI] [PubMed] [Google Scholar]
- 35.Turchetti G., Bellelli S., Amato M., On Behalf of the Tuscany CKD Study Group The social cost of chronic kidney disease in Italy. Eur J Health Econ. 2017;18(7):847–858. doi: 10.1007/s10198-016-0830-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Global Initiative for Asthma, https://ginasthma.org. (Accessed 19 July 2018).
- 37.Heffler E., Blasi F., Latorre M., SANI Network The severe asthma Network in Italy (SANI): findings and perspectives. J Allergy Clin Immunol Pract. 2018 doi: 10.1016/j.jaip.2018.10.016. [DOI] [PubMed] [Google Scholar]
- 38.Ruggeri I., Bragato D., Colombo G.L., Valla E., Di Matteo S. Cost and appropriateness of treating asthma with fixed-combination drugs in local health care units in Italy. Clinicoecon Outcomes Res. 2012;4:375–382. doi: 10.2147/CEOR.S36499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sarnes E., Crofford L., Watson M., Dennis G., Kan H., Bass D. Incidence and US costs of corticosteroid-associated adverse events: a systematic literature review. Clin Therapeut. 2011;33(10):1413–1432. doi: 10.1016/j.clinthera.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 40.Lefebvre P., Duh M.S., Lafeuille M.H. Acute and chronic systemic corticosteroid-related complications in patients with severe asthma. J Allergy Clin Immunol. 2015;136(6):1488–1495. doi: 10.1016/j.jaci.2015.07.046. [DOI] [PubMed] [Google Scholar]
- 41.Luskin A.T., Antonova E.N., Broder M.S., Chang E.Y., Omachi T.A., Ledford D.K. Health care resource use and costs associated with possible side effects of high oral corticosteroid use in asthma: a claims-based analysis. Clinicoecon Outcomes Res. 2016;8:641–648. doi: 10.2147/CEOR.S115025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heffler E., Bagnasco D., Canonica G.W. Strategies to reduce corticosteroid-related adverse events in asthma. Curr Opin Allergy Clin Immunol. 2018 doi: 10.1097/ACI.0000000000000493. [DOI] [PubMed] [Google Scholar]
- 43.Mosbech H., Deckelmann R., de Blay F. Standardized quality (SQ) house dust mite sublingual immunotherapy tablet (ALK) reduces inhaled corticosteroid use while maintaining asthma control: a randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol. 2014;134(3):568–575. doi: 10.1016/j.jaci.2014.03.019. [DOI] [PubMed] [Google Scholar]
- 44.Bhutani M., Yang W.H., Hébert J., de Takacsy F., Stril J.L. The real world effect of omalizumab add on therapy for patients with moderate to severe allergic asthma: the ASTERIX Observational study. PLoS One. 2017;12(8):e0183869. doi: 10.1371/journal.pone.0183869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bel E.H., Wenzel S.E., Thompson P.J., SIRIUS Investigators Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014;371(13):1189–1197. doi: 10.1056/NEJMoa1403291. [DOI] [PubMed] [Google Scholar]
- 46.Nair P., Wenzel S., Rabe K.F., ZONDA Trial Investigators Oral glucocorticoid-sparing effect of benralizumab in severe asthma. N Engl J Med. 2017;376(25):2448–2458. doi: 10.1056/NEJMoa1703501. [DOI] [PubMed] [Google Scholar]
- 47.Rabe K.F., Nair P., Brusselle G. Efficacy and safety of dupilumab in glucocorticoid-dependent severe asthma. N Engl J Med. 2018;378(26):2475–2485. doi: 10.1056/NEJMoa1804093. [DOI] [PubMed] [Google Scholar]
- 48.Canonica G.W., Ferrando M., Baiardini I. Asthma: personalized and precision medicine. Curr Opin Allergy Clin Immunol. 2018;18(1):51–58. doi: 10.1097/ACI.0000000000000416. [DOI] [PubMed] [Google Scholar]
- 49.Dal Negro R.W., Pradelli L., Tognella S., Micheletto C., Iannazzo S. Cost-utility of add-on omalizumab in difficult-to-treat allergic asthma in Italy. Eur Ann Allergy Clin Immunol. 2011;43(2):45–53. [PubMed] [Google Scholar]
- 50.Sullivan S.D., Turk F. An evaluation of the cost-effectiveness of omalizumab for the treatment of severe allergic asthma. Allergy. 2008;63(6):670–684. doi: 10.1111/j.1398-9995.2008.01723.x. [DOI] [PubMed] [Google Scholar]
- 51.McQueen R.B., Sheehan D.N., Whittington M.D., van Boven J.F.M., Campbell J.D. Cost-Effectiveness of biological asthma treatments: a systematic review and recommendations for future economic evaluations. Pharmacoeconomics. 2018 doi: 10.1007/s40273-018-0658-x. [DOI] [PubMed] [Google Scholar]
- 52.Jeffery M.M., Shah N.D., Karaca-Mandic P., Ross J.S., Rank M.A. Trends in omalizumab utilization for asthma: evidence of suboptimal patient selection. J Allergy Clin Immunol Pract. 2017 doi: 10.1016/j.jaip.2017.07.034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the Severe Asthma Network in Italy (SANI) but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of SANI.