Abstract
Background
Systemic autoinflammatory diseases (SAIDs) are rare debilitating disorders of which there is limited awareness and a significant delay in diagnosis. There is no uniform approach in the diagnosis and treatment of these disorders and the real life state of SAID patient care is poorly characterized. The aim of this study was to obtain data on the epidemiology, state of care and the perception of physicians who are involved in the care of SAID patients.
Methods
We performed a questionnaire-based survey and contacted 134 university departments of dermatology, pediatrics, rheumatology and other SAID departments of tertiary care in German-speaking countries.
Results
A total of 37 departments participated in the survey. The majority of departments managed both adult and pediatric patients with a variety of monogenic and polygenic/acquired SAIDs. For monogenic SAIDs such as cryopyrin-associated periodic syndromes (CAPS) and familial Mediterranean fever (FMF), the diagnostic and treatment strategies were similar among the departments. The diagnostic work-up included inflammatory markers and genetic testing, the first line treatment interleukin-1 (IL-1) blockers for CAPS and colchicine for FMF. For polygenic/acquired SAIDs, we observed a significant heterogeneity in diagnostic and therapeutic approaches. As a major unmet need, diagnostic delay was identified with a median time to diagnosis of 2 (range 1–5) years. The overall state of care for SAID patients was rated to be excellent or good by only 12% of departments, and to be poor or non-sufficient by 40% of departments.
Conclusion
This study demonstrates a high need to improve the state of care and to harmonize diagnostic and treatment strategies for SAID patients.
Keywords: Autoinflammatory disease, State of care, Survey
Abbreviations: AOSD, Adult-onset Still's disease; ARC2, Autoinflammation Reference Center Charité; BD, Behçet's disease; CAPS, Cryopyrin-associated periodic syndrome; CBC, Complete blood count; CRMO, Chronic recurrent osteomyelitis; CRP, C-reactive protein; FMF, Familial Mediterranean fever; HIDS, Hyper IgD syndrome; IL-1β, Interleukin-1β; MKD, Mevalonate kinase deficiency; MWS, Muckle-Wells syndrome; NSAIDs, Nonsteroidal anti-inflammatory drugs; PAPA, Pyogenic arthritis pyoderma gangrenosum and acne syndrome; PG, Pyoderma gangrenosum; PRAAS, Proteasome-associated autoinflammatory syndrome; SAA, Serum amyloid A; SAIDs, Systemic autoinflammatory diseases; SchS, Schnitzler's syndrome; SJIA, Systemic juvenile idiopathic arthritis; TNF, Tumor necrosis factor; TRAPS, TNF-receptor-associated periodic syndrome
Background
Systemic autoinflammatory diseases (SAIDs) are a cluster of monogenic and polygenic/acquired inflammatory diseases associated with dysfunctions in the innate immune system.1 Their phenotype covers fever episodes and skin symptoms besides musculoskeletal involvement and variable neurologic, gastrointestinal and hematologic abnormalities.2
Most SAIDs are rare entities. Their prevalence is estimated to range from 1 to 3 per million for Cryopyrin-associated periodic syndrome (CAPS),3 whilst in the case of Schnitzler's syndrome (SchS), there are only around 300 cases reported in the literature.4 Familial Mediterranean fever (FMF) shows a somewhat higher prevalence (1–2 per 1000) in Eastern Mediterranean countries, but presents with low frequencies in other geographic regions (e.g. 48 per 1 million children in Germany).5, 6, 7 The Eurofever registry (printo.it/eurofever), the largest international epidemiologic data collection of SAIDs, recently listed a total number of 1880 confirmed patients not including systemic juvenile idiopathic arthritis (SJIA) and other polygenic/acquired SAIDs.8 AID-Net, a translational research network and registry for SAID patients in Germany, recorded 117 monogenic SAID patients during the first 9 months after initiation of the project.9
SAIDs are debilitating disorders of which there is limited awareness, a significant delay in diagnosis and high rates of misdiagnoses.8 These obstacles result in insufficient treatment, marked quality of life impairment, high morbidity and eventually mortality in a large proportion of patients.10
Despite the recent development of clinical diagnostic criteria for selected SAIDs,3 there are still major challenges including atypical or overlapping phenotypes as well as phenotypically clear cases with mutation-negative status.11 In addition, for many SAIDs approved treatment options are still lacking.12 Overall, the real life state of SAID patient care remains poorly characterized.
To address these gaps of knowledge and to provide information on management and perception (e.g. expenditure of time and costs and patient characteristics that might affect care) of SAID patients by their treating physicians, we initiated a survey-based study on the state of care in German-speaking countries. The main objective of this study was to obtain data on: (i) the epidemiology of SAID patients seen at departments of tertiary care, (ii) the diagnostic and (iii) therapeutic approaches used in clinical practice, and (iv) the problems and unmet needs in the management of SAIDs.
Methods
Study design and participating departments
This cross-sectional study was conducted between April 2014 and March 2016. In the first step, we identified potential German-speaking departments of tertiary care for SAID patient care in Germany, Austria and Switzerland. This included the review of published lists of all university-based departments of dermatology, pediatrics and rheumatology in these countries as well as the identification of further non-university departments of tertiary care from the published list of the German Society of Rheumatology. In total, the respective heads of 134 departments (meaning one physician per department) were asked for their interest in participating in this study and were provided a letter of information and invitation by mail.
Questionnaire development
The questionnaire was developed after a thorough literature review by SAID experts, including rheumatologists, pediatricians and dermatologists. The final questionnaire consisted of 39 questions with Likert scale answer options, quantitative questions, yes/no lists and additional free text [See Additional File 1].
The questionnaire was divided into 6 different sections: (i) general data on the participating clinic and/or department; (ii) general questions on SAID patients and patient characteristics; (iii) questions on the diagnostic work up; (iv) questions on the therapeutic approach; (v) general questions on the current state of SAID patient care; and (vi) questions regarding the need for guidelines in SAID patient care.
The following 11 diseases were assessed: CAPS, FMF, Hyper IgD syndrome (HIDS)/Mevalonate kinase deficiency (MKD), TNF-receptor-associated periodic syndrome (TRAPS), Pyogenic arthritis pyoderma gangrenosum and acne syndrome (PAPA), Chronic recurrent osteomyelitis (CRMO), SchS, Adult-onset Still's disease (AOSD), SJIA, Pyoderma gangrenosum (PG), and Behcet's disease (BD).
Statistical analysis
All data from the completed paper survey questionnaires were transferred to an electronic databank (IBM SPSS Statistics version 23). For statistical analysis, descriptive measures (mean with standard deviation, median with the first and third quartile range, proportions) were applied as appropriate, and graphical representations and a table were employed to summarize the data. There were only few missing data observed in the completed questionnaires. These were not replaced.
Results
Participating departments
Of the 134 invited departments, 37 were interested in participating and returned the completed questionnaire, whereas 18 rejected participation due to lack of interest or lack of experience in managing these patients. Seventy-nine departments did not reply for unknown reasons. In case of non-reply, all departments were contacted at least two times by mail.
Of the received questionnaires, 13 were from dermatology, 13 from pediatric and 11 from rheumatology departments. The majority of departments (n = 31; 83.8%) were located in Germany, 3 departments (8.1%) were in Austria and 3 (8.1%) were in Switzerland. Nearly all participating departments (n = 35; 94.6%) belonged to university hospitals. The mean number of hospital beds in the respective departments was 58.9 ± 44.2. In 9 hospitals, different disciplines (meaning more than one department) participated in the survey.
Dermatologists, pediatricians and rheumatologists manage different SAIDs
The median number of patients seen per department within the last 12 months of the observation period was highest for FMF, followed by SJIA and BD. The lowest number of patients was observed for HIDS. Monogenic SAIDs as well as CRMO and SJIA were primarily seen by pediatricians. Dermatologists usually took care of PG and SchS patients and rheumatologists particularly looked after BD and AOSD patients [Table 1]. Interdisciplinary patient care was frequently implemented for SAIDs such as BD, SchS and PAPA syndrome (86.2%, 78.3% and 75% of departments respectively). The youngest age of onset was reported for HIDS patients and the oldest age for PG patients. Of note, female gender was predominant in all SAIDs [Table 1].
Table 1.
Epidemiology of selected SAIDs as reported by the participating departments.
| SAID | median number of patients seen within the last 12 months |
median age of onset (years) | mean percentages for females | |||
|---|---|---|---|---|---|---|
| by all disciplines | by pediatricians | by dermatologists | by rheumatologists | |||
| CAPS | 2 (1–3.75) | 2 (1–3.5) | 1 (0.5–3) | 3 (1–4) | 20 (10–30) | 64.7 ± 28.8 |
| FMF | 10 (3–20) | 20 (10–52) | 2 (0.5–5) | 6 (3–13) | 14.5 (10–29.75) | 56.3 ± 28.9 |
| HIDS/MKD | 0 (0–1) | 1 (0–2) | 0 (0–0.75) | 0 (0–1) | 8 (2–24) | 61.1 ± 48.6 |
| TRAPS | 1 (1–2) | 1.5 (0.75–2.25) | 1 (0–1.5) | 1 (1–1.75) | 18 (6.5–32.5) | 64.4 ± 44.6 |
| PAPA | 1 (0–2) | 2 (0–6) | 1.5 (0–2.25) | 0 (0–1) | 25 (7–36.25) | 56.5 ± 36.9 |
| CRMO | 4.5 (1.75–15) | 15 (4.5–20) | 0 (0–4.5) | 3 (2–4.75) | 12 (10–26) | 72.8 ± 20.2 |
| SchS | 1 (0–2) | 0 (0–0) | 2 (1–3) | 1 (0–1) | 50 (40–50) | 52.4 ± 40.0 |
| AOSD | 4 (0.75–10) | 0 (0–2.5) | 1 (1–4) | 10 (6–15) | 31.5 (24.75–40) | 73.3 ± 19.1 |
| SJIA | 5 (2–12) | 12 (2–20) | 5 (0.5–9) | 5 (2–7) | 9 (6.25–12) | 62.3 ± 25.7 |
| PG | 3 (0–10.5) | 0 (0–0.25) | 12 (4–20) | 2 (0–4) | 55 (40–60) | 60.7 ± 25.4 |
| BD | 5 (1–10) | 1 (0.5–2) | 5 (2–10) | 10 (4–15) | 30 (15–35) | 53.6 ± 34.2 |
SAIDs are associated with delay in diagnosis
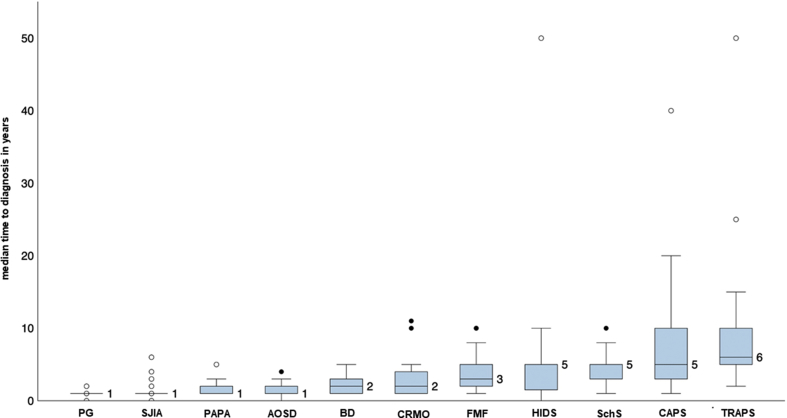
The median time to diagnosis showed great intra-individual variability and differed between the diseases as well. The median time to diagnosis for all SAIDs was 2 (interquartile range 1–5) years and 5 (interquartile range 1.9–6.3) years vs. 1 (interquartile range 0.5–3) year for monogenic SAIDs and polygenic/acquired SAIDs, respectively. The highest diagnostic delay was observed in TRAPS, CAPS, HIDS and SchS with a median of 5–6 years [Fig. 1].
Fig. 1.
Time to diagnosis. The participating departments were asked to rate the time from the start of first symptoms to the final diagnosis for the different diseases. The bars represent the median time to diagnosis in years with interquartile ranges.
Inflammation markers and genetic testing are common diagnostic tools in the work-up of SAID patients
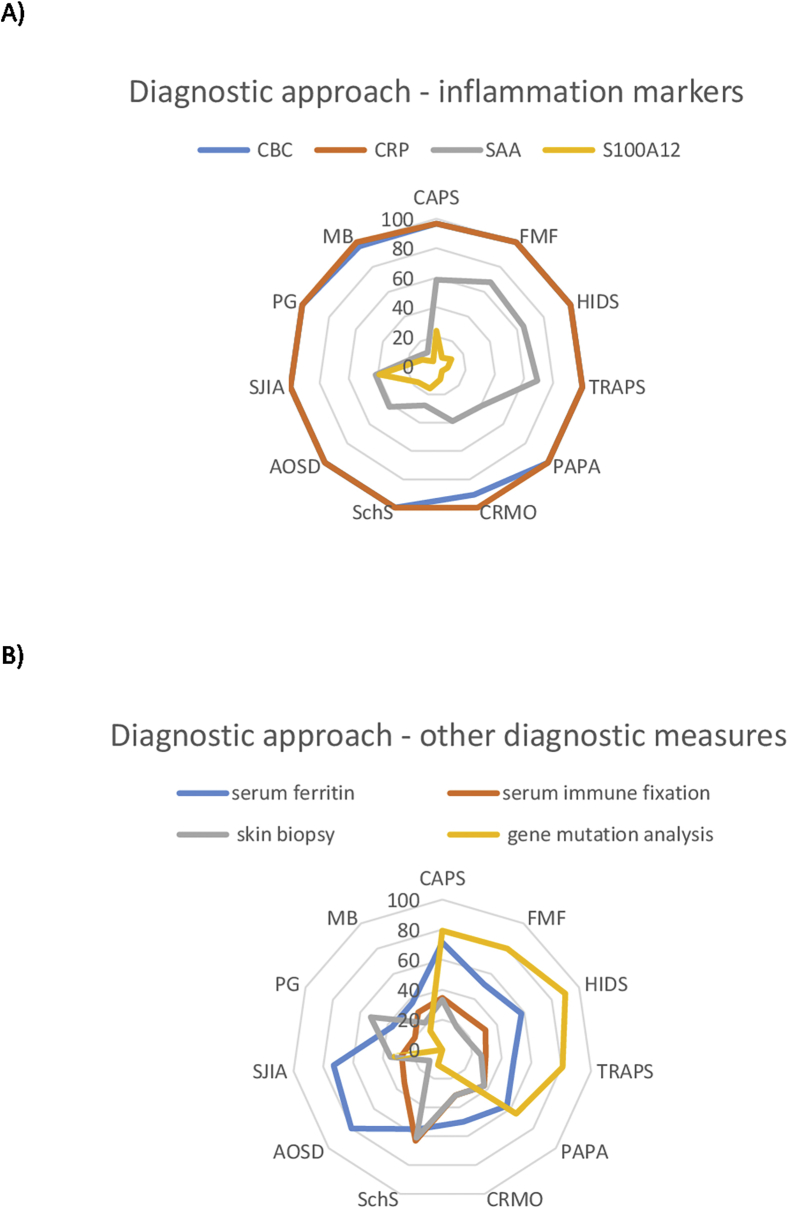
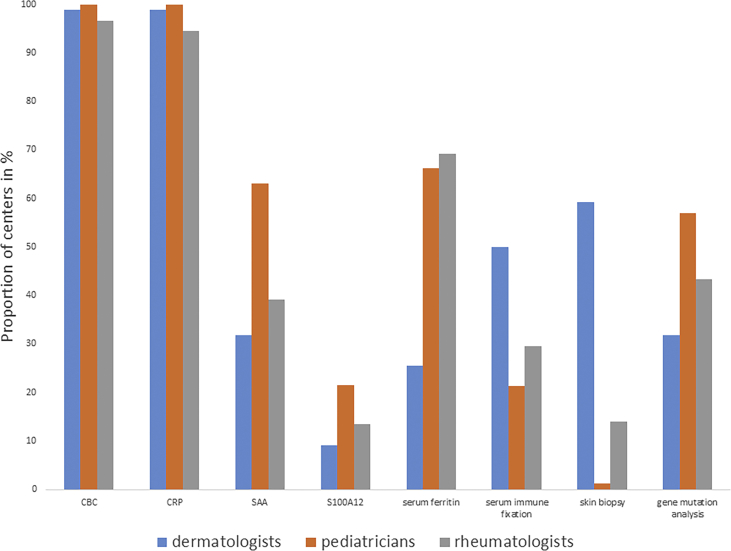
Complete blood count (CBC) and C-reactive protein (CRP) were performed in almost all patients with suspected or known SAIDs. Serum amyloid A (SAA) was determined more frequently in monogenic than polygenic/acquired SAIDs (60.1% vs. 29.4%). SAA and S100A12 or S100 A8/9 levels were studied particularly in SJIA (39.1% and 41.7%) and performed most frequently by pediatricians [Fig. 2, Fig. 3]. Thirty-five of 37 participating departments (94.3%) reported to regularly use genetic testing in the diagnostic work-up of patients. Genetic testing was done in suspected monogenic SAIDs namely, HIDS, TRAPS, FMF, CAPS and PAPA [Fig. 2B]. Pediatricians (63.1%) most frequently requested genetic analyses followed by rheumatologists (43.3%) and dermatologists (31.8%) [Fig. 3]. Thirteen (39.4%) participating departments experienced obstacles in performing genetic analyses, with high costs being the most common reported issue. In addition, a lack in specificity or sensitivity of the methods (e.g. detection of genetic polymorphisms or non-detection of genetic mosaicisms) leading to over- or underdiagnosis was mentioned. Serum ferritin, a biomarker for disease activity in SJIA and AOSD, and serum immune fixation, indicated in SchS to detect paraproteins that confirm the diagnosis, were also included in the diagnostic part of the questionnaire.13, 14 In our study, ferritin levels were primarily analyzed by rheumatologists and pediatricians (69.2% and 66.3%) in cases of suspected or confirmed AOSD, SJIA and CAPS. Immune fixation was particularly evaluated in suspected cases of SchS (63.2%) and by dermatologists (50%) in this survey [Fig. 2, Fig. 3].
Fig. 2.
Diagnostic approach. The participating departments were asked for their diagnostic approach in patients with suspected SAIDs. Participating departments had to pick all applicable diagnostic measures for the different SAIDs. The results are expressed as the proportion of departments that chose the different diagnostic parameters. CBC: Complete blood count; CRP: C-reactive protein; SAA: Serum amyloid A; S100A12: S100 calcium-binding protein A12.
Fig. 3.
Diagnostic measures distributed by each discipline. The participating departments were asked for their diagnostic approach in patients with suspected SAIDs. Participating departments had to pick diagnostic measures for the different SAIDs. The results are expressed as the proportion of departments distributed by each discipline that chose the different diagnostic parameters.
Skin biopsies are commonly performed by dermatologists
Patients with SAIDs presenting at the skin (e.g. urticarial, pustular or ulcerative lesions) often show neutrophil-rich dermal infiltrates upon skin biopsy. Although these findings are non-specific, they may guide the differential diagnosis and exclude other diseases with similar cutaneous phenotype.15, 16 Dermatologists performed this procedure most frequently (59.3%), followed by rheumatologists (13.9%) and pediatricians (1.2%). Skin biopsies were commonly performed, if SchS or PG were suspected [Fig. 2, Fig. 3].
A variety of tools are used to assess SAID disease activity
All three disciplines emphasized the importance of history taking, physical examination data, laboratory parameters (CRP, ferritin, SAA etc.), imaging and/or photo records to assess disease activity in SAID patients. However, there was great heterogeneity in the use of clinical scores. Dermatologists reported monitoring of disease activity primarily by general quality measures such as the Physician Global Assessment (PGA) and Visual Analog Scale (VAS). Pediatricians referred to the Auto-Inflammatory Diseases Activity Index (AIDAI), Childhood Health Assessment Questionnaire (CHAQ), American College of Rheumatology (ACR) Pediatric score, Disease Activity Score 28 (DAS28), Juvenile Arthritis Disease Activity Score (JADAS) and also PGA and VAS. Rheumatologists reported the use of AIDAI, DAS28 and Birmingham vasculitis activity score (BVAS).
Non-specific clinical symptoms and lack of diagnostic criteria are challenges in diagnosing SAIDs
The top three reported difficulties and challenges in the diagnostic approach of SAIDs were: (1) atypical or no obvious clinical symptoms at presentation; (2) difficulties in differentiating from other entities because of overlapping symptoms; and (3) lack of practical diagnostic criteria in selected diseases. Further problems were the unavailability of full panel laboratory services as well as financial restrictions reported by some departments, which hampered the performance of expensive diagnostic tests such as genetic analyses.
Glucocorticoids are commonly used in selected SAIDs
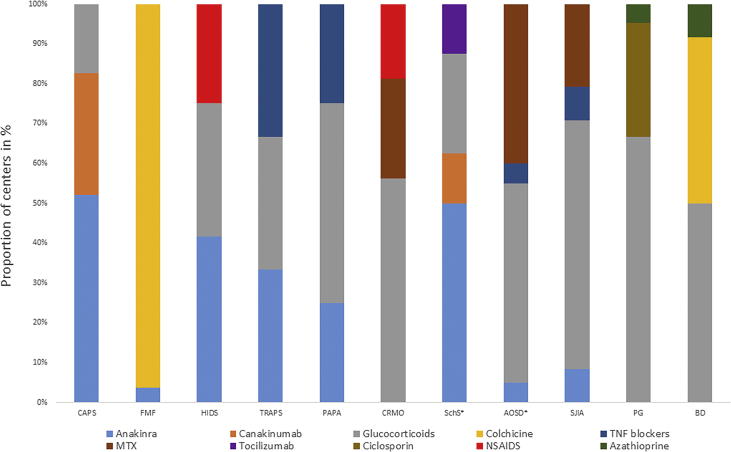
At the time of the survey study, approved treatment options, namely cytokine blockade, were available for CAPS (anti-IL-1) and SJIA (anti-IL-1/anti-IL-6) patients. In agreement with the existing guidelines, glucocorticoids were perceived as the first line treatment in several SAIDs by the majority of departments. The most frequently reported diseases, which were initially treated with glucocorticoids, according to the participants' responses, included PG (66.7%), SJIA (62.5%) and CRMO (52.9% of departments) [Fig. 4].
Fig. 4.
First line treatment choices. The participating departments were asked for their treatment of first choice for patients with the different SAIDs. The results show the top 3 first line treatment options in different SAIDs reported by the departments. * Four treatment options are shown because of equal frequency.
IL-1 blockers are perceived as first line treatment for CAPS by most departments
The IL-1 receptor antagonist anakinra was reportedly used as first-line treatment in monogenic SAIDs, especially in CAPS followed by HIDS and TRAPS (52%, 35.7% and 33.3% of departments). Conversely, anakinra was rarely used as first-line therapy in polygenic/acquired diseases except in SchS (50% of departments), whereas canakinumab, a fully human monoclonal antibody to IL-1β, was prescribed as first-line medication only in CAPS and SchS (29.2% and 11.8% of departments). Tumor necrosis factor (TNF) blockers were considered as first choice in TRAPS, PAPA, SJIA and AOSD (33.3%, 17.6%, 8.3% and 5% of departments). Lastly, tocilizumab was regarded as first-line therapy only in SchS and by very few physicians (11.8% of departments).
Other reported first-line treatments included colchicine, which was the drug of choice for FMF and BD (96.4% and 38.5% of departments). Also, non-steroidal anti-inflammatory drugs (NSAIDs) in HIDS and CRMO, methotrexate in AOSD, CRMO and SJIA as well as ciclosporin A in PG and azathioprine in BD and PG were named [Fig. 4].
SAID patient care is often evaluated as poor and insufficient
The general state of care for SAID patients was rated to be excellent or good by only 11.5% of departments, satisfying or sufficient by 48.5% and to be poor or non-sufficient by 40% of departments.
The participating departments were asked to compare the general conditions in SAID patient care with the situation of their other patients. Notably, the expenditure of time was reported to be above average by 80.6%. The drug and laboratory costs in managing SAIDs were noted to be more expensive compared with other diseases by 91.7% and 75.0% of departments, respectively. In contrast, the frequency of follow-up visits was in an average range by the majority of departments (70.3%). In a further set of questions, the participating departments were asked for characteristics of SAID patients that might affect care. Interestingly, 36% of departments regarded patient guidance to be difficult. However, none reported a poor patient compliance and only a single department stated that the patients seemed unsatisfied with the current treatment (2.9%). Half of the departments perceived problems with organizing interdisciplinary patient care and communication between the disciplines. In addition, 60% of departments indicated problems in the treatment of SAID patients. Here, the reimbursement for biologics, particularly in cases with off-label use, was the main issue of concern.
Guidelines for SAID patient care are essential
There were only few existing guidelines regarding the management of selected SAIDs, namely FMF, SJIA, PG and BD when this survey was conducted.17, 18, 19, 20, 21, 22 The majority of departments (83.5%) saw a need for guideline development in all SAIDs. Among them, AOSD was the most frequently stated (95%), followed by CRMO (91.7%) and PAPA syndrome (87.5%). A need for optimization of existing guidelines was stated by more than half (59.3%) of the participating departments. The diseases named most often were CRMO (71.4%), followed by HIDS (66.7%) and CAPS (64.3%).
Discussion
Systematic research in SAIDs represents a major challenge due to the rarity of the diseases. Thanks to the implementation of two European registries, EUROFEVER and AID-Net, essential data on the epidemiology, clinical presentation and treatment responses in SAIDs were obtained.8, 9 With this study, we provide further data on the real-life state of care and unmet needs in managing SAID patients.
Our findings emphasize the rarity of SAIDs even in departments of tertiary care and showed that most participating departments managed a variety of different autoinflammatory diseases and patients. Among hereditary monogenic SAIDs, FMF was the most prevalent disease, whereas the least one was HIDS. This was concordant to the data of previously published studies derived from the EUROFEVER registry.8, 12 Interestingly, the female predominance in this study was higher than previously reported in the EUROFEVER registry. The reason for this discrepancy remains unclear. It may be speculated that the higher number of females in our study is caused by a greater willingness of women to seek medical care as compared to men. Also, geographic differences in gender predominance may play a role as the majority of patients included in the EUROFEVER registry were derived from Italy. As expected, of all disciplines, pediatricians were the ones primarily involved in managing monogenic SAIDs, while all three disciplines, i.e., dermatologists, pediatricians and rheumatologists, contributed to the management of certain polygenic/acquired SAIDs.
The diagnostic delay caused by limited disease awareness is known to be a major issue in SAID management. This was confirmed by our survey. Consistent with earlier reports, the diagnostic delay was usually longer in monogenic than polygenic/acquired diseases,8 and the median time to diagnosis was slightly reduced (e.g. 5 years in this study vs. 7.3 years for monogenic SAIDs in previous reports).8 Diagnostic delay is known to result in quality of life impairment and long-term sequelae such as secondary amyloidosis.10 Thus, our study underlines the high need to increase awareness for these rare disorders. Fortunately, a reduction in time to diagnosis of hereditary monogenic SAIDs was demonstrated over the last decade; e.g. patients who were born after the year 2000 had less diagnostic delay compared to those who were born before the 1970s,8 which may be explained by improved availability and better access to genetic analyses together with a wider recognition of these conditions. Provisional classification criteria were recently proposed to pre-select potential patients eligible for genetic analyses and/or to classify patients with suspected autoinflammatory periodic fever diseases.11 These will help to reduce diagnostic delay and enable proper treatment for future SAID patients.
There was overall agreement between departments to use laboratory inflammation markers such as CBC and CRP on a routine basis. Notably, the use of genetic analyses to confirm a diagnosis of monogenic SAID was reported by nearly all departments (94.3%), although the availability, high costs and interpretation of genetic tests were acknowledged as critical drawbacks by many of them. We did not ask for genetic test results as this would require individual patient data and we were particularly interested in learning which diagnostic and management tools were used. During recent years, reports on atypical clinical phenotypes and the increased use of genetic tests resulted in reports of genetic variants or polymorphisms (e.g. the R92Q variant in TRAPS or V198 M in CAPS), for which their clinical relevance often remained unclear. Current efforts to analyze genotype-phenotype associations from large databases such as the EUROFEVER registry may contribute to better guide diagnosis and treatment decisions in hereditary SAIDs.23, 24, 25, 26
Disease activity in SAID patients varies considerably. Besides inter-individual differences, there are also intra-individual changes, e.g. higher disease activity in winter as compared to summer time. As variations in disease activity may require treatment changes, the assessment of disease activity should be embedded in the clinical routine. The participating departments reported the use of various clinical assessment tools. This largely reflects the current situation that there is no consensus on how to evaluate and monitor disease activity in SAID patients. So far, few disease-specific activity scores were developed, namely the auto-inflammatory diseases activity index (AIDAI) in hereditary recurrent fever syndromes,27 Schnitzler Activity Score (SchAS) in SchS28 and Juvenile Arthritis Disease Activity Score (JADAS) in SJIA29 and Muckle-Wells-syndrome disease activity score (MWS-DAS) in MWS.30 Due to the limited patient numbers, validation of these tools remains a major challenge. Another issue in SAIDs is organ damage such as hearing loss or kidney failure because of secondary amyloidosis. Organ damage is not captured by assessing disease activity, and it often develops as a consequence of diagnostic delay. To address this, the autoinflammatory disease damage index (ADDI)31 was recently established. Also, attempts to create specific health-related quality of life instruments in SAIDs such as the juvenile auto-inflammatory disease multidimensional assessment report (JAIMAR)32 have to be acknowledged. Further development and harmonization of SAID-specific clinical assessment tools will be important to improve patient care and future research in this field.
Licensed treatment options for SAIDs were limited at the time of this study and still are, although recently the use of canakinumab has been approved for FMF, TRAPS and HIDS.33 Hence, the therapeutic approaches used by participating departments showed substantial heterogeneity. Interestingly, glucocorticoids remained within the top three first line therapies in all SAIDs, except FMF. The abundant use of glucocorticoids may be explained by 1) the existing guideline recommendations; 2) the overall availability and great experience with glucocorticoids comparing to other treatment options and 3) the limited access to drugs such as cytokine blockers in off-label indications as well as iv) cases in which treatment was started before the diagnosis was confirmed. The use of IL-1 blockers, in particular anakinra, as first choice treatment reported by the majority of departments in CAPS, HIDS, TRAPS and SchS is mirrored by the efficacy of these drugs in clinical trials34, 35 and available data from the EUROFEVER registry.12 In FMF, although not approved, there was wide consensus among the participating physicians to use colchicine as first line treatment. This was affirmed by multiple lines of evidence showing a benefit of colchicine in FMF treatment.12, 36, 37, 38
In general, the data from our study on treatment decisions demonstrate the common use of off-label therapies in SAIDs and emphasize the need for management guidelines. Lately, several initiatives started to implement practical management guidelines for specific SAIDs such as CAPS, FMF, HIDS, TRAPS and SJIA/AOSD,34, 39, 40, 41, 42, 43, 44, 45 and further efforts to optimize existing guidelines are currently ongoing. Nonetheless, the rarity of the diseases and limited data from large scale, randomized controlled trials remain major challenges within this task.34, 46
There were some limitations of our study. First, there was a selection bias of participants, who were recruited from departments of Dermatology, Pediatrics and Rheumatology, whereas other disciplines were not involved. Although the included disciplines focused on different diseases, it cannot be excluded that single patient numbers were reported by more than one department. Furthermore, only German-speaking departments were involved, so the data may not be representative of other countries. Novel emerging SAIDs such as NOD2-associated autoinflammatory disease (Yao syndrome)47, 48 or less common disorders including NLRP12-associated autoinflammatory disease49 and proteasome-associated autoinflammatory syndrome (PRAAS)50 have not been included in this study. Also, the retrospective study design and data obtained relied on the estimations by the participating physicians, which may have produced inaccuracies. Finally, the results should be interpreted with care as only 28% of the departments invited participated actively in this survey, suggesting that non-participating institutions may be less experienced in treating SAID patients. However, comparing this response rate to other questionnaire-based surveys in much more common diseases, such as urticaria in which responses lay between 9%51 and 40%,52 the return rate in our study involving rare diseases was considered acceptable.
Conclusion
We observed great heterogeneity in the diagnostic work-up and treatment of SAIDs, especially in polygenic/acquired SAIDs. Our project demonstrates a high need to improve the real-life state of care by harmonizing diagnostic and treatment strategies for SAID patients. Specialized departments with interdisciplinary care and the close collaboration with international SAID registries may contribute to optimize the future state of care for these patients.
Declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Availability of data and material
The datasets generated during the current study are available from the corresponding author on reasonable request
Funding
This study was supported in part by grants from Novartis Pharma GmbH. The sponsor did not participate in the design, analysis or interpretation of the study. Also, the sponsor was not involved in writing of the study report and in the decision to submit this article for publication.
Competing interests
MC has no conflict of interest.
KW received honoraria and/or consultancy fees from Novartis outside of those of this publication.
EF received institutional research funding and/or honoraria for lectures/consulting from Novartis, Pfizer, MSD, Abbvie, Sobi and Roche outside of those of this publication.
TK received institutional research funding and/or honoraria for lectures/consulting from Novartis, Sobi and Roche outside of those of this publication.
MM received institutional research funding and/or honoraria for lectures/consulting from Novartis, Regeneron and Roche outside of those of this publication.
JKD received grant support and consultants/speakers fees from Novartis and Sobi outside of those of this publication.
KK received honoraria and/or consultancy fees from Novartis, Roche and SOBI on topics outside of those of this publication.
Authors' contributions
KK, KW and MM designed the study. MC and KK acquired and analyzed the data. All authors substantially contributed to the interpretation of data. MC and KK drafted the first version of the article. All authors critically revised the manuscript and gave final approval of the version to be published.
Acknowledgements
We would like to thank the following departments for participating in this study: Dermatologische Poliklinik, Universitätsspital Basel, Basel; Rheumatologie, Klinik für Kinder-und Jugendmedizin, Autoinflammation Reference Center Tübingen, Universitätsklinikum Tübingen, Tübingen; Universitätsklinik für Dermatologie, Venerologie und Allergologie, Medizinische Universität Innsbruck, Innsbruck; Sektion Pädiatrische Infektiologie und Rheumatologie, Zentrum für Kinder-und Jugendmedizin, Universitätsklinikum Freiburg, Freiburg; Ambulanz für Pädiatrische Hämato-Onkologie, Universitätsklinik für Kinder-und Jugendheilkunde, Landeskrankenhaus-Universitätsklinikum Graz, Graz; Klinik für Kinder-und Jugendmedizin, Universitätsklinikum Schleswig-Holstein, Campus Lübeck, Lübeck; Kinderklinik, Pädiatrische Rheumatologie und Immunologie, Universitätsklinikum Carl Gustav Carus Dresden, Dresden; Poliklinik für Kinder-und Jugendmedizin, Universitätsklinikum Hamburg-Eppendorf, Hamburg; Poliklinik für Rheumatologie, Heinrich-Heine-Universität Düsseldorf, Düsseldorf; Dermatologie, Universitätsklinikum Regensburg, Regensburg; Hautklinik, Universitätsklinikum Gieβen und Marburg, Marburg; Klinik für Dermatologie, Allergologie und Venerologie, Universitätsklinikum Schleswig-Holstein, Campus Lübeck, Lübeck; Universitäts-Hautklinik Kiel, Kiel; Arbeitsbereich Rheumatologie, Universitätsklinikum Halle, Halle; Pädiatrische Immunologie und Rheumatologie, Zentrum für Kinder-und Jugendmedizin, Universitätsmedizin Mainz, Mainz; Klinik und Poliklinik für Dermatologie, Universitätsklinikum Carl Gustav Carus Dresden, Dresden; Neuropädiatrie, Entwicklungsneurologie und Sozialpädiatrie, Universitätsklinikum Essen, Essen; Clinical Research Center, Haut-und Poliklinik, Universitätsmedizin der Johannes Gutenberg-Universität Mainz, Mainz; Abteilung für Rheumatologie und Immunologie, Universitätsklinik für Innere Medizin, Landeskrankenhaus-Universitätsklinikum Graz, Graz; Universitätsspital Basel, Basel; Dermatologische Klinik, Universitätsspital Zürich und Kinderspital Zürich, Zürich; Ambulanz für Rheumatologie, Sektion Pädiatrische Infektiologie und Rheumatologie, Zentrum für Kinder-und Jugendmedizin, Universitätsklinikum Freiburg, Freiburg; Dermatologie, Universitätsklinikum Regensburg, Regensburg; I. Medizinische Klinik, Abteilung Rheumatologie, Universitätsmedizin der Johannes Gutenberg-Universität Mainz, Mainz; Universitäts-Hautklinik, Universitätsklinikum Tübingen, Tübingen; Klinik für Gastroenterologie und Rheumatologie, Universitätsklinikum Leipzig, Leipzig; Kinder und Poliklinik für Allgemeine Pädiatrie, Universitätsklinikum Bonn, Bonn; Klinik und Poliklinik für Dermatologie und Allergologie am Biederstein, Technische Universität München, München; Osteologie/Pädiatrische Rheumatologie, Universitätsklinik Köln, Köln; 4. Medizin (Rheumatologie/Immunologie/Nephrologie), Asklepios Klinik Altona, Hamburg; Klinik für Dermatologie, Allergologie und Venerologie, Charité-Universitätsmedizin Berlin, Berlin; Rheumatologie, Charité-Universitätsmedizin Berlin, Berlin; Medizinische Klinik II, Rheumatologie, Universitätsklinikum Frankfurt, Frankfurt; Medizin VI, Abteilung für Rheumatologie und Klinische Immunologie, Klinik für Rheumatologie und Klinische Immunologie, Universitätsklinikum Freiburg, Freiburg; Kinderrheumatologie, Kinderklinik, Charité-Universitätsmedizin Berlin, Berlin; Klinik für Rheumatologie, Universitätsklinikum Schleswig-Holstein, Campus Lübeck, Lübeck.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.waojou.2019.100019.
Contributor Information
Mati Chuamanochan, Email: Mati.c@cmu.ac.th.
Karsten Weller, Email: karsten.weller@charite.de.
Eugen Feist, Email: eugen.feist@charite.de.
Tilmann Kallinich, Email: tilmann.kallinich@charite.de.
Marcus Maurer, Email: marcus.maurer@charite.de.
Jasmin Kümmerle-Deschner, Email: jasmin.kuemmerle-deschner@med.uni-tuebingen.de.
Karoline Krause, Email: karoline.krause@charite.de.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Grateau G., Hentgen V., Stojanovic K.S., Jeru I., Amselem S., Steichen O. How should we approach classification of autoinflammatory diseases? Nat Rev Rheumatol. 2013;9(10):624–629. doi: 10.1038/nrrheum.2013.101. [DOI] [PubMed] [Google Scholar]
- 2.Cush J.J. Autoinflammatory syndromes. Dermatol Clin. 2013;31(3):471–480. doi: 10.1016/j.det.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuemmerle-Deschner J.B., Ozen S., Tyrrell P.N. Diagnostic criteria for cryopyrin-associated periodic syndrome (CAPS) Ann Rheum Dis. 2017;76(6):942–947. doi: 10.1136/annrheumdis-2016-209686. [DOI] [PubMed] [Google Scholar]
- 4.Gusdorf L., Asli B., Barbarot S. Schnitzler syndrome: validation and applicability of diagnostic criteria in real-life patients. Allergy. 2017;72(2):177–182. doi: 10.1111/all.13035. [DOI] [PubMed] [Google Scholar]
- 5.Alghamdi M. Familial Mediterranean fever, review of the literature. Clin Rheumatol. 2017;36(8):1707–1713. doi: 10.1007/s10067-017-3715-5. [DOI] [PubMed] [Google Scholar]
- 6.Ben-Chetrit E., Touitou I. Familial mediterranean Fever in the world. Arthritis Rheum. 2009;61(10):1447–1453. doi: 10.1002/art.24458. [DOI] [PubMed] [Google Scholar]
- 7.Lainka E., Bielak M., Lohse P. Familial Mediterranean fever in Germany: epidemiological, clinical, and genetic characteristics of a pediatric population. Eur J Pediatr. 2012;171(12):1775–1785. doi: 10.1007/s00431-012-1803-8. [DOI] [PubMed] [Google Scholar]
- 8.Toplak N., Frenkel J., Ozen S. An international registry on autoinflammatory diseases: the Eurofever experience. Ann Rheum Dis. 2012;71(7):1177–1182. doi: 10.1136/annrheumdis-2011-200549. [DOI] [PubMed] [Google Scholar]
- 9.Lainka E., Bielak M., Hilger V. Translational research network and patient registry for auto-inflammatory diseases. Rheumatology (Oxford) 2011;50(1):237–242. doi: 10.1093/rheumatology/keq270. [DOI] [PubMed] [Google Scholar]
- 10.Lachmann H.J., Goodman H.J., Gilbertson J.A. Natural history and outcome in systemic AA amyloidosis. N Engl J Med. 2007;356(23):2361–2371. doi: 10.1056/NEJMoa070265. [DOI] [PubMed] [Google Scholar]
- 11.Federici S., Sormani M.P., Ozen S. Evidence-based provisional clinical classification criteria for autoinflammatory periodic fevers. Ann Rheum Dis. 2015;74(5):799–805. doi: 10.1136/annrheumdis-2014-206580. [DOI] [PubMed] [Google Scholar]
- 12.Ter Haar N., Lachmann H., Ozen S. Treatment of autoinflammatory diseases: results from the Eurofever Registry and a literature review. Ann Rheum Dis. 2013;72(5):678–685. doi: 10.1136/annrheumdis-2011-201268. [DOI] [PubMed] [Google Scholar]
- 13.Gerfaud-Valentin M., Jamilloux Y., Iwaz J., Seve P. Adult-onset Still's disease. Autoimmun Rev. 2014;13(7):708–722. doi: 10.1016/j.autrev.2014.01.058. [DOI] [PubMed] [Google Scholar]
- 14.Simon A., Asli B., Braun-Falco M. Schnitzler's syndrome: diagnosis, treatment, and follow-up. Allergy. 2013;68(5):562–568. doi: 10.1111/all.12129. [DOI] [PubMed] [Google Scholar]
- 15.Bolukbasi B., Krause K. Cutaneous manifestations of systemic autoinflammatory disorders. Clin Dermatol. 2015;33(5):520–526. doi: 10.1016/j.clindermatol.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 16.Ruocco E., Sangiuliano S., Gravina A.G., Miranda A., Nicoletti G. Pyoderma gangrenosum: an updated review. J Eur Acad Dermatol Venereol. 2009;23(9):1008–1017. doi: 10.1111/j.1468-3083.2009.03199.x. [DOI] [PubMed] [Google Scholar]
- 17.Hentgen V., Grateau G., Kone-Paut I. Evidence-based recommendations for the practical management of familial mediterranean fever. Semin Arthritis Rheum. 2013;43(3):387–391. doi: 10.1016/j.semarthrit.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 18.Beukelman T., Patkar N.M., Saag K.G. 2011 American College of Rheumatology recommendations for the treatment of juvenile idiopathic arthritis: initiation and safety monitoring of therapeutic agents for the treatment of arthritis and systemic features. Arthritis Care Res (Hoboken). 2011;63(4):465–482. doi: 10.1002/acr.20460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ringold S., Weiss P.F., Beukelman T. 2013 update of the 2011 American College of Rheumatology recommendations for the treatment of juvenile idiopathic arthritis: recommendations for the medical therapy of children with systemic juvenile idiopathic arthritis and tuberculosis screening among children receiving biologic medications. Arthritis Rheum. 2013;65(10):2499–2512. doi: 10.1002/art.38092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reichrath J., Bens G., Bonowitz A., Tilgen W. Treatment recommendations for pyoderma gangrenosum: an evidence-based review of the literature based on more than 350 patients. J Am Acad Dermatol. 2005;53(2):273–283. doi: 10.1016/j.jaad.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 21.Hatemi G., Silman A., Bang D. EULAR recommendations for the management of Behcet disease. Ann Rheum Dis. 2008;67(12):1656–1662. doi: 10.1136/ard.2007.080432. [DOI] [PubMed] [Google Scholar]
- 22.Hatemi G., Silman A., Bang D. Management of Behcet disease: a systematic literature review for the European League against Rheumatism evidence-based recommendations for the management of Behcet disease. Ann Rheum Dis. 2009;68(10):1528–1534. doi: 10.1136/ard.2008.087957. [DOI] [PubMed] [Google Scholar]
- 23.Papa R., Doglio M., Lachmann H.J. A web-based collection of genotype-phenotype associations in hereditary recurrent fevers from the Eurofever registry. Orphanet J Rare Dis. 2017;12(1):167. doi: 10.1186/s13023-017-0720-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levy R., Gerard L., Kuemmerle-Deschner J. Phenotypic and genotypic characteristics of cryopyrin-associated periodic syndrome: a series of 136 patients from the Eurofever Registry. Ann Rheum Dis. 2015;74(11):2043–2049. doi: 10.1136/annrheumdis-2013-204991. [DOI] [PubMed] [Google Scholar]
- 25.Ter Haar N.M., Jeyaratnam J., Lachmann H.J. The phenotype and genotype of Mevalonate kinase deficiency: a series of 114 cases from the eurofever registry. Arthritis Rheum. 2016;68(11):2795–2805. doi: 10.1002/art.39763. [DOI] [PubMed] [Google Scholar]
- 26.Jeske M., Lohse P., Kallinich T. Genotype-phenotype and genotype-origin correlations in children with mediterranean fever in Germany - an AID-net study. Klin Pädiatr. 2013;225(6):325–330. doi: 10.1055/s-0033-1355372. [DOI] [PubMed] [Google Scholar]
- 27.Piram M., Kone-Paut I., Lachmann H.J. Validation of the auto-inflammatory diseases activity index (AIDAI) for hereditary recurrent fever syndromes. Ann Rheum Dis. 2014;73(12):2168–2173. doi: 10.1136/annrheumdis-2013-203666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krause K., Tsianakas A., Wagner N. Efficacy and safety of canakinumab in Schnitzler syndrome: a multicenter randomized placebo-controlled study. J Allergy Clin Immunol. 2017;139(4):1311–1320. doi: 10.1016/j.jaci.2016.07.041. [DOI] [PubMed] [Google Scholar]
- 29.Consolaro A., Ruperto N., Bazso A. Development and validation of a composite disease activity score for juvenile idiopathic arthritis. Arthritis Rheum. 2009;61(5):658–666. doi: 10.1002/art.24516. [DOI] [PubMed] [Google Scholar]
- 30.Kuemmerle-Deschner J.B., Wittkowski H., Tyrrell P.N. Treatment of Muckle-Wells syndrome: analysis of two IL-1-blocking regimens. Arthritis Res Ther. 2013;15(3):R64. doi: 10.1186/ar4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ter Haar N.M., Annink K.V., Al-Mayouf S.M. Development of the autoinflammatory disease damage index (ADDI) Ann Rheum Dis. 2017;76(5):821–830. doi: 10.1136/annrheumdis-2016-210092. [DOI] [PubMed] [Google Scholar]
- 32.Konukbay D., Gattorno M., Yildiz D. A novel assessment tool for clinical care of patients with autoinflammatory disease: juvenile autoinflammatory disease multidimensional assessment report. Clin Exp Rheumatol. 2016;34(6 Suppl 102):129–135. [PubMed] [Google Scholar]
- 33.De Benedetti F., Gattorno M., Anton J. Canakinumab for the treatment of autoinflammatory recurrent fever syndromes. N Engl J Med. 2018;378(20):1908–1919. doi: 10.1056/NEJMoa1706314. [DOI] [PubMed] [Google Scholar]
- 34.ter Haar N.M., Oswald M., Jeyaratnam J. Recommendations for the management of autoinflammatory diseases. Ann Rheum Dis. 2015;74(9):1636–1644. doi: 10.1136/annrheumdis-2015-207546. [DOI] [PubMed] [Google Scholar]
- 35.Cavalli G., Dinarello C.A. Treating rheumatological diseases and co-morbidities with interleukin-1 blocking therapies. Rheumatology (Oxford) 2015;54(12):2134–2144. doi: 10.1093/rheumatology/kev269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Slobodnick A., Shah B., Krasnokutsky S., Pillinger M.H. Update on colchicine, 2017. Rheumatology (Oxford) 2018;57(suppl_1):i4–i11. doi: 10.1093/rheumatology/kex453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goldstein R.C., Schwabe A.D. Prophylactic colchicine therapy in familial Mediterranean fever. A controlled, double-blind study. Ann Intern Med. 1974;81(6):792–794. doi: 10.7326/0003-4819-81-6-792. [DOI] [PubMed] [Google Scholar]
- 38.Dinarello C.A., Wolff S.M., Goldfinger S.E., Dale D.C., Alling D.W. Colchicine therapy for familial mediterranean fever. A double-blind trial. N Engl J Med. 1974;291(18):934–937. doi: 10.1056/NEJM197410312911804. [DOI] [PubMed] [Google Scholar]
- 39.Terreri M.T., Bernardo W.M., Len C.A. Guidelines for the management and treatment of periodic fever syndromes: cryopyrin-associated periodic syndromes (cryopyrinopathies - CAPS) Rev Bras Reumatol Engl Ed. 2016;56(1):44–51. doi: 10.1016/j.rbre.2015.08.020. [DOI] [PubMed] [Google Scholar]
- 40.Giancane G., Ter Haar N.M., Wulffraat N. Evidence-based recommendations for genetic diagnosis of familial Mediterranean fever. Ann Rheum Dis. 2015;74(4):635–641. doi: 10.1136/annrheumdis-2014-206844. [DOI] [PubMed] [Google Scholar]
- 41.Ozen S., Demirkaya E., Erer B. EULAR recommendations for the management of familial Mediterranean fever. Ann Rheum Dis. 2016;75(4):644–651. doi: 10.1136/annrheumdis-2015-208690. [DOI] [PubMed] [Google Scholar]
- 42.Terreri M.T., Bernardo W.M., Len C.A. Guidelines for the management and treatment of periodic fever syndromes familial Mediterranean fever. Rev Bras Reumatol Engl Ed. 2016;56(1):37–43. doi: 10.1016/j.rbre.2015.08.019. [DOI] [PubMed] [Google Scholar]
- 43.Mimura T., Kondo Y., Ohta A. Evidence-based clinical practice guideline for adult Still's disease. Mod Rheumatol. 2018:1–22. doi: 10.1080/14397595.2018.1465633. [DOI] [PubMed] [Google Scholar]
- 44.Hinze C.H., Holzinger D., Lainka E. Practice and consensus-based strategies in diagnosing and managing systemic juvenile idiopathic arthritis in Germany. Pediatr Rheumatol Online J. 2018;16(1):7. doi: 10.1186/s12969-018-0224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maverakis E., Ma C., Shinkai K. Diagnostic criteria of ulcerative pyoderma gangrenosum: a Delphi consensus of international experts. JAMA Dermatol. 2018;154(4):461–466. doi: 10.1001/jamadermatol.2017.5980. [DOI] [PubMed] [Google Scholar]
- 46.Barry R.J., Markandey B., Malhotra R. Evidence-based practice in Behcet's disease: identifying areas of unmet need for 2014. Orphanet J Rare Dis. 2014;9:16. doi: 10.1186/1750-1172-9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McDonald C., Shen M., Johnson E.E., Kabi A., Yao Q. Alterations in nucleotide-binding oligomerization domain-2 expression, pathway activation, and cytokine production in Yao syndrome. Autoimmunity. 2018;51(2):53–61. doi: 10.1080/08916934.2018.1442442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yao Q., Shen B. A systematic analysis of treatment and outcomes of NOD2-associated autoinflammatory disease. Am J Med. 2017;130(3):365 e13–365 e18. doi: 10.1016/j.amjmed.2016.09.028. [DOI] [PubMed] [Google Scholar]
- 49.Shen M., Tang L., Shi X., Zeng X., Yao Q. NLRP12 autoinflammatory disease: a Chinese case series and literature review. Clin Rheumatol. 2017;36(7):1661–1667. doi: 10.1007/s10067-016-3410-y. [DOI] [PubMed] [Google Scholar]
- 50.Feist E., Brehm A., Kallinich T., Kruger E. [Clinical aspects and genetics of proteasome-associated autoinflammatory syndromes (PRAAS)] Z Rheumatol. 2017;76(4):328–334. doi: 10.1007/s00393-017-0264-x. [DOI] [PubMed] [Google Scholar]
- 51.Weller K., Viehmann K., Brautigam M. Management of chronic spontaneous urticaria in real life--in accordance with the guidelines? A cross-sectional physician-based survey study. J Eur Acad Dermatol Venereol. 2013;27(1):43–50. doi: 10.1111/j.1468-3083.2011.04370.x. [DOI] [PubMed] [Google Scholar]
- 52.Weller K., Schoepke N., Krause K., Ardelean E., Brautigam M., Maurer M. Selected urticaria patients benefit from a referral to tertiary care centres--results of an expert survey. J Eur Acad Dermatol Venereol. 2013;27(1):e8–16. doi: 10.1111/j.1468-3083.2011.04387.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during the current study are available from the corresponding author on reasonable request