Abstract
Background
Spontaneous coronary artery dissection (SCAD) is an infrequent and often misdiagnosis of a non-atherosclerotic cause of acute coronary syndrome (ACS). It is an important cause of ACS in young women, responsible for up to 25% of all cases in women <50 years of age without cardiovascular risk factors. Clinical presentation ranges from ST-segment-elevation myocardial infarction (MI) to ventricular fibrillation and sudden death. The treatment of patients with SCAD is a challenge and the ideal management strategy has yet to be determined.
Case summary
A 42-year-old woman without family history of cardiac disease and neither traditional atherosclerotic risk factors presented to our centre with an anterior acute ST-segment-elevation MI secondary to multiple spontaneous dissections of the left main, anterior descending, and ramus intermedius coronary arteries. Stenting was performed in the left anterior descending coronary artery and left main coronary artery to resolve its occlusion. Fibromuscular dysplasia was confirmed via computed tomography angiography.
Discussion
More cases are now being identified of SCAD due to increased clinical index of suspicion, earlier use of invasive angiography, and intracoronary imaging in patients presenting with acute chest pain. Despite this, the absence of previous cardiovascular risk factors and the ignorance of this pathology delay the start of an adequate medical treatment and the performance of a cardiac catheterization. Prognostic data are limited, partly because of its underdiagnosis and lack of prospective studies, so its knowledge is necessary to improve the prognosis of these patients.
Keywords: Spontaneous coronary artery dissection, Acute coronary syndrome, Fibromuscular dysplasia, Case report
Learning points
Spontaneous coronary artery dissection (SCAD) is an often misdiagnosed non-atherosclerotic cause of acute coronary syndrome in women presenting with myocardial infarction without traditional cardiovascular risk factors.
Predisposing factors such connective disorders, autoimmune and inflammatory disorders, and notably fibromuscular dysplasia have all been reported SCAD.
Intravascular ultrasound or optical coherence tomography can play an important role in the diagnosis of SCAD.
Treatment remains a challenge and the ideal management strategy has yet to be determined (conservative vs. invasive management).
Introduction
Spontaneous coronary artery dissection (SCAD) is an uncommon and often misdiagnosed non-atherosclerotic cause of acute coronary syndrome (ACS). Clinical presentation ranges from ST-segment-elevation myocardial infarction (MI) to ventricular fibrillation and sudden death. Predisposing factors such connective disorders, autoimmune and inflammatory disorders, and notably fibromuscular dysplasia (FMD) have all been reported1,2; however, the treatment of patients with SCAD remains a challenge and the ideal management strategy has yet to be determined.
Timeline
| Day | Events |
|---|---|
| 1 | Sudden, acute, and oppressive thoracic pain and subsequent assistance by the emergency medical services. |
| An electrocardiogram showed 3- to 4-mm ST-segment elevations in leads V2 through V6. Diagnosis of anterior ST-elevation myocardial infarction. | |
| Emergent coronary angiography revealed a possible intimal dissection of the left anterior descending coronary artery involving the first diagonal branch artery, the ramus intermedius coronary artery with an extension to the left main coronary artery. | |
| Stenting was performed in the left anterior descending coronary artery and left main coronary artery. | |
| Transferred to the coronary care unit in stable condition for observation. | |
| 2 | Transthoracic echocardiogram revealed an apical thrombus and severe left ventricular dysfunction without pericardial effusion. Anticoagulation was initiated. |
| 5 | Transferred from coronary care unit to the cardiology ward. |
| 8 | Screened for cerebrovascular, renal, and iliac fibromuscular dysplasia via computed tomography angiography, which showed renal involvement. |
| 12 | Patient was discharged under medical treatment and close medical follow-up by the heart failure team. |
Case presentation
A woman, 42-year-old former smoker without atherosclerotic risk factors, family history of cardiac disease, sudden cardiac death, autoimmune disease, or regular medication, required the emergency medical services due to acute and pressure-like chest pain of high intensity associated with diaphoresis and mild shortness of breath with a duration of more than 20 min unrelated to emotional stress or intense exercise at that time.
On physical examination, the patient was slightly anxious with stable vital signs (heart rate 90 b.p.m., blood pressure 122/67 mmHg, and respiratory rate 19/min with an oxygen saturation of 98% on ambient air). Normal S1 and S2 heart sounds were present, no murmurs, or gallops. There were no signs of heart failure present.
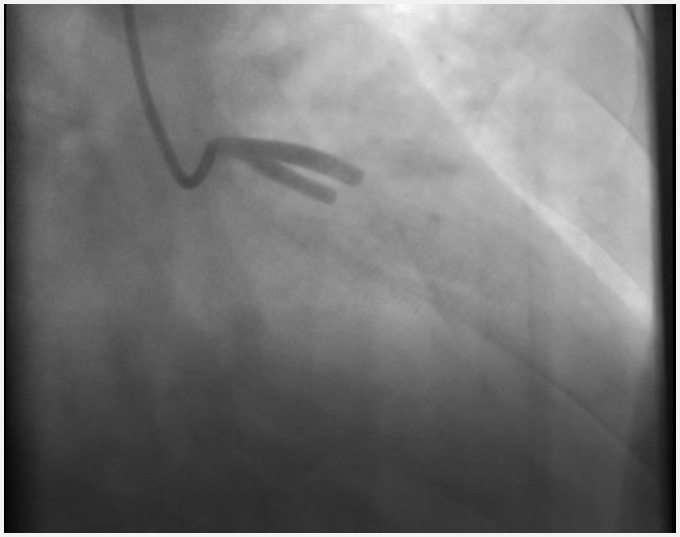
Figure 1.
Coronary angiogram in the right anterior oblique 25° and caudal 25° position. No coronary flow in left anterior descending coronary artery and ramus intermedius coronary artery.
An electrocardiogram (ECG) revealed sinus rhythm and 3- to 4-mm ST-segment elevations in leads V2 through V6. General treatment measures—300 mg of aspirin, 600 mg of clopidogrel, one dose of 0.4 mg of sublingual nitroglycerine, and an unfractionated heparin drip—were initiated. The patient was transferred to our cardiology unit by the emergency services for an emergent coronary angiography, which showed a dual lumen defect (TIMI 0), suggestive of a possible intimal dissection of the left anterior descending coronary artery involving the first diagonal branch artery, the ramus intermedius coronary artery with an extension to the left main coronary artery (Type 1 SCAD). Given the presence of involvement of proximal segments, a guide was passed to the anterior descending artery, which allowed the recovery of coronary flow and two stents were placed in the proximal and mid left anterior descending coronary artery after balloon dilation in each one. An intravascular ultrasound (IVUS) demonstrated a near-circumferential haematoma extending deeply into the media of the left main coronary artery. Based on this finding, drug-eluting stent implantation over the dissection was performed with the support of a guidewire located in the anterior descending artery and another in the circumflex artery to obtain more stabilization for its placement. Normal blood flow was restored with no residual stenosis (TIMI 3). During the procedure, she received electrical defibrillation due to two episodes of ventricular fibrillation.
After catheterization, the patient was without chest pain and a normalization of the ST-segment with anterior Q-waves (V1–4) with mild T-wave inversion was observed in the ECG. She was sent to the coronary care unit in stable condition for observation. Haematological examination indicated a marked peak in cardiac troponin T (cTnT) to 117 ng/mL (reference range 0–0.05). Full blood count, kidney function, and electrolytes were normal. A transthoracic echocardiogram showed an apical thrombus, severe left ventricular dysfunction (left ventricular ejection fraction 30%) with anterior wall akinesia. Anticoagulation therapy was initiated. The patient remained asymptomatic and was discharged under medical treatment—antiaggregant treatment (aspirin plus clopidogrel), vitamin K antagonist, beta-blockade, ACE inhibitor, spironolactone, and a high-dose statin—and close medical follow-up by the heart failure team. Due to the strong association between SCAD and FMD, a complete screening for FMD took place computed tomography angiography with its presence in renal (typical ‘string-of-beads’ aspect of the right renal artery), lower limb (‘string-of-beads’ appearance on right femoral artery), and brain arteries were confirmed. At 3-month follow-up after discharge, a repeat transthoracic echocardiogram revealed persistent severe left ventricular dysfunction (left ventricular ejection fraction 30%) without apical thrombus. Subsequently, the patient has not had additional medical events, remaining without symptoms (New York Heart Association I) after 1 year of follow-up.
Figure 2.
Coronary angiogram in the right anterior oblique 25° and caudal 25° position. After guide wire passes through, it showed multiple radiolucent lumen suggestive of possible type I intimal dissection of the left anterior descending coronary artery involving the first diagonal branch artery, the ramus intermedius coronary artery with extension to the left main coronary artery.
Figure 3.
Intravascular ultrasound showing dissection and intramural haematoma starting from the distal left main coronary artery and progressing into the ostium of the left anterior descending. Intramural haematoma (arrow) in the media of the arterial wall resulted in compression of the real lumen.
Figure 4.
Right anterior oblique 25° caudal 25° view. A drug-eluting stent was successfully implanted over the dissection involving the left main artery and the proximal portion of the left anterior descending coronary artery. Normal blood flow was restored with no residual stenosis.
Discussion
Spontaneous coronary artery dissection is an infrequent cause of ACS that is defined as a non-traumatic and non-iatrogenic tearing of the coronary wall. In the past, the prevalence of SCAD ranged from 0.1% to 1%, but nowadays, contemporary case series estimate that it is the underlying cause of 1.7% to 4% of all cases of ACS, and accounts for 0.5% of sudden cardiac deaths. This increase in prevalence is due to a more extensive use of intracoronary imaging, such as IVUS or optical coherence tomography (OCT).3
The pathophysiology of SCAD involves the sudden disruption of the coronary artery wall, resulting in a separation of the inner intimal lining from the outer vessel wall. The trigger is thought to be either an intimal tear or bleeding from the vasa vasorum, resulting in intramural haematoma. Pressure-driven expansion of the haematoma forms a false lumen leading to external pressure on the true coronary lumen.
Spontaneous coronary artery dissection is commonly associated with a predisposing arteriopathy and precipitating stress factors. The most common identified arteriopathies are connective disorders, autoimmune and inflammatory disorders, and notably FMD.4 Recently, Saw et al.5 identified lesions of FMD in at least one extra coronary vascular bed in 31.3% of cases in its large multicentre prospective study and an incomplete screening or no-screening for FMD took place including involvement of the renal, iliac, and cervicocephalic arteries took place in 45.2% of its population.
Multivessel SCAD is unusual and have only been described in approximately 10% of patients. In all, three different angiographic patterns of SCAD have been described6:
Type 1 (evident arterial wall stain): the most common pattern. Contrast dye staining of the arterial wall with multiple radiolucent lumens.
Type 2 (diffuse stenosis of varying severity): Often missed or misdiagnosed. There is an appreciable abrupt change in arterial calibre and usually involves the mid to distal segments.
Type 3 (mimic atherosclerosis): this appearance is similar to atherosclerosis with focal or tubular stenosis.
Intravascular imaging modalities, notably IVUS or OCT, aid diagnosis, and are essential for the diagnosis of Type 2 and Type 3 SCAD.6
The use of medical therapy for the treatment of SCAD is largely empirical, with no randomized clinical trials having been carried out. Initial treatment is that standard to all ACS patients including the use of dual antiplatelet agents (aspirin and clopidogrel), heparin, and beta-blockers to preserve the patency of the true lumen and prevent thrombotic occlusion.7
The choice to revascularize depends on the patient’s clinical status and affected coronary anatomy. Distal dissections, for example, can be left untreated if coronary flow is good, but indications for revascularization include complete vessel occlusion, left main stem involvement, ongoing ischaemia, haemodynamic instability, and malignant ventricular arrhythmias. A conservative approach to stent implantation is mainly preferred, with stenting of the proximal segment to close the dissection entrance and to limit the risk of dissection propagation.4,7,8 In these procedures, it is a challenge to advance the coronary guidewire into the true lumen, but both IVUS and OCT can help with this, and ensure optimal sizing, positioning, and placing of coronary stents. Coronary bypass grafting is reserved for patients with left main stem dissections or when percutaneous coronary intervention has been unsuccessful (TIMI 1 or 2) or is not technically possible in high-volume centres.7
The short- and long-term prognosis, and risks of SCAD recurrence are highly relevant. In one of the largest reported cohorts of prospectively followed patients from SCAD registries, long-term adverse events were common, with a recurrent MI event rate of 16.8%, of which 10.4% were due to recurrent SCAD. The presence of hypertension and beta-blocker use were associated with an increased and decreased risk of recurrent SCAD, respectively.5 In another smaller cohort, severe coronary tortuosity (defined as >2 consecutive curvatures >180°) was also borderline associated with a higher risk of reoccurrence.8
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The author/s confirm that written consent for submission and publication of this case report including image(s) and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: none declared.
Supplementary Material
References
- 1. Saw J, Aymong E, Mancini GJ, Sedlak T, Starovoytov A, Ricci D.. Nonatherosclerotic coronary artery disease in young women. Can J Cardiol 2014;30:814–819. [DOI] [PubMed] [Google Scholar]
- 2. Henkin S, Negrotto SM, Tweet MS, Kirmani S, Deyle DR, Gulati R, Olson TM, Hayes SN.. Spontaneous coronary artery dissection and its association with heritable connective tissue disorders. Heart 2016;102:876–881. [DOI] [PubMed] [Google Scholar]
- 3. Vanzetto G, Berger-Coz E, Barone-Rochette G, Chavanon O, Bouvaist H, Hacini R, Blin D, Machecourt J.. Prevalence, therapeutic management and medium-term prognosis of spontaneous coronary artery dissection: results from a database of 11,605 patients. Eur J Cardiothorac Surg 2009;35:250–254. [DOI] [PubMed] [Google Scholar]
- 4. Saw J, Aymong E, Sedlak T, Buller CE, Starovoytov A, Ricci D, Robinson S, Vuurmans T, Gao M, Humphries K, Mancini GBJ.. Spontaneous coronary artery dissection: association with predisposing arteriopathies and precipitating stressors. Circ Cardiovasc Interv 2014;7:645–655. [DOI] [PubMed] [Google Scholar]
- 5. Saw J, Starovoytov A, Humphries K, Sedlak T, Prakash R, Starovoytov A, et al. Canadian Spontaneous Coronary Artery Dissection Cohort Study Oral Session. ESC Congress Munich; 2018, 26 August 2018. [Google Scholar]
- 6. Saw J. Coronary angiogram classification of spontaneous coronary artery dissection. Catheter Cardiovasc Interv 2014;84:1115–1122. [DOI] [PubMed] [Google Scholar]
- 7. Tweet M, Eleid M, Best P, Lennon R, Lerman A, Rihal C, Holmes D, Hayes S, Gulati R. Spontaneous coronary artery dissection: revascularization versus conservative therapy. Circ Cardiovas Interv 2014;7:777–786. [DOI] [PubMed] [Google Scholar]
- 8. Eleid MF, Guddeti RR, Tweet MS, Lerman A, Singh M, Best PJ, Vrtiska T, Prasad M, Rihal C, Hayes S, Gulati R. Coronary artery tortuosity in spontaneous coronary artery dissection: angiographic characteristics and clinical implications. Circ Cardiovasc Interv 2014;7:656–662. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.