Abstract
Aim:
To explore inflammatory biomarkers secreted by adipose stem cells (ASCs) in omental, retroperitoneal and subcutaneous adipose tissues of women with endometrial cancer.
Patients & Methods:
ASCs were collected from 22 women, aged 35-83 years, undergoing hysterectomy for endometrial cancer. Angiopoietin-2, EGF, IL-8, leptin, VEGFA, VEGFC and VEFGD levels in the ASC-conditioned media were analyzed by Luminex.
Results:
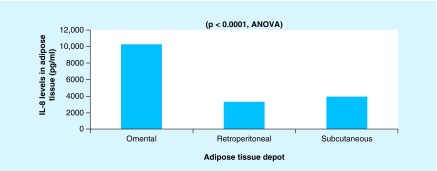
We found a significant difference between the three depots for IL-8 (p < 0.0001), with the highest levels of IL-8 in the omental depot. VEGFA levels were highest in the retroperitoneal depot.
Conclusion:
This is one of the first studies to explore biomarker expression in ASC-conditioned media in adipose tissue. ASC characteristics may be important to evaluate in relation to cancer risk.
Keywords: : adipogenenesis, adipose derived stem cells, adipose tissue, biomarkers, endometrial cancer, IL-8, inflammation, metabolic disease, obesity, prevention
Endometrial cancer (EC) is the most common gynecologic cancer in the developed world, with incidence and mortality increasing over the past decade and expected to continue increasing in the decades to come [1–4]. Approximately 61,380 new EC cases and 10,920 deaths are expected in USA in 2017 [5]. Increasing rate of obesity is thought to be the primary driver of increasing EC incidence in US population [6]. Prospective studies indicate that EC risk increases 1.6-fold for each additional 5 kg/m2 in BMI, reaching 9.1-fold higher risk at a BMI of 42 kg/m2 [7,8]. It has been recently suggested that adipose tissue dysfunction as a unifying causal factor linking obesity and cancer [9]; with adipose stromal cells shown to promote vascularization and proliferation of endometrial tumors in mice [10]. However, the exact cellular mechanisms linking adipose tissue to EC have yet to be explored. Our recent research suggests that adipose derived adipose stem cells (ASCs) might play an important role in EC carcinogenenesis [11,12], and characteristics of these cells may be different in cancer patients in comparison with cancer-free controls [12]. Adipose tissue is an abundant source of multipotent cells, similar to bone marrow mesenchymal stem cells, which are present within the stromal vascular fraction of adipose tissue. A primary mechanism through which ASCs may contribute to EC carcinogenesis is through the secretion of growth factors [11,12]. ASCs are extremely proliferative, multipotent cells and are known to secrete multiple growth factors including IGF, vascular endothelial growth factors VEGF and HGF [13]. Specifically, HGF is known to downregulate expression of transforming growth factor TGF-β, which has been verified as a signaling pathway that is also downregulated in endometrial tissue of EC patients [14]. Moreover, previous research proposed that omental ASCs recruited to tumors express specific factors that enhance tumor vascularization, promoting survival and proliferation of tumor cells [10]; however, none of the existing studies explored biomarker levels in all the three depots of adipose tissue in the same patient.
Adipose tissue is divided into two major depots: visceral fat (omental, mesenteric, and retroperitoneal) and subcutaneous (sc.) fat. Not much is known about the role of each depot in the development of EC; however, research suggests that adipose tissue is associated with EC through chronic low-grade inflammation resulting from increased secretion of proinflammatory biomarkers, such as cytokines and chemokines [15,16]. Gene set enrichment analysis comparisons of both omental and sc. adipose tissue from matched EC and non-EC subjects has shown that significant metabolic changes occur within omental, but not sc. adipose tissue in patients with EC [13,14]. Further, gene set enrichment analysis has also showed a strong upregulation of the peroxisome proliferator-activated pathway in omental and endometrial tissues in patients with EC, an indication of cells in the early commitment stages of adipogenesis, including preadipocytes and adipose stem cells [14]. Thus, it is likely that adipose stem cells (or preadipocytes) have an important role in the microenvironment of omental adipose tissue, and communicate bidirectionally with cancer cells during EC development [10,13].
Adipose tissue-based biomarkers have been investigated in relation to breast cancer, guided by the hypothesis that the proximity of breast adipose tissue to the tumor of interest makes it a valuable tissue depot to investigate for malignancy development [17]. To our knowledge, adipose tissue biomarkers, especially ASC-derived, have rarely been explored in the context of EC development, despite the fact that emerging literature suggests that they may play an important role [11,12,16]. Thus, studying functional characteristics of adipose tissues can potentially provide more information about local inflammatory, metabolic, and hormonal characteristics of the tumor milieu. For example, IL-8 is a proinflammatory cytokine with well-defined functions in tumor-associated inflammation. IL-8 is expressed by many tumor cells types, including endometrial cancer [18], with a statistically significant correlation reported between EC myometrial invasion and IL-8 levels [19]. Furthermore, an improved understanding of the underlying biologic mechanisms associated with the adipose tissue and EC risk may hold a key to better preventative strategies and improved screening programs for EC in obese women.
The goal of this pilot investigation was to conduct one of the first comparisons of the inflammatory biomarkers expression in ASC-conditioned media from three depots of adipose tissue (from sc., omental, and retroperitoneal sites) in women with EC.
Materials & methods
Patient population
A convenience sample of 22 women, aged 35–83, undergoing hysterectomy was recruited based on the prior EC diagnosis. Inclusion criteria for this study were: female, age 18 years or older, BMI ≥18, and had previously signed informed consent for the tissue banking protocol. Women who were suspected of having metastatic disease were excluded from this study as the goal of this pilot study was to explore adipose tissue characteristics that are specific to early disease before major complications such as metastatic disease occurs. Each eligible participant was asked to sign an informed consent for adipose tissue collection after a full explanation of the purpose of the study and the procedures used was provided. Samples from the three different adipose tissue depots were collected: retroperitoneal tissues were extracted from the waste materials generally discarded after surgery, whereas omental and sc. tissues were specifically obtained for the purpose of this study. This study was approved by the University of Pittsburgh Human Research Protection Office (PRO13080544), which allowed for the collection of nondiscarded adipose tissue, including tissues from the retroperitoneal and omental depots. Participants of this study also signed the Prognostic Marker (IRB0406147) protocol, allowing collection of any tissues or bodily fluids which were discarded by the Department of Pathology for any patients undergoing care at Magee-Womens Hospital. Sample size was dictated by the preliminary/exploratory nature of this pilot investigation. Data related to diagnosis, treatment, comorbid conditions, and demographics were abstracted from the patient electronic medical records.
ASC isolation
ASCs were isolated from the sc., omental, and retroperitoneal adipose tissues, which were harvested during the hysterectomy surgery. At least 2 g of adipose tissue were collected from each depot. The adipose tissue was finely minced in 50 ml conical tubes and digested in 30 mls of Type II collagenase solution (Worthington Biochemical Corp, NJ, USA), as per published protocols [20]. The tissue was gently shaken in a 37°C water bath for approximately 30 min, filtered to remove large debris, and centrifuged at 1000 rpm for 10 min. The cellular pellet was re-suspended in erythrocyte lysis buffer, and centrifuged again at 1000 rpm for 10 min. The resulting cellular pellet was plated and cultured on tissue culture-treated flasks in ASC plating medium (DMEM/F12 with phenol red, 10% fetal bovine serum [FBS], 1% penicillin/streptomycin, 1% fungizone, and 0.001% dexamethasone). After overnight incubation, the cells that adhered to flask surface were considered to be ASCs [20], and were further culture-expanded. Nonadherent cells and debris were removed, and the cell medium was replaced with fresh ASC plating medium. After 1–2 days, ASCs were maintained and expanded until near confluence (passage 0), then harvested, frozen, and stored at -80°C until further use.
The authors collaborated with the Adipose Stem Cell Center at the University of Pittsburgh to conduct this study. Methods used for isolation of stem cells were identical to those employed in the previous studies [12,21]. Cell populations were evaluated by morphology and population doubling rate, and compared with other published studies. The cell populations used in this study were identical to those that we have performed flow cytometry quantification on in the past. Further, we have published studies showing that stem cell populations obtained from adipose tissue vary significantly in terms of CD34+ cells immediately after isolation [22], but culturing reduces heterogeneity, and populations become much more uniform with culture as the cell surface marker profiles change [23]
ASC preparation for Luminex
Frozen ASCs were quickly thawed in a 37°C water bath, and transferred dropwise to prewarmed ASC plating medium at a 9:1 ratio to cryomedia, thus the final DMSO concentration was 1%. Cells were incubated overnight at 37°C and 5% CO2 after which time the media was replaced with 10 ml of fresh ASC plating media. The ASCs remained in culture for 72 h, then lifted with trypsin, centrifuged, resuspended in 4 ml ASC medium (2% FBS, devoid of Phenol Red), and counted using a hemocytometer. ASCs from the each depot were plated in duplicate at a density of 50,000 cells/well (2% FBS, 2 ml/well) in 6-well plates, and allowed to expand for 48 h (37°C and 5% CO2). After 48 h, 1.5 ml of conditioned media was removed from each well (approximately 75–85% ASC confluency), and stored in cryovials at -80°C until analysis with Luminex.
Luminex
The biomarkers investigated in this study (angiopoietin-2, EGF, IL-8, leptin, VEGFA, VEGFC, and VEGFD) have been chosen based on the association with a proinflammatory milieu, as well as with EC risks in previously published cohort and cross-sectional studies [24–27]. These seven biomarkers were analyzed by xMAP™ immunoassays, and measured in the culture medium. Each bead-based assay was validated in comparison with appropriate standard ELISA based on the same antibody pair, and demonstrated 89–98% correlation. Assays were performed according to the manufacturers’ protocols as previously described [28]. Samples were analyzed using the Bio-Plex suspension array system (Bio-Rad Laboratories, CA, USA). For each analyte, 100 beads were analyzed, and the means were calculated. Analysis of experimental data was performed using four-parametric-curve fitting to the standard analyte curves. Quality controls were supplied by the Luminex kit manufacturer, and plain media was used as the blank and as the matrix for both standard and quality controls.
Statistics
Basic descriptive statistics were used to summarize the characteristics of the study population. Biomarker levels were log-transformed to normalize their distributions, and the mean levels of biomarkers were computed as a continuous average. One-way analysis of variance (ANOVA) was used to compare the mean levels of biomarkers among the three depots. A one-way analysis of covariance (ANCOVA) was conducted to determine if there were statistically significant differences between the three adipose depots on mean levels of biomarkers while controlling for age and BMI. All statistical analyses were done with SAS version 9.4 (SAS Institute Inc., NC, USA). Two-sided α level was set at 0.05.
Results
This pilot study included 22 EC patients aged 35–83 (mean age 64.82 years [SD: 12.39]) that were scheduled for hysterectomy at Magee-Womens Hospital of the UPMC Health System. The mean BMI was 36.90 kg/m2 (SD: 7.48; range: 21.8–58.9), and 94.12% of the women were of European–American decent. One participant had Type 2 diabetes, and 8 participants (36.36%) had hypertension.
All of the 22 participants had EC confirmed by the review of the final pathology records. Angiopoietin-2, EGF, leptin, VEGFD, and approximately 20% of VEGFC levels were below Luminex detection limits in the ASCs extracted from three depots. ANOVA analysis suggested that there was a significant difference between the ASCs from the three depots for IL-8, (p < 0.0001) (Figure 1). ANCOVA analysis suggested that there is a significant effect of adipose tissue depot on IL-8 level after controlling for patients’ age and BMI (F[461] = 7.55; p < 0.0001). Age (p = 0.8462) and BMI (p = 0.5024) were not associated with IL-8 levels between adipose depots. Highest levels of IL-8 was observed in the ASCs from the omental depot, and the lowest levels in the ASCs from the retroperitoneal depot. VEGFA levels were highest in the ASCs extracted from the retroperitoneal depot and lowest in the ASCs from the sc. depot; however, these differences were not statistically significant. Table 1 summarizes baseline characteristics of the study participants.
Figure 1. . IL-8 levels in the three depots of the adipose tissue.
AA: African–American; ANOVA: Analysis of variance; EA: European–American.
Table 1. . Baseline characteristics of study participants.
| n = 22 | Mean (SD) |
|---|---|
| Age, years | 64.82 (12.39) |
| BMI, kg/m2 | 36.90 (7.48) |
| IL-8o | 10027 (5071) |
| IL-8r | 3292 (2291) |
| IL-8s | 4083 (2986) |
| VEGFAo | 404 (374) |
| VEGFAr | 481 (335) |
| VEGFAs | 355 (165) |
| Race | |
| EA | 16 (94.12) |
| AA | 1 (5.88) |
| Missing | 5 |
| T2DM | |
| Yes | 1 (4.55) |
| No | 21 (95.45) |
| Hypertension | |
| Yes | 8 (36.36) |
| No | 14 (63.64) |
AA:African–American; BMI: Body mass index; EA: European–Amercan; IL:Interleukin; SD: Standard deviation; T2DM: Type 2 diabetes mellitus.
Discussion
Luminex measurements in ASC-conditioned media from all of the three anatomical locations demonstrated that the differences were observed in IL-8 levels, which were highest in the omental depot and lowest in the retroperitoneal depot in EC patients. IL-8 is a proinflammatory chemokine whose expression correlates with the angiogenic, tumorigenic, and metastatic potential of several in vivo tumor models [29]. Increasing IL-8 levels have been linked to the increase in obesity and waist circumference [30], as well as with EC progression, suggesting that IL-8 is an important marker to investigate in future research on ACSs and EC. Interestingly, neither patient age nor BMI were significantly associated with the differences in in IL-8 levels among the three adipose tissue depots.
In breast cancer, it has been suggested that ASCs might play a crucial role in the tumor growth and progression by inducing regulatory molecules, and promoting an anti-inflammatory reaction within the tumor microenvironment [17]. Thus, it is possible that the risk of hormone sensitive cancers can be predicted by evaluating the functional characteristics of ASCs, as a preliminary evidence suggests that ASCs increase vascularization and growth of endometrial tumors [31]. Moreover, ASCs metabolically alter cancer cells toward enhanced survival by increasing nitric oxide synthesis and reducing oxidative stress in cancer cells [13].
Adipose tissue does not serve merely as an energy storage site, but also as the largest endocrine organ that actively secretes adipokines and inflammatory cytokines [32,33], which often trigger a state of low-grade inflammation [34]. Adipose tissue inflammation occurs when macrophages are recruited, which leads to an imbalance in adipokines, and the uncontrolled release of inflammatory cytokines [34–36]. Obesity-associated chronic inflammation has been strongly linked to the development of metabolic diseases such as Type 2 diabetes and cardiovascular disease, as well as EC [35]. Recently published studies have indicated that it is the visceral fat, rather than the sc. fat, that is strongly linked to the development of metabolic diseases [32,33,36,37]. Of the three depots of visceral fat, the omental depot appears to be the most inflammatory, while the sc. fat is the least inflammatory [32,38]. Our finding that IL-8 has a higher expression in ASCs from the omental depot, and VEGFA expression was highest (albeit not statistically significant) in the retroperitoneal depot, is consistent with previously published studies on the inflammatory nature of visceral adipose tissue.
Strengths of this study include the collection of adipose tissue samples from the three depots of each subject, which is very novel. Additionally, the uniform protocol for tissue collection and processing of tissue samples has been used. Weaknesses of this study include small sample size and inability to include cancer free controls in this early investigation. Further, we acknowledge, that some ASC heterogeneity may exist between patients.
Adipose tissue is the potentially attractive target to evaluate biomarkers in relation to cancer risk, and more research needs to be done in this area. While we previously demonstrated the role of ASCs in tissue regeneration [39], ASCs may also play an important role in influencing the loco-regional immune inflammation in EC. ASCs can be differentiated into multiple lineages under specific culture conditions [40], including adipocytes, which results in the potential use of ASCs for multiple clinical applications, including cancer detection and control. Thus, one important future direction of this research is to focus on comparative studies of ASC differentiation, targeting patients with EC and cancer-free controls.
Consistently with our previous investigations on EC risk [41–45], future research in this area will need to focus on how the patient factors including age, race, cancer status, and time from cancer diagnosis are associated with the changes in inflammatory factors associated with ASCs. Expanding the work on EC cell lines in relation ASCs will also be important for improving our understanding of this area [12]. Additionally, since obesity has been reported to be an important determinant of stem cell function [46], it is important to investigate the exact nature of relationship between increases in BMI and ASC function. Also, investigating the relationship between ASC status and age is an important consideration for the future research work in this area.
Conclusion
This study is the first to compare adipose tissue from multiple anatomical locations in cancer patients. Our results suggest that the differences in IL-8 production from different sites can differentially influence the loco-regional tumor-associated inflammation. In the future, identifying biological characteristics associated with EC development, such as novel biomarkers in obese women, would be critical for development of EC screening and prevention, especially for women in high-risk categories. This pilot study opens up new opportunities in the area of ASC research in EC.
Summary points.
Endometrial cancer (EC) is the most common gynecologic malignancy in the USA that is strongly associated with obesity.
The exact mechanisms linking adipose tissue to endometrial cancer have yet to be explored.
Based on our previous research and evidence published by others, we believe that adipose stem cells may be an important component of EC etiology.
We conducted one of the first comparisons of the inflammatory biomarker levels in ASC-conditioned media from the three depots of adipose tissue (subcutaneous, omental, and retroperitoneal) in women with EC.
We found a significant difference between the three depots for IL-8 (p < 0.0001), with the highest levels of IL-8 in the omental depot, and the lowest levels in the retroperitoneal depot.
Adipose tissue is an attractive target to evaluate biomarkers in relation to cancer risk.
Footnotes
Financial & competing interests disclosure
This work was supported by a grant from the Scaife Foundation and departmental funds from the Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh School of Medicine. This project used the UPMC Hillman Cancer Center Luminex Core Laboratory that is supported in part by award P30CA047904. The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Wartko P, Sherman ME, Yang HP, Felix AS, Brinton LA, Trabert B. Recent changes in endometrial cancer trends among menopausal-age US women. Cancer Epidemiol. 2013;37(4):374–377. doi: 10.1016/j.canep.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morice P, Leary A, Creutzberg C, Abu-Rustum N, Darai E. Endometrial cancer. Lancet. 387(10023):1094–1108. doi: 10.1016/S0140-6736(15)00130-0. [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J. Clin. 2015;65(1):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 4.Sheikh MA, Althouse AD, Freese KE, et al. USA endometrial cancer projections to 2030: should we be concerned? Future Oncol. 2014;10(16):2561–2568. doi: 10.2217/fon.14.192. [DOI] [PubMed] [Google Scholar]; • Demonstrates future increase in endometrial cancer (EC) incidence.
- 5.ACS. 2017. www. cancer.org/cancer/endometrial-cancer/about/key-statistics.html
- 6.Mackintosh ML, Crosbie EJ. Obesity-driven endometrial cancer: is weight loss the answer? BJOG. 2013;120(7):791–794. doi: 10.1111/1471-0528.12106. [DOI] [PubMed] [Google Scholar]
- 7.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371(9612):569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 8.Schmandt RE, Iglesias DA, Co NN, Lu KH. Understanding obesity and endometrial cancer risk: opportunities for prevention. Am. J. Obstet. Gynecol. 2011;205(6):518–525. doi: 10.1016/j.ajog.2011.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Kruijsdijk RC, van der Wall E, Visseren FL. Obesity and cancer: the role of dysfunctional adipose tissue. Cancer Epidemiol. Biomarkers Prev. 2009;18(10):2569–2578. doi: 10.1158/1055-9965.EPI-09-0372. [DOI] [PubMed] [Google Scholar]
- 10.Klopp AH, Zhang Y, Solley T, et al. Omental adipose tissue-derived stromal cells promote vascularization and growth of endometrial tumors. Clin Cancer Res. 2012;18(3):771–782. doi: 10.1158/1078-0432.CCR-11-1916. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Describes the possible mechanism of adipose-derived stem cell and EC link.
- 11.Freese KE, Kokai L, Edwards RP, et al. Adipose-derived stems cells and their role in human cancer development, growth, progression and metastasis: a systematic review. Cancer Res. 2015;75(7):1161–1168. doi: 10.1158/0008-5472.CAN-14-2744. [DOI] [PubMed] [Google Scholar]; •• Provides a conceptual framework of the link between cancer and adipose-derived stem cells.
- 12.Linkov F, Kokai L, Edwards R, et al. The role of adipose-derived stem cells in endometrial cancer proliferation. Scand. J. Clin. Lab. Invest. Suppl. 2014;244:54–58. doi: 10.3109/00365513.2014.936682. [DOI] [PubMed] [Google Scholar]; •• Describes one of the first laboratory investigations of a adipose-derived stem cells from EC patients.
- 13.Salimian B, Caneba C, Nowicka A, et al. Nitric oxide mediates metabolic coupling of omentum-derived adipose to ovarian and endometrial cancer cells. Cancer Res. 2014;75(2):456–471. doi: 10.1158/0008-5472.CAN-14-1337. [DOI] [PubMed] [Google Scholar]
- 14.Modesitt SC, Hsu JY, Chowbina SR, Lawrence RT, Hoehn KL. Not all fat is equal: differential gene expression and potential therapeutic targets in subcutaneous adipose, visceral adipose and endometrium of obese women with and without endometrial cancer. Int. J. Gynecol. Cancer. 2012;22(5):732–741. doi: 10.1097/IGC.0b013e3182510496. [DOI] [PubMed] [Google Scholar]
- 15.Friedenreich CM, Langley AR, Speidel TP, et al. Case–control study of inflammatory markers and the risk of endometrial cancer. Eur. J. Cancer Prev. 2013;22(4):374–379. doi: 10.1097/CEJ.0b013e32835b3813. [DOI] [PubMed] [Google Scholar]
- 16.Prieto-Hontoria PL, Perez-Matute P, Fernandez-Galilea M, Bustos M, Martinez JA, Moreno-Aliaga MJ. Role of obesity-associated dysfunctional adipose tissue in cancer: a molecular nutrition approach. Biochim. Biophys. Acta. 2011;1807(6):664–678. doi: 10.1016/j.bbabio.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 17.Razmkhah M, Jaberipour M, Ghaderi A. Bcl-2 and Fas expressions correlate with proliferative specificity of adipose-derived stem cells (ASCs) in breast cancer. Immunol. Invest. 2011;40(3):290–298. doi: 10.3109/08820139.2010.540892. [DOI] [PubMed] [Google Scholar]
- 18.Ewington L, Taylor A, Sriraksa R, Horimoto Y, Lam EW, El-Bahrawy MA. The expression of interleukin-8 and interleukin-8 receptors in endometrial carcinoma. Cytokine. 2012;59(2):417–422. doi: 10.1016/j.cyto.2012.04.036. [DOI] [PubMed] [Google Scholar]
- 19.Fujimoto J, Aoki I, Khatun S, Toyoki H, Tamaya T. Clinical implications of expression of interleukin-8 related to myometrial invasion with angiogenesis in uterine endometrial cancers. Ann. Oncol. 2002;13(3):430–434. doi: 10.1093/annonc/mdf078. [DOI] [PubMed] [Google Scholar]
- 20.Brayfield CA, Marra KG, Rubin JP. Adipose tissue regeneration. Curr. Stem Cell Res. Ther. 2010;5(2):116–121. doi: 10.2174/157488810791268582. [DOI] [PubMed] [Google Scholar]
- 21.Kokai LE, Traktuev DO, Zhang L, et al. Adipose stem cell function maintained with age: an intrasubject study of long-term cryopreserved cells. Aesthet. Surg. J. 2017;37(4):454–463. doi: 10.1093/asj/sjw197. [DOI] [PubMed] [Google Scholar]
- 22.Philips BJ, Grahovac TL, Valentin JE, et al. Discussion: prevalence of endogenous CD34+ adipose stem cells predicts human fat graft retention in a xenograft model. Plast. Reconstr. Surg. 2013;132(4):845–858. doi: 10.1097/PRS.0b013e31829fe5b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mitchell JB, McIntosh K, Zvonic S, et al. Immunophenotype of human adipose-derived cells: temporal changes in stromal-associated and stem cell-associated markers. Stem Cells. 2006;24(2):376–385. doi: 10.1634/stemcells.2005-0234. [DOI] [PubMed] [Google Scholar]
- 24.Dossus L, Lukanova A, Rinaldi S, et al. Hormonal, metabolic and inflammatory profiles and endometrial cancer risk within the EPIC cohort: a factor analysis. Am. J. Epidemiol. 2013;177(8):787–799. doi: 10.1093/aje/kws309. [DOI] [PubMed] [Google Scholar]
- 25.Zhang B, Shao X, Zhou J, Qiu J, Wu Y, Cheng J. YT521 promotes metastases of endometrial cancer by differential splicing of vascular endothelial growth factor A. Tumour Biol. 2016;37:15543. doi: 10.1007/s13277-015-3908-y. [DOI] [PubMed] [Google Scholar]
- 26.Dznelashvili N, Kasradze D, Tavartkiladze A. Expression of epidermal growth factor receptor and concentrations of epidermal growth factor and melatonin in endometrial carcinoma. Georgian Med. News. 2014;(235):17–24. [PubMed] [Google Scholar]
- 27.Ryan AJ, Susil B, Jobling TW, Oehler MK. Endometrial cancer. Cell Tissue Res. 2005;322(1):53–61. doi: 10.1007/s00441-005-1109-5. [DOI] [PubMed] [Google Scholar]
- 28.Gorelik E, Landsittel DP, Marrangoni AM, et al. Multiplexed immunobead-based cytokine profiling for early detection of ovarian cancer. Cancer Epidemiol. Biomarkers Prev. 2005;14(4):981–987. doi: 10.1158/1055-9965.EPI-04-0404. [DOI] [PubMed] [Google Scholar]
- 29.Waugh DJ, Wilson C. The interleukin-8 pathway in cancer. Clin. Cancer Res. 2008;14(21):6735–6741. doi: 10.1158/1078-0432.CCR-07-4843. [DOI] [PubMed] [Google Scholar]
- 30.Kim CS, Park HS, Kawada T, et al. Circulating levels of MCP-1 and IL-8 are elevated in human obese subjects and associated with obesity-related parameters. Int. J. Obes. 2006;30(9):1347–1355. doi: 10.1038/sj.ijo.0803259. [DOI] [PubMed] [Google Scholar]
- 31.Klopp AH, Zhang Y, Solley T, et al. Omental adipose tissue-derived stromal cells promote vascularization and growth of endometrial tumors. Clin. Cancer Res. 2012;18(3):771–782. doi: 10.1158/1078-0432.CCR-11-1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fain JN. Release of inflammatory mediators by human adipose tissue is enhanced in obesity and primarily by the nonfat cells: a review. Mediators Inflamm. 2010;2010:513948. doi: 10.1155/2010/513948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hung CS, Lee JK, Yang CY, et al. Measurement of visceral fat: should we include retroperitoneal fat? PLoS ONE. 2014;9(11):e112355. doi: 10.1371/journal.pone.0112355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poulain-Godefroy O, Lecoeur C, Pattou F, Fruhbeck G, Froguel P. Inflammation is associated with a decrease of lipogenic factors in omental fat in women. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008;295(1):R1–R7. doi: 10.1152/ajpregu.00926.2007. [DOI] [PubMed] [Google Scholar]
- 35.Bjorndal B, Burri L, Staalesen V, Skorve J, Berge RK. Different adipose depots: their role in the development of metabolic syndrome and mitochondrial response to hypolipidemic agents. J. Obes. 2011;2011:490650. doi: 10.1155/2011/490650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bigornia SJ, Farb MG, Mott MM, et al. Relation of depot-specific adipose inflammation to insulin resistance in human obesity. Nutr. Diabetes. 2012;2:e30. doi: 10.1038/nutd.2012.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ibrahim MM. Subcutaneous and visceral adipose tissue: structural and functional differences. Obes. Rev. 2010;11(1):11–18. doi: 10.1111/j.1467-789X.2009.00623.x. [DOI] [PubMed] [Google Scholar]
- 38.Fain JN, Sacks HS, Bahouth SW, Tichansky DS, Madan AK, Cheema PS. Human epicardial adipokine messenger RNAs: comparisons of their expression in substernal, subcutaneous and omental fat. Metab. Clin. Exp. 2010;59(9):1379–1386. doi: 10.1016/j.metabol.2009.12.027. [DOI] [PubMed] [Google Scholar]
- 39.Kokai LE, Marra K, Rubin JP. Adipose stem cells: biology and clinical applications for tissue repair and regeneration. Transl. Res. 2014;163(4):399–408. doi: 10.1016/j.trsl.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 40.Tsuji W, Rubin JP, Marra KG. Adipose-derived stem cells: implications in tissue regeneration. World J. Stem Cells. 2014;6(3):312–321. doi: 10.4252/wjsc.v6.i3.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Linkov F, Elishaev E, Gloyeske N, et al. Bariatric surgery-induced weight loss changes immune markers in the endometrium of morbidly obese women. Surg. Obes. Relat. Dis. 2014;10(5):921–926. doi: 10.1016/j.soard.2014.03.023. [DOI] [PubMed] [Google Scholar]
- 42.Linkov F, Goughnour SL, Edwards RP, et al. Endometrial cancer associated biomarkers in bariatric surgery candidates: exploration of racial differences. Surg. Obes. Relat. Dis. 2017;13(5):862–868. doi: 10.1016/j.soard.2017.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Linkov F, Goughnour SL, Ma Tianzhou, et al. Changes in inflammatory endometrial cancer risk biomarkers in individuals undergoing surgical weight loss. Gynecol. Oncol. 2017;147(1):133–138. doi: 10.1016/j.ygyno.2017.07.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Linkov F, Maxwell GL, Felix AS, et al. Longitudinal evaluation of cancer-associated biomarkers before and after weight loss in RENEW study participants: implications for cancer risk reduction. Gynecol. Oncol. 2012;125(1):114–119. doi: 10.1016/j.ygyno.2011.12.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Linkov F, Yurkovetsky Z, Lokshin A. Biomarker approaches to the development of cancer screening tests: can cancer blood tests become a routine health check-up? Future Oncol. 2007;3(3):295–298. doi: 10.2217/14796694.3.3.295. [DOI] [PubMed] [Google Scholar]
- 46.Pachon-Pena G, Serena C, Ejarque M, et al. Obesity determines the immunophenotypic profile and functional characteristics of human mesenchymal stem cells from adipose tissue. Stem Cells Transl. Med. 2016;5(4):464–475. doi: 10.5966/sctm.2015-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]