Abstract
Volatile anesthetics depress neurotransmitter release in a brain region- and neurotransmitter-selective manner by unclear mechanisms. Voltage-gated sodium channels (Navs), which are coupled to synaptic vesicle exocytosis, are inhibited by volatile anesthetics through reduction of peak current and modulation of gating. Subtype-selective effects of anesthetics on Nav might contribute to observed neurotransmitter-selective anesthetic effects on release. We analyzed anesthetic effects on Na+ currents mediated by the principal neuronal Nav subtypes Nav1.1, Nav1.2, and Nav1.6 heterologously expressed in ND7/23 neuroblastoma cells using whole-cell patch-clamp electrophysiology. Isoflurane at clinically relevant concentrations induced a hyperpolarizing shift in the voltage dependence of steady-state inactivation and slowed recovery from fast inactivation in all three Nav subtypes, with the voltage of half-maximal steady-state inactivation significantly more positive for Nav1.1 (−49.7 ± 3.9 mV) than for Nav1.2 (−57.5 ± 1.2 mV) or Nav1.6 (−58.0 ± 3.8 mV). Isoflurane significantly inhibited peak Na+ current (INa) in a voltage-dependent manner: at a physiologically relevant holding potential of −70 mV, isoflurane inhibited peak INa of Nav1.2 (16.5% ± 5.5%) and Nav1.6 (18.0% ± 7.8%), but not of Nav1.1 (1.2% ± 0.8%). Since Nav subtypes are differentially expressed both between neuronal types and within neurons, greater inhibition of Nav1.2 and Nav1.6 compared with Nav1.1 could contribute to neurotransmitter-selective effects of isoflurane on synaptic transmission.
Introduction
Volatile anesthetics have been in clinical use for over 170 years, but their mechanisms of action are poorly understood (Hemmings et al., 2005). They have well described effects on multiple protein targets involved in neurotransmission, including ligand-gated and voltage-gated ion channels (Hemmings et al., 2005; Franks, 2006), but the contributions of these molecular actions to their specific neurophysiological effects are less clear. Volatile anesthetics induce a therapeutic state of unconsciousness, amnesia, and immobility, but they can also produce serious side effects, including cardiovascular and respiratory depression and developmental neurotoxicity (Hemmings et al., 2005; Franks, 2006; Jevtovic-Todorovic, 2016). Understanding the effect-specific cellular and molecular targets for volatile anesthetics is therefore critical for potential mechanism-based development of novel anesthetics or approaches to mitigating toxicities.
Voltage-gated Na+ channels (Navs) are crucial for mediating cellular membrane excitability, including initiation and propagation of action potentials (APs) (Hodgkin and Huxley, 1952; Catterall et al., 2005; Hu et al., 2009; Clay, 2013). They are also targets for the effects of volatile anesthetics (Rehberg et al., 1996; Hemmings, 2009; Herold and Hemmings, 2012; Herold et al., 2014; Covarrubias et al., 2015). Multiple Nav subtypes are inhibited by volatile anesthetics (Ouyang et al., 2003, 2009; Shiraishi and Harris, 2004; OuYang and Hemmings, 2007), which can lead to reduced presynaptic AP amplitude in rat calyceal neurons (Wu et al., 2004), isolated neurohypophysial nerve terminals (Ouyang et al., 2003), and inhibition of neurotransmitter release (Westphalen and Hemmings, 2003a, 2006; Wu et al., 2004). Modulation of Nav function also contributes to general anesthetic potency in vivo. Activation of Nav in the spinal cord by intrathecal infusion of veratridine reduces the immobilizing effect of isoflurane in rats; conversely, blocking Nav by intrathecal infusion of the highly selective Nav blocker tetrodotoxin (TTX) enhances the immobilizing effect of isoflurane (Zhang et al., 2008, 2010).
Volatile anesthetics inhibit neurotransmitter release in a brain region- and neurotransmitter-selective manner (Westphalen and Hemmings, 2006; Westphalen et al., 2010, 2011; Baumgart et al., 2015). For example, isoflurane inhibits release of glutamate more potently than release of GABA from cultured hippocampal neurons (Westphalen and Hemmings, 2006; Baumgart et al., 2015) and differentially inhibits neurotransmitter release between brain regions and the spinal cord (Westphalen et al., 2010, 2011). Nav subtypes show subcellular, regional, and neurotransmitter-selective expression in the central nervous system (CNS) (Lai and Jan, 2006; Ogiwara et al., 2007; Lorincz and Nusser, 2008b; Johnson et al., 2017). Given their sensitivity to volatile anesthetics, the differential effects of anesthetics on neurotransmitter release might be explained by their selective inhibition of specific presynaptic Nav subtypes. Of the nine identified Nav subtypes (Nav1.1–Nav1.9), Nav1.1, Nav1.2, and Nav1.6 are highly expressed in the CNS (Black and Waxman, 1996; Wood and Baker, 2001). Volatile anesthetics display differential subtype-selective inhibition of Nav1.2 (one of the predominant brain subtypes), Nav1.4 (the predominant skeletal muscle subtype), and Nav1.5 (the predominant cardiac subtype) (OuYang and Hemmings, 2007). However, the role of subtype-selective effects of volatile anesthetics on neuronal Nav subtypes in their presynaptic effects is unknown. We therefore examined the effects of isoflurane on the three major neuronal Nav subtypes expressed in the adult mammalian brain using whole-cell patch-clamp electrophysiology in a homogenous neuronal cell line. Selective inhibition of Nav subtypes would provide a neurophysiological basis for Nav inhibition in the region- and neurotransmitter-selective effects of volatile anesthetics on neuronal excitability and synaptic transmission.
Materials and Methods
Materials.
Isoflurane was obtained from Abbott Laboratories (North Chicago, IL). TTX was from Sankyo Kasei (Tokyo, Japan). All other compounds for solution preparation were from Sigma-Aldrich (St. Louis, MO).
cDNA Constructs.
Na+ currents from three neuronal Nav subtypes (Nav1.1, Nav1.2, and Nav1.6) were recorded and analyzed by heterologous expression of the respective subtype in a neuronal background using the ND7/23 neuroblastoma cell line (Herold et al., 2009). Each subtype was rendered TTX resistant by mutation of a single amino acid located in the extracellular toxin binding domain that has been shown to have no effect on gating properties, channel kinetics, or anesthetic sensitivity (Herzog et al., 2003; Leffler et al., 2005; Cestèle et al., 2013; Purtell et al., 2015). We refer to the mutant channels as Nav1.1, Nav1.2, and Nav1.6 below. Wild-type human Nav1.1 (accession number NM_001165963), kindly provided by A. L. George, Jr. (Northwestern University, Evanston, IL), was mutated to F383S (Cestèle et al., 2013). Wild-type rat Nav1.2 (accession number NM_012647), kindly provided by W. Catterall (University of Washington, Seattle, WA), was mutated to F385S (Leffler et al., 2005; Purtell et al., 2015). Wild-type mouse Nav1.6 (accession number NM_001077499), kindly provided by S. G. Waxman (Yale University, New Haven, CT), was mutated to Y371S (Herzog et al., 2003).
Cell Culture and Transfection.
Rodent ND7/23 neuroblastoma cells (Sigma-Aldrich) were plated on 12-mm glass coverslips and incubated at 37°C in a humidified atmosphere of 5% CO2/95% O2 in Dulbecco’s modified Eagle’s medium supplemented with 10% (v/v) fetal bovine serum, 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin (Sigma-Aldrich). This cell line provides a neuronal background for expression of Nav by providing critical factors such as auxiliary subunits that facilitate functional expression of the channel protein (Herold et al., 2009). Transfected TTX-resistant Nav subtypes were studied in the presence of 250 nM TTX to block endogenous Na+ currents (Herold et al., 2009; Purtell et al., 2015). ND7/23 cells were cotransfected with 2.5 μg Nav1.1, Nav1.2, or Nav1.6 cDNA together with 0.7 μg pEGFP-N1 cDNA (Clontech, Mountain View, CA) using Lipofectamine LTX (Invitrogen, Carlsbad, CA) to allow identification of enhanced green fluorescent protein–transfected cells by fluorescence microscopy. Electrophysiological studies were conducted 48 hours after transfection.
Measurement of Sodium Currents.
Whole-cell patch-clamp recordings of Na+ currents (INa) were obtained at room temperature (23–24°C) using an Axopatch 200B amplifier (Axon Instruments, Burlingame, CA) digitized with a Digidata 1321A interface and analyzed using pClamp 10.2 software (Axon Instruments). Whole-cell INa was sampled at 50 kHz and low-pass filtered at 10 kHz. Pipettes were filled with internal solution as follows: 120 mM CsF, 10 mM NaCl, 10 mM HEPES, 10 mM EGTA, 10 mM TEA-Cl, 1 mM CaCl2, and 1 mM MgCl2 adjusted to pH 7.3 (by CsOH) and to 334 mOsm/kg (with sucrose). Pipette resistance when filled was 1.5–3.0 MΩ. The external solution contained the following: 140 mM NaCl, 10 mM HEPES, 3 mM KCl, 1 mM MgCl2, 2 mM CaCl2, 20 mM TEA-Cl, 5 mM d-glucose, and 0.00025 mM TTX adjusted to pH 7.4 (with NaOH) and to 330 mOsm/kg (with sucrose). Liquid-junction potential was not corrected. To minimize space-clamp and series resistance errors, only cells expressing 1–8 nA peak current were analyzed. Capacitance transients were cancelled and voltage error was minimized using 70%–80% series resistance correction. Series resistance was typically 2–5 MΩ; data were discarded if series resistance was >8 MΩ. Recordings began 5 minutes after attaining the whole-cell patch to allow equilibration of pipette solution and cytosol. Leak currents were subtracted using the P/4 method (Bezanilla and Armstrong, 1977) applied after test pulses. Stimulation protocols were applied in control external solution containing 250 nM TTX, and again after a 2-minute perfusion with external solution containing isoflurane.
Simulation of Isoflurane Effects on APs.
A computational model using NEURON software 7.4 (http://www.neuron.yale.edu/neuron/) was used to simulate the effects of isoflurane on Nav1.1, Nav1.2, and Nav1.6 function by adjusting reported Nav electrophysiological parameters (Herzog et al., 2001) to the parameters we obtained. Simulation of APs was modified from the model (Akemann et al., 2009) to be mediated by Nav1.1, Nav1.2, or Nav1.6, with soma length and width set as 25 μm and Ra as 80 Ω/cm. The electrophysiological properties of original pas (passive) and hh (Hodgkin-Huxley) channels were set to default NEURON values, and resting membrane potential of the soma was set at −70 mV. Because experimental recordings were performed at 23 to 24°C, temperature-dependent effects of isoflurane on simulated Nav kinetics were modeled as described (Collins and Rojas, 1982) based on the kinetic model of m3h, where Q10 = 2.34 for m and Q10 = 2.9 for h to simulate APs mediated by Nav1.1, Nav1.2, or Nav1.6 at 37°C. The relationship between AP amplitude and probability of transmitter release was modeled using a previously established nerve terminal model (Graham and Redman, 1994). The simulation of excitatory postsynaptic currents (EPSCs) was modified from Graham et al. (2001) and based on the observed effects of isoflurane on AP amplitude and firing frequency mediated by Nav1.1, Nav1.2, or Nav1.6 at 37°C. The control presynaptic stimulus was 100 milliseconds at 100 Hz. The stimulus under isoflurane was based on the effects of isoflurane on AP frequency mediated by each Nav subtype.
Isoflurane Application.
A saturated stock solution of isoflurane (Abbott Laboratories) in external solution (12 to 13 mM) was diluted to the desired final concentrations in gas-tight glass syringes. For electrophysiological recording, isoflurane solutions were perfused using a pressure-driven microperfusion system (ALA BPS-8; ALA Scientific, Westbury, NY) with the perfusion pipette tip positioned 100–150 μm away from the recorded cell. Isoflurane concentrations sampled at the perfusion pipette tip were determined by gas chromatography using a Shimadzu GC-2010 Plus gas chromatograph (Shimadzu, Tokyo, Japan) after extraction into octane (Herold et al., 2009). Isoflurane (0.3 mM) was used as the predicted minimum alveolar concentration (MAC; equivalent to the EC50 for immobilization) in rats after temperature adjustment to 23°C (Taheri et al., 1991; Ouyang et al., 2009).
Data and Statistical Analysis.
Data were analyzed using GraphPad Prism 6 (GraphPad Software Inc., San Diego, CA) and SPSS 22.0 (SPSS Science Software Inc., Chicago, IL). Conductance (G) values were derived from the I-V relationship using the equation G = I/(V − Vrev), where I is the peak INa at a given voltage (V) and Vrev is the measured Na+ reversal potential. Voltage of half-maximal activation (V1/2act) was obtained by fitting data for each cell to a Boltzmann equation of the form G/Gmax = 1/[1 + exp (V1/2act − V/k)], where G/Gmax is the normalized fractional conductance and k is the slope factor. Voltage of half-maximal fast inactivation (V1/2inact) was measured by fitting normalized steady-state INa values to a Boltzmann equation of the form INa/INa max = 1/[1 + exp (V1/2inact − V/k)]. Half-maximal inhibitory concentration (IC50) values were obtained by least-squares fitting to the Hill equation: Y = 1/(1 + 10([logIC50 − X] × h), where Y is the effect, X is the measured concentration of isoflurane, and h is the Hill slope. Time course data were fitted to the monoexponential function Y = exp (−τ × n) + AP, where τ is the time constant, AP is the plateau, and n is stimulus number based on complete recovery time. Data (expressed as means ± S.D.) were assessed for normality using the Shapiro–Wilk test and analyzed using the two-tailed paired t test or ANOVA with post hoc testing as indicated, with P < 0.05 as the threshold for statistical significance.
Results
Neuronal Nav Subtypes have Distinct Electrophysiological Properties.
Voltage of half-maximal activation (V1/2act) did not differ between Nav1.1, Nav1.2, and Nav1.6 under control conditions (Table 1). Voltage of half-maximal inactivation (V1/2inact) for Nav1.1 was significantly more depolarized than for Nav1.2 or Nav1.6 (Table 1). From a hyperpolarized holding potential of −100 mV, recovery from fast inactivation (τ) was significantly slower for Nav1.2 than for Nav1.6. From a physiologic holding potential of −70 mV, τ for recovery was significantly slower for Nav1.2 than for Nav1.1 or Nav1.6 (Table 2).
TABLE 1.
Effects of isoflurane on voltage-dependent activation and inactivation of neuronal Nav subtypes
Data are expressed as means ± S.D. Numbers in parentheses are the percentage change compared with the control.
| Subtype | V1/2act |
V1/2inact |
||||
|---|---|---|---|---|---|---|
| CTL | ISO | Δ | CTL | ISO | Δ | |
| mV | ||||||
| Nav1.1 | −14.4 ± 3.4 | −15.4 ± 3.3 | −1.0 ± 1.1 (7) | −49.7 ± 3.9*,** | −54.6 ± 4.9*** | −4.9 ± 2.7 (10) |
| Nav1.2 | −15.8 ± 1.4 | −16.5 ± 0.8 | −0.6 ± 0.7 (4) | −57.5 ± 1.2* | −61.3 ± 1.4*** | −3.8 ± 1.7 (7) |
| Nav1.6 | −15.3 ± 2.0 | −16.3 ± 2.1 | −0.9 ± 0.5 (7) | −58.0 ± 3.8** | −64.2 ± 5.3*** | −6.2 ± 2.3 (11) |
CTL, control; ISO, isoflurane at 0.48 ± 0.03 mM (∼1.6 MAC).
P < 0.05 (Nav1.1 vs. Nav1.2); **P < 0.05 (Nav1.1 vs. Nav1.6); ***P < 0.05 (CTL vs. ISO).
TABLE 2.
Effects of isoflurane on neuronal Nav subtype recovery from fast inactivation
Data are expressed as means ± S.D. Numbers in parentheses are the percentage change compared with the control.
| Subtype |
τrecovery, Vh −100 mV |
τrecovery, Vh −70 mV |
||||
|---|---|---|---|---|---|---|
| CTL | ISO | Δ | CTL | ISO | Δ | |
| ms | ||||||
| Nav1.1 | 1.6 ± 0.2 | 1.9 ± 0.3** | 0.3 ± 0.2 (16) | 6.1 ± 0.8*** | 7.4 ± 1.2** | 1.3 ± 0.7 (21) |
| Nav1.2 | 1.9 ± 0.5* | 2.2 ± 0.6** | 0.3 ± 0.2 (17) | 8.4 ± 2.4*,*** | 10.1 ± 2.9** | 1.7 ± 0.8 (20) |
| Nav1.6 | 1.2 ± 0.2* | 1.7 ± 0.2** | 0.4 ± 0.3 (38) | 4.7 ± 0.7* | 6.6 ± 1.0** | 1.8 ± 0.4 (39) |
CTL, control; ISO, isoflurane at 0.58 ± 0.04 mM (∼1.9 MAC); τrecovery, half-time for recovery form inactivation; Vh, holding potential.
P < 0.05 (Nav1.2 vs. Nav1.6); **P < 0.05 (CTL vs. ISO); ***P < 0.05 (Nav1.1 vs. Nav1.2).
Isoflurane Does Not Affect Sodium Channel Activation.
Channel activation was evoked by a series of 10-millisecond voltage steps from –70 to +60 mV preceded by a 100-millisecond prepulse to –100 mV (Purtell et al., 2015). Isoflurane (0.48 ± 0.03 mM; ∼1.6 MAC) did not significantly affect current-voltage (I-V) relationships or voltage dependence of half-maximal activation (V1/2act) of Nav1.1 (n = 6), Nav1.2 (n = 6), or Nav1.6 (n = 5) (all P > 0.05 by paired two-tailed t test) (Fig. 1). Peak INa was elicited at 0 mV for all three Nav subtypes for both control and isoflurane conditions. Values of V1/2act for the control and 1.6 MAC isoflurane conditions are shown in Table 1.
Fig. 1.
Effects of isoflurane on Nav activation. Isoflurane did not significantly affect the voltage of half-maximal activation (V1/2act) of Nav1.1 (n = 6), Nav1.2 (n = 6), or Nav1.6 (n = 5). See Table 1 for individual values. Channels were activated by a series of voltage steps from −70 to +60 mV preceded by a 100-millisecond prepulse to −100 mV. (A–C) Representative traces for the control (CTL) or isoflurane (ISO) (0.48 mM, 1.6 MAC) for Nav1.1, Nav1.2, and Nav1.6. (D–F) Current-voltage relationships (I-V) for Nav1.1, Nav1.2, and Nav1.6. (G–I) Activation curves for Nav1.1, Nav1.2, and Nav1.6, for the control or isoflurane (0.48 mM isoflurane). (J–L) Voltage of half-maximum activation (V1/2act) for Nav1.1, Nav1.2, and Nav1.6 for the control or isoflurane (0.48 mM). Data are expressed as means ± S.D. n.s., not significant by paired t test (P > 0.05).
Isoflurane Enhances Voltage-Dependent Inactivation.
The effect of isoflurane on steady-state fast inactivation was determined by a double pulse protocol with a 300-millisecond prepulse ranging from −110 to −10 mV in 10-mV steps, followed by depolarization to 0 mV to elicit peak INa. Normalized INa/INa max values reflected the fraction of channels inactivated during the prepulse. Isoflurane (0.48 ± 0.03 mM; ∼1.6 MAC) shifted the voltage dependence of steady-state inactivation toward more hyperpolarized potentials for Nav1.1 (n = 6), Nav1.2 (n = 6), and Nav1.6 (n = 5) (all P < 0.05, by paired t test) (Fig. 2, G–I). The magnitude of the voltage shift was similar for all three Nav subtypes tested (Table 1).
Fig. 2.
Effects of isoflurane on steady-state fast inactivation of neuronal Nav subtypes. Isoflurane (0.48 mM; 1.6 MAC) shifted the voltage of half-maximal inactivation (V1/2inact) in the hyperpolarizing direction for all three isoforms (n = 6 for Nav1.1 and Nav1.2; n = 5 for Nav1.6; P < 0.05 by two-tailed, paired t test). Steady-state inactivation was determined by eliciting currents at 0 mV after a 300-millisecond prepulse to voltages of −110 to −10 mV in 10-mV steps (see inset). (A–C) Representative families of current traces in the absence (CTL) or presence of isoflurane (ISO) for Nav1.1, Nav1.2, and Nav1.6. (D–F) Isoflurane shifted the voltage dependence of inactivation of Nav1.1, Nav1.2, and Nav1.6 in the hyperpolarized direction. Data are expressed as means ± S.D. (G–I) Effects of isoflurane on the voltage of half-maximal inactivation (V1/2inact). *P < 0.05 by two-tailed, paired t test.
Isoflurane Differentially Inhibited Peak INa.
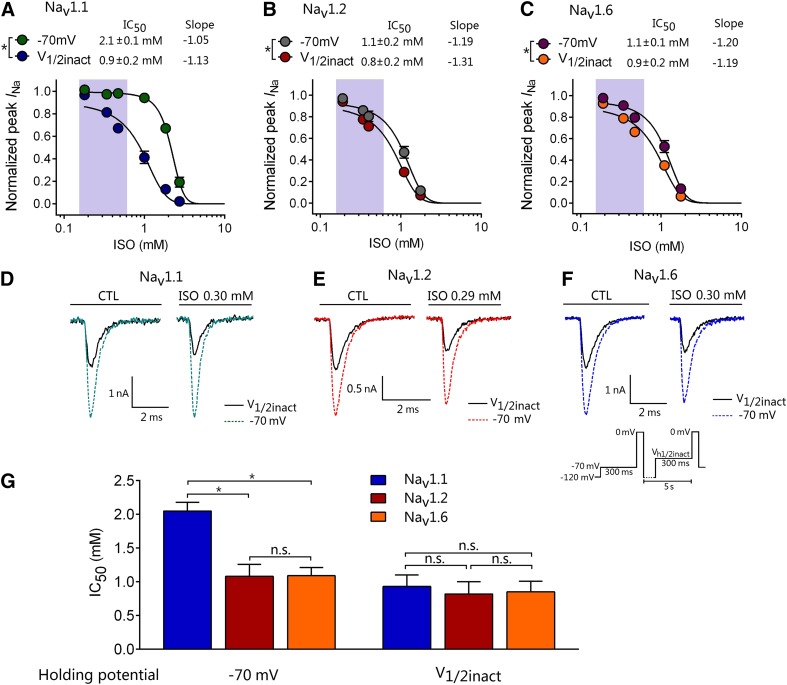
Inhibition by isoflurane was greater for Nav1.2 and Nav1.6 than for Nav1.1 at a physiologically relevant holding potential of −70 mV. We tested the degree of Nav inhibition from holding potentials (Vh) of −120, −110, or −70 mV or V1/2inact (voltage of half-maximal inactivation measured for each individual cell prior to the control recording). V1/2inact was −51.2 ± 1.2, −58.8 ± 1.8, or −59.0 ± 1.3 mV for Nav1.1, Nav1.2, or Nav1.6, respectively (Fig. 3). From a holding potential of −120 mV, isoflurane (0.49 ± 0.03 mM; ∼1.6 MAC) did not inhibit peak INa for any Nav subtype. From a holding potential of −110 mV, isoflurane inhibited peak INa of Nav1.2 by 8.9% ± 2.9% and of Nav1.6R by 8.8% ± 3.9%, whereas Nav1.1 was not significantly inhibited (Fig. 4). From a physiologic holding potential of −70 mV, isoflurane inhibited peak INa of Nav1.2 by 16.5% ± 5.5% and of Nav1.6 by 18.0% ± 7.8%, with no effect on Nav1.1. From a holding potential of V1/2inact, isoflurane significantly inhibited peak INa of all three Nav subtypes to a similar extent (by ∼30%). The concentration-dependent inhibition of peak INa by isoflurane was well fitted to a Hill equation, and potency for inhibition was voltage dependent (Fig. 5, A–C). From a holding potential of −70 mV, Nav1.2 and Nav1.6 were more sensitive to isoflurane (IC50 = 1.0 ± 0.2 and 1.1 ± 0.1 mM, respectively) compared with Nav1.1 (IC50 = 2.0 ± 0.1 mM; P < 0.05 by ANOVA, Fig. 5). From a holding potential of V1/2inact, the IC50 values of isoflurane for all three Nav subtypes were similar (0.9 ± 0.2 mM for Nav1.1, 0.8 ± 0.2 mM for Nav1.2, and 0.9 ± 0.2 mM for Nav1.6; P > 0.05 by ANOVA).
Fig. 3.
Inhibition of peak Na+ current (INa) during wash-in and washout of isoflurane. Effect of 0.49 mM (1.6 MAC) isoflurane with an alternating pulse protocol to elicit peak INa by a prepulse to −110 mV, −70 mV, or V1/2inact (voltage of half-maximal inactivation) (n = 6). V1/2inact was determined for each individual cell prior to control recording. (A) Representative traces for Nav1.1 for the control (black traces) or isoflurane (ISO; colored traces). No inhibition was observed for Nav1.1 from a holding potential (Vh) of −110 or −70 mV. (B) Representative traces for Nav1.2 for the control or isoflurane. (C) Representative traces for Nav1.6 for the control or isoflurane. (D–F) Inhibition by isoflurane of Nav subtypes. Data are expressed as means ± S.D.
Fig. 4.
Inhibition of peak Na+ current (INa) by isoflurane. From a holding potential (Vh) of −110 mV, isoflurane (0.49 ± 0.03 mM; ∼1.6 MAC) inhibited peak INa of Nav1.2 and Nav1.6, but not of Nav1.1. From a holding potential of −70 mV, isoflurane inhibited peak INa of Nav1.2 and Nav1.6, but not of Nav1.1. From a holding potential of V1/2inact (voltage of half-maximal inactivation), isoflurane inhibited peak INa of Nav1.1, Nav1.2, and Nav1.6 with similar efficacy (P > 0.05 by ANOVA). Data are expressed as means ± S.D. (n = 5 to 6). *P < 0.05 vs. Nav1.1. n.s., not significant (P > 0.05).
Fig. 5.
Concentration-dependent effects of isoflurane on Nav subtypes. IC50 values for isoflurane inhibition of Nav subtypes from holding potentials of −70 mV or V1/2inact. (A–C) Data for concentration-dependent inhibition of peak INa by isoflurane were well fitted to a Hill equation with significant voltage-dependent inhibition (P < 0.05 by paired t test). The shaded area indicates the clinical concentration range of isoflurane (0.15–0.6 mM; 0.5–2.0 MAC). (D–F) Representative Na+ current traces for the control or isoflurane (ISO). (G) From a holding potential of −70 mV, Nav1.2 (n = 20) and Nav1.6 (n = 17) were more sensitive to isoflurane inhibition compared with Nav1.1 (n = 19). From a holding potential of V1/2inact, IC50 values were similar for all three Nav subtypes. *P < 0.05 by ANOVA with post hoc Bonferroni correction. Data are expressed as means ± S.D. (n = 3–5 for each point). n.s., not significant (P > 0.05).
Isoflurane Differential Affects Recovery from Fast Inactivation.
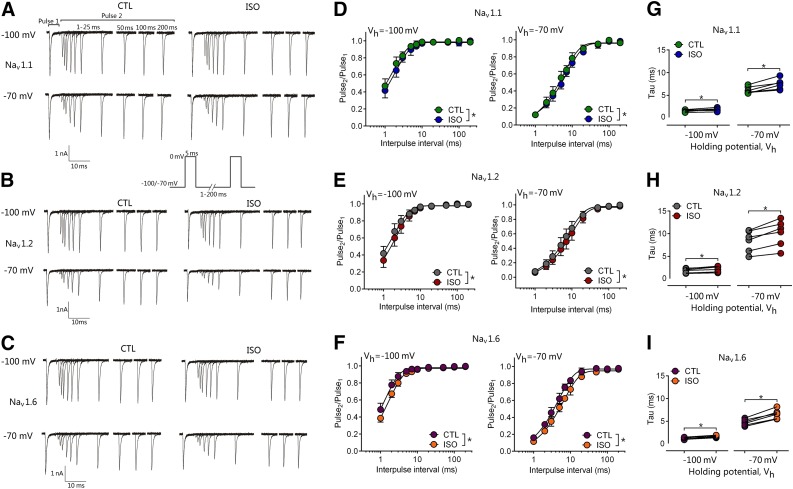
Neuronal firing frequency depends in part on the speed of Nav gating through its resting, open, and inactivated states, which is voltage dependent. We measured the effects of isoflurane on the time course of recovery from the fast-inactivated state. Peak INa was recorded in response to two 5-millisecond pulses to 0 mV, where the duration between pulses varied from 1 to 200 milliseconds. Recovery time courses were well fitted by a monoexponential function in control and isoflurane conditions, indicating that channels predominantly entered a single fast-inactivated state. Isoflurane (0.58 ± 0.04 mM; ∼1.9 MAC) slowed the recovery time course (recovery) of Nav1.1, Nav1.2, and Nav1.6 (Table 2). Isoflurane significantly increased the time required for full channel recovery at a hyperpolarized holding potential of −100 mV (Fig. 6) in Nav1.1, Nav1.2, and Nav1.6 (all n = 6, paired two-tailed t test). These effects were enhanced at a holding potential of −70 mV in Nav1.1, Nav1.2, and Nav1.6 (all n = 6, paired t test).
Fig. 6.
Effect of isoflurane on recovery from fast inactivation. Isoflurane at 0.58 mM (1.9 MAC) significantly slowed recovery from fast inactivation. (A–C) Representative traces recorded in the absence (CTL, left) or presence of 0.58 mM isoflurane (ISO, right) for Nav1.1 (A), Nav1.2 (B), and Nav1.6 (C). Currents were evoked by a paired-pulse protocol in which the time between the two 5-millisecond pulses (to 0 mV) was varied from 1 to 200 milliseconds. Holding potentials (Vh) were −100 mV (upper trace) or −70 mV (lower trace). (D–F) Normalized peak current (Pulse2/Pulse1) plotted against duration of the interpulse interval for a Vh of −100 mV (left) or −70 mV (right) for Nav1.1 (D), Nav1.2 (E), and Nav1.6 (F). (G–I) Time constant (τ) for recovery from inactivation determined from monoexponential fits of data from individual cells in the absence (CTL) or presence of isoflurane from a holding potential of −100 mV (n = 6) or −70 mV (n = 6). Data are presented as means ± S.D. *P < 0.05 vs. CTL by two-tailed, paired t test.
Effects of Isoflurane on Simulated APs and Synaptic Transmission.
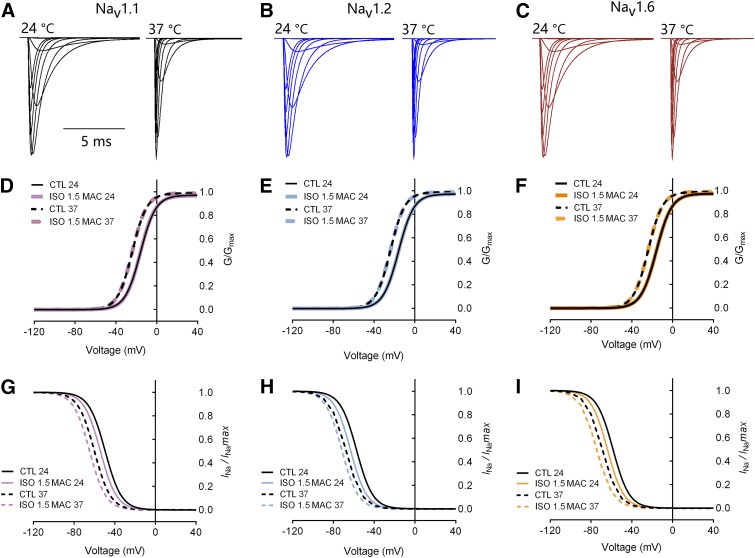
We used the NEURON algorithm to simulate the voltage dependence of Nav1.1-, Nav1.2-, and Nav1.6-mediated Na+ currents in a model neuron based on the experimentally derived gating properties of each channel (Fig. 7, A–C). Simulated voltage-dependent activation and inactivation values of Nav1.1, Nav1.2, and Nav1.6 were similar to those obtained experimentally by patch-clamp electrophysiological recordings (Fig. 7, D–F). Values of V1/2act for Nav1.1, Nav1.2, and Nav1.6 were all −15 mV, and values of V1/2inact for Nav1.1, Nav1.2, and Nav1.6 were −50, −58, and −58 mV, respectively.
Fig. 7.
Simulation of Na+ currents mediated by Nav1.1, Nav1.2, or Nav1.6. (A–C) Representative INa for each neuronal Nav subtype. Simulation of Hodgkin-Huxley model Na+ channel gating by NMODL. Currents at 24°C (left) were similar to INa recorded experimentally and the currents at 37°C (right) were corrected. The electrophysiological parameters of Nav at 24°C were based on empirical data obtained from recordings. Temperature correction of Nav was based on the kinetic model of m3h, where Q10 = 2.34 for m and Q10 = 2.9 for h (Collins and Rojas, 1982). (D–F) Simulated activation curves for Nav1.1 (D), Nav1.2 (E), and Nav1.6 (F) in the absence (CTL; black traces) or presence (ISO; colored traces) of isoflurane. The simulated currents at both 24°C and 37°C are shown. (G–I) Simulated inactivation curves for Nav1.1 (G), Nav1.2 (H), and Nav1.6 (I) in the absence (black traces) or presence (colored traces) of isoflurane. The simulated currents at both 24°C and 37°C are shown. CTL, control; ISO, isoflurane.
We also performed simulations to determine the temperature dependence of isoflurane effects on macroscopic Nav gating properties. After temperature correction to 37°C, values of V1/2act for Nav1.1, Nav1.2, and Nav1.6 were all −23 mV, and values of V1/2inact for Nav1.1, Nav1.2, and Nav1.6 were −60, −68, and −69 mV, respectively (Fig. 7, G–I). At a temperature of 24°C, isoflurane (0.48 mM; ∼1.6 MAC) shifted the inactivation curves of Nav1.1, Nav1.2, and Nav1.6 in the hyperpolarized direction to V1/2inact values of −55, −61, and −64 mV, respectively, with no effect on V1/2act (Fig. 7, G–I). At a temperature of 37°C, isoflurane (∼1.6 MAC) shifted the inactivation curves of Nav1.1, Nav1.2, and Nav1.6 in the hyperpolarized direction to V1/2inact values of −65, −72, and −75 mV, respectively, with no effect on V1/2act (Fig. 7, G–I).
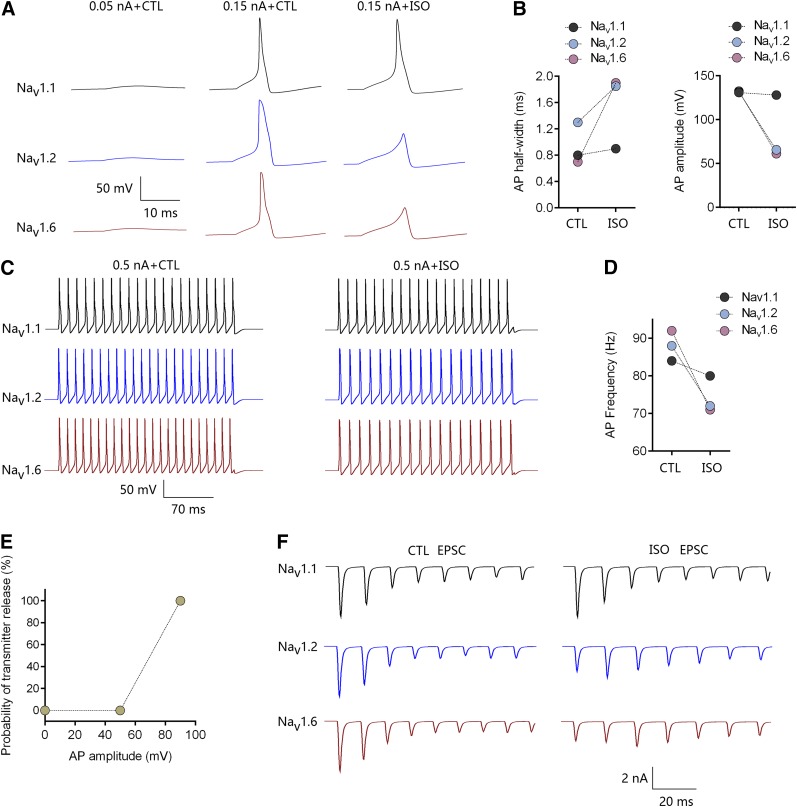
Inhibition by isoflurane of Nav1.1, Nav1.2, and Nav1.6 from a holding potential of −70 mV was used for simulation of single APs at 37°C. For an AP evoked by a current stimulus of 0.15 nA (Fig. 8A), the simulated AP half-width was greater for Nav1.2 (1.3 milliseconds) than for Nav1.6 (0.7 milliseconds) or Nav1.1 (0.8 milliseconds). Isoflurane inhibited AP initiation mediated by Nav1.2 or Nav1.6, whereas APs mediated by Nav1.1 were minimally affected (Fig. 8B). When AP trains were evoked with a longer and larger-amplitude depolarizing stimulus (250-millisecond current injection at 0.5 nA, Fig. 8C), the simulated AP frequency was higher for Nav1.6 (92 Hz) than for Nav1.1 (84 Hz) or Nav1.2 (88 Hz). Isoflurane reduced AP frequency to 80 Hz (−5.0%), 72 Hz (−18.2%), or 71 Hz (−22.8%) for Nav1.1, Nav1.2, and Nav1.6, respectively (Fig. 8D). Simulation of EPSCs was based on the effects of isoflurane on AP amplitude and firing frequency as a prediction of effects on synaptic transmission. Isoflurane depressed single AP amplitude from 131, 132, and 132 mV to 128, 66, and 61 mV for Nav1.1, Nav1.2, and Nav1.6, respectively (Fig. 8B). The depression by isoflurane of EPSCs was significant for Nav1.2- and Nav1.6-mediated transmission but minimal for Nav1.1-mediated transmission (Fig. 8F).
Fig. 8.
Simulated effects of isoflurane on action potentials and synaptic transmission mediated by Nav1.1, Nav1.2, or Nav1.6. Channel gating kinetics were temperature corrected to 37°C. (A) Effects of isoflurane (ISO) on single AP morphology evoked by 0.15 nA for 10 milliseconds. (B) Effects of isoflurane on half-width and amplitude of single APs mediated by Nav1.1, Nav1.2, or Nav1.6. (C) Effects of isoflurane on AP trains evoked by a larger stimulus of 0.5 nA for 250 milliseconds. (D) Effects of isoflurane on AP frequency mediated by Nav1.1, Nav1.2, or Nav1.6. (E) The relationship between AP amplitude and probability of transmitter release was modified from an established nerve terminal model (Graham and Redman, 1994). (F) Simulated effects of isoflurane on postsynaptic EPSCs mediated by Nav1.1, Nav1.2, or Nav1.6.
Discussion
In a combined electrophysiological and simulation study, we determined the major neuronal Nav subtype-specific effects of isoflurane on channel gating properties, AP firing, and synaptic transmission. Although voltage-dependent activation of Nav was similar between the three major neuronal subtypes, their voltage dependence of inactivation differed, with that of Nav1.1 being more positive compared with Nav1.2 and Nav1.6. This is consistent with previous reports that the V1/2inact of Nav1.1 is more positive than that of Nav1.6 when heterologously expressed in human embryonic kidney cells (Patel et al., 2015; Fruscione et al., 2018). These differences in inactivation gating lead to greater isoflurane-induced fast inactivation and inhibition of peak INa for Nav1.2 and Nav1.6 compared with Nav1.1 at a physiologic holding potential. The greater anesthetic sensitivity of Nav1.2 and Nav1.6 provides a plausible mechanism for brain region- and neurotransmitter-selective effects of isoflurane on synaptic transmission due to differential expression of Nav subtypes between neuron subtypes and within neuronal compartments (Wood and Baker, 2001; Johnson et al., 2017).
All clinically used volatile anesthetics inhibit the major Nav subtypes, including brain Nav1.2, skeletal muscle Nav1.4, and cardiac Nav1.5 (OuYang and Hemmings, 2007). Inhibition by volatile anesthetics of Nav1.5 contributes to their modulation of electrophysiological properties including APs in cardiomyocytes (Eskinder et al., 1993; Raatikainen et al., 1998). Of the nine identified Nav subtypes (Nav1.1–Nav1.9), Nav1.1, Nav1.2, and Nav1.6 are highly expressed in the CNS (Black and Waxman, 1996; Wood and Baker, 2001). Advantages of expressing neuronal Nav subtypes in the ND7/23 neuroblastoma/dorsal root ganglion hybridoma cell line include robust expression in the presence of critical auxiliary subunits and other neuronal signaling pathways, minimal space-clamp issues compared with mature isolated neurons facilitating reliable voltage-control during electrophysiological recordings (Herold et al., 2009), and expression and function in a uniform neuronal background minimizing effects of neuronal subtype heterogeneity (John et al., 2004; Rogers et al., 2016).
Even small reductions in INa have large electrophysiological consequences due to nonlinear coupling (Fujiwara et al., 1988; Goldin, 2001; Engel and Jonas, 2005). Small differences in presynaptic AP shape can produce large changes in the timing and magnitude of presynaptic Ca2+ entry because the kinetics of Ca2+ channels are strongly voltage dependent (Clark et al., 1996; Clarke et al., 2016). Thus, AP-evoked Ca2+ entry can have a steep dependence on AP shape, which can translate into significant changes in Ca2+ entry and even more dramatic changes in transmitter release and postsynaptic currents (Bean, 2007). Volatile anesthetics inhibit Nav and APs at clinically relevant concentrations (Westphalen and Hemmings, 2003b, 2006; Wu et al., 2004; Herold and Hemmings, 2012). Based on our findings, the simulated AP half-width is greater for Nav1.2 and Nav1.1 than for Nav1.6, whereas the AP train frequency is higher for Nav1.6 than for Nav1.1 and Nav1.2, consistent with Nav1.6 as an important contributor to fast repetitive firing (Khaliq et al., 2003; Brackenbury et al., 2010). Our findings provide a mechanistic basis for previous observations that isoflurane inhibits neurotransmitter release and spontaneous firing patterns of hippocampal neurons in an activity-dependent manner (Fujiwara et al., 1988; Goldin, 2001; Wu et al., 2004; Purtell et al., 2015).
Isoflurane suppressed peak Na+ current, produced a hyperpolarizing shift in the voltage dependence of fast inactivation, and slowed recovery from fast inactivation of brain Nav subtypes at a physiologically relevant membrane potential. These effects might involve more than one site of interaction of isoflurane with Nav since volatile anesthetics appear to have multiple sites of interaction with voltage-gated ion channels including Nav (Raju et al., 2013; Spurny et al., 2013; Sand et al., 2017). At a hyperpolarized holding potential, none of the Nav subtypes were inhibited by isoflurane, consistent with a low affinity of isoflurane for the resting state of Nav. From hyperpolarized or physiologic holding potentials, isoflurane did not inhibit Nav1.1-mediated peak INa but did inhibit Nav1.2 and Nav1.6 at a physiologic membrane potential. Further studies are required to determine whether differences in anesthetic binding sites contribute to this subtype-selective sensitivity to isoflurane.
All neuronal Nav subtypes were inhibited to a similar degree from holding potentials near their V1/2inact, a potential at which 50% of channels are closed or inactivated, indicating that isoflurane has similar quantitative effects on Nav1.1-, Nav1.2-, and Nav1.6-mediated currents when currents are measured at equivalent states of inactivation. Since the V1/2inact is more positive for Nav1.1 than for Nav1.2 or Nav1.6, isoflurane showed greater inhibition of Nav1.2 and Nav1.6 compared with Nav1.1 at a holding potential of −70 mV, supporting preferential interaction with inactivated channels. At this holding potential, Nav1.2 and Nav1.6 have a higher proportion of channels in the inactivated state compared with Nav1.1, leading to preferential inhibition of Nav1.2 and Nav1.6 by isoflurane. Thus, the differential sensitivity of neuronal Nav subtypes to isoflurane can be explained by differences in voltage-dependent gating rather than by subtype-specific differences in drug sensitivity per se. These findings suggest conserved sites of interaction between isoflurane and the major neuronal Nav subtypes, which are highly homologous (Black and Waxman, 1996; Wood and Baker, 2001). Identification of their pharmacologically relevant binding site(s) should clarify whether volatile anesthetics interact with structurally homologous sites in Nav (Sand et al., 2017).
Neuronal excitability and synaptic transmission show physiologic and pharmacological differences between brain regions and between neurons of different neurotransmitter phenotype (Pinheiro and Mulle, 2008; Spruston, 2008). Some of this heterogeneity could derive from differences in relative expression of various neuronal Nav subtypes having distinct voltage-dependent gating properties, with physiologic and pharmacological implications (Lai and Jan, 2006; Ogiwara et al., 2007; Lorincz and Nusser, 2008b; Johnson et al., 2017). Specific Nav subtypes are selectively expressed on presynaptic and postsynaptic glutamatergic synapses in the hippocampus, consistent with subtype-specific roles in neurotransmitter release and synaptic plasticity (Johnson et al., 2017). Most excitatory neurons express a high density of Nav1.6 and Nav1.2 (Whitaker et al., 2000; Tian et al., 2014), whereas Nav1.1 is preferentially expressed in inhibitory GABAergic interneurons (Ogiwara et al., 2007; Lorincz and Nusser, 2008a).
Neurotransmitter phenotype-specific expression and sensitivity to isoflurane inhibition of neuronal Nav subtypes could underlie the greater isoflurane sensitivity of Nav-dependent release of glutamate compared with GABA (Westphalen and Hemmings, 2006; Baumgart et al., 2015; Purtell et al., 2015). Further studies of the relationship between the functionally selective effects of isoflurane on Nav subtypes and various pharmacological endpoints might provide leads for the development of more selective and potentially safer general anesthetic agents that selectively target specific Nav subtypes. The function and gating kinetics of neuronal Nav are also modulated by phosphorylation, calmodulin binding (Cantrell and Catterall, 2001; Pitt and Lee, 2016; Yan et al., 2017), and auxiliary subunits (Wimmer et al., 2015), which could further modulate Nav sensitivity to general anesthetics.
This study has certain limitations. We used well characterized Nav subtypes from different mammalian species (human, rat, and mouse), since their amino acid sequences are >98% conserved; species-specific differences in Nav properties and anesthetic sensitivity are possible but unlikely and have not been reported (Whitaker et al., 2001; Lewis and Raman, 2011; Carrasco et al., 2017). The single point mutations to produce TTX resistance introduce a difference from wild-type channels with possible unanticipated effects on drug binding. However, these mutations are in the extracellular toxin binding domain and have not been shown to affect gating properties, channel kinetics, or anesthetic sensitivity compared with native hippocampal neuron channels (Herzog et al., 2003; Leffler et al., 2005; Purtell et al., 2015; Carrasco et al., 2017).
Although our simulation studies support our conclusions, the functional predictions have not been validated in intact neurons. Recordings of native INa from intact neurons are not an ideal model for comparing Nav subtypes, since it is impossible to isolate the individual subtype and selectively expressing TTX-resistant subtypes in cultured neurons is complicated by the heterogeneity of neuron types. All electrophysiological recordings were performed at room temperature (23 to 24°C), as are most electrophysiological studies because of recording stability and reliability. Although we used a simulated model to correct the temperature to 37°C, the effects of isoflurane at 37°C were not measured directly, and whether isoflurane would more potently inhibit Nav at 37°C is therefore unclear. Since the voltage dependence of Nav inactivation was hyperpolarized at the physiologic temperature of 37°C, inhibition by isoflurane of Na+ currents would be enhanced compared with 24°C, with perhaps more marked effects on excitability and AP properties. Although all volatile anesthetics tested inhibit Nav (OuYang and Hemmings, 2007), we only tested isoflurane and cannot extrapolate these effects to other clinically used volatile anesthetics, and it is possible that agent-specific effects exist.
In conclusion, Na+ currents mediated by the principal neuronal Nav subtypes Nav1.1, Nav1.2, and Nav1.6 exhibit different sensitivities to inhibition by isoflurane, leading to reduced inhibition of Nav1.1 compared with Nav1.2 and Nav1.6. This differential sensitivity can be explained by fundamental differences in voltage-dependent gating properties between channel subtypes. Thus, differences in the expression of specific Nav subtypes between brain regions, neurons, and synapses could underlie brain region- and neurotransmitter-selective effects of isoflurane, and perhaps other volatile anesthetics, on CNS function.
Abbreviations
- AP
action potential
- CNS
central nervous system
- EPSC
excitatory postsynaptic current
- INa
sodium current
- MAC
minimum alveolar concentration
- Nav
voltage-gated sodium channel
- TTX
tetrodotoxin
- V1/2act
voltage of half-maximal activation
- V1/2inact
voltage of half-maximal inactivation
Authorship Contributions
Participated in research design: Zhou, Herold, Hemmings.
Conducted experiments: Zhou, Johnson.
Contributed new reagents or analytic tools: Zhou, Herold.
Performed data analysis: Zhou, Herold, Hemmings.
Wrote or contributed to the writing of the manuscript: Zhou, Johnson, Herold, Hemmings.
Footnotes
This work was supported by the National Institutes of Health National Institute of General Medical Sciences [Grant GM-58055] (to H.C.H.)] and the Weill Cornell Medicine Department of Anesthesiology.
References
- Akemann W, Lundby A, Mutoh H, Knöpfel T. (2009) Effect of voltage sensitive fluorescent proteins on neuronal excitability. Biophys J 96:3959–3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgart JP, Zhou ZY, Hara M, Cook DC, Hoppa MB, Ryan TA, Hemmings HC., Jr (2015) Isoflurane inhibits synaptic vesicle exocytosis through reduced Ca2+ influx, not Ca2+-exocytosis coupling. Proc Natl Acad Sci USA 112:11959–11964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean BP. (2007) The action potential in mammalian central neurons. Nat Rev Neurosci 8:451–465. [DOI] [PubMed] [Google Scholar]
- Bezanilla F, Armstrong CM. (1977) Inactivation of the sodium channel. I. Sodium current experiments. J Gen Physiol 70:549–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black JA, Waxman SG. (1996) Sodium channel expression: a dynamic process in neurons and non-neuronal cells. Dev Neurosci 18:139–152. [DOI] [PubMed] [Google Scholar]
- Brackenbury WJ, Calhoun JD, Chen C, Miyazaki H, Nukina N, Oyama F, Ranscht B, Isom LL. (2010) Functional reciprocity between Na+ channel Nav1.6 and beta1 subunits in the coordinated regulation of excitability and neurite outgrowth. Proc Natl Acad Sci USA 107:2283–2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantrell AR, Catterall WA. (2001) Neuromodulation of Na+ channels: an unexpected form of cellular plasticity. Nat Rev Neurosci 2:397–407. [DOI] [PubMed] [Google Scholar]
- Carrasco DI, Vincent JA, Cope TC. (2017) Distribution of TTX-sensitive voltage-gated sodium channels in primary sensory endings of mammalian muscle spindles. J Neurophysiol 117:1690–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall WA, Goldin AL, Waxman SG. (2005) International Union of Pharmacology. XLVII. Nomenclature and structure-function relationships of voltage-gated sodium channels. Pharmacol Rev 57:397–409. [DOI] [PubMed] [Google Scholar]
- Cestèle S, Schiavon E, Rusconi R, Franceschetti S, Mantegazza M. (2013) Nonfunctional NaV1.1 familial hemiplegic migraine mutant transformed into gain of function by partial rescue of folding defects. Proc Natl Acad Sci USA 110:17546–17551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RB, Bouchard RA, Giles WR. (1996) Action potential duration modulates calcium influx, Na(+)-Ca2+ exchange, and intracellular calcium release in rat ventricular myocytes. Ann N Y Acad Sci 779:417–429. [DOI] [PubMed] [Google Scholar]
- Clarke SG, Scarnati MS, Paradiso KG. (2016) Neurotransmitter release can be stabilized by a mechanism that prevents voltage changes near the end of action potentials from affecting calcium currents. J Neurosci 36:11559–11572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay JR. (2013) A comparative analysis of models of Na+ channel gating for mammalian and invertebrate nonmyelinated axons: relationship to energy efficient action potentials. Prog Biophys Mol Biol 111:1–7. [DOI] [PubMed] [Google Scholar]
- Collins CA, Rojas E. (1982) Temperature dependence of the sodium channel gating kinetics in the node of Ranvier. Q J Exp Physiol 67:41–55. [DOI] [PubMed] [Google Scholar]
- Covarrubias M, Barber AF, Carnevale V, Treptow W, Eckenhoff RG. (2015) Mechanistic insights into the modulation of voltage-gated ion channels by inhalational anesthetics. Biophys J 109:2003–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel D, Jonas P. (2005) Presynaptic action potential amplification by voltage-gated Na+ channels in hippocampal mossy fiber boutons. Neuron 45:405–417. [DOI] [PubMed] [Google Scholar]
- Eskinder H, Supan FD, Turner LA, Kampine JP, Bosnjak ZJ. (1993) The effects of halothane and isoflurane on slowly inactivating sodium current in canine cardiac Purkinje cells. Anesth Analg 77:32–37. [DOI] [PubMed] [Google Scholar]
- Franks NP. (2006) Molecular targets underlying general anaesthesia. Br J Pharmacol 147 (Suppl 1):S72–S81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fruscione F, Valente P, Sterlini B, Romei A, Baldassari S, Fadda M, Prestigio C, Giansante G, Sartorelli J, Rossi P, et al. (2018) PRRT2 controls neuronal excitability by negatively modulating Na+ channel 1.2/1.6 activity. Brain 141:1000–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara N, Higashi H, Nishi S, Shimoji K, Sugita S, Yoshimura M. (1988) Changes in spontaneous firing patterns of rat hippocampal neurones induced by volatile anaesthetics. J Physiol 402:155–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin AL. (2001) Resurgence of sodium channel research. Annu Rev Physiol 63:871–894. [DOI] [PubMed] [Google Scholar]
- Graham B, Redman S. (1994) A simulation of action potentials in synaptic boutons during presynaptic inhibition. J Neurophysiol 71:538–549. [DOI] [PubMed] [Google Scholar]
- Graham B, Wong AY, Forsythe ID. (2001) A computational model of synaptic transmission at the calyx of Held. Neurocomputing 34–40:37–42. [Google Scholar]
- Hemmings HC., Jr (2009) Sodium channels and the synaptic mechanisms of inhaled anaesthetics. Br J Anaesth 103:61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmings HC, Jr, Akabas MH, Goldstein PA, Trudell JR, Orser BA, Harrison NL. (2005) Emerging molecular mechanisms of general anesthetic action. Trends Pharmacol Sci 26:503–510. [DOI] [PubMed] [Google Scholar]
- Herold KF, Hemmings HC., Jr (2012) Sodium channels as targets for volatile anesthetics. Front Pharmacol 3:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herold KF, Nau C, Ouyang W, Hemmings HC., Jr (2009) Isoflurane inhibits the tetrodotoxin-resistant voltage-gated sodium channel Nav1.8. Anesthesiology 111:591–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herold KF, Sanford RL, Lee W, Schultz MF, Ingólfsson HI, Andersen OS, Hemmings HC., Jr (2014) Volatile anesthetics inhibit sodium channels without altering bulk lipid bilayer properties. J Gen Physiol 144:545–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog RI, Cummins TR, Ghassemi F, Dib-Hajj SD, Waxman SG. (2003) Distinct repriming and closed-state inactivation kinetics of Nav1.6 and Nav1.7 sodium channels in mouse spinal sensory neurons. J Physiol 551:741–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog RI, Cummins TR, Waxman SG. (2001) Persistent TTX-resistant Na+ current affects resting potential and response to depolarization in simulated spinal sensory neurons. J Neurophysiol 86:1351–1364. [DOI] [PubMed] [Google Scholar]
- Hodgkin AL, Huxley AF. (1952) A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol 117:500–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W, Tian C, Li T, Yang M, Hou H, Shu Y. (2009) Distinct contributions of Na(v)1.6 and Na(v)1.2 in action potential initiation and backpropagation. Nat Neurosci 12:996–1002. [DOI] [PubMed] [Google Scholar]
- Jevtovic-Todorovic V. (2016) General anesthetics and neurotoxicity: how much do we know? Anesthesiol Clin 34:439–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John VH, Main MJ, Powell AJ, Gladwell ZM, Hick C, Sidhu HS, Clare JJ, Tate S, Trezise DJ. (2004) Heterologous expression and functional analysis of rat Nav1.8 (SNS) voltage-gated sodium channels in the dorsal root ganglion neuroblastoma cell line ND7-23. Neuropharmacology 46:425–438. [DOI] [PubMed] [Google Scholar]
- Johnson KW, Herold KF, Milner TA, Hemmings HC, Jr, Platholi J. (2017) Sodium channel subtypes are differentially localized to pre- and post-synaptic sites in rat hippocampus. J Comp Neurol 525:3563–3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaliq ZM, Gouwens NW, Raman IM. (2003) The contribution of resurgent sodium current to high-frequency firing in Purkinje neurons: an experimental and modeling study. J Neurosci 23:4899–4912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai HC, Jan LY. (2006) The distribution and targeting of neuronal voltage-gated ion channels. Nat Rev Neurosci 7:548–562. [DOI] [PubMed] [Google Scholar]
- Leffler A, Herzog RI, Dib-Hajj SD, Waxman SG, Cummins TR. (2005) Pharmacological properties of neuronal TTX-resistant sodium channels and the role of a critical serine pore residue. Pflugers Arch 451:454–463. [DOI] [PubMed] [Google Scholar]
- Lewis AH, Raman IM. (2011) Cross-species conservation of open-channel block by Na channel β4 peptides reveals structural features required for resurgent Na current. J Neurosci 31:11527–11536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorincz A, Nusser Z. (2008a) Cell-type-dependent molecular composition of the axon initial segment. J Neurosci 28:14329–14340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorincz A, Nusser Z. (2008b) Specificity of immunoreactions: the importance of testing specificity in each method. J Neurosci 28:9083–9086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogiwara I, Miyamoto H, Morita N, Atapour N, Mazaki E, Inoue I, Takeuchi T, Itohara S, Yanagawa Y, Obata K, et al. (2007) Nav1.1 localizes to axons of parvalbumin-positive inhibitory interneurons: a circuit basis for epileptic seizures in mice carrying an Scn1a gene mutation. J Neurosci 27:5903–5914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OuYang W, Hemmings HC., Jr (2007) Isoform-selective effects of isoflurane on voltage-gated Na+ channels. Anesthesiology 107:91–98. [DOI] [PubMed] [Google Scholar]
- Ouyang W, Herold KF, Hemmings HC., Jr (2009) Comparative effects of halogenated inhaled anesthetics on voltage-gated Na+ channel function. Anesthesiology 110:582–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang W, Wang G, Hemmings HC., Jr (2003) Isoflurane and propofol inhibit voltage-gated sodium channels in isolated rat neurohypophysial nerve terminals. Mol Pharmacol 64:373–381. [DOI] [PubMed] [Google Scholar]
- Patel RR, Barbosa C, Xiao Y, Cummins TR. (2015) Human Nav1.6 channels generate larger resurgent currents than human Nav1.1 channels, but the Navβ4 peptide does not protect either isoform from use-dependent reduction. PLoS One 10:e0133485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro PS, Mulle C. (2008) Presynaptic glutamate receptors: physiological functions and mechanisms of action. Nat Rev Neurosci 9:423–436. [DOI] [PubMed] [Google Scholar]
- Pitt GS, Lee SY. (2016) Current view on regulation of voltage-gated sodium channels by calcium and auxiliary proteins. Protein Sci 25:1573–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purtell K, Gingrich KJ, Ouyang W, Herold KF, Hemmings HC., Jr (2015) Activity-dependent depression of neuronal sodium channels by the general anaesthetic isoflurane. Br J Anaesth 115:112–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raatikainen MJ, Trankina MF, Morey TE, Dennis DM. (1998) Effects of volatile anesthetics on atrial and AV nodal electrophysiological properties in Guinea pig isolated perfused heart. Anesthesiology 89:434–442. [DOI] [PubMed] [Google Scholar]
- Raju SG, Barber AF, LeBard DN, Klein ML, Carnevale V. (2013) Exploring volatile general anesthetic binding to a closed membrane-bound bacterial voltage-gated sodium channel via computation. PLoS Comput Biol 9:e1003090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehberg B, Xiao YH, Duch DS. (1996) Central nervous system sodium channels are significantly suppressed at clinical concentrations of volatile anesthetics. Anesthesiology 84:1223–1233; discussion 27A. [DOI] [PubMed] [Google Scholar]
- Rogers M, Zidar N, Kikelj D, Kirby RW. (2016) Characterization of endogenous sodium channels in the ND7-23 neuroblastoma cell line: implications for use as a heterologous ion channel expression system suitable for automated patch clamp screening. Assay Drug Dev Technol 14:109–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sand RM, Gingrich KJ, Macharadze T, Herold KF, Hemmings HC., Jr (2017) Isoflurane modulates activation and inactivation gating of the prokaryotic Na+ channel NaChBac. J Gen Physiol 149:623–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraishi M, Harris RA. (2004) Effects of alcohols and anesthetics on recombinant voltage-gated Na+ channels. J Pharmacol Exp Ther 309:987–994. [DOI] [PubMed] [Google Scholar]
- Spruston N. (2008) Pyramidal neurons: dendritic structure and synaptic integration. Nat Rev Neurosci 9:206–221. [DOI] [PubMed] [Google Scholar]
- Spurny R, Billen B, Howard RJ, Brams M, Debaveye S, Price KL, Weston DA, Strelkov SV, Tytgat J, Bertrand S, et al. (2013) Multisite binding of a general anesthetic to the prokaryotic pentameric Erwinia chrysanthemi ligand-gated ion channel (ELIC). J Biol Chem 288:8355–8364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taheri S, Halsey MJ, Liu J, Eger EI, 2nd, Koblin DD, Laster MJ. (1991) What solvent best represents the site of action of inhaled anesthetics in humans, rats, and dogs? Anesth Analg 72:627–634. [DOI] [PubMed] [Google Scholar]
- Tian C, Wang K, Ke W, Guo H, Shu Y. (2014) Molecular identity of axonal sodium channels in human cortical pyramidal cells. Front Cell Neurosci 8:297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphalen RI, Hemmings HC., Jr (2003a) Effects of isoflurane and propofol on glutamate and GABA transporters in isolated cortical nerve terminals. Anesthesiology 98:364–372. [DOI] [PubMed] [Google Scholar]
- Westphalen RI, Hemmings HC., Jr (2003b) Selective depression by general anesthetics of glutamate versus GABA release from isolated cortical nerve terminals. J Pharmacol Exp Ther 304:1188–1196. [DOI] [PubMed] [Google Scholar]
- Westphalen RI, Hemmings HC., Jr (2006) Volatile anesthetic effects on glutamate versus GABA release from isolated rat cortical nerve terminals: 4-aminopyridine-evoked release. J Pharmacol Exp Ther 316:216–223. [DOI] [PubMed] [Google Scholar]
- Westphalen RI, Kwak NB, Daniels K, Hemmings HC., Jr (2011) Regional differences in the effects of isoflurane on neurotransmitter release. Neuropharmacology 61:699–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphalen RI, Yu J, Krivitski M, Jih TY, Hemmings HC., Jr (2010) Regional differences in nerve terminal Na+ channel subtype expression and Na+ channel-dependent glutamate and GABA release in rat CNS. J Neurochem 113:1611–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker WR, Clare JJ, Powell AJ, Chen YH, Faull RL, Emson PC. (2000) Distribution of voltage-gated sodium channel alpha-subunit and beta-subunit mRNAs in human hippocampal formation, cortex, and cerebellum. J Comp Neurol 422:123–139. [DOI] [PubMed] [Google Scholar]
- Whitaker WR, Faull RL, Waldvogel HJ, Plumpton CJ, Emson PC, Clare JJ. (2001) Comparative distribution of voltage-gated sodium channel proteins in human brain. Brain Res Mol Brain Res 88:37–53. [DOI] [PubMed] [Google Scholar]
- Wimmer VC, Harty RC, Richards KL, Phillips AM, Miyazaki H, Nukina N, Petrou S. (2015) Sodium channel β1 subunit localizes to axon initial segments of excitatory and inhibitory neurons and shows regional heterogeneity in mouse brain. J Comp Neurol 523:814–830. [DOI] [PubMed] [Google Scholar]
- Wood JN, Baker M. (2001) Voltage-gated sodium channels. Curr Opin Pharmacol 1:17–21. [DOI] [PubMed] [Google Scholar]
- Wu XS, Sun JY, Evers AS, Crowder M, Wu LG. (2004) Isoflurane inhibits transmitter release and the presynaptic action potential. Anesthesiology 100:663–670. [DOI] [PubMed] [Google Scholar]
- Yan H, Wang C, Marx SO, Pitt GS. (2017) Calmodulin limits pathogenic Na+ channel persistent current. J Gen Physiol 149:277–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Guzinski M, Eger EI, 2nd, Laster MJ, Sharma M, Harris RA, Hemmings HC., Jr (2010) Bidirectional modulation of isoflurane potency by intrathecal tetrodotoxin and veratridine in rats. Br J Pharmacol 159:872–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Sharma M, Eger EI, 2nd, Laster MJ, Hemmings HC, Jr, Harris RA. (2008) Intrathecal veratridine administration increases minimum alveolar concentration in rats. Anesth Analg 107:875–878. [DOI] [PMC free article] [PubMed] [Google Scholar]