Key Points
Question
In high-risk patients with aortic stenosis, does the mechanically expanded valve continue to be as safe as a self-expanding valve at 2 years, are there differences in clinical outcomes, and does paravalvular leak remain lower?
Findings
In this analysis of the randomized clinical trial, at 2 years, mortality and all stroke were similar between valves. Disabling stroke and paravalvular leak were less while pacemaker requirement and valve thrombosis were more frequent after using the mechanically expanded valve.
Meaning
Mechanically expanded valves are an alternative to self-expanding valves in this high-risk population.
Abstract
Importance
To our knowledge, REPRISE III is the first large randomized comparison of 2 different transcatheter aortic valve replacement platforms: the mechanically expanded Lotus valve (Boston Scientific) and self-expanding CoreValve (Medtronic).
Objective
To evaluate outcomes of Lotus vs CoreValve after 2 years.
Design, Setting, and Participants
A total of 912 patients with high/extreme risk and severe, symptomatic aortic stenosis enrolled between September 22, 2014, and December 24, 2015, were randomized 2:1 to receive Lotus (607 [66.6%]) or CoreValve (305 [33.4%] at 55 centers in North America, Europe, and Australia. The first 2-year visit occurred on October 17, 2016, and the last was conducted on April 12, 2018. Clinical and echocardiographic assessments are complete through 2 years and will continue annually through 5 years.
Main Outcomes and Measures
All-cause mortality and all-cause mortality or disabling stroke at 2 years. Other clinical factors included overall stroke, disabling stroke, repeated procedures, rehospitalization, valve thrombosis, and pacemaker implantation. Echocardiographic analyses included effective orifice area, mean gradient, and paravalvular leaks (PVLs).
Results
Of 912 participants, the mean (SD) age was 82.8 (7.3) years, 465 (51%) were women, and the mean (SD) Society of Thoracic Surgeons predicted risk of mortality was 6.8% (4.0%). At 2 years, all-cause death was 21.3% with Lotus vs 22.5% with CoreValve (hazard ratio [HR], 0.94; 95% CI, 0.69-1.26; P = .67) and all-cause mortality or disabling stroke was 22.8% with Lotus and 27.0% with CoreValve (HR, 0.81; 95% CI, 0.61-1.07; P = .14). Overall stroke was 8.4% vs 11.4% (HR, 0.75; 95% CI, 0.48-1.17; P = .21); disabling stroke was more frequent with CoreValve vs Lotus (4.7% Lotus vs 8.6% CoreValve; HR, 0.53; 95% CI, 0.31-0.93; P = .02). More Lotus patients received a new permanent pacemaker (41.7% vs 26.1%; HR, 1.87; 95% CI, 1.41-2.49; P < .01) or had a valve thrombosis (3.0% vs 0.0%; P < .01) compared with CoreValve. More patients who received CoreValve experienced a repeated procedure (0.6% Lotus vs 2.9% CoreValve; HR, 0.19; 95% CI, 0.05-0.70; P < .01), valve migration (0.0% vs 0.7%; P = .05), or embolization (0.0% vs 2.0%; P < .01) than Lotus. Valve areas remained significantly larger and the mean gradient was lower with CoreValve than Lotus (valve area, mean [SD]: Lotus, 1.53 [0.49] cm2 vs CoreValve, 1.76 [0.51] cm2; P < .01; valve gradient, mean [SD]: Lotus, 13.0 [6.7] mm Hg vs 8.1 [3.7] mm Hg; P < .01). Moderate or greater PVL was more frequent with CoreValve (0.3% Lotus vs 3.8% CoreValve; P < .01) at 2 years. Larger improvements in New York Heart Association (NYHA) functional class were observed with Lotus compared with CoreValve (improved by ≥1 NYHA class: Lotus, 338 of 402 [84.1%] vs CoreValve, 143 of 189 [75.7%]; P = .01; improved by ≥2 NYHA classes: 122 of 402 [37.3%] vs 65 of 305 [21.3%]).
Conclusions and Relevance
After 2 years, all-cause mortality rates, mortality or disabling stroke were similar between Lotus and CoreValve. Disabling stroke, functional class, valve migration, and PVL favored the Lotus arm whereas valve hemodynamics, thrombosis, and new pacemaker implantation favored the CoreValve arm.
Trial Registration
clinicaltrials.gov Identifier: NCT02202434
This randomized clinical trial examines 2-year outcomes in all-cause mortality rates and all-cause mortality or disabling stroke for high-risk patients with aortic stenosis in North America, Europe, and Australia who received a mechically expanding valve vs a self-expanding valve.
Introduction
Transcatheter aortic valve replacement (TAVR) has been compared with surgery in randomized clinical trials in the high-risk aortic stenosis (AS) population and found at 1 and 2 years to be noninferior1,2 or superior to surgery.3,4 To our knowledge, the Repositionable Percutaneous Replacement of Stenotic Aortic Valve Through Implantation of Lotus Valve System–Randomized Clinical Evaluation (REPRISE III) trial is the first large, core-laboratory adjudicated randomized trial comparison between TAVR valves comparing the mechanically expanded Lotus valve (Boston Scientific) and the self-expanding CoreValve (Medtronic).5 In this high-risk population, the mechanically expanded Lotus valve showed no difference in the 30-day primary safety composite end point of all-cause mortality, stroke, life-threatening bleeding, stage 2/3 acute kidney injury, and major vascular complications and was superior for the 1-year primary effectiveness end point of all-cause mortality, disabling stroke, and moderate or greater paravalvular leaks (PVLs).5 The comparator valve in this trial, the self-expanding CoreValve, showed superior survival when randomized against surgery at 1 and 2 years, making the durability of clinical outcomes with Lotus important.3,4 This post hoc analysis examines the 2-year outcomes of the REPRISE III trial to confirm these outcomes.
Methods
Study Design and Methods
REPRISE III is a multicenter, randomized clinical trial comparing the Lotus valve with the CoreValve. The study design, methods, patient selection, and 1-year results have been described previously.5,6 Eligible patients had severe native AS with a valve area 1.0 cm2 or less (or aortic valve area index ≤0.6 cm2/m2) and a mean pressure gradient of 40 mm Hg or more or jet velocity of 4.0 m/s or more. Patients were required to have a Society of Thoracic Surgeons predicted risk of mortality of 8% or greater or another indicator of high or extreme risk. Agreement by the local heart team regarding risk and suitability for TAVR and eligibility for an available size of both valves were required. All patients were reviewed by the REPRISE III case review committee to confirm eligibility.5
Patients (N = 912) were randomly assigned to receive Lotus or CoreValve (2 Lotus [607 (66.6%)]:1 CoreValve [305 (33.4%)]) at 55 centers in North American, Europe, and Australia. Clinical and echocardiographic assessments occurred at 2 years (and will continue annually to 5 years; Figure 1). Institutional review boards at each site approved the protocol. All patients provided written informed consent. Race/ethnicity was reported previously.5 All data were analyzed by independent core laboratories and a clinical events committee adjudicated all major clinical events. The data and study protocol for this clinical trial (Supplement 1 and Supplement 2) may be made available to other researchers in accordance with the Boston Scientific Data Sharing Policy (Supplement 3).
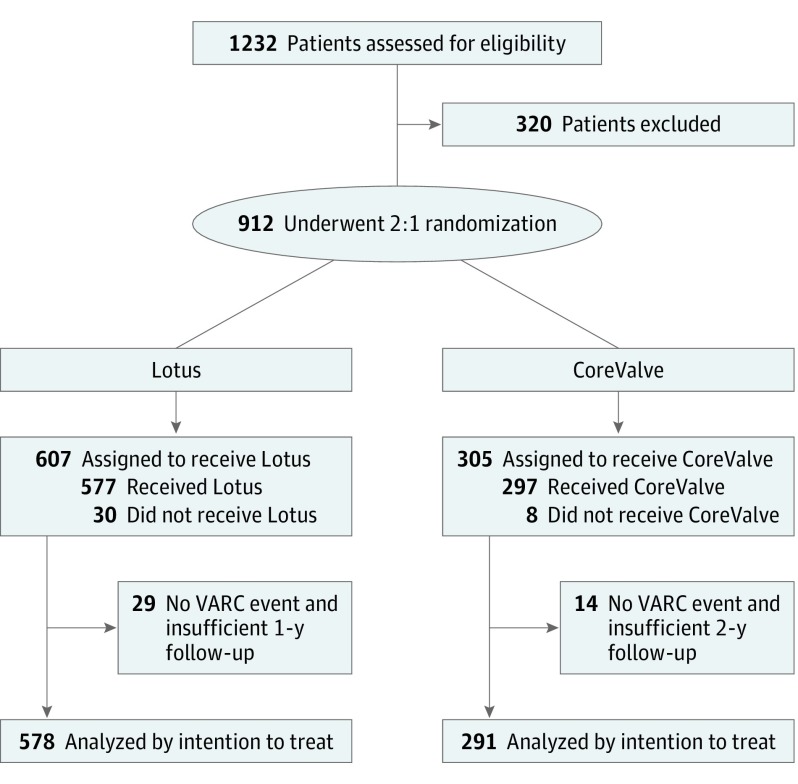
Figure 1. Study Flow Diagram.
VARC indicates Valve Academic Research Consortium.
Study End Points
All-cause mortality and all-cause mortality or disabling stroke were evaluated at 2 years. Other 2-year clinical outcomes that were evaluated include all stroke, disabling stroke, valve reintervention, rehospitalization, valve thrombosis, and the need for new pacemakers. Valve hemodynamics and PVL were assessed. Functional status was evaluated by New York Heart Association (NYHA) classification. Additional end points were based on the Valve Academic Research Consortium end points and definitions.7,8 The protocol required neurological examinations by a neurology professional in all patients at baseline, discharge, 1 year, and following any suspected stroke. Echocardiographic parameters, including aortic regurgitation, mean aortic gradient, and effective orifice area, were analyzed by an independent core laboratory (MedStar Health Research Institute).
Statistical Analysis
The intention-to-treat patient population was analyzed in this article. Continuous variables were estimated as mean (SD) and compared with the t test. Discrete variables were reported as counts and percentages and differences were assessed by means of the χ2 or Fisher exact tests. No adjustments for multiple tests other than the hierarchical testing strategy used for the primary and secondary end points were performed; analyses other than the primary and secondary analyses should be considered exploratory. Cumulative event rates were estimated by the Kaplan-Meier method. Statistical analyses were performed with SAS software, version 9.2 or later (SAS Institute). Statistical significance was set at P = .05.
Results
A total of 912 patients were randomized in REPRISE III (mean [SD] age, 82.8 [7.3] years; 465 women [51%]); patient selection and characteristics have been previously described (eTable 1 in Supplement 4).5 The mean (SD) Society of Thoracic Surgeons risk was 6.7 (4.0%) for Lotus and 6.9 (4.1%) for CoreValve and both groups were highly symptomatic, with 433 of 607 (71.3%) in the Lotus group and 207 of 305 (67.9%) of the CoreValve group having a NYHA functional class III or IV. No significant differences in baseline characteristics were seen in these randomized groups.5 The 2-year intention-to-treat analyses included 586 patients who received Lotus (95.2%) and 291 patients who received CoreValve (95.4%) who either had a Valve Academic Research Consortium event or clinical follow-up after 730 days (Figure 1).
All-Cause Mortality and All-Cause Mortality or Disabling Stroke at 2 Years
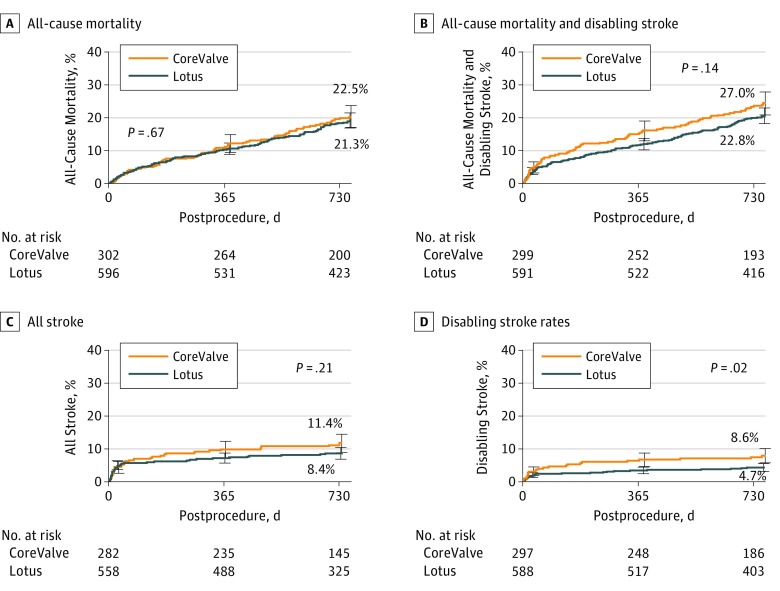
All-cause mortality occurred in 123 Lotus cases (21.3%) and 65 CoreValve cases (22.5%) at 2 years (P = .67; Figure 2A). All-cause mortality or disabling stroke occurred in 132 Lotus cases (22.8%) and 79 CoreValve cases (27.0%) at 2 years (P = .14; Figure 2B).
Figure 2. Cumulative Event Curve for Death and Stroke.
Two-year all-cause death (A), all-cause death and disabling stroke (B), all stroke (C), and disabling stroke rates in the intention-to-treat patient population (D). Event rates were calculated with Kaplan-Meier methods. Error bars indicate the standard error.
Other Clinical End Points at 2 Years
Overall stroke occurred in 48 Lotus cases (8.4%) and 32 CoreValve cases (11.4%; P = .21; Table and Figure 2C). Disabling stroke occurred in 26 Lotus cases (4.7%) and 24 CoreValve cases (8.6%; P = .02; Table and Figure 2D). Additional clinical end points are shown in the Table and eTable 2 in Supplement 4. Repeated procedures occurred in 3 Lotus cases (0.6%; all due to PVL) and 8 CoreValve cases (2.9%; P < .01; 6 due to PVL, 1 valve dislodgement/migration, and 1 unknown). Rehospitalization occurred in 98 Lotus cases (18.4%) and 52 CoreValve cases (18.9%; P = .62). Valve thrombosis occurred in 16 Lotus cases (3.0%) and 0 CoreValve cases (0%; P < .01); 5 cases occurred between years 1 and 2. Valve thrombosis was detected during routine echocardiographic follow-up as an increase in the transvalvular gradient in all but 1 case. In 12 of 16 patients (75%), subsequent cardiac computed tomography results showed findings consistent with valve thrombosis. No patients were receiving oral anticoagulants at the time of the event; 14 of 16 patients were treated with anticoagulants (1 patient was not a good candidate; there was unknown treatment in 1 patient). Clinical valve thrombosis resolved with oral anticoagulation in 12 of 16 cases (75%); the other 4 cases were detected at the 2-year follow-up visit and although 3 patients were treated with anticoagulants, the response to therapy is not yet available. None of the patients with valve thrombosis died or had a stroke associated with the thrombosis event or required a repeated valvular intervention. Pacemakers occurred in 202 Lotus cases (41.7%) and 61 CoreValve cases (26.1%; P < .01). Between 1 and 2 years, 6 patients who received CoreValve (2.9%) received new pacemakers compared with 1 patient who received Lotus (0.2%; P < .01; eTable 2 in Supplement 4).
Table. VARC-2 End Points at 2 Years Postrandomizationa.
| Characteristic | No. (%) | Hazard Ratio (95% CI) | |
|---|---|---|---|
| CoreValve (n = 305) | Lotus (n = 607) | ||
| All-cause mortality or disabling stroke | 79 (27.0) | 132 (22.8) | 0.81 (0.61-1.07) |
| All-cause mortality | 65 (22.5) | 123 (21.3) | 0.94 (0.69-1.26) |
| Cardiovascular mortality | 45 (15.9) | 79 (14.2) | 0.87 (0.60-1.25) |
| Stroke | 32 (11.4) | 48 (8.4) | 0.75 (0.48-1.17) |
| Disabling | 24 (8.6) | 26 (4.7) | 0.53 (0.31-0.93) |
| Nondisabling | 8 (2.8) | 23 (4.0) | 1.46 (0.65-3.27) |
| Major vascular complications | 19 (6.4) | 45 (7.5) | 1.21 (0.71-2.06) |
| Permanent pacemaker implantation | |||
| All patients | 61 (21.1) | 202 (34.2) | 1.85 (1.39-2.46) |
| Pacemaker-naive patients | 61 (26.1) | 202 (41.7) | 1.87 (1.41-2.49) |
| Bleeding | 60 (20.5) | 125 (21.7) | 1.07 (0.79-1.45) |
| Life-threatening or disabling | 35 (12.3) | 71 (12.4) | 1.03 (0.69-1.55) |
| Myocardial infarction | 17 (6.3) | 32 (6.1) | 0.93 (0.52-1.68) |
| Repeated procedure for valve-related dysfunction | 8 (2.9) | 3 (0.6) | 0.19 (0.05-0.70) |
| TAVR | 6 (2.2) | 0 (0.0) | Undefined |
| Surgical AVR | 1 (0.5) | 3 (0.6) | 1.48 (0.15-14.18) |
| Hospitalizationb | 52 (18.9) | 98 (18.4) | 0.92 (0.66-1.29) |
| New onset of atrial fibrillation/flutter | 14 (4.7) | 39 (6.6) | 1.42 (0.77-2.62) |
| Prosthetic aortic valve thrombosis | 0 (0.0) | 16 (3.0) | Undefined |
Abbreviations: NYHA, New York Heart Association; TAVR, transcatheter aortic valve replacement; VARC, Valve Academic Research Consortium.
Time-to-event rates in the intention-to-treat patient population.
Hospitalization for valve-related symptoms or worsening congestive heart failure (NYHA class III or IV).
Valve Performance at 2 Years
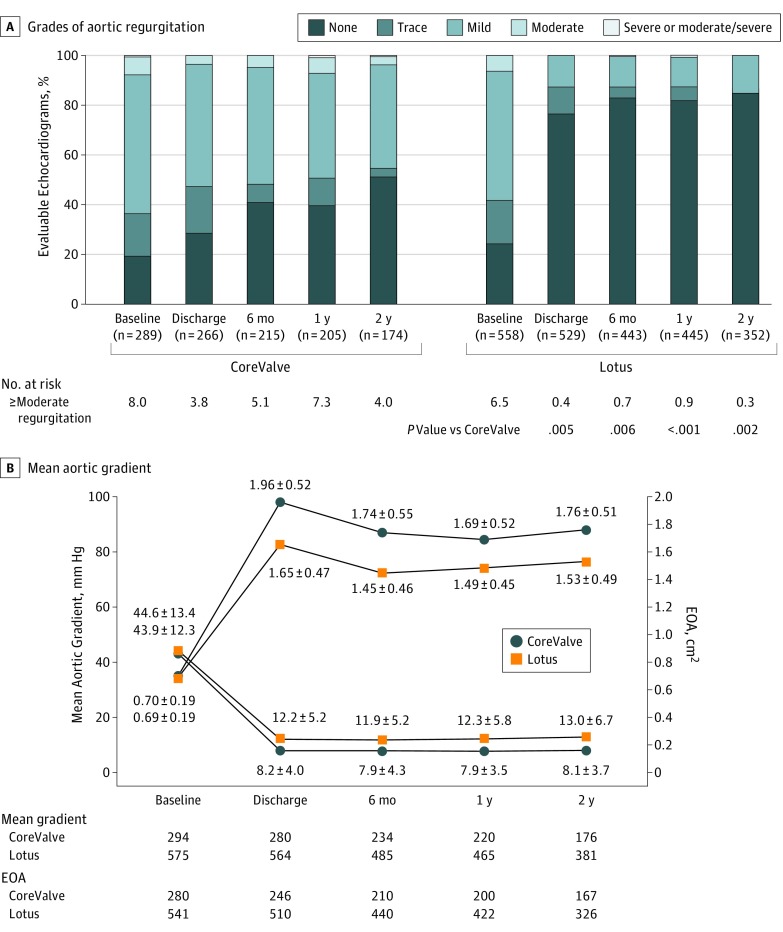
At 2 years, moderate or greater PVL remained more frequent with CoreValve (4.0% CoreValve vs 0.3% Lotus, P < .01) at 2 years (Figure 3A). There was no or trace/trivial PVL at 2 years in 95 of 174 patients (54.6%) who received CoreValve and 304 or 352 patients (86.4%) who received Lotus at 2 years as assessed by the echocardiographic core laboratory. The effective orifice area (EOA) improved significantly in both groups. In the CoreValve cohort, EOA improved from a mean (SD) of 0.70 (0.19) cm2 at baseline to 1.96 (0.52) cm2 at discharge and was 1.76 (0.51) cm2 at 2 years. In the Lotus cohort, EOA improved from a mean (SD) of 0.69 (0.19) cm2 to 1.65 (0.47) cm2 at discharge and was 1.53 (0.49) cm2 at 2 years (Figure 3B). The mean (SD) aortic gradients decreased with CoreValve from 43.9 (12.3) mm Hg to 8.2 (4.0) mm Hg at discharge and remained at that level through 2 years (8.1 [3.7] mm Hg); the mean (SD) gradients with Lotus also stably improved from 44.6 (13.4) mm Hg at baseline to 12.2 (5.3) mm Hg at discharge and 13.0 (6.7) mm Hg at 2 years (Figure 3B). The EOA and mean gradient in Lotus were significantly lower and higher, respectively, than with CoreValve at each point.
Figure 3. Hemodynamic Parameters to 2 Years.
A, Grades of aortic regurgitation are presented over time in the intention-to-treat patient population. Patients who received a CoreValve implant (compared with Lotus, right). B, Aortic regurgitation is only shown at baseline; paravalvular leak is shown at 1 and 2 years. Mean aortic gradient (circles) and effective orifice area (EOA; squares) in CoreValve and Lotus between baseline and 2 years.
NYHA Functional Status at 2 Years
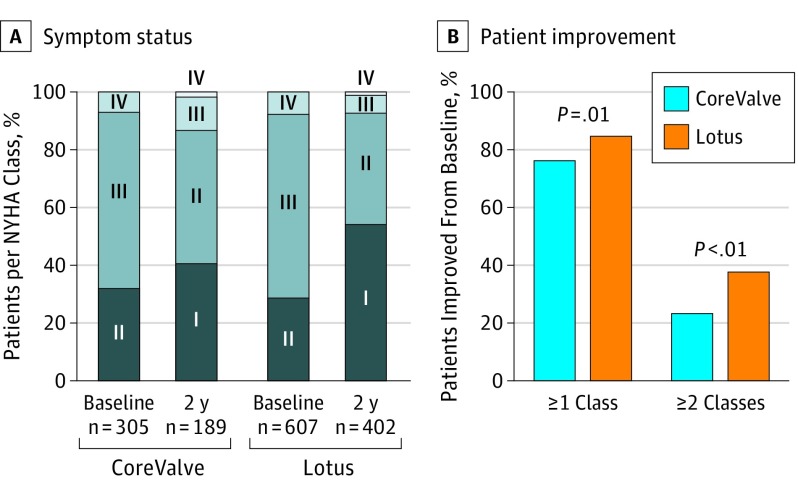
At baseline, most patients in either cohort had AS that was NYHA functional class III or IV (CoreValve, 207 [67.9%]; Lotus, 433 [71.3%]; Figure 4A). During follow-up, there were steady and stable improvements overall such that 164 of 189 patients who received CoreValve (86.6%) and 373 of 402 patients who received Lotus (92.8%) had AS that was functional class I or II at 2 years. More patients’ AS improved by 1 or more or 2 or more NYHA classes with Lotus compared with CoreValve (improved by 1 or more NYHA class: Lotus, 338 of 402 [84.1%] vs CoreValve, 143 of 189 [75.7]; P = .01; improved by 2 or more NYHA classes: 122 of 402 [37.3%] vs 65 of 305 [21.3%]; Figure 4B).
Figure 4. Symptom Status to 2 Years.
A, Symptom status according to New York Heart Association (NYHA) class shown at baseline and 2 years in the intention-to-treat patient population. B, The percentage of patients whose aortic stenosis improved at least 1 or at least 2 NYHA classes between baseline and 2 years is shown.
Discussion
The primary finding at 2 years is that, compared with the self-expanding CoreValve, the mechanically expandable Lotus valve has similar survival. Additionally, the composite of all-cause mortality or disabling stroke was also similar. Transcatheter aortic valve replacement with the self-expanding CoreValve in the high-risk population was seen to have superior survival to surgery at 1 and 2 years.3,4 Although a numerical survival advantage of 6.2% persisted for TAVR at 3 years, survival was no longer statistically superior.9 At 5-year follow-up, TAVR and surgery had similar survival rates in this trial.10 Survival at 2 years was dependent on how well AS was relieved in combination with the patient’s intrinsic risk level once procedural mortality was considered. The 2-year survival for the CoreValve high-risk randomized clinical trial and this trial are very similar (22.2% vs 22.5%, respectively) suggesting we recruited a similar risk population to the CoreValve high-risk trial (eFigure in Supplement 4). The 2-year survival rate of Lotus compared with a valve that was already shown to be superior to surgery at 2 years is encouraging.
Stroke has been a concern for TAVR. The Placement of Aortic Transcatheter Valve Trial (PARTNER) IA trial initially found that TAVR had a higher risk of stroke compared with surgery. A recent propensity-matched analysis of the PARTNER trials found a lower risk of early major stroke after transfemoral TAVR vs surgery.2,11 The composite of death and disabling stroke being similar at 2 years between these valves is therefore reassuring. Stroke was also examined as overall stroke and disabling stroke individually. Overall stroke at 2 years in the CoreValve trial for TAVR vs surgery was 10.9% vs 16.6% (P = .05), trending in favor of TAVR.4 All stroke in this trial for Lotus vs CoreValve was 8.4% vs 11.4% (P = .75). For the CoreValve trial at 2 years, disabling stroke for TAVR vs surgery was 6.8% vs 9.8% (P = .25)4 and in this trial for Lotus vs CoreValve was 4.7% vs 8.6% (P = .53). Because stroke is one of the more feared complications of both TAVR and surgery, these similar results to the self-expanding valve that tested well compared with surgery support the safety of the Lotus valve. The mechanism for the higher stroke rate in the CoreValve arm is unknown but could be due to the larger frame size and its interaction with the anatomy or the play of chance. Valve-related reinterventions at 2 years for Lotus vs CoreValve were 0.6% and 2.9% (P < .01). Most of these occurred in the first year and were catheter-based and related to moderate or severe PVL with no difference in the rate of rehospitalization. Rehospitalization for worsening NYHA class remained similar between the groups at 2 years. Although total pacemaker implantations were higher for Lotus at both 1 and 2 years, beyond the first year, the pacemaker requirement was actually less for Lotus (0.6% vs 2.9% CoreValve). The depth of valve implantation of the Lotus valve was directly related to risk for receiving a pacemaker.12 Valve thrombosis was mainly detected during routine follow-up and was higher at 2 years in Lotus (3.0% vs 0%; P < .01); 4 of 16 patients (25%) experienced clinical symptoms. In 11 cases, patients were treated with anticoagulants and the thrombosis resolved. In 4 cases, patients were treated with anticoagulants but information on response to therapy is not available, although none of these patients died or had a stroke. One patient was not able to take anticoagulants.
The self-expanding CoreValve has better forward flow dynamics, EOA, and mean gradient than the Lotus valve. The CoreValve demonstrated superior forward flow dynamics compared with surgery in the high-risk3 and intermediate-risk randomized trials.4 The self-expanding valve has a supra-annular valve design that helps explain these results. Core laboratory-adjudicated forward flow dynamics for the supra-annular CoreValve or Sapien 3 (S3; Edwards Lifesciences) at 30 days demonstrated a Doppler velocity index ranging from 0.42 to 0.40, respectively, in the smallest to largest S3 valves and 0.61 to 0.58 in the smallest to largest Evolut valves.13 The 30-day results from REPRISE III show a Doppler velocity index ranging from 0.45 to 0.48 and 0.65 to 0.58 going from the smallest to largest valves for Lotus and CoreValve, respectively.6 These results are similar to those seen at 2 years in this trial and suggest similar flow characteristics between the intra-annular S3 and Lotus valves. The Lotus valve had a lower PVL rate than the CoreValve at all points. Both valves showed significant improvement in NYHA at 2 years, with greater improvement seen in the Lotus group.
The CoreValve has received US Food and Drug Administration approval and has been widely adopted with good clinical results in real-world use as reported from the Transcatheter Valve Therapy registry.14 The longer-term results from REPRISE III validate good outcomes with the Lotus valve, which compares favorably with a currently approved predicate device.
Limitations
Lotus was compared, in part, with an early-generation CoreValve device that has been largely replaced by a newer-generation valve that may have less PVL. The durability of bioprosthetic valve performance will require longer-term follow-up. The current pacemaker rate is higher than what we consider acceptable as we move toward lower-risk patients. Changes in implantation technique and location, as well as improvements in valve design, may lower pacemaker rates.
Conclusions
After 2 years, REPRISE III demonstrates that all-cause mortality rates and all-cause mortality or disabling stroke with Lotus or CoreValve were similar. Disabling stroke, functional class, valve malpositioning, and PVL favored the Lotus arm, whereas valve hemodynamics, thrombosis, and new pacemaker implantation favored the CoreValve arm.
Trial Protocol.
Statistical Analysis Plan.
Data Sharing Statement.
eTable 1. Key baseline characteristics in REPRISE III
eTable 2. VARC-2 events between 0-1 and 1-2 years
eFigure. Mortality at 2 years in high-risk TAVR randomized trials
References
- 1.Kodali SK, Williams MR, Smith CR, et al. ; PARTNER Trial Investigators . Two-year outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med. 2012;366(18):1686-1695. doi: 10.1056/NEJMoa1200384 [DOI] [PubMed] [Google Scholar]
- 2.Smith CR, Leon MB, Mack MJ, et al. ; PARTNER Trial Investigators . Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364(23):2187-2198. doi: 10.1056/NEJMoa1103510 [DOI] [PubMed] [Google Scholar]
- 3.Adams DH, Popma JJ, Reardon MJ, et al. ; U.S. CoreValve Clinical Investigators . Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med. 2014;370(19):1790-1798. doi: 10.1056/NEJMoa1400590 [DOI] [PubMed] [Google Scholar]
- 4.Reardon MJ, Adams DH, Kleiman NS, et al. . 2-Year outcomes in patients undergoing surgical or self-expanding transcatheter aortic valve replacement. J Am Coll Cardiol. 2015;66(2):113-121. doi: 10.1016/j.jacc.2015.05.017 [DOI] [PubMed] [Google Scholar]
- 5.Feldman TE, Reardon MJ, Rajagopal V, et al. . Effect of mechanically expanded vs self-expanding transcatheter aortic valve replacement on mortality and major adverse clinical events in high-risk patients with aortic stenosis: the REPRISE III randomized clinical trial. JAMA. 2018;319(1):27-37. doi: 10.1001/jama.2017.19132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asch FM, Vannan MA, Singh S, et al. . Hemodynamic and echocardiographic comparison of the Lotus and CoreValve transcatheter aortic valves in patients with high and extreme surgical risk: an analysis from the REPRISE III randomized controlled trial. Circulation. 2018;137(24):2557-2567. doi: 10.1161/CIRCULATIONAHA.118.034129 [DOI] [PubMed] [Google Scholar]
- 7.Kappetein AP, Head SJ, Généreux P, et al. . Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. J Am Coll Cardiol. 2012;60(15):1438-1454. doi: 10.1016/j.jacc.2012.09.001 [DOI] [PubMed] [Google Scholar]
- 8.Leon MB, Piazza N, Nikolsky E, et al. . Standardized endpoint definitions for transcatheter aortic valve implantation clinical trials: a consensus report from the Valve Academic Research Consortium. J Am Coll Cardiol. 2011;57(3):253-269. doi: 10.1016/j.jacc.2010.12.005 [DOI] [PubMed] [Google Scholar]
- 9.Deeb GM, Reardon MJ, Chetcuti S, et al. ; CoreValve US Clinical Investigators . 3-Year outcomes in high-risk patients who underwent surgical or transcatheter aortic valve replacement. J Am Coll Cardiol. 2016;67(22):2565-2574. doi: 10.1016/j.jacc.2016.03.506 [DOI] [PubMed] [Google Scholar]
- 10.Gleason TG, Reardon MJ, Popma JJ, et al. . 5-Year outcomes of self-expanding transcatheter versus aortic valve replacement in high-risk patients. J Am Coll Cardiol. 2018;72(22):2696. doi: 10.1016/j.jacc.2018.08.2146 [DOI] [PubMed] [Google Scholar]
- 11.Kapadia SR, Huded CP, Kodali SK, et al. ; PARTNER Trial Investigators . Stroke after surgical versus transfemoral transcatheter aortic valve replacement in the PARTNER trial. J Am Coll Cardiol. 2018;72(20):2415-2426. doi: 10.1016/j.jacc.2018.08.2172 [DOI] [PubMed] [Google Scholar]
- 12.Falk V, Wöhrle J, Hildick-Smith D, et al. . Safety and efficacy of a repositionable and fully retrievable aortic valve used in routine clinical practice: the RESPOND Study. Eur Heart J. 2017;38(45):3359-3366. doi: 10.1093/eurheartj/ehx297 [DOI] [PubMed] [Google Scholar]
- 13.Hahn RT, Leipsic J, Douglas PS, et al. . Comprehensive echocardiographic assessment of normal transcatheter valve function. JACC Cardiovasc Imaging. 2018;12(1):25-34. doi: 10.1016/j.jcmg.2018.04.010 [DOI] [PubMed] [Google Scholar]
- 14.Sorajja P, Kodali S, Reardon MJ, et al. . Outcomes for the commercial use of self-expanding prostheses in transcatheter aortic valve replacement: a report from the STS/ACC TVT Registry. JACC Cardiovasc Interv. 2017;10(20):2090-2098. doi: 10.1016/j.jcin.2017.07.027 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol.
Statistical Analysis Plan.
Data Sharing Statement.
eTable 1. Key baseline characteristics in REPRISE III
eTable 2. VARC-2 events between 0-1 and 1-2 years
eFigure. Mortality at 2 years in high-risk TAVR randomized trials