Key Points
Question
What were the characteristics of International Study of Comparative Health Effectiveness With Medical and Invasive Approaches (ISCHEMIA) trial participants, and did qualification by stress imaging or nonimaging exercise tolerance test influence risk profiles?
Findings
In this analysis of randomized clinical trial data from the ISCHEMIA trial, randomized participants (n = 5179) were a median age of 64 years, 41% had diabetes, 19% had prior myocardial infarction, and 90% had prior angina. Stress imaging was the qualifying test for 75% (86% with moderate or severe ischemia); of those with coronary computed tomography angiography, 79% had multivessel, 87% had left anterior descending coronary artery, and 47% had proximal left anterior descending coronary artery disease.
Meaning
The International Study of Comparative Health Effectiveness With Medical and Invasive Approaches trial randomized stable patients with moderate or severe ischemia and predominantly multivessel and/or left anterior descending coronary artery disease
Abstract
Importance
It is unknown whether coronary revascularization, when added to optimal medical therapy, improves prognosis in patients with stable ischemic heart disease (SIHD) at increased risk of cardiovascular events owing to moderate or severe ischemia.
Objective
To describe baseline characteristics of participants enrolled and randomized in the International Study of Comparative Health Effectiveness With Medical and Invasive Approaches (ISCHEMIA) trial and to evaluate whether qualification by stress imaging or nonimaging exercise tolerance test (ETT) influenced risk profiles.
Design, Setting, and Participants
The ISCHEMIA trial recruited patients with SIHD with moderate or severe ischemia on stress testing. Blinded coronary computed tomography angiography was performed in most participants and reviewed by a core laboratory to exclude left main stenosis of at least 50% or no obstructive coronary artery disease (CAD) (<50% for imaging stress test and <70% for ETT). The study included 341 enrolling sites (320 randomizing) in 38 countries and patients with SIHD and moderate or severe ischemia on stress testing. Data presented were extracted on December 17, 2018.
Main Outcomes and Measures
Enrolled, excluded, and randomized participants’ baseline characteristics. No clinical outcomes are reported.
Results
A total of 8518 patients were enrolled, and 5179 were randomized. Common reasons for exclusion were core laboratory determination of insufficient ischemia, unprotected left main stenosis of at least 50%, or no stenosis that met study obstructive CAD criteria on study coronary computed tomography angiography. Randomized participants had a median age of 64 years, with 1168 women (22.6%), 1726 nonwhite participants (33.7%), 748 Hispanic participants (15.5%), 2122 with diabetes (41.0%), and 4643 with a history of angina (89.7%). Among the 3909 participants randomized after stress imaging, core laboratory assessment of ischemia severity (in 3901 participants) was severe in 1748 (44.8%), moderate in 1600 (41.0%), mild in 317 (8.1%) and none or uninterpretable in 236 (6.0%), Among the 1270 participants who were randomized after nonimaging ETT, core laboratory determination of ischemia severity (in 1266 participants) was severe (an eligibility criterion) in 1051 (83.0%), moderate in 101 (8.0%), mild in 34 (2.7%) and none or uninterpretable in 80 (6.3%). Among the 3912 of 5179 randomized participants who underwent coronary computed tomography angiography, 79.0% had multivessel CAD (n = 2679 of 3390) and 86.8% had left anterior descending (LAD) stenosis (n = 3190 of 3677) (proximal in 46.8% [n = 1749 of 3739]). Participants undergoing ETT had greater frequency of 3-vessel CAD, LAD, and proximal LAD stenosis than participants undergoing stress imaging.
Conclusions and Relevance
The ISCHEMIA trial randomized an SIHD population with moderate or severe ischemia on stress testing, of whom most had multivessel CAD.
Trial Registration
ClinicalTrials.gov Identifier: NCT01471522
This analysis of data from the International Study of Comparative Health Effectiveness With Medical and Invasive Approaches trial (ISCHEMIA) tdescribes baseline characteristics of participants enrolled and randomized in the trial and evaluates whether qualification by stress imaging or nonimaging exercise tolerance test influenced risk profiles.
Introduction
Prior strategy trials in patients with stable ischemic heart disease (SIHD) that evaluated routine revascularization added to optimal medical therapy (OMT), as compared with an initial conservative strategy of OMT alone, did not show a reduction in death or myocardial infarction (MI) with early revascularization.1,2,3 While these trials used more intensive medical therapy in contrast to trials conducted in the prior era, cardiac catheterization was performed prior to randomization such that the coronary anatomy was known to the physician, patient, and study team and may have introduced bias in patient selection and subsequent treatment. Moreover, patients were not selected based on a predefined threshold of baseline ischemia. It is possible that randomization prior to cardiac catheterization (to minimize selection bias) and inclusion of patients at higher cardiovascular risk (such as those with moderate or severe ischemia) may increase the likelihood of demonstrating that an initial invasive strategy improves prognosis in patients with SIHD, if any such benefit exists.
The primary aim of the International Study of Comparative Health Effectiveness with Medical and Invasive Approaches (ISCHEMIA) trial is to assess whether, in addition to OMT, an initial invasive strategy of cardiac catheterization and revascularization when feasible reduces clinical events as compared with an initial conservative strategy of OMT, with catheterization and revascularization reserved for failure of medical therapy, in patients with SIHD with moderate or severe ischemia on stress testing. The purpose of this report is to (1) outline reasons for exclusion of those who were enrolled but not randomized, (2) describe the baseline characteristics of the randomized population, and (3) describe the cohort randomized following stress imaging tests and nonimaging exercise tolerance tests (ETT).
Methods
Details of the study design have been published.4 The primary end point is the composite of cardiovascular death, MI, hospitalization for unstable angina, hospitalization for heart failure, or resuscitated cardiac arrest, all strictly defined and blindly adjudicated. A major secondary end point is the composite of cardiovascular death or MI. The first enrollment was in July 2012, with sequential initiation of 341 sites in 38 countries that enrolled and 320 sites that randomized participants (see eTable 1 and eTable 2 in Supplement 1 for a list of site personnel, committees, and study center personnel); the last randomization was on January 31, 2018. The projected mean follow-up is approximately 3.5 years, with a range of 1.5 to 7 years. The data in this manuscript are from a data extract on December 17, 2018. The data will not be final until database lock and therefore are subject to change. The formal trial protocols are detailed in Supplement 2. The study was approved by the New York University School of Medicine institutional review board on March 29, 2012. In addition, each enrolling site obtained approval from its local or national institutional review board or ethics committee that holds an institutional review board organization number from the US Department of Health and Human Services Office for Human Research Protections and complies with the Code of Federal Regulations title 45, part 46. Every participant was required to provide written consent prior to enrollment and randomization.
Patient Eligibility Criteria
Patients with SIHD and moderate or severe ischemia on clinically indicated stress imaging or severe ischemia on nonimaging ETT were eligible if they were clinically stable, including medically controlled angina or silent ischemia. Key exclusion criteria were estimated glomerular filtration rate (eGFR) less than 30 mL/min/1.73 m2, MI or unstable angina within 2 months, left ventricular ejection fraction less than 35%, unprotected left main stenosis of at least 50%, New York Heart Association class III to IV heart failure or exacerbation of chronic heart failure within 6 months, or unacceptable angina despite maximally tolerated medical therapy. The latter were evaluated primarily prior to enrollment, with confirmation before randomization when participants were asked about the frequency of angina. At least daily angina without ability to further titrate medical or antianginal therapy excluded the participant from randomization. Alternatively, prior to randomization, individuals were asked “How bothersome is it for you to take your pills for chest pain, chest tightness, or angina as prescribed?” Of 6 potential responses, participants who chose “extremely bothersome” were excluded. Assessment of eligibility based on ischemia severity was made locally, but stress studies were also read by independent core laboratories for each modality (nuclear, echocardiography, cardiac magnetic resonance, and ETT) either before or after randomization. Core laboratories educated sites about the target level of ischemia during site training. As detailed in the protocol and in the trial design report,4 the Clinical Coordinating Center (CCC) designated sites that were permitted to randomize participants based on site interpretation of the stress test result (ie, before stress core laboratory review). These sites were advised to submit stress test results for core laboratory review before randomization whenever there was local uncertainty about ischemia severity. Remaining sites were advised to delay randomization until the stress core laboratory confirmed moderate or severe ischemia and to exclude any enrolled participants with insufficient ischemia based on the interpretation of the core laboratory (eTable 3 in Supplement 1).5 To reduce workflow complexity and therefore enhance enrollment, in October 2014, many more sites were given permission to randomize participants based on accruing data on concordance with core laboratory interpretation. Therefore, some enrolled participants were excluded from randomization owing to insufficient ischemia as determined by core laboratory review, and some randomized participants were determined to have less than moderate ischemia by core laboratories after randomization, which was consistent with the protocol. Sites were monitored for concordance of stress test interpretation with the core laboratories. Corrective actions were taken for sites with discordant reads, including an education session with the core laboratory and/or requirement to wait for confirmation of ischemia severity before randomizing future participants. This operational workflow was intended to assure that most participants were considered by independent experts to have moderate or severe ischemia and to preserve trial power.
Coronary anatomic entry criteria were incorporated into the trial as follows: blinded coronary computed tomographic angiography (CCTA) was performed in most participants with eGFR of at least 60 mL/min/1.73 m2 who qualified based on stress imaging. Coronary CTA was not required in all participants, eg, those with eGFR less than 60 mL/min/1.73m2, known coronary anatomy, or other reasons with a low index of suspicion of left main stenosis.4 Study staff, participants, and their physicians were advised whether the blinded CCTA core-laboratory interpretation demonstrated anatomic eligibility, but no further details regarding extent and severity of coronary artery disease (CAD) were provided unless they were excluded from randomization.
Anatomic eligibility criteria after imaging stress tests required at least 50% stenosis in at least 1 major coronary artery. To avoid false-positives among participants who underwent nonimaging ETT and to maximize enrollment of patients at increased risk, either CCTA or, in a small number of participants, cardiac catheterization within the prior year was required for these participants. In addition, eligibility criteria for participants enrolled based on ETT required all of the following: (1) history of stable or exercise test-induced typical angina; (2) an interpretable resting electrocardiogram (eg, no resting ST-segment depression ≥1 mm and no left ventricular hypertrophy with repolarization abnormalities); (3) at least 2 leads showing new exercise-induced ST-segment depression of at least 1.5 mm or a single lead of at least 2 mm, or exercise-induced ST-segment elevation of at least 1.5 mm in a noninfarct territory, as compared to the baseline tracing, occurring at an early stage (≤7 METS) or at heart rate less than 75% of age-predicted maximum; and (4) demonstration of at least 70% stenosis in a coronary artery serving a large myocardial region, defined as the proximal or mid left anterior descending (LAD), proximal or mid right coronary artery, or proximal left circumflex or equivalent.4
Coronary Computed Tomography Angiography Analysis
Anatomic eligibility was assessed at an independent CCTA core laboratory prior to randomization, defined as the absence of at least 50% unprotected left main stenosis and presence of obstructive CAD using the stress test modality–specific definitions listed previously, based on a consensus of 2 readers, with a third reader as needed to resolve discrepancies. To maximize data on coronary anatomy, we subsequently collected available CCTA images (nonstudy CCTA) that were performed less than 1 year prior to enrollment for CCTA core laboratory review (n = 148). For each arterial segment, percent stenosis was categorized as 0%, 1% to 24%, 25% to 49%, 50% to 69%, 70% to 100%, or uninterpretable for severity of stenosis by the core laboratory. The principles guiding core laboratory review were to maintain participant safety with regard to exclusion of significant left main CAD and to diagnose the presence or absence of obstructive CAD in other major coronary arteries. The CCTA core laboratory director reviewed all cases to confirm the absence of significant unprotected left main stenosis. The core laboratory readers made their best attempt to characterize stenosis in any scans of suboptimal quality in this trial population, which included patients with conditions that could limit scan quality (eg, difficulty with breath holding or extensive coronary calcification).
The number of vessels diseased was determined for CCTA studies in which at least all of the following segments were interpretable for percent stenosis: left main, proximal and mid LAD, first diagonal, proximal left circumflex, first obtuse marginal, proximal and mid right coronary artery, and the posterior descending artery. In addition, the CCTA was included in the assessment of number of vessels diseased if the right coronary artery was evaluable and the left main or both the LAD and left circumflex had at least 50% stenosis. Coronary CTA studies were categorized as showing multivessel disease if the left main coronary artery had at least 50% stenosis and/or at least 2 of the LAD, left circumflex, and right coronary artery had at least 50% stenosis, even if 1 of these 3 coronary arteries could not be assessed for percent stenosis.
Randomization
Enrolled participants who met all eligibility criteria were randomized to an invasive or conservative strategy via an interactive web response system (IXRS; Almac Group). Details of the 2 strategies have been published.4
Statistical Analysis
Baseline characteristics are presented as medians (25th, 75th percentiles) of continuous variables and as counts (percentages) of categorical variables. Characteristics of randomized participants vs those excluded after enrollment and between participants qualifying on the basis of stress imaging vs ETT are shown in the tables. Hypothesis testing of between-group differences was not performed because these groups were known to differ as a result of different selection criteria. All analyses were performed using SAS, version 9.4 (SAS Institute Inc).
Results
Study Population
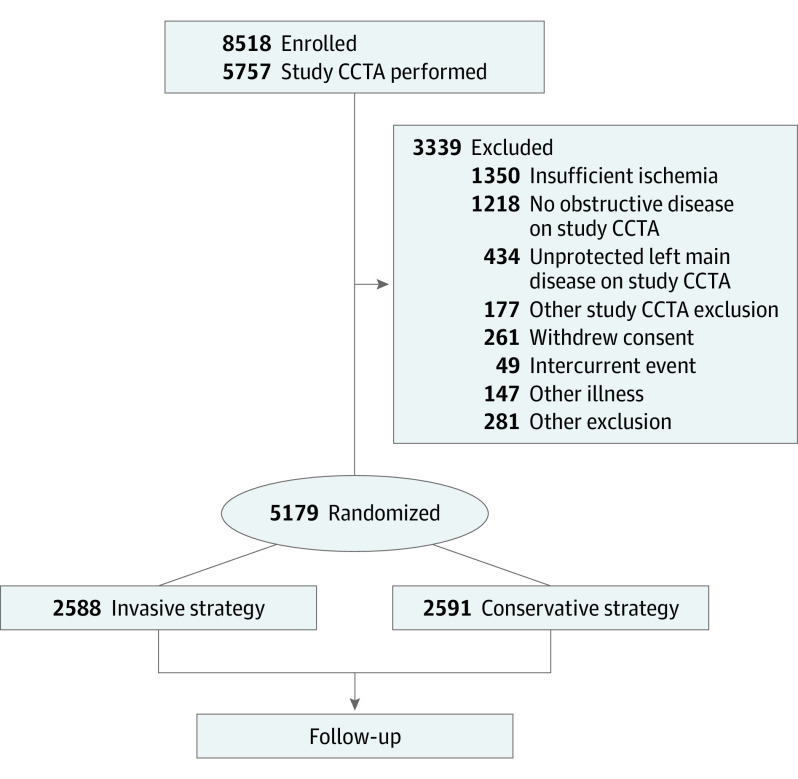
Of 8518 participants enrolled, 3339 failed eligibility screening and 5179 were randomized (61%): 2588 to the invasive and 2591 to the conservative strategy groups. Baseline characteristics of participants who were enrolled, excluded after enrollment, and randomized are shown in Table 1, with reasons for screen failure depicted in the Figure. The most common reasons for ineligibility in excluded participants were unprotected left main stenosis of at least 50% (n = 434), no significant obstructive CAD on study CCTA (n = 1218) defined according to imaging vs nonimaging stress test modality, or insufficient ischemia (n = 1350), as determined by the designated core laboratories when stress test results were reviewed prior to randomization. Randomized participants had similar demographics compared with eligible, nonrandomized participants, except the proportion of women was higher among those who were excluded after enrollment than among those who were randomized (n = 1094 [33%] vs n = 1168 [23%]) (Table 1). These differences were largely owing to higher frequency of insufficient ischemia on stress testing and of no obstructive disease on CCTA among women. The remaining description pertains to the randomized cohort.
Table 1. Enrolled, Excluded, and Randomized Participant Baseline Characteristics.
| Characteristic | No./Total No. (%) | ||
|---|---|---|---|
| Enrolled (n = 8518) | Excluded (n = 3339) | Randomized (n = 5179) | |
| Demographics | |||
| Age at enrollment, y | |||
| No. | 8518 | 3339 | 5179 |
| Median (IQR) | 63 (57-70) | 62 (55-68) | 64 (58-70) |
| Female | 2262/8518 (26.6) | 1094/3339 (32.8) | 1168/5179 (22.6) |
| Race | |||
| American Indian or Alaskan Native | 20/8433 (0.2) | 7/3304 (0.2) | 13/5129 (0.3) |
| Asian | 2568/8433 (30.5) | 1083/3304 (32.8) | 1485/5129 (29.0) |
| Native Hawaiian or other Pacific Islander | 19/8433 (0.2) | 7/3304 (0.2) | 12/5129 (0.2) |
| Black or African American | 410/8433 (4.9) | 206/3304 (6.2) | 204/5129 (4.0) |
| White | 5395/8433 (64.0) | 1992/3304 (60.3) | 3403/5129 (66.3) |
| Multiple races reported | 21/8433 (0.2) | 9/3304 (0.3) | 12/5129 (0.2) |
| Ethnicity | |||
| Hispanic or Latino | 1102/7924 (13.9) | 354/3109 (11.4) | 748/4815 (15.5) |
| Not Hispanic or Latino | 6822/7924 (86.1) | 2755/3109 (88.6) | 4067/4815 (84.5) |
| Clinical history | |||
| Hypertension | 6031/8474 (71.2) | 2242/3313 (67.7) | 3789/5161 (73.4) |
| Diabetes | 3128/8517 (36.7) | 1006/3338 (30.1) | 2122/5179 (41.0) |
| Prior myocardial infarction | 1354/8419 (16.1) | 364/3258 (11.2) | 990/5161 (19.2) |
| Cigarette smoking | |||
| Never | 3903/8276 (47.2) | 1694/3103 (54.6) | 2209/5173 (42.7) |
| Former | 3327/8276 (40.2) | 1002/3103 (32.3) | 2325/5173 (44.9) |
| Current | 1046/8276 (12.6) | 407/3103 (13.1) | 639/5173 (12.4) |
| Laboratory values | |||
| Estimated GFR from enrollment, mL/min | |||
| No. | 8518 | 3339 | 5179 |
| Median (IQR) | 82 (69-97) | 83 (72-98) | 81 (67-97) |
| Baseline ECG core laboratory interpretation | |||
| Q waves: meets UMI criteriaa | 555/4769 (11.6) | NA | 555/4769 (11.6) |
| ST-segment depression ≥0.5mmb | 514/4922 (10.4) | NA | 514/4922 (10.4) |
| T-wave inversion ≥1 mm | 399/4923 (8.1) | NA | 399/4923 (8.1) |
| Qualifying stress test core laboratory interpretation | |||
| Ischemia severity by imaging modality | |||
| Stress imaging overall | 5940/8517 (69.7) | 2031/3338 (60.8) | 3909/5179 (75.5) |
| Severity | |||
| Severe | 2279/5897 (38.6) | 531/1996 (26.6) | 1748/3901 (44.8) |
| Moderate | 2178/5897 (36.9) | 578/1996 (29.0) | 1600/3901 (41.0) |
| Mild | 812/5897 (13.8) | 495/1996 (24.8) | 317/3901 (8.1) |
| None | 607/5897 (10.3) | 381/1996 (19.1) | 226/3901 (5.8) |
| Uninterpretable | 21/5897 (0.4) | 11/1996 (0.6) | 10/3901 (0.3) |
| Exercise tolerance test | 2577/8517 (30.3) | 1307/3338 (39.2) | 1270/5179 (24.5) |
| Severity | |||
| Severe | 1715/2441 (70.3) | 664/1175 (56.5) | 1051/1266 (83.0) |
| Moderate | 345/2441 (14.1) | 244/1175 (20.8) | 101/1266 (8.0) |
| Mild | 109/2441 (4.5) | 75/1175 (6.4) | 34/1266 (2.7) |
| None | 108/2441 (4.4) | 80/1175 (6.8) | 28/1266 (2.2) |
| Uninterpretable | 164/2441 (6.7) | 112/1175 (9.5) | 52/1266 (4.1) |
| Nuclear | 3843/8517 (45.1) | 1276/3338 (38.2) | 2567/5179 (49.6) |
| Severity | |||
| Severe | 1177/3817 (30.8) | 207/1254 (16.5) | 970/2563 (37.8) |
| Moderatec | 1588/3817 (41.6) | 386/1254 (30.8) | 1202/2563 (46.9) |
| Mild | 565/3817 (14.8) | 352/1254 (28.1) | 213/2563 (8.3) |
| None | 468/3817 (12.3) | 298/1254 (23.8) | 170/2563 (6.6) |
| Uninterpretable | 19/3817 (0.5) | 11/1254 (0.9) | 8/2563 (0.3) |
| Echo | 1784/8517 (20.9) | 699/3338 (20.9) | 1085/5179 (20.9) |
| Severity | |||
| Severe | 926/1769 (52.3) | 300/688 (43.6) | 626/1081 (57.9) |
| Moderatec | 500/1769 (28.3) | 184/688 (26.7) | 316/1081 (29.2) |
| Mild | 218/1769 (12.3) | 130/688 (18.9) | 88/1081 (8.1) |
| None | 123/1769 (7.0) | 74/688 (10.8) | 49/1081 (4.5) |
| Uninterpretable | 2/1769 (0.1) | 0/688 (0.0) | 2/1081 (0.2) |
| CMR | 313/8517 (3.7) | 56/3338 (1.7) | 257/5179 (5.0) |
| Severity | |||
| Severe | 176/311 (56.6) | 24/54 (44.4) | 152/257 (59.1) |
| Moderate | 90/311 (28.9) | 8/54 (14.8) | 82/257 (31.9) |
| Mild | 29/311 (9.3) | 13/54 (24.1) | 16/257 (6.2) |
| None | 16/311 (5.1) | 9/54 (16.7) | 7/257 (2.7) |
| Uninterpretable | 0/311 (0.0) | 0/54 (0.0) | 0/257 (0.0) |
| CCTA findingsd | |||
| Any obstructive disease ≥50% stenosis by CCTA | 4672/5402 (86.5) | 840/1566 (53.6) | 3832/3836 (99.9) |
| Multivessel disease ≥50% stenosis by CCTA | 3348/5111 (65.5) | 669/1721 (38.9) | 2679/3390 (79.0) |
| Vessels ≥50% stenosis by CCTA | |||
| 0 | 730/4375 (16.7) | 726/1389 (52.3) | 4/2986 (0.1) |
| 1 | 785/4375 (17.9) | 88/1389 (6.3) | 697/2986 (23.3) |
| 2 | 1081/4375 (24.7) | 143/1389 (10.3) | 938/2986 (31.4) |
| ≥3 | 1779/4375 (40.7) | 432/1389 (31.1) | 1347/2986 (45.1) |
| Specific native vessels with ≥50% stenosis by CCTA | |||
| Left main | 501/5757 (8.7) | 461/1912 (24.1) | 40/3845 (1.0) |
| Left anterior descending | 3893/5417 (71.9) | 703/1740 (40.4) | 3190/3677 (86.8) |
| Proximal LAD | 2248/5633 (39.9) | 499/1894 (26.3) | 1749/3739 (46.8) |
| Left circumflex | 2889/5172 (55.9) | 535/1677 (31.9) | 2354/3495 (67.4) |
| Right coronary artery | 2844/4956 (57.4) | 533/1597 (33.4) | 2311/3359 (68.8) |
Abbreviations: CCTA, coronary computed tomographic angiography; CMR, cardiac magnetic resonance; ECG, electrocardiogram; ETT, exercise tolerance test; GFR, glomerular filtration rate; IQR, interquartile range; LAD, left anterior descending; MI, myocardial infarction; NA, not applicable; UMI, universal definition of myocardial infarction.
Meets universal definition of MI in 2 leads in at least 1 territory (anterior, inferior, or lateral).
Protocol criteria to enter through the ETT pathway required absence of resting ST-segment depression of at least 1 mm at the time of ETT. The baseline prerandomization ECG was not always acquired on the day of the ETT.
The determination of moderate ischemia by the core laboratory included ancillary criteria (eg, heart rate and workload) for 26 randomized and 4 excluded participants who were enrolled after stress nuclear and for 30 randomized and 6 excluded participants who were enrolled after stress echocardiography.
Includes study and nonstudy CCTAs. Enrolled participants with study CCTA: 5757. Enrolled participants with nonstudy CCTA: 148. Randomized participants with any study or nonstudy CCTA: 3912. These numbers represent 50% stenosis criteria for all stress test modalities, in contrast to the Figure, which shows excluded participants based on those who did not meet the differential 50% and 70% stenosis criteria for imaging and nonimaging stress tests, respectively.
Figure. Participant Flow From Enrollment to Randomization.
Those who were excluded after consent and enrollment are indicated in the right side box. An enrolled participant may have more than 1 reason for being excluded before randomization and therefore may be counted in more than 1 screen failure category (n = 594). Coronary computed tomography angiography (CCTA) was not required in all participants, eg, those with estimated glomerular filtration rate of less than 60 mL/min/1.73 m2 or catheterization or CCTA within the prior year. To maximize information about baseline coronary anatomy, we subsequently collected available CCTA images that were performed less than 1 year prior to enrollment for CCTA core laboratory review (n = 148). No obstructive disease was defined as no epicardial coronary artery stenosis at least 50% on study CCTA for participants who were enrolled after stress imaging or no stenosis at least 70% in specified segments for participants who were enrolled after exercise tolerance test.4 Unprotected left main disease was defined as at least 50% stenosis on study CCTA. Incidental findings were defined as incidental findings on study CCTA of sufficient clinical importance that site investigators did not find randomization appropriate.
Baseline Characteristics
Clinical characteristics of the randomized population are shown in Table 2, stratified according to whether the patient qualified for the trial by stress imaging or ETT. Median age was 64 years, with a high prevalence of diabetes (n = 2122 of 5179 [41.0%]) and hypertension (n = 3789 of 5161 [73.4%]). Overall, 990 of 5161 (19.2%) had prior MI. Left ventricular ejection fraction was normal in most participants. Most participants had a history of angina prior to enrollment, with only 536 of 5179 (10.3%) reporting no history of angina, ie, silent ischemia, and 4138 of 5177 (79.9%) reporting angina within the last month (Table 3). The median Seattle Angina Questionnaire angina frequency score (range 0-100, higher more favorable) was 80. New or worsening angina was reported in the prior 3 months in 1358 of 4637 participants (29.3%), with new onset of angina within that time in 858 of 4918 (17.4%). Only 206 of 5179 (4.0%) had a history of heart failure. Study team assessment of heart failure symptoms, eg, dyspnea on exertion, in all randomized participants the month prior to enrollment revealed that 2015 of 5179 (38.9%) had New York Heart Association class I to II symptoms.
Table 2. Randomized Participant Baseline Characteristics and Clinical History Intent-to-Treat Population, Imaging Stress Test vs Exercise Tolerance Test.
| Characteristic | No./ Total No. (%) | ||
|---|---|---|---|
| Total (n = 5179) | Imaging Stress Test (n = 3909) | Exercise Tolerance Test (n = 1270) | |
| Demographics | |||
| Age at randomization, y | |||
| No. | 5179 | 3909 | 1270 |
| Median (IQR) | 64 (58-70) | 66 (59-72) | 60 (54-66) |
| Female | 1168/5179 (22.6) | 924/3909 (23.6) | 244/1270 (19.2) |
| Race | |||
| American Indian or Alaskan Native | 13/5129 (0.3) | 11/3865 (0.3) | 2/1264 (0.2) |
| Asian | 1485/5129 (29.0) | 557/3865 (14.4) | 928/1264 (73.4) |
| Native Hawaiian or other Pacific Islander | 12/5129 (0.2) | 10/3865 (0.3) | 2/1264 (0.2) |
| Black or African American | 204/5129 (4.0) | 192/3865 (5.0) | 12/1264 (0.9) |
| White | 3403/5129 (66.3) | 3087/3865 (79.9) | 316/1264 (25.0) |
| Multiple races reported | 12/5129 (0.2) | 8/3865 (0.2) | 4/1264 (0.3) |
| Ethnicity | |||
| Hispanic or Latino | 748/4815 (15.5) | 682/3687 (18.5) | 66/1128 (5.9) |
| Not Hispanic or Latino | 4067/4815 (84.5) | 3005/3687 (81.5) | 1062/1128 (94.1) |
| Clinical history | |||
| Hypertension | 3789/5161 (73.4) | 3060/3895 (78.6) | 729/1266 (57.6) |
| Diabetes | 2122/5179 (41.0) | 1579/3909 (40.4) | 543/1270 (42.8) |
| Diabetes treatment | |||
| Insulin treated | 491/2110 (23.3) | 424/1574 (26.9) | 67/536 (12.5) |
| Noninsulin diabetes medication | 1351/2110 (64.0) | 962/1574 (61.1) | 389/536 (72.6) |
| None/diet controlled | 268/2110 (12.7) | 188/1574 (11.9) | 80/536 (14.9) |
| Prior myocardial infarction | 990/5161 (19.2) | 887/3894 (22.8) | 103/1267 (8.1) |
| Cigarette smoking | |||
| Never | 2209/5173 (42.7) | 1535/3906 (39.3) | 674/1267 (53.2) |
| Former | 2325/5173 (44.9) | 1876/3906 (48.0) | 449/1267 (35.4) |
| Current | 639/5173 (12.4) | 495/3906 (12.7) | 144/1267 (11.4) |
| Family history of premature coronary heart disease | 1170/4490 (26.1) | 914/3324 (27.5) | 256/1166 (22.0) |
| Prior cardiac catheterizationa | 1898/5179 (36.6) | 1607/3909 (41.1) | 291/1270 (22.9) |
| Time from most recent cardiac catheterization to enrollment, y | |||
| No. | 1726 | 1447 | 279 |
| Median (IQR) | 1.7 (0.2-4.9) | 1.9 (0.2-5.4) | 0.3 (0.0-3.4) |
| Prior percutaneous coronary intervention | 1050/5175 (20.3) | 923/3906 (23.6) | 127/1269 (10.0) |
| Time from most recent PCI to randomization, y | |||
| No. | 920 | 806 | 114 |
| Median (IQR) | 4.2 (2.1-7.8) | 4.2 (2.1-7.8) | 4.4 (2.5-8.0) |
| Prior coronary artery bypass graft | 200/5179 (3.9) | 190/3909 (4.9) | 10/1270 (0.8) |
| Time from most recent CABG to randomization, y | |||
| No. | 158 | 148 | 10 |
| Median (IQR) | 8.5 (4.4-14.4) | 8.8 (4.2-14.5) | 7.1 (5.3-14.0) |
| Prior MI or prior PCI or prior CABG | 1566/5161 (30.3) | 1378/3895 (35.4) | 188/1266 (14.8) |
| Prior heart failure | 206/5179 (4.0) | 184/3909 (4.7) | 22/1270 (1.7) |
| Atrial fibrillation/atrial flutter | 221/5173 (4.3) | 211/3903 (5.4) | 10/1270 (0.8) |
| Noncardiac vascular and comorbidity history | |||
| Prior carotid artery surgery or stent, prior stroke, or prior TIA | 378/5165 (7.3) | 334/3898 (8.6) | 44/1267 (3.5) |
| Prior stroke | 150/5178 (2.9) | 135/3908 (3.5) | 15/1270 (1.2) |
| Prior peripheral artery disease | 205/5168 (4.0) | 195/3902 (5.0) | 10/1266 (0.8) |
| Prior surgery or percutaneous procedure for PAD | 98/5159 (1.9) | 91/3893 (2.3) | 7/1266 (0.6) |
Abbreviations: CABG, coronary artery bypass graft surgery; IQR, interquartile range; MI, myocardial infarction; PAD, peripheral artery disease; PCI, percutaneous coronary intervention.
Cardiac catheterization prior to enrollment.
Table 3. Randomized Participant Angina and Heart Failure History Intent-to-Treat Population, Imaging Stress Test vs Exercise Tolerance Test.
| Characteristic | No./Total No. (%) | ||
|---|---|---|---|
| Total (n = 5179) | Imaging Stress Test (n = 3909) | Exercise Tolerance Test (n = 1270) | |
| Baseline Seattle Angina Questionnaire Angina Frequency scale | |||
| No. | 5108 | 3861 | 1247 |
| Median (IQR) | 80 (70-100) | 90 (70-100) | 80 (60-90) |
| Baseline Seattle Angina Questionnaire Angina Frequency scale score | |||
| Daily (0-30) | 116/5108 (2.3) | 105/3861 (2.7) | 11/1247 (0.9) |
| Weekly (31-60) | 995/5108 (19.5) | 615/3861 (15.9) | 380/1247 (30.5) |
| Monthly (61-99) | 2241/5108 (43.9) | 1641/3861 (42.5) | 600/1247 (48.1) |
| No angina in past month (100) | 1756/5108 (34.4) | 1500/3861 (38.9) | 256/1247 (20.5) |
| Participant has ever had angina | 4643/5179 (89.7) | 3386/3909 (86.6) | 1257/1270 (99.0) |
| Angina during the past mo | |||
| None | 1039/5177 (20.1) | 971/3908 (24.8) | 68/1269 (5.4) |
| CCS Angina Class I | 1384/5177 (26.7) | 1131/3908 (28.9) | 253/1269 (19.9) |
| CCS Angina Class II | 2524/5177 (48.8) | 1612/3908 (41.2) | 912/1269 (71.9) |
| CCS Angina Class III | 230/5177 (4.4) | 194/3908 (5.0) | 36/1269 (2.8) |
| CCS Angina Class IV | 0/5177 | 0/3908 | 0/1269 |
| New onset of angina during the past 3 mo | 858/4918 (17.4) | 547/3668 (14.9) | 311/1250 (24.9) |
| Angina began or became more frequent during the past 3 mo | 1358/4637 (29.3) | 961/3380 (28.4) | 397/1257 (31.6) |
| Prior heart failure | 206/5179 (4.0) | 184/3909 (4.7) | 22/1270 (1.7) |
| Ejection fractiona | |||
| No. | 4637 | 3673 | 964 |
| Median (IQR) | 60 (55-65) | 60 (55-65) | 60 (60-65) |
| Heart failure status over the past mo | |||
| None | 3164/5179 (61.1) | 2305/3909 (59.0) | 859/1270 (67.6) |
| NYHA Class I | 999/5179 (19.3) | 820/3909 (21.0) | 179/1270 (14.1) |
| NYHA Class II | 1016/5179 (19.6) | 784/3909 (20.1) | 232/1270 (18.3) |
| NYHA Class III | 0/5179 | 0/3909 | 0/1270 |
| NYHA Class IV | 0/5179 | 0/3909 | 0/1270 |
Abbreviations: CCS, Canadian Cardiovascular Society; IQR, interquartile range.
Site-reported value, if available. If not available, then core laboratory entered value.
Stress Test Findings
Of those randomized, the qualifying stress test modality was stress imaging in 3909 (75%); the remainder were nonimaging ETTs. Core laboratories judged that the trial-required level of ischemia was met (moderate or severe for imaging test results and severe for ETT) in 4399 of 5167 randomized participants (85%). Among the cohort randomized via stress imaging, 1748 of 3901 had severe ischemia (44.8%), 1600 of 3901 had moderate ischemia (41.0%), 317 of 3901 had mild ischemia (8.1%), and 236 of 3901 had no ischemia or were uninterpretable on core laboratory review (6.0%). Among the cohort randomized via ETT, 1051 of 1266 (83.0%) had severe ischemia, 101 of 1266 (8.0%) had moderate ischemia, 34 of 1266 (2.7%) had mild ischemia, and 80 of 1266 (6.3%) had no ischemia or were uninterpretable on core laboratory review. A higher proportion of participants who had ETT had severe ischemia because we defined the criteria to qualify by ETT as severe ischemia (Table 1 and Table 4).
Table 4. Randomized Participant Stress Test and Coronary Computed Tomographic Angiography Findings Intent-to-Treat Population, Imaging Stress Test vs Exercise Tolerance Test.
| Characteristic, Stress Test Summary | No./Total No. (%) | ||
|---|---|---|---|
| Total (n = 5179) | Imaging Stress Test (n = 3909) | Exercise Tolerance Test (n = 1270) | |
| Core laboratory summary, baseline ischemia | |||
| Severe | 2799/5167 (54.2) | 1748/3901 (44.8) | 1051/1266 (83.0) |
| Moderate | 1701/5167 (32.9) | 1600/3901 (41.0) | 101/1266 (8.0) |
| Mild | 351/5167 (6.8) | 317/3901 (8.1) | 34/1266 (2.7) |
| None | 254/5167 (4.9) | 226/3901 (5.8) | 28/1266 (2.2) |
| Uninterpretable | 62/5167 (1.2) | 10/3901 (0.3) | 52/1266 (4.1) |
| CCTA findingsa | |||
| Any obstructive disease ≥50% stenosis by CCTA | 3832/3836 (99.9) | 2785/2786 (>99.9) | 1047/1050 (99.7) |
| Multivessel disease ≥50% stenosis by CCTA | 2679/3390 (79.0) | 1923/2441 (78.8) | 756/949 (79.7) |
| Vessels ≥50% stenosis by CCTA | |||
| 0 | 4/2986 (0.1) | 1/2147 (0.0) | 3/839 (0.4) |
| 1 | 697/2986 (23.3) | 508/2147 (23.7) | 189/839 (22.5) |
| 2 | 938/2986 (31.4) | 699/2147 (32.6) | 239/839 (28.5) |
| ≥3 | 1347/2986 (45.1) | 939/2147 (43.7) | 408/839 (48.6) |
| Specific native vessels with ≥50% stenosis by CCTA | |||
| Left main | 40/3845 (1.0) | 37/2794 (1.3) | 3/1051 (0.3) |
| Left anterior descending | 3190/3677 (86.8) | 2286/2659 (86.0) | 904/1018 (88.8) |
| Proximal LAD | 1749/3739 (46.8) | 1189/2711 (43.9) | 560/1028 (54.5) |
| Left circumflex | 2354/3495 (67.4) | 1690/2546 (66.4) | 664/949 (70.0) |
| Right coronary artery | 2311/3359 (68.8) | 1668/2417 (69.0) | 643/942 (68.3) |
Abbreviations: CCTA, coronary computed tomographic angiography; LAD, left anterior descending.
Includes study and nonstudy CCTAs.
Coronary Artery Anatomic Findings
Coronary CTA was not performed in some randomized participants owing to low eGFR (n = 568 of 5179 [11.0%]) known coronary anatomy (n = 570 of 5179 [11.0%]), or other reasons (n = 129 of 5179 [2.5%]). In the randomized cohort that had CCTA reviewed by the core laboratory (3912 of 5179 [76%], includes study and nonstudy CCTA), 99.9% (n = 3832 of 3836) had at least 50% stenosis of at least 1 vessel and 79.0% (n = 2679 of 3390) had multivessel CAD. Among the 2986 participants with CCTA evaluable for number of vessels diseased, 1-vessel, 2-vessel, and 3-vessel CAD was observed in 697 (23.3%), 938 (31.4%), and 1347 (45.1%) participants, respectively; 86.8% had LAD stenosis (n = 3190 of 3677), and 46.8% had proximal LAD stenosis (n = 1749 of 3739) (Table 4).
Stress Imaging vs ETT
As compared with those who qualified based on stress imaging, those randomized based on ETT were younger, less often of white race and non-Hispanic ethnicity owing to more frequent use of ETT at Asian sites, and had fewer comorbidities including a lower prevalence of hypertension, prior MI, prior PCI, prior coronary artery bypass grafting, and prior stroke (Table 4). However, a higher proportion of participants randomized based on ETT had 3-vessel disease and disease in the LAD, including the proximal LAD, compared with those who qualified via stress imaging (Table 4).
Vital Signs, Laboratory Tests, and Medications
Median baseline systolic blood pressure was 130 mm Hg (interquartile range [IQR], 120-142 mm Hg), and median low-density lipoprotein cholesterol level was 83 mg/dL (IQR, 63-111 mg/dL; to convert to micromoles per liter, multiply by 0.0259). Most participants had normal eGFR, with median 81 mL/min/1.73m2 (IQR, 67-97 mL/min/1.73m2) (eTable 4 in Supplement 1). Medication use at baseline is shown in eTable 5 in Supplement 1. Almost all were receiving antiplatelet or anticoagulant therapy, statin therapy, and at least 1 antihypertensive/anti-ischemic/antianginal medication.
Discussion
The ISCHEMIA trial randomized an SIHD population with median age of 64 years, 23% of whom were women (n = 1168 of 5179) and 41% of whom had diabetes (n = 2122 of 5179). Most participants (n = 3909 [75%]) had stress imaging tests, with confirmation of severe ischemia in 1748 of 3901 (44.8%) and moderate ischemia in 1600 of 3901 (41.0%) by independent imaging core laboratories. Eligibility for randomization by nonimaging ETT criteria required anginal symptoms, ischemia at an early workload, and at least 70% stenosis in a major non–left main coronary artery. Consequently, those randomized after ETT had more extensive anatomic CAD. The independent core laboratory confirmed that ETT trial entry criteria were met in 83%. Consistent with the design to recruit patients with at least moderate ischemia on stress imaging and severe ischemia with ETT, of all randomized participants who underwent CCTA, most had multivessel CAD and/or LAD stenosis, including proximal LAD in nearly half. Of note, those randomized following ETT had slightly higher rates of 3-vessel disease, LAD, and proximal LAD stenosis on CCTA than those randomized based on stress imaging, with a similar overall rate of multivessel CAD, thereby confirming that the eligibility criteria required for participants who underwent ETT achieved the objective of randomizing patients with at least as much CAD as those randomized based on imaging. Those randomized following ETT more often had angina compared with participants undergoing stress imaging because angina was required for enrollment following ETT. Finally, core laboratories judged that the trial-required level of ischemia was met in 85% of participants, despite frequent randomization before core laboratory review.
The most common reasons for exclusion in ISCHEMIA were associated with the key entry criteria for stress testing and CCTA. Consistent with the high degree of ischemia required for study entry, 8% of enrolled participants who had a study CCTA failed eligibility owing to significant unprotected left main coronary artery disease. Even with this degree of ischemia, a modest proportion had no stenosis of 50% or greater on CCTA, highlighting the differences between anatomic evidence of epicardial coronary artery disease and physiologic evidence of ischemia; false-positive test results may have also contributed to this finding. Women were more likely than men to be excluded from randomization after enrollment, particularly owing to nonobstructive CAD.
Most previous studies have demonstrated that the extent and severity of ischemia is associated with an increased risk for death and MI, and that revascularization is associated with better prognosis in these patients.6,7,8,9,10,11,12 For example, Hachamovitch et al6 reported an observational study of 13 969 patients with a range of core laboratory quantitated inducible ischemia by single-photon emission computed tomography and mean 8.7-year follow-up. They reported improved survival for those selected for revascularization compared with those who did not undergo revascularization but only for those with at least 10% ischemia.6 Studies using other stress modalities reported similar findings of an association of more ischemia and higher risk of death or MI.11,13,14,15,16,17,18 However, even in propensity-matched cohorts there is residual confounding, and a clinical trial was needed to verify this finding. Moreover, these patients did not receive OMT by contemporary standards, while in ISCHEMIA, the median baseline low-density lipoprotein cholesterol level was 83 mg/dL, with high rates of use of aspirin and potent statins, and this was prior to intensification of medical therapy when needed to achieve risk factor goals.
The principal objective of ISCHEMIA is to determine whether an initial invasive strategy of cardiac catheterization followed by routine revascularization, if feasible, plus OMT in participants with moderate or severe ischemia will reduce adverse ischemia-related events by a clinically meaningful amount compared with an initial conservative strategy of OMT alone, with catheterization and revascularization reserved for failure of medical therapy. In prior trials testing the incremental value of routine revascularization for SIHD, patients were enrolled without a specified level or quantitative threshold of ischemia required on a stress test.1,2,3 In the ISCHEMIA trial, to maximize applicability of study results globally, participants could qualify based on stress nuclear, echocardiographic or cardiac magnetic resonance, or nonimaging ETT. These modalities detect ischemia in inherently different ways; for example, nuclear imaging and cardiac magnetic resonance definitions used in the trial assess perfusion, while echo assesses wall motion.
Numerous studies have also reported an association between the presence of more extensive anatomic CAD, including proximal LAD stenosis, and higher risk of death or MI.19,20,21 However, prior randomized trials in patients with SIHD failed to demonstrate that routine revascularization reduced that risk. In these trials, patients were selected for enrollment after diagnostic catheterization. A possible explanation, in part, for the neutral findings of those trials is that they effectively (although not necessarily intentionally) limited inclusion of higher-risk patients or that knowledge of coronary anatomy prior to randomization introduced potential bias by excluding higher-risk patients who might have derived benefit from a strategy of routine revascularization. In contrast, ISCHEMIA was designed to assess whether an initial invasive strategy improves clinical outcomes in stable patients at higher risk of cardiovascular events based on more severe ischemia, without contemporaneous knowledge of their coronary anatomy.
One of the key design objectives of ISCHEMIA was to select a population that would include participants who, if randomized to the conservative strategy, would be less likely to undergo elective catheterization and revascularization after randomization (ie, crossover). Thus, ISCHEMIA seeks to study the prognostic benefits of catheterization and revascularization separated to the extent possible from the use of these procedures for symptom control. Hence, most ISCHEMIA participants had mild-to-moderate angina at baseline, 10% had no history of angina, and approximately 20% had silent ischemia at the time of randomization. The baseline median Seattle Angina Questionnaire angina frequency score of 80 is consistent with monthly angina.22
Limitations
The data in this article are from a data extract on December 17, 2018. The data will not be final until database lock and, therefore, are subject to change.
Conclusions
Based on the demographic and clinical profile of participants randomized in ISCHEMIA, the results will inform the care of a broad range of patients with SIHD with moderate or severe ischemia on stress testing, including those with multivessel CAD and angina who can be treated medically without revascularization for symptom control. For such patients, the trial will provide robust and contemporary scientific evidence regarding the risk of clinically important events with a routine early invasive vs initial conservative management strategy.
eTable 1. Site Personnel
eTable 2. Committees, CCC, Study Centers Personnel
eTable 3. Ischemia Definitions
eTable 4. Randomized Participant Baseline Vital Signs and Laboratory Values Intent-to-Treat Population
eTable 5. Randomized Participant Baseline Medications Intent-to-Treat Population
Trial Protocol
Data Sharing Statement.
References
- 1.Boden WE, O’Rourke RA, Teo KK, et al. ; COURAGE Trial Research Group . Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356(15):1503-1516. doi: 10.1056/NEJMoa070829 [DOI] [PubMed] [Google Scholar]
- 2.Frye RL, August P, Brooks MM, et al. ; BARI 2D Study Group . A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med. 2009;360(24):2503-2515. doi: 10.1056/NEJMoa0805796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Bruyne B, Pijls NH, Kalesan B, et al. ; FAME 2 Trial Investigators . Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N Engl J Med. 2012;367(11):991-1001. doi: 10.1056/NEJMoa1205361 [DOI] [PubMed] [Google Scholar]
- 4.Maron DJ, Hochman JS, O’Brien SM, et al. ; ISCHEMIA Trial Research Group . International Study of Comparative Health Effectiveness with Medical and Invasive Approaches (ISCHEMIA) trial: rationale and design. Am Heart J. 2018;201:124-135. doi: 10.1016/j.ahj.2018.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shaw LJ, Berman DS, Picard MH, et al. ; National Institutes of Health/National Heart, Lung, and Blood Institute-Sponsored ISCHEMIA Trial Investigators . Comparative definitions for moderate-severe ischemia in stress nuclear, echocardiography, and magnetic resonance imaging. JACC Cardiovasc Imaging. 2014;7(6):593-604. doi: 10.1016/j.jcmg.2013.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hachamovitch R, Hayes SW, Friedman JD, Cohen I, Berman DS. Comparison of the short-term survival benefit associated with revascularization compared with medical therapy in patients with no prior coronary artery disease undergoing stress myocardial perfusion single photon emission computed tomography. Circulation. 2003;107(23):2900-2907. doi: 10.1161/01.CIR.0000072790.23090.41 [DOI] [PubMed] [Google Scholar]
- 7.Hachamovitch R, Rozanski A, Shaw LJ, et al. Impact of ischaemia and scar on the therapeutic benefit derived from myocardial revascularization vs. medical therapy among patients undergoing stress-rest myocardial perfusion scintigraphy. Eur Heart J. 2011;32(8):1012-1024. doi: 10.1093/eurheartj/ehq500 [DOI] [PubMed] [Google Scholar]
- 8.Hachamovitch R, Kang X, Amanullah AM, et al. Prognostic implications of myocardial perfusion single-photon emission computed tomography in the elderly. Circulation. 2009;120(22):2197-2206. doi: 10.1161/CIRCULATIONAHA.108.817387 [DOI] [PubMed] [Google Scholar]
- 9.Sorajja P, Chareonthaitawee P, Rajagopalan N, et al. Improved survival in asymptomatic diabetic patients with high-risk SPECT imaging treated with coronary artery bypass grafting. Circulation. 2005;112(9)(suppl):I311-I316. [DOI] [PubMed] [Google Scholar]
- 10.Johnson NP, Tóth GG, Lai D, et al. Prognostic value of fractional flow reserve: linking physiologic severity to clinical outcomes. J Am Coll Cardiol. 2014;64(16):1641-1654. doi: 10.1016/j.jacc.2014.07.973 [DOI] [PubMed] [Google Scholar]
- 11.Yao SS, Bangalore S, Chaudhry FA. Prognostic implications of stress echocardiography and impact on patient outcomes: an effective gatekeeper for coronary angiography and revascularization. J Am Soc Echocardiogr. 2010;23(8):832-839. doi: 10.1016/j.echo.2010.05.004 [DOI] [PubMed] [Google Scholar]
- 12.Hachamovitch R, Rozanski A, Hayes SW, et al. Predicting therapeutic benefit from myocardial revascularization procedures: are measurements of both resting left ventricular ejection fraction and stress-induced myocardial ischemia necessary? J Nucl Cardiol. 2006;13(6):768-778. doi: 10.1016/j.nuclcard.2006.08.017 [DOI] [PubMed] [Google Scholar]
- 13.Zagatina A, Krylova L, Vareldzhan Y, Tyurina TV, Clitsenko O, Zhuravskaya N. Comparison of 5-year outcomes for patients with coronary artery disease in groups with and without revascularization with different results of stress echocardiography. Cardiol Res. 2013;4(4-5):152-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaibazzi N, Porter T, Lorenzoni V, et al. Effect of coronary revascularization on the prognostic value of stress myocardial contrast wall motion and perfusion imaging. J Am Heart Assoc. 2017;6(6):e006202. doi: 10.1161/JAHA.117.006202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bourque JM, Beller GA. Value of exercise ECG for risk stratification in suspected or known CAD in the era of advanced imaging technologies. JACC Cardiovasc Imaging. 2015;8(11):1309-1321. doi: 10.1016/j.jcmg.2015.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mark DB, Hlatky MA, Harrell FE Jr, Lee KL, Califf RM, Pryor DB. Exercise treadmill score for predicting prognosis in coronary artery disease. Ann Intern Med. 1987;106(6):793-800. doi: 10.7326/0003-4819-106-6-793 [DOI] [PubMed] [Google Scholar]
- 17.Weiner DA, Ryan TJ, McCabe CH, et al. Prognostic importance of a clinical profile and exercise test in medically treated patients with coronary artery disease. J Am Coll Cardiol. 1984;3(3):772-779. doi: 10.1016/S0735-1097(84)80254-5 [DOI] [PubMed] [Google Scholar]
- 18.Weiner DA, Ryan TJ, McCabe CH, et al. Value of exercise testing in determining the risk classification and the response to coronary artery bypass grafting in three-vessel coronary artery disease: a report from the Coronary Artery Surgery Study (CASS) registry. Am J Cardiol. 1987;60(4):262-266. doi: 10.1016/0002-9149(87)90224-4 [DOI] [PubMed] [Google Scholar]
- 19.Mancini GBJ, Hartigan PM, Shaw LJ, et al. Predicting outcome in the COURAGE trial (Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation): coronary anatomy versus ischemia. JACC Cardiovasc Interv. 2014;7(2):195-201. doi: 10.1016/j.jcin.2013.10.017 [DOI] [PubMed] [Google Scholar]
- 20.Califf RM, Phillips HR III, Hindman MC, et al. Prognostic value of a coronary artery jeopardy score. J Am Coll Cardiol. 1985;5(5):1055-1063. doi: 10.1016/S0735-1097(85)80005-X [DOI] [PubMed] [Google Scholar]
- 21.Serruys PW, Onuma Y, Garg S, et al. Assessment of the SYNTAX score in the Syntax study. EuroIntervention. 2009;5(1):50-56. doi: 10.4244/EIJV5I1A9 [DOI] [PubMed] [Google Scholar]
- 22.Spertus JA, Winder JA, Dewhurst TA, et al. Development and evaluation of the Seattle Angina Questionnaire: a new functional status measure for coronary artery disease. J Am Coll Cardiol. 1995;25(2):333-341. doi: 10.1016/0735-1097(94)00397-9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Site Personnel
eTable 2. Committees, CCC, Study Centers Personnel
eTable 3. Ischemia Definitions
eTable 4. Randomized Participant Baseline Vital Signs and Laboratory Values Intent-to-Treat Population
eTable 5. Randomized Participant Baseline Medications Intent-to-Treat Population
Trial Protocol
Data Sharing Statement.