Key Points
Question
Are there any survival differences in women and men undergoing septal myectomy for hypertrophic cardiomyopathy?
Findings
In this study of clinical data collected from 1961 to 2016, women were significantly older at the time of surgery, but adjusted statistics of survival were similar between women and men.
Meaning
This study suggests that clinicians should focus on early identification of disease in both women and men and promptly refer patients who do not respond to medical treatment for surgical evaluation.
This study of clinical data collected from 1961 to 2016 compares the preoperative characteristics and survival of women vs men undergoing septal myectomy for obstructive hypertrophic cardiomyopathy.
Abstract
Importance
Recent data indicate that women with hypertrophic cardiomyopathy (HCM) are older and more symptomatic at presentation and have worse clinical outcomes than men. However, to our knowledge, there are no large studies of the association of patient sex with outcomes after surgical myectomy.
Objective
To analyze preoperative characteristics and overall survival of women and men undergoing septal myectomy for obstructive HCM.
Design, Setting, and Participants
This retrospective, single-center study included the clinical data of adult patients who underwent septal myectomy from January 1961 through April 2016. Data analysis occurred from December 2017 to December 2018.
Exposures
Septal myectomy.
Main Outcomes and Measures
Survival.
Results
A total of 2506 adults were included; 1379 patients (55.0%) were men. At the time of surgery, women were older, with median (IQR) age of 59.5 (46.6-68.2) years vs 52.9 (42.9-62.7) years in men (P < .001). Women were more likely to have New York Heart Association class III or IV status at presentation (women, 1023 [90.8%]; men, 1169 [84.8%]; P < .001) and more severe obstructive physiology, as reflected in higher resting left ventricular outflow tract gradients (women, 67.0 [36.0-97.0] mm Hg; men, 50.0 [23.0-81.0] mm Hg; P < .001). Women also had a greater likelihood of having moderate or severe mitral regurgitation (606 [55.2%]) than men (581 [43.1%]; P < .001) and higher right ventricular systolic pressure (women, 36.0 [30.0-46.0] mm Hg; men, 33.0 [28.0-39.0] mm Hg; P < .001). The unadjusted overall survival was lower in women, corresponding to a median 3.9-year shorter survival than men (median [IQR] survival time: women, 18.2 [12.1-27.2] years; men, 22.1 [15.1-32.5] years; P < .001). In a multivariable Cox regression analysis, however, the association between sex and mortality was attenuated and not significant after controlling for other baseline variables (hazard ratio, 0.98 [95% CI, 0.76-1.26]; P = .86). Among the covariates in the model, older age at surgery (adjusted hazard ratio [aHR], 3.09 [95% CI, 2.12-4.52]; P < .001), higher body mass index (aHR, 1.22 [95% CI, 0.90-1.66]; P < .001), greater NYHA class (aHR, 2.31 [95% CI, 1.03-5.15]; P = .04), and presence of diabetes prior to surgery (aHR, 1.57 [95% CI, 1.10-2.24]; P = .01) were each independently associated with increased mortality. Operations performed later in the study period (2013 vs 2004) were associated with decreased mortality (aHR, 0.82 [95% CI, 0.55-1.22]; P = .001).
Conclusions and Relevance
In this large cohort of surgical patients with obstructive HCM, we observed significant differences at clinical presentation between women and men, in that women were older and more symptomatic. However, after adjustment for important baseline prognostic factors, there was no survival difference after septal myectomy by sex. Improved care of women with obstructive HCM should focus on early identification of disease and prompt surgical referral of appropriate patients who do not respond to medical treatment.
Introduction
Hypertrophic cardiomyopathy (HCM) is the most common monogenic heart disease, with a prevalence that may be as high as 1:200.1,2 Hypertrophic cardiomyopathy can be managed effectively both medically and surgically and should now be considered as a treatable genetic disease associated with a normal life span and low disease-associated mortality.3 Septal reduction therapy is useful for patients with medically refractory heart failure symptoms owing to obstructive HCM. Extended transaortic septal myectomy and alcohol septal ablation reduce left ventricular outflow tract (LVOT) obstruction and improve functional status.4
There has been accumulating interest in sex-specific differences and outcomes in a variety of cardiovascular diseases, including HCM, and most studies have shown that women experience worse outcomes than men.5,6,7,8,9,10,11,12,13,14,15,16 Women with HCM are diagnosed at an older age, tend to be more symptomatic at presentation, have more obstructive physiology, and have reduced survival compared with men.5,17 However, the delay in presentation may not necessarily result in worse survival, and survival differences after myectomy to provide durable relief of LVOT obstruction are less well understood. We hypothesized that patients with obstructive HCM undergoing extended transaortic septal myectomy would demonstrate sex-specific differences in clinical characteristics preoperatively, but that outcomes after surgery would be similar between women and men. We therefore sought to analyze preoperative characteristics, postoperative outcomes, and overall survival of both women and men undergoing extended transaortic septal myectomy for obstructive HCM.
Methods
Study Population
This study included adult patients (≥18 years) who underwent extended transaortic septal myectomy at Mayo Clinic in Rochester, Minnesota from January 1961 through April 2016. We reviewed available clinical and echocardiographic data from all eligible patients and stratified the study cohort by sex. Informed consent was obtained for the use of patient medical records for research purposes in accordance with Minnesota law, and the study was approved by the Mayo Clinic Institutional Review Board.
Survival data were collated from the electronic medical record and multiple national death databases. This involved linking our database with the Social Security Death Index, state death records, and other data sources via Accurint (LexisNexis). Patients not indicated as deceased in these records were assumed to still be alive and were censored 1 month prior to the date last searched (predominantly from December 2017 to January 2018). For a limited number of patients in whom a link could not be established, their vital status was determined by use of the electronic medical records and censoring at last follow-up visit. In addition, the cause of death was ascertained by merging the study subset of deceased patients with the National Death Index.
Echocardiographic Evaluation
Information from transthoracic echocardiographic examination was available in patients from November 1974. Because of the long time frame of this study, some patients were evaluated prior to the availability of certain echocardiographic parameters. Left ventricular outflow tract gradients were obtained by continuous-wave Doppler interrogation of the LVOT from an apical window and calculated using the modified Bernoulli equation (gradient = 4VLVOT2, where VLVOT is peak LVOT velocity). Provoked gradients were determined by provocative maneuvers (ie, Valsalva maneuver, amyl nitrite inhalation, and exercise). Ejection fraction, left ventricular (LV) cavity size, LV mass, and wall thickness were determined as previously described.18 Mitral regurgitation was graded as none to trivial (0), mild (1), moderate (2), moderately severe (3), or severe (4) after analyzing jet area and width, and spectral Doppler intensity, as well as regurgitation quantitation with the continuity and/or proximal isovelocity surface area method, as appropriate.19 Mitral regurgitation could not be quantitated in all patients due to jet eccentricity and LVOT turbulence merging with the regurgitant jet flow convergence. Right ventricular systolic pressure was estimated in a standard procedure as per American Society of Echocardiography guidelines.20 Information from the most recent Doppler echocardiograms performed before septal myectomy were compared with those obtained postoperatively prior to hospital dismissal.
Statistical Methods
Descriptive statistics used to summarize baseline data included median (interquartile range [IQR]) for continuous variables and number (percentage) for categorical or ordinal variables. Statistical tests for assessing baseline differences by sex were based on the Wilcoxon-Kruskal-Wallis test for continuous or ordinal variables and the Pearson χ2 test for categorical variables. Unadjusted survival time was summarized with Kaplan-Meier survival probabilities and quartile estimates; follow-up time was described with quartiles by the same estimator, except that the codes for the event or censoring indicator were reversed. Multivariable Cox proportional hazards modeling was used to assess the partial effect of sex on time until death in the presence of known important baseline prognostic factors. Model assumptions of proportional hazards and additivity, specifically for nonadditivity of effects by sex, were assessed with global (overall) tests and further by partial tests, if warranted. Independent variables that violated proportional hazards (atrial fibrillation and left ventricular ejection fraction) were entered into the model as stratification variables rather than as covariates as a way to adjust for outcome without the need to assume proportional hazards. The Cox model was also used to analyze postoperative length of stay, with in-hospital deaths censored and tied responses handled exactly in the model. In addition, analysis of covariance ordinal regression based on the proportional-odds model was applied to postoperative echocardiographic data to compare baseline-adjusted responses between men and women. In all these regression models, continuous covariates were fitted using restricted cubic splines with 4 knots to relax linearity assumptions and to allow nonlinear effects. Effects of variables are estimated with adjusted hazard ratios (and 95% CIs). For continuous variables, hazard ratios were calculated comparing the 75th percentile with the 25th percentile. Since these variables are modeled nonlinearly, general tests of association can easily generate significant P values, even when the confidence interval of a hazard ratio contains 1.0. Statistical significance was defined as P ≤ .05, and analyses were performed using SAS version 9.4 (SAS Institute Inc) and R version 3.2.3 (R Foundation for Statistical Computing) from December 2017 to December 2018.
Results
Study Population
A total of 2540 patients underwent septal myectomy at the study institution from January 1961 to April 2016. Thirty-four patients who did not consent for research were excluded from this analysis. The study group was therefore 2506 patients, of whom 1379 patients (55.0%) were men. The number of patients increased markedly over the course of the study (n = 49 in the 1960-1970s, 76 in the 1980s, 267 in the 1990s, 985 in the 2000s, and 1129 from 2010 to 2016), with 84.4% of patients having their surgery in or after 2000. The median (IQR) age was 55.6 (44.5-65.6) years. A total of 2192 patients (87.4%) presented with New York Heart Association (NYHA) class III or IV symptoms. (Patients with class I or II symptoms preoperatively had troublesome syncope or extremely high gradients that led to activity limitations.21)
Clinical Characteristics
Patient demographics are shown by sex in Table 1. At the time of surgery, women were older, with a median (IQR) age of 59.5 (46.6-68.2) years vs 52.9 (42.9-62.7) years for men (P < .001), and were more symptomatic, with higher likelihood of being in NYHA class III or IV at presentation (women, 1023 [90.8%]; men, 1169 [84.8%]; P < .001). Women had greater prevalence of systemic hypertension (586 [52.0%]) than men (652 [47.3%]; P = .02), were more likely to have a family history of HCM (239 [21.3%]) than men (236 [17.2%]; P = .008), and were more likely to have had previous septal reduction therapy with either alcohol septal reduction or transaortic septal myectomy (women 62 [5.5%]; men, 41 [3.0%]; P < .002). There were no sex differences in the other comorbidities, nor were there differences in the use of any preoperative medical therapy between women and men.
Table 1. Baseline, Preoperative Echocardiographic, and Operative Characteristics According to Sexa.
| Variable | No. | Patients, No. (%) | P Value | |
|---|---|---|---|---|
| Women | Men | |||
| Year of operation | 2506 | 1127 | 1379 | NA |
| 1960-1979 | 49 | 22 (2.0) | 27 (2.0) | .81 |
| 1980-1989 | 76 | 36 (3.2) | 40 (2.9) | |
| 1990-1999 | 267 | 141 (12.5) | 126 (9.1) | |
| 2000-2009 | 985 | 414 (36.7) | 571 (41.4) | |
| 2010-2016 | 1129 | 514 (45.6) | 615 (44.6) | |
| Age, median (IQR), y | 2506 | 59.5 (46.6-68.2) | 52.9 (42.9-62.7) | <.001 |
| BMI, median (IQR) | 2506 | 29.3 (25.0-34.5) | 30.0 (27.0-33.6) | .006 |
| New York Heart Association class III or IV | 2506 | 1023 (90.8) | 1169 (84.8) | <.001 |
| Comorbidities | ||||
| Hypertension | 2506 | 586 (52.0) | 652 (47.3) | .02 |
| Diabetes mellitus | 2506 | 110 (9.8) | 121 (8.8) | .40 |
| Dyslipidemia | 2506 | 671 (59.5) | 868 (62.9) | .08 |
| Near syncope | 2506 | 400 (35.5) | 493 (35.8) | .91 |
| Syncope | 2506 | 219 (19.5) | 252 (18.3) | .45 |
| Atrial fibrillation | 2506 | 204 (18.1) | 282 (20.4) | .14 |
| Nonsustained ventricular tachycardia | 2506 | 91 (8.1) | 139 (10.1) | .08 |
| Implantable cardioverter defibrillator use | 2506 | 144 (12.8) | 196 (14.2) | .30 |
| Family history | ||||
| Hypertrophic cardiomyopathy | 2506 | 239 (21.3) | 236 (17.2) | .008 |
| Sudden cardiac death | 2506 | 187 (16.7) | 203 (14.8) | .19 |
| Medication use | ||||
| β-Blocker | 2506 | 894 (79.3) | 1103 (80.0) | .68 |
| Calcium channel blockers | 2506 | 440 (39.1) | 513 (37.2) | .34 |
| Angiotensin converting enzyme inhibitors/angiotensin receptor blockers | 2506 | 169 (15.0) | 199 (14.4) | .69 |
| Disopyramide | 2506 | 113 (10.0) | 115 (8.3) | .14 |
| Amiodarone | 2506 | 62 (5.5) | 55 (4.0) | .07 |
| LVOT gradient, mm Hg, median (IQR) | ||||
| Resting gradient | 2343 | 67 (36-97) | 50 (23-81) | <.001 |
| Provoked gradient | 1300 | 81 (64-208) | 74 (55-100) | <.001 |
| LV ejection fraction | 2388 | 73 (69-75) | 70 (67-75) | <.001 |
| LV end diastolic dimension, mm | 2269 | 43 (40-47) | 47 (43-51) | <.001 |
| Anteroseptal wall thickness, mm | 2394 | 19 (17-23) | 20 (18-23) | <.001 |
| Posterior wall thickness, mm | 2256 | 13 (11-15) | 14 (12-15) | <.001 |
| LV mass, g | 2059 | 262 (213-325) | 328 (259-400) | <.001 |
| LV mass index, g/m2 | 2063 | 143 (116-177) | 151 (124-185) | <.001 |
| Right ventricular systolic pressure, mm Hg | 1704 | 36 (30-46) | 33 (28-39) | <.001 |
| Moderate or greater mitral regurgitation | 2446 | 606 (55.2) | 581 (43.1) | <.001 |
| Concomitant coronary artery bypass grafting | 2470 | 80 (7.2) | 140 (10.3) | .008 |
| Concomitant valve operation | 2470 | 451 (40.7) | 509 (37.4) | .009 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); LV, left ventricle; LVOT, left ventricular outflow tract; NA, not applicable.
P values for continuous variables are from the Wilcoxon rank sum test; categorical variables, Pearson χ2 test. Ordinal variables were dichotomized at a relevant cutoff level for descriptive purposes, whereas for statistical testing the full ordinal scale was analyzed by Wilcoxon rank sum test.
The distribution of women vs men undergoing myectomy has remained fairly stable through time (for example, 1960-1979: 22 of 1127 women [2.0%]; 27 of 1379 men [2.0%]; 2010-2016, 514 of 1127 women [45.6%]; 615 of 1379 men [44.6%]; P = .81 across all 5 decades; Table 1), and additional demographic data stratified by decade are shown in the eTable and the eFigure in the Supplement. In more recent years, the median age of both women and men has increased, and preoperative resting gradient has decreased (eTable in the Supplement).
Echocardiographic Evaluation
Preoperative echocardiographic data are shown in Table 1. Women had more severe obstructive physiology as reflected in greater median (IQR) resting LVOT gradients (women, 67 [36-97] mm Hg; men, 50 [23-81] mm Hg; P < .001) and median (IQR) provoked LVOT gradients (women, 81 [64-208] mm Hg; men, 74 [55-100] mm Hg; P < .001) among those subsets of patients with available measurements. The proportion of individuals found to have moderate or severe mitral valve regurgitation was greater in women (606 [55.2%]) than men (581 [43.1%]; P < .001).
Septal hypertrophy, measured as median (IQR) absolute anteroseptal thickness (women, 19 [17-23] mm; men, 20 [18-23] mm) and median (IQR) posterior wall thicknesses (women, 13 [11-15] mm; men, 14 [12-15] mm), was less pronounced in women (both P < .001). The proportion of women with extreme anteroseptal wall thickness (≥30 mm) was slightly lower (women, 47 [4.4%]; men, 85 [6.4%]; P < .001). Women also had significantly smaller LV mass (women, 262 [213-325] g; men, 328 [259-400] g; P < .001) and LV end-diastolic dimensions (women, 43 [40-47] mm; men, 47 [43-51] mm; Table 1). In patients with right ventricular systolic pressure assessment (n = 1704; 68.0%), women demonstrated higher estimated right ventricular systolic pressure (36.0 [30.0-46.0] mm Hg) than men (33.0 [28.0-39.0] mm Hg; P < .001).
The median (IQR) interval from operation to the predischarge echocardiogram was 4 (3-5) days. Table 2 shows observed changes in echocardiographic parameters, as well as expected mean changes for women and men with baseline measurement and other covariates, adjusted to their median levels. After extended transaortic septal myectomy, there was a significant reduction in LVOT gradient in both women and men, with a similar magnitude of expected mean change after baseline adjustment (−50.3 [95% CI, −51.5 to −49.1]; −50.4 [95% CI, −51.4 to −49.4]; P = .88). There was no significant difference in reduction of anteroseptal wall thickness, but adjusted difference in reduction of posterior wall thickness was greater in women than men (−0.9 [95% CI, −1.2 to −0.6; −0.5 [95% CI, −0.7 to −0.2]; P = .006).
Table 2. Changes in Echocardiographic Measurements According to Sexa.
| Measurement | No. | Women | Men | P Value |
|---|---|---|---|---|
| Left ventricular outflow tract gradient, mm Hg | ||||
| Preoperative measurement, median (IQR) | 2124 | 67.0 (34.0-96.0) | 49.0 (21.0-78.0) | NAa |
| Observed change, median (IQR) | −60.0 (−88.0 to −26.0) | −41.0 (−71.0 to −16.0) | NAa | |
| Expected mean change (95% CI) | −50.3 (−51.5 to −49.1) | −50.4 (−51.4 to −49.4) | .88 | |
| Anteroseptal wall thickness, mm | ||||
| Preoperative measurement, median (IQR) | 1700 | 19.0 (17.0-22.0) | 20.0 (18.0-23.0) | NAa |
| Observed change, median (IQR) | −4.0 (−7.0 to −2.0) | −4.0 (−7.0 to −2.0) | NAa | |
| Predicted mean change (95% CI) | −4.9 (−5.4 to −4.4) | −4.7 (−5.1 to −4.2) | .36 | |
| Posterior wall thickness, mm | ||||
| Preoperative measurement, median (IQR) | 1525 | 12.0 (11.0-14.0) | 13.0 (12.0-15.0) | NAa |
| Observed change, median (IQR) | −1.0 (−2.0 to 1.0) | −1.0 (−2.0 to 1.0) | NAa | |
| Predicted mean change (95% CI) | −0.9 (−1.2 to −0.6) | −0.5 (−0.7 to −0.2) | .006 |
Abbreviations: IQR, interquartile range; NA, not applicable.
Formal statistical testing was limited to comparisons of the baseline-adjusted change in outcome, given the potential differences in baseline values by sex. For each echocardiogram outcome, comparison of predischarge improvement by sex was based on semiparametric analysis of covariance with the use of a proportional odds ordinal logistic regression model adjusting for preoperative values as well as patient age, height, and weight. The mean change expected from the model is the improvement for 2 patients, one a woman and the other a man, with the same values of the baseline covariates. (Each was held constant to the median so as to resemble the typical patient.)
Clinical Outcomes
All patients underwent septal myectomy. Interestingly, women were less likely to undergo concomitant coronary artery bypass grafting (women, 80 [7.2%]; men, 140 [10.3%]; P = .008), but the percentage who underwent concomitant valve operations was not significantly different (women, 451 [40.7%]; men, 509 [37.4%]; P = .10).
Early postoperative deaths (within 30 days of operation) were observed in 21 participants (0.8%) over the course of the study. As time progressed, early mortality decreased markedly, from 4 of 49 (8.2%) in the 1960s and 1970s to 3 of 1129 (0.3%) in the present decade, with declining percentages throughout the study period. Early mortality occurred in 13 of 1127 women (1.2%) and 8 of 1379 men (0.6%; P = .12). Postoperative median (IQR) length of hospital stay was longer in women than men (6 [5-7] days vs 5 [4-7] days; P < .001 after adjusting for age, year of operation, and diabetes status). Risk of postoperative stroke was slightly but nonsignificantly higher in women (12 of 1125 [1.1%] vs 6 of 1377 [0.4%]; P = .07), whereas early arrhythmias after surgery, including atrial fibrillation and ventricular tachycardia, were similar among the 2 sexes (atrial fibrillation: women, 334 of 1125 [29.7%]; men, 419 of 1377 [30.4%]; P = .70; ventricular tachycardia: women, 26 of 1125 [2.3%]; men, 35 of 1377 [2.5%]; P = .71).
During a median (IQR) follow-up of 8.2 (3.1-13.2) years, a total of 445 patients died. The 1-year, 5-year, and 10-year survival estimates were 98.3% (95% CI, 97.7%-98.8%), 94.2% (95% CI, 93.1%-95.2%), and 84.6% (95% CI, 82.6%-86.4%), respectively, and median (IQR) survival time was 20.8 (13.6-30.8) years. The cause of mortality was known in 371 of the 445 deaths (83.4%). Deaths associated with cardiovascular conditions accounted for 1124 of 197 deaths (58.2%) in women and 99 of 174 deaths (56.9%) in men (P = .81).
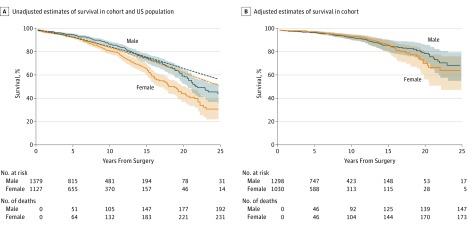
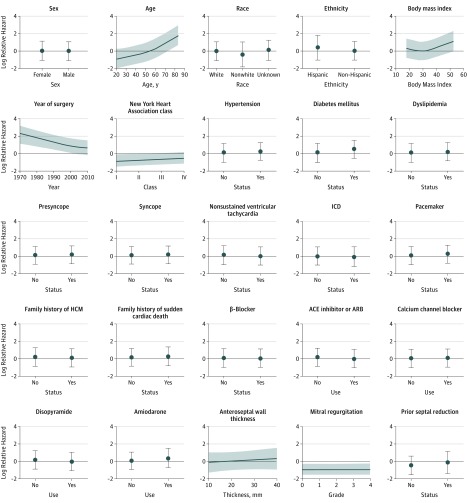
In the unadjusted analysis (Figure 1A), women had lower survival than men after myectomy, corresponding to an apparent 3.9-year shorter median survival time (median [IQR] survival time: men, 22.1 [15.1-32.5] years; women, 18.2 [12.1-27.2] years). These estimates were significantly worse than those of age-matched women in the general US population (238 observed vs 152.3 expected deaths; P < .001), whereas observed and population-based survival estimates for men varied considerably over time but did not have significantly different means (207 observed vs 184.7 expected deaths; P = .10) (Figure 1A). In the multivariable-adjusted Cox analysis, the association between sex and adjusted mortality was attenuated and not significant after controlling for all other covariates included in the model (hazard ratio [HR], 0.98 [95% CI, 0.76-1.26]; P = .86) (Table 3 and Figure 1B). Furthermore, a global test of all interaction terms between sex and the other covariates suggested no important sex-specific effects on mortality risk (χ224 = 27.2; P = .29). Independent factors associated with mortality (Table 3 and Figure 2) were older age at surgery (adjusted HR, 3.09 [95% CI, 2.12-4.52]; P < .001), higher body mass index (adjusted HR, 1.22 [95% CI, 0.90-1.66]; P < .001), greater NYHA class (adjusted HR, 2.31 [95% CI, 1.03-5.15]; P = .04), and presence of diabetes prior to surgery (adjusted HR, 1.57 [95% CI, 1.10-2.24]; P = .01). In addition, myectomy performed more recently was independently associated with decreased mortality (adjusted hazard ratio, 0.82 [95% CI, 0.55-1.22]; P = .001).
Figure 1. Unadjusted and Adjusted Survival in Women and Men.
A, Unadjusted sex-specific estimates of survival compared with corresponding age-matched, sex-matched US population rates. Blue indicates male; orange, female; dotted lines, expected values; solid lines, mean observed values; shaded areas, 95% CIs. B, Adjusted (Cox-Kalbfleisch-Prentice) estimates of survival for women and men in a combined sample of 2328 patients (92.9% of the entire cohort) who had complete information with respect to the baseline adjustment factors. Survival curves are adjusted to the median levels of continuous covariates and modal categories of categorical covariates from the combined sample; the resulting difference is nonsignificant. Blue indicates male; orange, female; solid lines, mean values; shaded areas, 95% CIs.
Table 3. Factors Associated With Mortality After Septal Myectomya.
| Variable | Comparisonb | Adjusted Hazard Ratio (95% CI)c | P Value |
|---|---|---|---|
| Age, y | 65.6:44.5 | 3.09 (2.12-4.52) | <.001 |
| Year of surgery | 2013:2004 | 0.82 (0.55-1.22) | .001 |
| BMI | 34:26 | 1.22 (0.90-1.66) | .001 |
| Diabetes mellitus | Yes:no | 1.57 (1.10-2.24) | .01 |
| New York Heart Association class | IV:I | 2.31 (1.03-5.15) | .04 |
| Amiodarone | Yes:no | 1.59 (1.00-2.54) | .05 |
| Pacemaker | Yes:no | 1.38 (0.98-1.95) | .07 |
| Nonsustained ventricular tachycardia | Yes:no | 0.70 (0.42-1.18) | .18 |
| Hypertension | Yes:no | 1.20 (0.91-1.57) | .19 |
| Disopyramide | Yes:no | 0.79 (0.52-1.18) | .25 |
| Use of angiotensin-converting enzyme inhibitors or angiotensin receptor blockers | Yes:no | 0.83 (0.59-1.17) | .29 |
| Presyncope | Yes:no | 1.12 (0.88-1.44) | .35 |
| Dyslipidemia | Yes:no | 1.12 (0.86-1.46) | .41 |
| Prior septal reduction | Yes:no | 1.27 (0.71-2.27) | .42 |
| Syncope | Yes:no | 1.11 (0.83-1.51) | .48 |
| Mitral valve regurgitation grade | Moderate:mild | 1.04 (0.93-1.16) | .49 |
| Race | Nonwhite:white | 0.68 (0.22-2.17) | .69 |
| Unknown:white | 1.17 (0.66-2.10) | ||
| β-Blocker | Yes:no | 0.95 (0.71-1.27) | .72 |
| Calcium-channel blocker | Yes:no | 1.04 (0.81-1.34) | .74 |
| Family history of hypertrophic cardiomyopathy | Yes:no | 0.94 (0.64-1.37) | .75 |
| Ethnicity | Hispanic:non-Hispanic | 1.17 (0.35-3.88) | .80 |
| Family history of sudden cardiac death | Yes:no | 1.06 (0.68-1.65) | .81 |
| Anteroseptal wall thickness, mm | 23:17 | 1.06 (0.80-1.40) | .84 |
| Sex | Female:male | 0.98 (0.76-1.26) | .86 |
| Implantable cardioverter defibrillator | Yes:no | 1.01 (0.56-1.81) | .97 |
Abbreviation: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared).
A multivariable Cox regression model was fitted for all-cause mortality on 2328 participants using the variables shown; we further adjusted for 2 nonmodeled variables (atrial fibrillation and left ventricular ejection fraction) by stratifying the model on levels of each (with left ventricular ejection fraction grouped into quartiles), owing to failure of the proportional hazards assumption.
Continuous variables were expanded into multiple terms using restricted cubic splines (using 4 knots) to allow for nonlinear effects. Owing to data discreteness, a linear association was assumed for ordinal scales of New York Heart Association and mitral regurgitation grade. The reference value is the righthand value in each pair.
Effects of variables are estimated with adjusted hazard ratios (and 95% CIs). For continuous variables, hazard ratios were calculated comparing the 75th percentile with the 25th percentile. Since these variables are modeled nonlinearly, general tests of association can easily generate a significant P value, even when the confidence interval of a hazard ratio contains 1.0.
Figure 2. Plot of Log Relative Hazard of Mortality for All Modeled Variables Included in the Multivariable Analysis.
This illustrates how the partial effect on risk changes when the factor plotted is varied, with all other factors not being plotted held fixed to constants based on their median value or modal category. ACE indicates angiotensin-converting enzyme; ARB, angiotensin II receptor blockers; HCM, hypertrophic cardiomyopathy; ICD, implantable cardioversion defibrillator.
Discussion
In this review of 2506 patients undergoing transaortic septal myectomy for obstructive HCM, we observed significant differences in preoperative characteristics between women and men. Importantly, women were older and more symptomatic than men at the time of surgery. However, there were no differences between the 2 sexes in early postoperative outcomes, including mortality and relief of LVOT obstruction. Factors associated with overall mortality included older age at surgery, greater body mass index, higher NYHA class, diabetes mellitus, and surgery performed earlier in the study period.
Overall survival after septal myectomy was worse in women than men. Indeed, outcome of women who underwent surgery was reduced compared with that expected in a matched US population. In contrast, for men, observed and expected survivals were similar (Figure 1A). In a previous study of patients who underwent septal myectomy at the Mayo clinic, 10-year survival of observed vs expected populations seemed comparable.22 The present investigation, however, extends follow-up beyond 10 years, and it is in this later postoperative period that survival appears reduced in surgical patients compared with a matched US population; these differences were observed in both sexes.
Although the unadjusted analysis demonstrated worse survival in women (Figure 1A), this difference was attenuated in an adjusted model (Figure 1B). These results differ from a report by Geske et al,5 who studied more than 3600 patients with HCM, most of whom had no LVOT obstruction. In that analysis, women had poorer survival than men, and septal reduction was performed in only 32%. These results also contrast with the investigation by Olivotto et al,17 who observed worse chances of survival in women with HCM; however, in their study, only 10% of patients had advanced (NYHA class III or IV) symptoms, and less than 30% had important LVOT obstruction.
In this large study of patients with HCM who underwent transaortic septal myectomy, female sex was not a factor independently associated with mortality (Table 3). This differs from the findings of Woo et al23 who reported that, among 338 patients with HCM undergoing septal myectomy, female sex was an important factor associated with late survival, with a hazard ratio for death of 2.5 in their multivariable analysis. The explanation for the different results is not clear. In both the Woo et al study23 and this analysis, older age was associated with reduced late survival, but other risk factors associated with survival differ from the present investigation. For example, left atrial diameter was independently associated with late mortality in the study from Toronto, Ontario, Canada, but in a recent investigation from the study clinic, left atrial size, as measured by left atrial volume index, was not associated with late death.24 Another difference in the studies was the finding in the patients that diabetes is an important factor associated with overall mortality.
Risk of 30-day mortality was 0.8% in this review, and there were no differences in early outcomes between women and men. These results are consistent with reports from other centers, where early operative mortality rates of less than 1% can be achieved when patients with HCM are treated by experienced teams.25,26 Indeed, some clinicians argue that HCM should be treated at experienced referral centers to achieve the best patient outcomes.27 Other early postoperative complications, including atrial fibrillation and stroke, were similar among the 2 sexes.
Hypertrophic cardiomyopathy is a genetic disease in which inheritance is not linked to sex chromosomes, and thus women and men should be affected equally. Lower proportions of women vs men in contemporary medical and surgical series of HCM may be because of underdiagnosis, which can be explained by multiple factors.5,17,28,29 First, studies suggest that women are less aware of their risks for cardiovascular disease and may not seek medical attention during earlier stages of the disease.30 Second, direct comparisons between women and men with similar cardiovascular risks have demonstrated that women are less likely to receive aggressive treatment.31 This may be owing to some degree of physician bias, and it is possible that surgical referral for septal reduction is less common in women with similar disease profile compared with men. Early and late results from the present study, however, show that regardless of potential referral bias, surgical myectomy is an excellent treatment option for women with obstructive HCM.
Another consideration is that healthy women have slightly thinner interventricular septa compared with healthy men,32 but the diagnostic criteria for HCM are identical for both sexes.33,34 The variations in septal thickness are especially important for patients undergoing echocardiographic screening for HCM. Thus, women may have relatively more severe hypertrophy when they first meet criteria for diagnosis of HCM. This potential lead-time bias could also contribute to survival differences observed in studies of women and men with HCM.
Limitations
This is a retrospective study from a single tertiary center. The analysis is limited by inherent selection bias. The results reported may not be generalizable to other centers.
Conclusions
In this large cohort of surgical patients with obstructive HCM, women were older and more symptomatic at clinical presentation compared with men. Women also had more severe obstructive physiology and diastolic dysfunction in association with smaller LV mass index on echocardiography. However, survival after septal myectomy was not associated with female sex after adjustment for important baseline prognostic factors. Improved care of women with obstructive HCM should focus on early identification of disease and prompt surgical referral of appropriate patients who do not respond to medical treatment.
eTable. Baseline Characteristics over the Study Period.
eFigure. Temporal trend in baseline characteristics by sex. Curve estimates trend over time stratified by sex using a locally weighted scatterplot smooth (loess).
References
- 1.Maron BJ, Maron MS. A discussion of contemporary nomenclature, diagnosis, imaging, and management of patients with hypertrophic cardiomyopathy. Am J Cardiol. 2016;118(12):1897-1907. doi: 10.1016/j.amjcard.2016.08.086 [DOI] [PubMed] [Google Scholar]
- 2.Semsarian C, Ingles J, Maron MS, Maron BJ. New perspectives on the prevalence of hypertrophic cardiomyopathy. J Am Coll Cardiol. 2015;65(12):1249-1254. doi: 10.1016/j.jacc.2015.01.019 [DOI] [PubMed] [Google Scholar]
- 3.Maron BJ, Rowin EJ, Casey SA, Maron MS. How hypertrophic cardiomyopathy became a contemporary treatable genetic disease with low mortality: shaped by 50 years of clinical research and practice. JAMA Cardiol. 2016;1(1):98-105. doi: 10.1001/jamacardio.2015.0354 [DOI] [PubMed] [Google Scholar]
- 4.Nishimura RA, Seggewiss H, Schaff HV. Hypertrophic obstructive cardiomyopathy: surgical myectomy and septal ablation. Circ Res. 2017;121(7):771-783. doi: 10.1161/CIRCRESAHA.116.309348 [DOI] [PubMed] [Google Scholar]
- 5.Geske JB, Ong KC, Siontis KC, et al. Women with hypertrophic cardiomyopathy have worse survival. Eur Heart J. 2017;38(46):3434-3440. doi: 10.1093/eurheartj/ehx527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dey S, Flather MD, Devlin G, et al. ; Global Registry of Acute Coronary Events investigators . Sex-related differences in the presentation, treatment and outcomes among patients with acute coronary syndromes: the Global Registry of Acute Coronary Events. Heart. 2009;95(1):20-26. doi: 10.1136/hrt.2007.138537 [DOI] [PubMed] [Google Scholar]
- 7.Warnes CA. Sex differences in congenital heart disease: should a woman be more like a man? Circulation. 2008;118(1):3-5. doi: 10.1161/CIRCULATIONAHA.108.785899 [DOI] [PubMed] [Google Scholar]
- 8.Hsich EM, Grau-Sepulveda MV, Hernandez AF, et al. Sex differences in in-hospital mortality in acute decompensated heart failure with reduced and preserved ejection fraction. Am Heart J. 2012;163(3):430-437, 437.e1-437.e3. doi: 10.1016/j.ahj.2011.12.013 [DOI] [PubMed] [Google Scholar]
- 9.Shapiro S, Traiger GL, Turner M, McGoon MD, Wason P, Barst RJ. Sex differences in the diagnosis, treatment, and outcome of patients with pulmonary arterial hypertension enrolled in the registry to evaluate early and long-term pulmonary arterial hypertension disease management. Chest. 2012;141(2):363-373. doi: 10.1378/chest.10-3114 [DOI] [PubMed] [Google Scholar]
- 10.Bucholz EM, Strait KM, Dreyer RP, et al. Editor’s choice-sex differences in young patients with acute myocardial infarction: a VIRGO study analysis. Eur Heart J Acute Cardiovasc Care. 2017;6(7):610-622. doi: 10.1177/2048872616661847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fairweather D, Cooper LT Jr, Blauwet LA. Sex and gender differences in myocarditis and dilated cardiomyopathy. Curr Probl Cardiol. 2013;38(1):7-46. doi: 10.1016/j.cpcardiol.2012.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Humphries KH, Kerr CR, Connolly SJ, et al. New-onset atrial fibrillation: sex differences in presentation, treatment, and outcome. Circulation. 2001;103(19):2365-2370. doi: 10.1161/01.CIR.103.19.2365 [DOI] [PubMed] [Google Scholar]
- 13.Habib RH, Zacharias A, Schwann TA, Riordan CJ, Durham SJ, Shah A. Sex differences in mortality after coronary artery bypass graft surgery. JAMA. 2004;292(1):40-41. [DOI] [PubMed] [Google Scholar]
- 14.O’Meara E, Clayton T, McEntegart MB, et al. ; CHARM Investigators . Sex differences in clinical characteristics and prognosis in a broad spectrum of patients with heart failure: results of the Candesartan in heart failure: assessment of reduction in mortality and morbidity (CHARM) program. Circulation. 2007;115(24):3111-3120. doi: 10.1161/CIRCULATIONAHA.106.673442 [DOI] [PubMed] [Google Scholar]
- 15.Simon T, Mary-Krause M, Funck-Brentano C, Jaillon P. Sex differences in the prognosis of congestive heart failure: results from the Cardiac Insufficiency Bisoprolol Study (CIBIS II). Circulation. 2001;103(3):375-380. doi: 10.1161/01.CIR.103.3.375 [DOI] [PubMed] [Google Scholar]
- 16.Raphael CE, Singh M, Bell M, et al. Sex differences in long-term cause-specific mortality after percutaneous coronary intervention: temporal trends and mechanisms. Circ Cardiovasc Interv. 2018;11(3):e006062. doi: 10.1161/CIRCINTERVENTIONS.117.006062 [DOI] [PubMed] [Google Scholar]
- 17.Olivotto I, Maron MS, Adabag AS, et al. Gender-related differences in the clinical presentation and outcome of hypertrophic cardiomyopathy. J Am Coll Cardiol. 2005;46(3):480-487. doi: 10.1016/j.jacc.2005.04.043 [DOI] [PubMed] [Google Scholar]
- 18.Lang RM, Bierig M, Devereux RB, et al. ; Chamber Quantification Writing Group; American Society of Echocardiography’s Guidelines and Standards Committee; European Association of Echocardiography . Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18(12):1440-1463. doi: 10.1016/j.echo.2005.10.005 [DOI] [PubMed] [Google Scholar]
- 19.Zoghbi WA, Enriquez-Sarano M, Foster E, et al. ; American Society of Echocardiography . Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003;16(7):777-802. doi: 10.1016/S0894-7317(03)00335-3 [DOI] [PubMed] [Google Scholar]
- 20.Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23(7):685-713. doi: 10.1016/j.echo.2010.05.010 [DOI] [PubMed] [Google Scholar]
- 21.Orme NM, Sorajja P, Dearani JA, Schaff HV, Gersh BJ, Ommen SR. Comparison of surgical septal myectomy to medical therapy alone in patients with hypertrophic cardiomyopathy and syncope. Am J Cardiol. 2013;111(3):388-392. doi: 10.1016/j.amjcard.2012.10.014 [DOI] [PubMed] [Google Scholar]
- 22.Ommen SR, Maron BJ, Olivotto I, et al. Long-term effects of surgical septal myectomy on survival in patients with obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol. 2005;46(3):470-476. doi: 10.1016/j.jacc.2005.02.090 [DOI] [PubMed] [Google Scholar]
- 23.Woo A, Williams WG, Choi R, et al. Clinical and echocardiographic determinants of long-term survival after surgical myectomy in obstructive hypertrophic cardiomyopathy. Circulation. 2005;111(16):2033-2041. doi: 10.1161/01.CIR.0000162460.36735.71 [DOI] [PubMed] [Google Scholar]
- 24.Nguyen A, Schaff HV, Nishimura RA, et al. Determinants of reverse remodeling of the left atrium after transaortic myectomy. Ann Thorac Surg. 2018;106(2):447-453. doi: 10.1016/j.athoracsur.2018.03.039 [DOI] [PubMed] [Google Scholar]
- 25.Maron BJ, Dearani JA, Ommen SR, et al. Low operative mortality achieved with surgical septal myectomy: role of dedicated hypertrophic cardiomyopathy centers in the management of dynamic subaortic obstruction. J Am Coll Cardiol. 2015;66(11):1307-1308. doi: 10.1016/j.jacc.2015.06.1333 [DOI] [PubMed] [Google Scholar]
- 26.Maron BJ. Controversies in cardiovascular medicine: surgical myectomy remains the primary treatment option for severely symptomatic patients with obstructive hypertrophic cardiomyopathy. Circulation. 2007;116(2):196-206. doi: 10.1161/CIRCULATIONAHA.107.691378 [DOI] [PubMed] [Google Scholar]
- 27.Maron BJ, Dearani JA, Maron MS, et al. Why we need more septal myectomy surgeons: an emerging recognition. J Thorac Cardiovasc Surg. 2017;154(5):1681-1685. doi: 10.1016/j.jtcvs.2016.12.038 [DOI] [PubMed] [Google Scholar]
- 28.Bos JM, Theis JL, Tajik AJ, Gersh BJ, Ommen SR, Ackerman MJ. Relationship between sex, shape, and substrate in hypertrophic cardiomyopathy. Am Heart J. 2008;155(6):1128-1134. doi: 10.1016/j.ahj.2008.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Y, Wang J, Zou Y, et al. Female sex is associated with worse prognosis in patients with hypertrophic cardiomyopathy in China. PLoS One. 2014;9(7):e102969. doi: 10.1371/journal.pone.0102969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mosca L, Mochari-Greenberger H, Dolor RJ, Newby LK, Robb KJ. Twelve-year follow-up of American women’s awareness of cardiovascular disease risk and barriers to heart health. Circ Cardiovasc Qual Outcomes. 2010;3(2):120-127. doi: 10.1161/CIRCOUTCOMES.109.915538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mosca L, Linfante AH, Benjamin EJ, et al. National study of physician awareness and adherence to cardiovascular disease prevention guidelines. Circulation. 2005;111(4):499-510. doi: 10.1161/01.CIR.0000154568.43333.82 [DOI] [PubMed] [Google Scholar]
- 32.Hindsø L, Fuchs A, Kühl JT, et al. Normal values of regional left ventricular myocardial thickness, mass and distribution-assessed by 320-detector computed tomography angiography in the Copenhagen General Population Study. Int J Cardiovasc Imaging. 2017;33(3):421-429. doi: 10.1007/s10554-016-1015-9 [DOI] [PubMed] [Google Scholar]
- 33.Elliott PM, Anastasakis A, Borger MA, et al. ; Authors/Task Force members . 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J. 2014;35(39):2733-2779. doi: 10.1093/eurheartj/ehu284 [DOI] [PubMed] [Google Scholar]
- 34.Gersh BJ, Maron BJ, Bonow RO, et al. ; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines; American Association for Thoracic Surgery; American Society of Echocardiography; American Society of Nuclear Cardiology; Heart Failure Society of America; Heart Rhythm Society; Society for Cardiovascular Angiography and Interventions; Society of Thoracic Surgeons . 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;124(24):2761-2796. doi: 10.1161/CIR.0b013e318223e230 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Baseline Characteristics over the Study Period.
eFigure. Temporal trend in baseline characteristics by sex. Curve estimates trend over time stratified by sex using a locally weighted scatterplot smooth (loess).