Key Points
Question
Does dual-target immuno-oncology therapy (anti–programmed death ligand 1 [PD-L1] and anticytotoxic T-lymphocyte associated protein 4) have clinically meaningful antitumor activity without compromising safety in patients with recurrent and/or metastatic head and neck squamous cell carcinoma (R/M HNSCC) and low or no tumor cell expression of PD-L1?
Findings
In this phase 2 randomized clinical trial of 267 patients, durvalumab monotherapy and durvalumab + tremelimumab demonstrated manageable toxic effects and clinically relevant overall survival for patients with R/M HNSCC and low or no PD-L1 tumor cell expression; durvalumab monotherapy and durvalumab + tremelimumab combination therapy showed similar efficacy.
Meaning
Treatment with durvalumab monotherapy and durvalumab + tremelimumab resulted in clinical benefit in patients with PD-L1–low/negative tumor cell expression, but no significant difference in efficacy or adverse effects was observed between the 2 regimens.
Abstract
Importance
Dual blockade of programmed death ligand 1 (PD-L1) and cytotoxic T-lymphocyte associated protein 4 (CTLA-4) may overcome immune checkpoint inhibition. It is unknown whether dual blockade can potentiate antitumor activity without compromising safety in patients with recurrent or metastatic head and neck squamous cell carcinoma (R/M HNSCC) and low or no PD-L1 tumor cell expression.
Objective
To assess safety and objective response rate of durvalumab combined with tremelimumab.
Design, Setting, and Participants
The CONDOR study was a phase 2, randomized, open-label study of Durvalumab, Tremelimumab, and Durvalumab in Combination With Tremelimumab in Patients With R/M HNSCC. Eligibility criteria included PD-L1–low/negative disease that had progressed after 1 platinum-containing regimen in the R/M setting. Patients were randomized (N = 267) from April 15, 2015, to March 16, 2016, at 127 sites in North America, Europe, and Asia Pacific.
Interventions
Durvalumab (20 mg/kg every 4 weeks) + tremelimumab (1 mg/kg every 4 weeks) for 4 cycles, followed by durvalumab (10 mg/kg every 2 weeks), or durvalumab (10 mg/kg every 2 weeks) monotherapy, or tremelimumab (10 mg/kg every 4 weeks for 7 doses then every 12 weeks for 2 doses) monotherapy.
Main Outcomes and Measures
Safety and tolerability and efficacy measured by objective response rate.
Results
Among the 267 patients (220 men [82.4%]), median age (range) of patients was 61.0 (23-82) years. Grade 3/4 treatment-related adverse events occurred in 21 patients (15.8%) treated with durvalumab + tremelimumab, 8 (12.3%) treated with durvalumab, and 11 (16.9%) treated with tremelimumab. Grade 3/4 immune-mediated adverse events occurred in 8 patients (6.0%) in the combination arm only. Objective response rate (95% CI) was 7.8% (3.78%-13.79%) in the combination arm (n = 129), 9.2% (3.46%-19.02%) for durvalumab monotherapy (n = 65), and 1.6% (0.04%-8.53%) for tremelimumab monotherapy (n = 63); median overall survival (95% CI) for all patients treated was 7.6 (4.9-10.6), 6.0 (4.0-11.3), and 5.5 (3.9-7.0) months, respectively.
Conclusions and Relevance
In patients with R/M HNSCC and low or no PD-L1 tumor cell expression, all 3 regimens exhibited a manageable toxicity profile. Durvalumab and durvalumab + tremelimumab resulted in clinical benefit, with minimal observed difference between the two. A phase 3 study is under way.
Trial Registration
clinicaltrials.gov Identifier: NCT02319044
This phase 2 randomized clinical trial compare the efficacy and toxicity profiles of durvalumab monotherapy and tremelimumab monotherapy against a combination of both in patients with recurrent or metastatic head and neck squamous cell carcinoma with low or no expression of programmed death ligand 1.
Introduction
Approximately 60% of patients with head and neck squamous cell carcinoma (HNSCC) present with locally advanced or metastatic disease; survival rates are low and many will experience relapse with locoregional recurrence, and/or metastatic disease.1,2 Patients with disease progression after first-line combination chemotherapy with or without biologic therapy have poor prognoses and are typically treated with single agents (eg, methotrexate, docetaxel, or cetuximab), which yield objective response rates (ORRs) of 4% to 13%.3,4,5,6
Agents targeting the anti–programmed death 1 (PD-1)/programmed death ligand 1 (PD-L1) pathway have shown promising activity in recurrent/metastatic (R/M) HNSCC with 2 PD-1 monoclonal antibodies (mAbs) approved for treatment of patients with platinum-refractory R/M HNSCC.7,8,9,10 Durvalumab, a human IgG1 mAb that blocks PD-L1 binding to PD-1 and CD80, has also shown antitumor activity as monotherapy in R/M HNSCC. In a phase 1/2 study to evaluate durvalumab,11 an ORR of 11% was achieved with durvalumab monotherapy; in the Phase II Study of Durvalumab Monotherapy in Treatment of Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Neck (HAWK),12 durvalumab demonstrated an ORR of 16.2% with a median duration of response of 10.3 months in patients with 25% or more tumor cells expressing PD-L1 (TC≥25%) whose disease progressed after 1 line of platinum-based therapy in the R/M setting.11,12
Expression of PD-L1 has been shown to be predictive of efficacy for PD-1/PD-L1 targeted therapy in several tumor types, including HNSCC.13,14,15,16 The PD-L1 cutoff of TC25% was derived from a phase 1/2 study of durvalumab monotherapy, in which response rates in R/M HNSCC were 18% in patients with PD-L1 expression TC≥25% and 8% in patients with PD-L1 expression TC<25%.16,17,18 Although there is a clear difference in efficacy in these 2 groups, which supports the use of the TC25% cutoff, durvalumab monotherapy did show modest activity for patients with PD-L1 expression TC<25%, which is consistent with other single-agent activity in patients with R/M HNSCC.
Dual-targeted immunotherapy is an approach that can improve on the efficacy of monotherapy, and combining anti–PD-L1 and anti–cytotoxic T-lymphocyte–associated protein 4 (CTLA-4) mAbs has shown enhanced preclinical and clinical antitumor activity over either agent alone, indicating that the 2 pathways are not redundant.7,19,20,21 The combination of PD-1/PD-L1 and CTLA-4 targeting agents has demonstrated synergistic antitumor activity regardless of PD-L1 expression levels.22 Furthermore, the combination of durvalumab and tremelimumab, a selective human IgG2 anti–CTLA-4 mAb, has specifically shown efficacy in patients with PD-L1–low/negative tumors.15,23,24,25 Based on these and other data,26,27 addition of an anti–CTLA-4 mAb to enhance antitumor activity of anti–PD-L1 agents in patients with R/M HNSCC and PD-L1–low/negative tumors warrants investigation.
Herein we present results from the phase 2, randomized, open-label study of Durvalumab, Tremelimumab, and Durvalumab in Combination With Tremelimumab in Patients With R/M HNSCC (CONDOR) study. To our knowledge, this is the first study to specifically focus on patients with PD-L1–low/negative (TC <25%) R/M HNSCC with disease progression on or after a single platinum-containing regimen in the R/M setting.
Methods
Patients
Patients were enrolled from April 15, 2015, to March 16, 2016, at 127 sites in 15 countries in North America, Europe, and the Asia Pacific region. Eligible patients had disease progression or recurrence during or after treatment with only 1 platinum-containing regimen for R/M disease and were 18 years or older with confirmed R/M HNSCC of the oropharynx, oral cavity, hypopharynx, or larynx. Other key eligibility criteria included provision of newly acquired (preferred) or archival tumor tissue (<3 years old) for PD-L1 expression, confirmed PD-L1–low/negative HNSCC (TC<25%, using the validated VENTANA PD-L1 [SP263] Assay [Ventana Medical Systems]), and Eastern Cooperative Oncology Group performance status of 0 (normal activity) or 1 (restricted activity) at enrollment. Key exclusion criteria can be found in the eAppendix in Supplement 1. Review and approval of the study and diagnostic testing by an institutional review board or ethics committee was obtained for each site. The full trial protocol is provided in Supplement 2. Written informed consent from participants and any additional locally required authorization was obtained before performing any protocol-related procedures.
Study Design and Treatment
CONDOR was a randomized, open-label, multicenter, global phase 2 study. Patients were stratified by human papillomavirus (HPV) status and smoking status and then randomized 2:1:1 to treatment with durvalumab + tremelimumab (20 mg/kg durvalumab every 4 weeks plus 1 mg/kg tremelimumab every 4 weeks) for 4 cycles followed by durvalumab (10 mg/kg every 2 weeks), durvalumab monotherapy (10 mg/kg every 2 weeks) or tremelimumab monotherapy (10 mg/kg every 4 weeks for 7 doses then every 12 weeks for 2 additional doses) for up to 12 months. All treatment was administered via intravenous infusion. Computer software programming generated and allocated blocked randomization codes, which were assigned sequentially within each stratum via an interactive voice–interactive web response system. All study centers used the same list to minimize treatment assignment imbalances. Treatment beyond progressive disease was permitted for up to 12 months if a patient continued to receive benefit from the assigned treatment, met criteria for treatment in the setting of progressive disease, and, in the case of combination treatment, only if progressive disease occurred during the monotherapy portion. Human papillomavirus status was evaluated in all patients regardless of tumor location either from medical records (when available) or assessed in licensed clinical laboratories per local standard procedures or central testing (if local testing was not available).
Study Assessments
The primary objective was the ORR of durvalumab + tremelimumab using blinded independent central review (BICR) assessment according to Response Evaluation Criteria in Solid Tumours Working Group (RECIST), version 1.1. Tumor assessments were performed by using computed tomography or magnetic resonance imaging every 8 weeks for the first 48 weeks, then every 12 weeks in patients with disease control after 12 months until confirmed progressive disease. Secondary outcomes were the ORR in the monotherapy arms, duration of response, best objective response, disease control rate, progression-free survival, overall survival (OS), and safety in all arms. Health-related quality of life was assessed and will be reported elsewhere. Treatment-related adverse events (TRAEs) were monitored and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03.28 Adverse events of special interest were defined as events with potential inflammatory or immune-mediated mechanisms that may require more frequent monitoring or interventions, such as steroid, immunosuppressant, or hormone therapy. Immune-mediated adverse events (imAEs) were adverse events of special interest considered to have immune-mediated mechanisms of action.
Statistical Analysis
The study planned to screen 384 patients to identify and randomize approximately 240 patients with PD-L1–low/negative disease and to evaluate at least 208 patients for primary and secondary end points. No formal statistical comparisons were planned, although a sample size of 104 was deemed to provide greater than 90% power to test the hypothesis that the ORR of greater than 13% assuming the true ORR was 27% in the evaluable analysis set of patients treated with combination therapy. Descriptive statistics were used for all variables, as appropriate, and were presented by treatment group. Efficacy data were summarized and analyzed based on either the evaluable analysis set (patients receiving assigned treatment and with tumor assessment with measurable disease at baseline according to BICR), full analysis set (patients randomized to treatment or “intention-to-treat” population), or both. Kaplan-Meier plots and median values of progression-free survival and OS end points were determined and presented using a 95% CI and 2-sided P value, unless otherwise stated. An exploratory analysis of ORR was performed for PD-L1 at cutoffs of TC≥1% and TC≥10%. Safety data were summarized based on the safety analysis set and for the first 12 months of treatment only. Statistical analyses were performed using SAS software (SAS Institute Inc).
Results
Patient Disposition and Baseline Characteristics
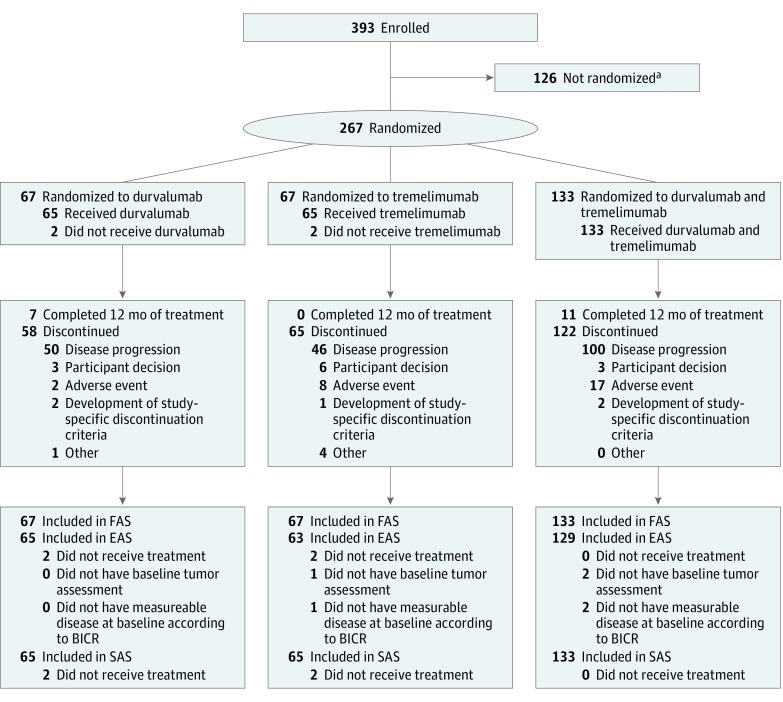
Data cutoff occurred March 31, 2017, approximately 12 months after the last patient entered the study. Two hundred sixty-seven patients were randomized: 133 to receive treatment with durvalumab + tremelimumab, 67 to receive durvalumab monotherapy, and 67 to receive tremelimumab monotherapy (Figure 1 and eTable in Supplement 1). A total of 257 patients received the assigned treatment and had tumor assessment with measurable disease at baseline according to BICR. Of these, 136 patients (52.9%) had PD-L1 TC<1%, 77 (30.0%) had PD-L1 TC≥1% but TC<10%, and 44 (17.1%) had PD-L1 TC≥10% but TC<25%, with a similar distribution among treatment arms. Overall, 212 patients (82.5%) had recurrence within 6 months after the last dose of a platinum-containing regimen, and 198 (74.2%) received cetuximab before study enrollment. Patients received a median of 2 previous lines of therapy: 121 (45.3%) received 1, 134 (50.2%) received 2, and 11 (4.1%) patients received 3 previous lines of therapy. Previous therapy included treatments with curative intent for locally advanced, neoadjuvant, adjuvant, and R/M disease or with concurrent chemotherapy (eg, chemoradiotherapy, radiotherapy, and/or surgery).
Figure 1. CONSORT Diagram.
All patients randomized are included in the full analysis set (FAS). EAS indicates evaluable analysis set; SAS, safety analysis set.
aThese patients did not meet all inclusion criteria, including programmed death ligand 1 low or no tumor expression, or met at least 1 of the exclusion criteria.
Baseline characteristics were generally well balanced among the 3 treatment arms and representative of a patient population with platinum-refractory R/M HNSCC (Table 1). One hundred seven tumors (40.1%) were located in the oropharynx, whereas 60 patients (22.5%) had primary tumors in the larynx, 54 (20.2%) in the oral cavity, and 43 (16.1%) in the hypopharynx. Human papillomavirus status was evaluated in all patients regardless of primary site: 75 (28.1%) patients had HPV, including 39 (29.3%) in the combination arm, 18 (26.9%) in the durvalumab arm, and 18 (26.9%) in the tremelimumab arm.
Table 1. Demographic and Disease Baseline Characteristics for Patients in the Full Analysis Set.
| Patient Characteristic | Treatment Group | All Patients (N = 267) | ||
|---|---|---|---|---|
| Durvalumab + tremelimumab (n = 133)a | Durvalumab (n = 67)a | Tremelimumab (n = 67)a | ||
| Age, median (range), y | 62 (26-81) | 62 (23-82) | 61 (42-77) | 61 (23-82) |
| Male, No. (%) | 113 (85.0) | 54 (80.6) | 53 (79.1) | 220 (82.4) |
| ECOG PS 1,b No. (%) | 93 (69.9) | 45 (67.2) | 48 (71.6) | 186 (69.7) |
| HPV positive,c No. (%) | 39 (29.3) | 18 (26.9) | 18 (26.9) | 75 (28.1) |
| Smoking/nicotine use, No (%) | ||||
| Current/former | 113 (85.0) | 58 (86.6) | 53 (79.1) | 224 (83.9) |
| >10 Pack-years | 81 (60.9) | 42 (62.7) | 40 (59.7) | 163 (61.0) |
| ≤10 Pack-years | 32 (24.1) | 16 (23.9) | 13 (19.4) | 61 (22.8) |
| Never | 20 (15.0) | 9 (13.4) | 14 (20.9) | 43 (16.1) |
| Previous therapy, No. (%) | ||||
| Cetuximab | 99 (74.4) | 50 (74.6) | 49 (73.1) | 198 (74.2) |
| Taxane | 59 (44.4) | 26 (38.8) | 26 (38.8) | 111 (41.6) |
| Chemotherapy (other)d | 97 (72.9) | 49 (73.1) | 51 (76.1) | 197 (73.8) |
| Radiotherapy | 117 (88.0) | 58 (86.6) | 62 (92.5) | 237 (88.8) |
| ≤6 mo From previous platinum therapy, No. (%) | 106 (82.8) | 53 (82.8) | 53 (81.5) | 212 (82.5) |
| Overall disease classification, No. (%) | ||||
| Locally advanced diseasee | 50 (37.6) | 22 (32.8) | 24 (35.8) | 96 (36.0) |
| Metastatic diseasef | 83 (62.4) | 45 (67.2) | 43 (64.2) | 171 (64.0) |
| Primary tumor location, No. (%) | ||||
| Oropharynx | 52 (39.1) | 25 (37.3) | 30 (44.8) | 107 (40.1) |
| Oral cavity | 24 (18.0) | 15 (22.4) | 15 (22.4) | 54 (20.2) |
| Hypopharynx | 25 (18.8) | 8 (11.9) | 10 (14.9) | 43 (16.1) |
| Larynx | 32 (24.1) | 17 (25.4) | 11 (16.4) | 60 (22.5) |
| Otherg | 0 | 2 (3.0) | 1 (1.5) | 3 (1.1) |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; FAS, full analysis set; HPV, human papillomavirus; PS, performance status.
Percentages are based on the number of patients with data for each baseline characteristic.
ECOG PS 1 indicates restricted activity.
Human papillomavirus status was evaluated in all patients.
All other chemotherapy and chemotherapy regimens, with the exception of platinum agents and taxanes.
Patients with recurrent disease.
Patients with metastatic disease at any site.
Other tumor locations include neck spinocellular carcinoma–larynx, lymph nodes from right submandibular area, and synchronous both oropharynx and hypopharynx.
Safety
Rates of TRAEs, but not of imAEs, were similar in the 3 arms (Table 2). Treatment-related AEs of any grade were seen in 77 patients (57.9%) in the combination arm, 41 (63.1%) in the durvalumab arm, and 36 (55.4%) in the tremelimumab arm. Overall, the most common TRAEs of any grade were diarrhea (36 patients [13.7%]), fatigue (24 [9.1%]), and asthenia (22 [8.4%]). Grade 3/4 TRAEs occurred in 21 patients (15.8%) in the combination arm, 8 (12.3%) in the durvalumab arm, and 11 (16.9%) in the tremelimumab arm. Treatment-related imAEs were reported in 26 patients (19.5%) in the combination arm and 5 (7.7%) in the durvalumab arm, but none in the tremelimumab arm. Grade 3/4 treatment-related imAEs were reported in 8 patients (6.0%) in the combination arm only. These adverse events were generally manageable, and were reversible in many cases. Bleeding events included hemorrhage in 1 patient (0.8%) in the combination arm, epistaxis in 3 patients (4.6%) in the combination arm and 1 (1.5%) in the durvalumab arm, and hemoptysis in 5 patients (3.8%) in the combination arm, 1 (1.5%) in the durvalumab arm, and 1 (1.5%) in the tremelimumab arm. Two bleeding events in the combination arm were considered treatment related, including 1 patient each with epistaxis and hemoptysis. Overall, 12 patients (4.6%) discontinued treatment owing to TRAEs. Common reasons were diarrhea (6 reports), dehydration (2 reports), and hepatobiliary disorders (2 reports). No patient from the durvalumab arm discontinued treatment owing to TRAEs.
Table 2. Treatment-Related Adverse Events in the Safety Analysis Set.
| Event | Treatment Group | All Patients (N = 263) | ||
|---|---|---|---|---|
| Durvalumab + Tremelimumab (n = 133) | Durvalumab (n = 65) | Tremelimumab (n = 65) | ||
| All-grade TRAEs, No. (%)a | 77 (57.9) | 41 (63.1) | 36 (55.4) | 154 (58.6) |
| Diarrhea | 19 (14.3) | 7 (10.8) | 10 (15.4) | 36 (13.7) |
| Asthenia | 13 (9.8) | 5 (7.7) | 4 (6.2) | 22 (8.4) |
| Hypothyroidism | 11 (8.3) | 7 (10.8) | 1 (1.5) | 19 (7.2) |
| Decreased appetite | 11 (8.3) | 2 (3.1) | 3 (4.6) | 16 (6.1) |
| Rash | 9 (6.8) | 1 (1.5) | 5 (7.7) | 15 (5.7) |
| Fatigue | 8 (6.0) | 12 (18.5) | 4 (6.2) | 24 (9.1) |
| Anemia | 8 (6.0) | 1 (1.5) | 0 | 9 (3.4) |
| Nausea | 7 (5.3) | 1 (1.5) | 5 (7.7) | 13 (4.9) |
| Pyrexia | 6 (4.5) | 0 | 4 (6.2) | 10 (3.8) |
| Pruritus | 5 (3.8) | 5 (7.7) | 3 (4.6) | 13 (4.9) |
| Vomiting | 2 (1.5) | 1 (1.5) | 5 (7.7) | 8 (3.0) |
| Grade 3/4 TRAEs, No. (%)b | 21 (15.8) | 8 (12.3) | 11 (16.9) | 40 (15.2) |
| Diarrhea | 4 (3.0) | 0 | 3 (4.6) | 7 (2.7) |
| Anemia | 3 (2.3) | 0 | 0 | 3 (1.1) |
| Asthenia | 3 (2.3) | 0 | 0 | 3 (1.1) |
| Fatigue | 1 (0.8) | 2 (3.1) | 1 (1.5) | 4 (1.5) |
| Treatment-related SAE, No. (%) | 17 (12.8) | 1 (1.5) | 8 (12.3) | 26 (9.9) |
| Treatment-related AESI, No. (%) | 47 (35.3) | 23 (35.4) | 20 (30.8) | 90 (34.2) |
| Treatment-related imAE, No. (%) | 26 (19.5) | 5 (7.7) | 0 (0.0) | 31 (11.8) |
| Grade 3/4 treatment-related imAE, No. (%) | 8 (6.0) | 0 (0.0) | 0 (0.0) | 8 (3.0) |
| TRAE leading to dose interruption, No. (%) | 11 (8.3) | 4 (6.2) | 6 (9.2) | 21 (8.0) |
| TRAE leading to dose discontinuation, No. (%) | 7 (5.3) | 0 | 5 (7.7) | 12 (4.6) |
| TRAE leading to death, No. (%) | 1 (0.8)c | 0 | 0 | 1 (0.4) |
Abbreviations: AESI, adverse event of special interest; imAE, immune-mediated adverse event; SAE, serious adverse event; SAS, safety analysis set; TRAEs, treatment-related adverse events.
Individual adverse events with incidence greater than 5% in any arm are reported.
Individual grade 3/4 adverse events with incidence greater than 2% in any arm are reported.
Grade 3 acute respiratory failure.
One treatment-related death occurred. A 72-year-old man in the combination arm experienced treatment-related grade 3 acute respiratory failure 38 days after his first and only administration of therapy and died 50 days after administration. The primary cause of death was squamous cell carcinoma disease progression.
Efficacy
A reduction in target lesion size from baseline was reported in 40 patients (31.0%) in the combination arm, 15 (23.1%) in the durvalumab arm, and 9 (14.3%) in the tremelimumab arm (eFigure in Supplement 1). For most patients, no new lesions were reported during the study.
Objective response rate by BICR in patients treated with durvalumab + tremelimumab was 7.8% (95% CI, 3.78%-13.79%) and was 9.2% (95% CI, 3.46%-19.02%) in the durvalumab arm and 1.6% (95% CI, 0.04%-8.53%) in the tremelimumab arm (Table 3); all were partial responses. In patients who responded, the median time to response (range) was 2.0 (2-6) months in the combination arm, 4.1 (2-6) months in the durvalumab arm, and 1.8 months (range not applicable; 1 patient responded) in the tremelimumab arm. At data cutoff, responses were ongoing in 5 of 10 patients (50%) in the combination arm, 4 of 6 (66.7%) in the durvalumab arm, and 1 of 1 (100%) in the tremelimumab arm. The median (interquartile range) duration of response was 9.4 (4.9-not applicable) months in the combination arm and had not been reached in the durvalumab or tremelimumab arms. The ORR for patients with PD-L1–negative disease (TC<1%) was 7.4% (5 of 68 patients) in the combination arm, and 8.8% (3 of 34) in the durvalumab arm. The ORR for patients with TC<10% was 6.8% (7 of 103 patients) in the combination arm and 8.9% (5 of 56) in the durvalumab arm.
Table 3. Objective Response Rate in the Evaluable Analysis Seta.
| Response | Treatment Group | All Patients (N = 257) | ||
|---|---|---|---|---|
| Durvalumab + Tremelimumab (n = 129) | Durvalumab (n = 65) | Tremelimumab (n = 63) | ||
| ORR, No. (%) [95% CI]a | 10 (7.8) [3.78-13.79] | 6 (9.2) [3.46-19.02] | 1 (1.6) [0.04-8.53] | 17 (6.6) |
| Odds ratio (95% CI)b | 1 [Reference] | 0.83 (0.29-2.53) | 5.21 (0.96-96.70) | NA |
| P value | .73 | .06 | ||
| Complete response, No. | 0 | 0 | 0 | 0 |
| Partial response, No. (%) | 10 (7.8) | 6 (9.2) | 1 (1.6) | 17 (6.6) |
| Stable disease ≥6 mo, No. (%) | 7 (5.4) | 4 (6.2) | 0 | 11 (4.3) |
| Progressive disease, No. (%) | 83 (64.3) | 42 (64.6) | 44 (69.8) | 169 (65.8) |
| DCR at 6 mo, No. (%)c | 17 (13.2) | 14 (21.5) | 1 (1.6) | 32 (12.5) |
| Median (range) time to response, mo | 2.0 (2-6) | 4.1 (2-6) | 1.8 (NA) | 3.5 (2-6) |
| Median (IQR) duration of response, mo | 9.4 (4.9-NA) | NA | NA | NA |
| Ongoing response at data cutoff, No. (%) | 5 (50.0) | 4 (66.7) | 1 (100) | 10 (58.8) |
Abbreviations: DCR, disease control rate; IQR, interquartile range; NA, not applicable; ORR, objective response rate.
ORR at 12 months of treatment based on blinded independent central review assessments using RECIST, version 1.1.
Odds ratio is a comparison between groups (combination vs monotherapy); an odds ratio greater than 1 favors the combination.
Patients who had a best objective response of complete response or partial response in the first 24 weeks or demonstrated stable disease for a minimum interval of 24 weeks following randomization.
Among patients with HPV-positive tumors, the ORR was 5.4% (95% CI, 0.66%-18.19%) in the combination arm and 16.7% (95% CI, 3.58%-41.42%) in the durvalumab arm. However, the 95% CIs for this subgroup were wide; therefore, this result should be interpreted with caution. The patient in the tremelimumab arm with partial response was HPV negative.
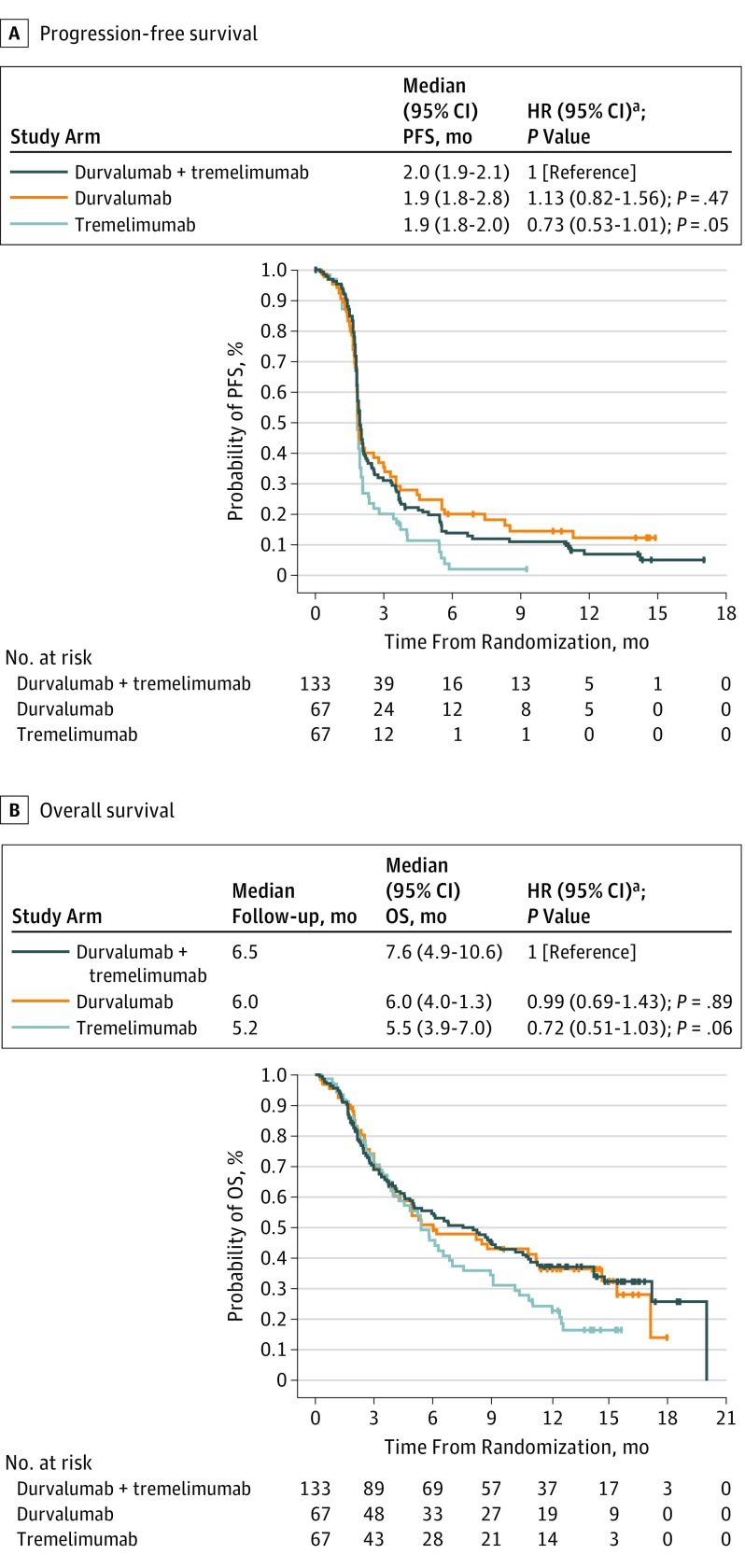
Median (95% CI) progression-free survival was 2.0 (1.9-2.1) months in the combination arm and 1.9 (1.8-2.8) months in the durvalumab arm and 1.9 (1.8-2.0) months in the tremelimumab arm (Figure 2A). Risk of progression for patients receiving combination therapy vs durvalumab monotherapy (hazard ratio [HR], 1.13; 95% CI, 0.82-1.56; P = .47) or tremelimumab monotherapy (HR, 0.73; 95% CI, 0.53-1.01; P = .05) was not statistically significant. The 6-month progression-free survival rate was higher in the combination arm (13.7%; 95% CI, 8.3%-20.4%) and durvalumab arm (20.0%; 95% CI, 11.3%-30.4%) than in the tremelimumab arm (1.9%; 95% CI, 0.2%-8.6%).
Figure 2. Kaplan-Meier Estimates of Progression-Free Survival and Overall Survival.
Hatching on plots indicates censored data.
aStratified log rank test.
Median (range) follow-up was 6.5 (0.2-20.0) months in the combination arm, 6.0 (0.3-18.0) months in the durvalumab arm, and 5.2 (0.3-16.0) months in the tremelimumab arm. Median (95% CI) OS were 7.6 (4.9-10.6) months in the combination arm, 6.0 (4.0-11.3) months in the durvalumab arm, and 5.5 (3.9-7.0) months in the tremelimumab arm (Figure 2B). Risk of death for patients receiving the combination vs durvalumab monotherapy (HR, 0.99; 95% CI, 0.69-1.43; P = .89) or tremelimumab monotherapy (HR, 0.72; 95% CI, 0.51-1.03; P = .06) was not statistically significant. The OS rate at 12 months was 37% in the combination arm, 36% in the durvalumab arm, and 24% in the tremelimumab arm.
Discussion
To our knowledge, CONDOR was the first study to exclusively focus on a population of patients with PD-L1–low/negative R/M HNSCC in whom disease progressed on or after 1 platinum-containing regimen in the R/M setting and the first to use an anti–CTLA-4 mAb for patients with HNSCC. Durvalumab monotherapy in the CONDOR study showed an ORR of 9.2%. This compares with an ORR of 14.6% for pembrolizumab vs 10.1% for investigators’ choice of therapy in all comers in the Keynote-040 study, and with an ORR of 13.3% for nivolumab vs 5.8% for investigators’ choice in all comers in the CheckMate-141 trial.29,30 Studies to date of anti–PD-1 immuno-oncology agents indicate that PD-L1 expression is associated with response rate. In the single-arm Keynote-055 study, pembrolizumab ORR was 12% in the subgroup with PD-L1 TC<1% vs 18% in the subgroup with PD-L1 TC≥1%.31 Similarly, in the Checkmate-141 study, nivolumab ORR was 12.3% vs 17% in the same subgroups. In contrast, in our study, ORR within each treatment group, and PD-L1 subgroup, was broadly similar irrespective of the PD-L1 expression grouping within patients with low or no expression of PD-L1. In the HAWK study, which enrolled only patients with PD-L1–high expression (TC≥25%), durvalumab ORR was 16.2%.12 Although cross-trial comparisons can be challenging, study populations among the Keynote-040, CheckMate-141, and CONDOR trials had several similarities. All studies enrolled patients with R/M HNSCC and disease progression after platinum-containing chemotherapy; Keynote-040 enrolled patients within 3 to 6 months of multimodal platinum-based therapy, and CheckMate-141 enrolled patients within 6 months after platinum-containing chemotherapy.32,33 More than 80% of patients in the CONDOR study had disease recurrence within 6 months of the last dose of a platinum-containing regimen. CONDOR, CheckMate-141, and Keynote-040 included similar percentages of HPV-positive patients (28.1%, 25.6%, and 24.0%, respectively).32,33 In addition, 74.2% of patients had previously received cetuximab in CONDOR, and 61.3% had previously received cetuximab in CheckMate-141.33 Keynote-040 and CheckMate-141 did enroll all comers in terms of PD-L1 expression; therefore, some patients had high PD-L1 expression according to the specific algorithms used for those studies.
The CONDOR study was not powered to compare the combination and monotherapy arms, but addition of tremelimumab did not appear to improve outcomes compared with single-agent durvalumab in patients with PD-L1–low/negative disease. However, both the combination and durvalumab monotherapy arms showed clinically relevant OS. In Keynote-040, median OS was 8.4 months for pembrolizumab vs 6.9 months for investigators’ choice of therapy, whereas, in patients with a combined positive score of 1 or higher (PD-L1 TC and immune cell expression ≥1), median OS was 8.7 months with pembrolizumab vs 7.1 months with investigators’ choice of therapy. In patients with a tumor proportion score of greater than 50%, median OS was 11.6 months with pembrolizumab vs 6.6 months with investigators’ choice of therapy.32 In CheckMate-141, median OS in response to nivolumab treatment was 8.2 months for patients with PD-L1–positive expression (≥1% tumor cell membrane staining) vs 6.5 months for patients with PD-L1–negative expression (<1% tumor cell membrane staining).33 Considering all comers in CheckMate-141, in which the median OS in response to nivolumab therapy was 7.7 months vs 5.1 months for the investigators’ choice of therapy, our study shows similar median survival for patients treated with durvalumab + tremelimumab (7.6 months) or durvalumab alone (6.0 months), indicating their potential utility irrespective of PD-L1 expression.
The rationale for combining anti–PD-L1 and anti–CTLA-4 mAbs has precedence in other tumor types, including PD-L1–low/negative tumors. The combination of nivolumab and ipilimumab has yielded higher ORRs compared with each monotherapy in melanoma.34 A dose of 20 mg/kg of durvalumab plus 1 mg/kg of tremelimumab was identified as the maximum tolerated dose for this combination in a phase 1b study in patients with non–small cell lung cancer, which demonstrated antitumor activity in patients with PD-L1–low/negative (TC<25%) expression.15 Higher dosages of tremelimumab were evaluated in this early-phase study but resulted in a higher frequency of TRAEs, grade 3 or 4 adverse events, and serious adverse events, without any increase in efficacy (ORR).15 Serum concentration levels of tremelimumab in combination with durvalumab did not show any marked difference between doses and T-cell proliferation, and activation markers for different doses of tremelimumab also did not show any marked difference in this study.15 It is therefore unlikely that the tremelimumab dose used in the combination arm in the present study limited treatment efficacy. The lack of efficacy of tremelimumab may be related to its mechanism of action, which as an IgG2 mAb does not cause lysis of regulatory T cells through antibody-dependent cell-mediated cytotoxicity, which is observed with ipilimumab.35 The clinical relevance of this difference remains uncertain.
All arms exhibited manageable safety profiles in a patient population with few treatment options. The durvalumab monotherapy safety profile is consistent with previous data,11,12 and the combination arm produced no additional safety concerns. Grade 3 or 4 TRAEs occurred in 15% of patients overall and imAEs reported in the study were typical of the PD-1/PD-L1/CTLA-4 class of immunotherapies, which have previously been associated with hypothyroidism, diarrhea, pneumonitis, and colitis.11,12,15,26,27,30,31,36,37 One treatment-related death was reported in a patient receiving combination therapy.
Limitations
The most apparent limitation to this study is that the study was not powered to compare the combination and monotherapy arms.
Conclusions
Durvalumab monotherapy showed a manageable toxicity profile and clinical benefit for patients with R/M HNSCC and low or no PD-L1 TC expression; durvalumab + tremelimumab demonstrated similar efficacy to durvalumab monotherapy. Although the CONDOR study was not powered to compare the combination and monotherapy arms, our findings in this particular study do not appear to support the hypothesis that tremelimumab combined with durvalumab exerts a synergistic therapeutic effect in this population with low or no expression of PD-L1. CONDOR is part of a broader comprehensive clinical program in HNSCC that includes patients with both PD-L1–high and PD-L1–low/negative disease. The CONDOR study is the first, to our knowledge, to focus on the use of an anti–PD-L1/CTLA-4 combination and its respective monotherapies in patients with PD-L1–low/negative disease. Continued efforts are needed to improve outcomes in this challenging patient population, perhaps in combination with additional or other therapeutics that can stimulate the immune system without adding significant toxicity. Further understanding of the immune landscape of this group of tumors is germane to designing rational combinations. The ongoing phase 3 study of Durvalumab Monotherapy and in Combination With Tremelimumab vs Standard of Care Therapy in Patients With Head and Neck Cancer (EAGLE) will assess the combination of durvalumab + tremelimumab as second-line treatment in patients with PD-L1–high (TC≥25%) and PD-L1–low/negative (TC<25%) R/M HNSCC.30
eAppendix. Eligibility Criteria
eTable. Patient Disposition
eFigure. Best Percentage Change in Tumor Size Based on BICR Assessment According to RECIST v1.1 (EAS)
Trial Protocol
Data Sharing Statement
References
- 1.Monnerat C, Faivre S, Temam S, Bourhis J, Raymond E. End points for new agents in induction chemotherapy for locally advanced head and neck cancers. Ann Oncol. 2002;13(7):995-1006. doi: 10.1093/annonc/mdf172 [DOI] [PubMed] [Google Scholar]
- 2.Vermorken JB, Specenier P. Optimal treatment for recurrent/metastatic head and neck cancer. Ann Oncol. 2010;21(suppl 7):vii252-vii261. doi: 10.1093/annonc/mdq453 [DOI] [PubMed] [Google Scholar]
- 3.Kushwaha VS, Gupta S, Husain N, et al. Gefitinib, methotrexate and methotrexate plus 5-Fluorouracil as palliative treatment in recurrent head and neck squamous cell carcinoma. Cancer Biol Ther. 2015;16(2):346-351. doi: 10.4161/15384047.2014.961881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stewart JS, Cohen EE, Licitra L, et al. Phase III study of gefitinib compared with intravenous methotrexate for recurrent squamous cell carcinoma of the head and neck [corrected]. J Clin Oncol. 2009;27(11):1864-1871. doi: 10.1200/JCO.2008.17.0530 [DOI] [PubMed] [Google Scholar]
- 5.Vermorken JB, Trigo J, Hitt R, et al. Open-label, uncontrolled, multicenter phase II study to evaluate the efficacy and toxicity of cetuximab as a single agent in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck who failed to respond to platinum-based therapy. J Clin Oncol. 2007;25(16):2171-2177. doi: 10.1200/JCO.2006.06.7447 [DOI] [PubMed] [Google Scholar]
- 6.Zenda S, Onozawa Y, Boku N, Iida Y, Ebihara M, Onitsuka T. Single-agent docetaxel in patients with platinum-refractory metastatic or recurrent squamous cell carcinoma of the head and neck (SCCHN). Jpn J Clin Oncol. 2007;37(7):477-481. doi: 10.1093/jjco/hym059 [DOI] [PubMed] [Google Scholar]
- 7.Allen CT, Clavijo PE, Van Waes C, Chen Z. Anti-tumor immunity in head and neck cancer: understanding the evidence, how tumors escape and immunotherapeutic approaches. Cancers (Basel). 2015;7(4):2397-2414. doi: 10.3390/cancers7040900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keck MK, Zuo Z, Khattri A, et al. Integrative analysis of head and neck cancer identifies two biologically distinct HPV and three non-HPV subtypes. Clin Cancer Res. 2015;21(4):870-881. doi: 10.1158/1078-0432.CCR-14-2481 [DOI] [PubMed] [Google Scholar]
- 9.KEYTRUDA® (pembrolizumab) [prescribing information]. Whitehouse Station, NJ: Merck & Co, Inc; 2017. [Google Scholar]
- 10.OPDIVO (nivolumab) [prescribing information]. Princeton, NJ: Bristol-Myers Squibb; 2017. [Google Scholar]
- 11.Segal NH, Ou S-HI, Balmanoukian AS, et al. Updated safety and efficacy of durvalumab (MEDI4736), an anti-PD-L1 antibody, in patients from a squamous cell carcinoma of the head and neck (SCCHN) expansion cohort [abstract 949O]. Ann Oncol. 2016;27(suppl 6):328-350. doi: 10.1093/annonc/mdw376.01 [DOI] [Google Scholar]
- 12.Zandberg D, Algazi A, Jimeno A, et al. Durvalumab for recurrent/metastatic (R/M) head and neck squamous cell carcinoma (HNSCC): preliminary results from a single-arm, phase 2 study. Paper presented at ESMO; September 8-12, 2017; Madrid, Spain. [Google Scholar]
- 13.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443-2454. doi: 10.1056/NEJMoa1200690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515(7528):563-567. doi: 10.1038/nature14011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Antonia S, Goldberg SB, Balmanoukian A, et al. Safety and antitumour activity of durvalumab plus tremelimumab in non-small cell lung cancer: a multicentre, phase 1b study. Lancet Oncol. 2016;17(3):299-308. doi: 10.1016/S1470-2045(15)00544-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rebelatto MMA, Sabalos C, Walker J, et al. Development of a PD-L1 companion diagnostic assay for treatment with MEDI4736 in NSCLC and SCCHN patients. J Clin Oncol. 2015;33(15)(suppl):8033-8033. doi: 10.1200/jco.2015.33.15_suppl.8033 [DOI] [Google Scholar]
- 17.Segal NH, Hamid O, Hwu W, et al. A phase I multi-arm dose-expansion study of the anti-programmed cell death-ligand-1 (PD-L1) antibody MEDI4736: preliminary data. Presented as a poster at: ESMO; September 26-30, 2014; Madrid, Spain. Poster 1058PD. [Google Scholar]
- 18.Wildsmith S, Scott M, Midha A, et al. PD-L1 expression in patients screened for phase 2 head an neck squamous cell carcinoma clinical studies (HAWK and CONDOR). Paper presented at: AACR Annual Meeting; April 14-17, 2018; Chicago, IL. [Google Scholar]
- 19.Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci U S A. 2010;107(9):4275-4280. doi: 10.1073/pnas.0915174107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mangsbo SM, Sandin LC, Anger K, Korman AJ, Loskog A, Tötterman TH. Enhanced tumor eradication by combining CTLA-4 or PD-1 blockade with CpG therapy. J Immunother. 2010;33(3):225-235. doi: 10.1097/CJI.0b013e3181c01fcb [DOI] [PubMed] [Google Scholar]
- 21.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252-264. doi: 10.1038/nrc3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369(2):122-133. doi: 10.1056/NEJMoa1302369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ribas A, Camacho LH, Lopez-Berestein G, et al. Antitumor activity in melanoma and anti-self responses in a phase I trial with the anti-cytotoxic T lymphocyte-associated antigen 4 monoclonal antibody CP-675,206. J Clin Oncol. 2005;23(35):8968-8977. doi: 10.1200/JCO.2005.01.109 [DOI] [PubMed] [Google Scholar]
- 24.Tarhini AA. Tremelimumab: a review of development to date in solid tumors. Immunotherapy. 2013;5(3):215-229. doi: 10.2217/imt.13.9 [DOI] [PubMed] [Google Scholar]
- 25.Balar AV, Mahipal A, Grande E, et al. Durvalumab + tremelimumab in patients with metastatic urothelial cancer. In: Proceedings of the 109th Annual Meeting of the American Association for Cancer Research Chicago, IL: AACR; 2018:78(13)(suppl):CT112-CT112. doi: 10.1158/1538-7445.AM2018-CT112 [DOI] [Google Scholar]
- 26.Kirkwood JM, Lorigan P, Hersey P, et al. Phase II trial of tremelimumab (CP-675,206) in patients with advanced refractory or relapsed melanoma. Clin Cancer Res. 2010;16(3):1042-1048. doi: 10.1158/1078-0432.CCR-09-2033 [DOI] [PubMed] [Google Scholar]
- 27.Ribas A, Kefford R, Marshall MA, et al. Phase III randomized clinical trial comparing tremelimumab with standard-of-care chemotherapy in patients with advanced melanoma. J Clin Oncol. 2013;31(5):616-622. doi: 10.1200/JCO.2012.44.6112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.US Dept of Health and Human Services Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/Archive/CTCAE_4.0_2009-05-29_QuickReference_8.5x11.pdf. Published May 28, 2009. Accessed October 1, 2018.
- 29.Cohen EE, Harrington KJ, Le Tourneau C, et al. Pembrolizumab (pembro) vs standard of care (SOC) for recurrent or metastatic head and neck squamous cell carcinoma (R/M HNSCC): phase 3 KEYNOTE-040 trial [abstract]. Ann Oncol. 2017;28(suppl 5):28. doi: 10.1093/annonc/mdx440 [DOI] [Google Scholar]
- 30.Ferris RL, Blumenschein G Jr, Fayette J, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375(19):1856-1867. doi: 10.1056/NEJMoa1602252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bauml J, Seiwert TY, Pfister DG, et al. Pembrolizumab for platinum- and cetuximab-refractory head and neck cancer: results from a single-arm, phase II study. J Clin Oncol. 2017;35(14):1542-1549. doi: 10.1200/JCO.2016.70.1524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Soulieres D, Cohen E, LeTourneau C, et al. Updated survival results of the KEYNOTE-040 study of pembrolizumab vs standard-of-care chemotherapy for recurrent or metastatic head and neck squamous cell carcinoma. In: Proceedings of the 109th Annual Meeting of the American Association for Cancer Research (AACR) Chicago, IL; AACR; 2018. [Google Scholar]
- 33.Ferris RL, Blumenschein G Jr, Fayette J, et al. Nivolumab vs investigator’s choice in recurrent or metastatic squamous cell carcinoma of the head and neck: 2-year long-term survival update of CheckMate 141 with analyses by tumor PD-L1 expression. Oral Oncol. 2018;81:45-51. doi: 10.1016/j.oraloncology.2018.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(1):23-34. doi: 10.1056/NEJMoa1504030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Romano E, Kusio-Kobialka M, Foukas PG, et al. Ipilimumab-dependent cell-mediated cytotoxicity of regulatory T cells ex vivo by nonclassical monocytes in melanoma patients. Proc Natl Acad Sci U S A. 2015;112(19):6140-6145. doi: 10.1073/pnas.1417320112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chow LQM, Haddad R, Gupta S, et al. Antitumor activity of pembrolizumab in biomarker-unselected patients with recurrent and/or metastatic head and neck squamous cell carcinoma: results from the phase Ib KEYNOTE-012 expansion cohort. J Clin Oncol. 2016;34(32):3838-3845. doi: 10.1200/JCO.2016.68.1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seiwert TY, Burtness B, Mehra R, et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol. 2016;17(7):956-965. doi: 10.1016/S1470-2045(16)30066-3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Eligibility Criteria
eTable. Patient Disposition
eFigure. Best Percentage Change in Tumor Size Based on BICR Assessment According to RECIST v1.1 (EAS)
Trial Protocol
Data Sharing Statement