Key Points
Question
Does local control of brain metastases differ between treatment with surgical resection vs stereotactic radiosurgery?
Findings
In this exploratory analysis of the European Organization for the Research and Treatment of Cancer 22952-26001 trial including 268 patients with brain metastases, those undergoing stereotactic radiosurgery exhibited similar local tumor control to patients undergoing surgical resection after adjustment for tumor size, neurologic status, metastasis site, number of metastases, and presence of extracranial disease. However, risk of early local recurrence was higher among patients undergoing surgery, whereas risk of late recurrence was higher among those undergoing stereotactic radiosurgery.
Meaning
Surgical resection and stereotactic radiosurgery achieve comparable local control of brain metastases; future study should identify whether any patients benefit from escalations in local therapy.
This exploratory secondary analysis of a randomized clinical trial evaluated local control of brain metastases among patients treated with stereotactic radiosurgery vs surgical resection in the European Organization for the Research and Treatment of Cancer 22952-26001 phase 3 trial.
Abstract
Importance
Brain metastases are a common source of morbidity for patients with cancer, and limited data exist to support the local therapeutic choice between surgical resection and stereotactic radiosurgery (SRS).
Objective
To evaluate local control of brain metastases among patients treated with SRS vs surgical resection within the European Organization for the Research and Treatment of Cancer (EORTC) 22952-26001 phase 3 trial.
Design, Setting, and Participants
This unplanned, exploratory analysis of the international, multi-institutional randomized clinical trial EORTC 22952-26001 (conducted from 1996-2007) was performed from February 9, 2017, through July 25, 2018. The EORTC 22952-26001 trial randomized patients with 1 to 3 brain metastases to whole-brain radiotherapy vs observation after complete surgical resection or before SRS. Patients in the present analysis were stratified but not randomized according to local modality (SRS or surgical resection) and treated per protocol with 1 to 2 brain metastases and tumors with a diameter of no greater than 4 cm.
Interventions
Surgical resection or SRS.
Main Outcomes and Measures
The primary end point was local recurrence of treated lesions. Cumulative incidence of local recurrence was calculated according to modality (surgical resection vs SRS) with competing risk regression to adjust for prognostic factors and competing risk of death.
Results
A total of 268 patients were included in the analysis (66.4% men; median age, 60.7 years [range, 26.9-81.1 years]); 154 (57.5%) underwent SRS and 114 (42.5%) underwent surgical resection. Median follow-up time was 39.9 months (range, 26.0-1982.0 months). Compared with the SRS group, patients undergoing surgical resection had larger metastases (median 28 mm [range, 10-40 mm] vs 20 mm [range, 4-40 mm]; P < .001), more frequently had 1 brain metastasis (112 [98.2%] vs 114 [74.0%]; P < .001), and differed in location (parietal, 21 [18.4%] vs 61 [39.6%]; posterior fossa, 30 [26.3%] vs 12 [7.8%]; P < .001). In adjusted models, local recurrence was similar between the SRS and surgical resection groups (hazard ratio [HR], 1.15; 95% CI, 0.72-1.83). However, when stratified by interval, patients with surgical resection had a much higher risk of early (0-3 months) local recurrence compared with those undergoing SRS (HR, 5.94; 95% CI, 1.72-20.45), but their risk decreased with time (HR for 3-6 months, 1.37 [95% CI, 0.64-2.90]; HR for 6-9 months, 0.75 [95% CI, 0.28-2.00]). At 9 months or longer, the surgical resection group had a lower risk of local recurrence (HR, 0.36; 95% CI, 0.14-0.93).
Conclusions and Relevance
In this exploratory analysis, local control of brain metastases was similar between SRS and surgical resection groups. Stereotactic radiosurgery was associated with improved early local control of treated lesions compared with surgical resection, although the relative benefit decreased with time.
Trial Registration
ClinicalTrials.gov Identifier: NCT00002899
Introduction
Brain metastases are a common source of cancer morbidity, accounting for approximately 140 000 cases annually in the United States.1 Local therapies, including stereotactic radiosurgery (SRS) and surgical resection, result in improved survival relative to whole-brain radiotherapy (WBRT) alone among patients with a single lesion.2,3 Outside recognized indications for surgery such as establishing diagnosis or relieving mass effect, little evidence is available to guide the therapeutic choice of SRS vs surgical resection in the treatment of patients with limited brain metastases.
The European Organization for the Research and Treatment of Cancer (EORTC) 22952-26001 phase 3 trial assessed the role of adjuvant WBRT after local therapy (SRS or surgical resection) in patients with 1 to 3 brain metastases.4 In the original trial, patients were stratified by SRS or surgical resection and had surveillance with neuroimaging while receiving per protocol treatment. The purpose of our study was to assess local control of treated brain metastases among patients in EORTC 22952-26001 by SRS vs surgical resection.
Methods
Study Design
We performed an unplanned, exploratory analysis of EORTC 22952-26001 data. We included patients treated per protocol with 1 to 2 brain metastases and tumor diameter of no greater than 4 cm. The original trial allocated patients with 1 to 3 brain metastases to WBRT vs observation after surgical resection or before SRS. Patients were stratified but not randomized according to SRS or surgical resection. Follow-up examinations, including imaging of the brain, were performed 8 weeks after SRS or surgical resection and every 3 months thereafter. Data were collected from February 9, 2017, through July 25, 2018. This study was approved by the local institutional ethics committees, and all patients provided written informed consent.
Statistical Analysis
We defined the primary end point as local recurrence of treated brain metastases; the protocol specified local neurologic progression as an increase on magnetic resonance imaging of more than 25% of the size (in the 2 largest perpendicular diameters) of 1 of the original brain metastases. We compared categorical variables using the χ2 test or Fisher exact test and continuous variables using unpaired 2-sample, 2-sided t tests. We calculated cumulative incidence of local recurrence according to local therapy (SRS vs surgical resection) and compared incidence between groups using the Gray test to account for competing risk of all-cause mortality. For reference, we calculated the cumulative incidences of distant neurologic progression and all-cause mortality. In adjusted models stratified by WBRT, we used competing risk regression to adjust for covariates and account for competing risk of all-cause mortality. Covariates included diameter of largest brain metastasis, neurologic status, metastasis site, presence of macroscopic tumor outside the brain, and number of brain metastases. We tested for violations of the proportional hazards assumption by adding an effect of log-time to the Fine and Gray multiple regression model, which demonstrated substantial violations of proportionality. For ease of interpretation, we allowed the hazard ratios (HRs) to vary in discrete intervals early in the study; we chose intervals of 0 to 3, 3 to 6, 6 to 9, and greater than 9 months based on the period when hazards changed most substantially and because including these intervals resulted in nonsignificance of the test for violations of proportionality. We defined time of randomization as time 0. We performed a sensitivity analysis defining time 0 as completion of local therapy, because patients were randomized after surgical resection and before SRS. We performed statistical analysis with SAS software (version 9.4; SAS Institute, Inc), considered α<.05 for statistical significance, and reported 2-sided P values.
Results
We included 268 patients for analysis (178 men [66.4%] and 90 women [33.6%]; median age, 60.7 years [range, 26.9-81.1 years]). Reasons for patient exclusion are illustrated in eFigure 1 in Supplement 1. The median follow-up among all living patients was 39.9 months (range, 26.0-1982.0 months) (41.7 months [range, 26.0-1657.0 months] for SRS and 39.6 months [range, 65.0-1982.0 months] for surgical resection groups). Compared with the SRS group, the surgical resection group had larger tumors (median, 28 mm [range, 10-40 mm] vs 20 mm [range, 4-40 mm]; P < .001), more frequent baseline neurologic deficits (62 [54.4%] vs 66 [42.8%]; P = .04), varied location of metastases (parietal, 21 [18.4%] vs 61 [39.6%]; posterior fossa, 30 [26.3%] vs 12 [7.8%]; P < .001), less frequent presence of macroscopic tumor outside of the brain (55 [48.2%] vs 87 [56.5%]; P = .04), and more frequently 1 brain metastasis (112 [98.2%] vs 114 [74.0%]; P < .001) (Table 1).
Table 1. Baseline Patient Characteristics.
| Characteristic | Treatment Groupa | P Value | |
|---|---|---|---|
| SRS (n = 154) | Surgical Resection (n = 114) | ||
| Age, median, y | 60.6 | 61.2 | .68 |
| Diameter of largest lesion, median (range), mm | 20 (4-40) | 28 (10-40) | <.001 |
| Sex, No. (%) | |||
| Male | 99 (64.3) | 79 (69.3) | .39 |
| Female | 55 (35.7) | 35 (30.7) | |
| WBRT, No. (%) | |||
| Observation | 75 (48.7) | 59 (51.8) | .62 |
| WBRT | 79 (51.3) | 55 (48.2) | |
| Performance status, No. (%)b | |||
| 0 | 68 (44.2) | 49 (43.0) | .94 |
| 1 | 67 (43.5) | 52 (45.6) | |
| 2 | 19 (12.3) | 13 (11.4) | |
| Neurologic status, No. (%) | |||
| No neurologic deficit | 88 (57.1) | 52 (45.6) | .04 |
| Some deficit, useful work | 37 (24.0) | 44 (38.6) | |
| Moderate impairment | 29 (18.8) | 18 (15.8) | |
| Primary tumor location, No. (%) | |||
| Breast | 18 (11.7) | 10 (8.8) | .25 |
| Colorectum | 10 (6.5) | 15 (13.2) | |
| Kidney | 17 (11.0) | 9 (7.9) | |
| Lung | 91 (59.1) | 67 (58.8) | |
| Melanoma | 9 (5.8) | 3 (2.6) | |
| Other | 9 (5.8) | 10 (8.8) | |
| Histologic finding, No. (%) | |||
| Adenocarcinoma | 82 (53.2) | 69 (60.5) | .71 |
| Squamous | 22 (14.3) | 15 (13.2) | |
| Melanoma | 9 (5.8) | 3 (2.6) | |
| Anaplastic | 3 (1.9) | 1 (0.9) | |
| Non–small cell | 28 (18.2) | 18 (15.8) | |
| Other | 10 (6.5) | 8 (7.0) | |
| Brain metastasis site, No. (%) | |||
| Frontal | 43 (27.9) | 42 (36.8) | <.001 |
| Parietal | 61 (39.6) | 21 (18.4) | |
| Temporal | 11 (7.1) | 5 (4.4) | |
| Occipital | 19 (12.3) | 14 (12.3) | |
| Posterior fossa | 12 (7.8) | 30 (26.3) | |
| Other | 8 (5.2) | 2 (1.8) | |
| Macroscopic tumor outside brain, No. (%) | |||
| Absent | 67 (43.5) | 55 (48.2) | .04 |
| Present | 87 (56.5) | 55 (48.2) | |
| Unknown | 0 | 4 (3.5) | |
| No. of metastases, No. (%) | |||
| 1 | 114 (74.0) | 112 (98.2) | <.001 |
| 2 | 40 (26.0) | 2 (1.8) | |
Abbreviations: SRS, stereotactic radiosurgery; WBRT, whole brain radiotherapy.
Percentages have been rounded and may not total 100.
Higher scores indicate worse performance status.
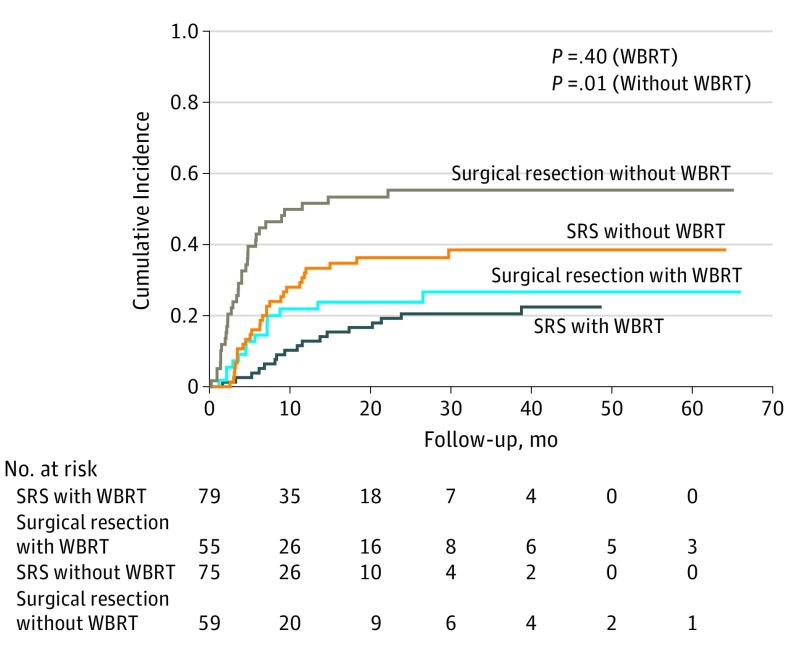
Unadjusted cumulative incidence of local recurrence was significantly higher among patients with surgical resection; however, local recurrence was similar between the SRS and surgical resection groups receiving WBRT (Figure and eTable 1 in Supplement 1). The adjusted risk of local brain metastasis recurrence is outlined in Table 2. Adjusted risk of local recurrence was similar after surgical resection and SRS (HR, 1.15; 95% CI, 0.72-1.83). However, the risk of local recurrence by modality varied according to time (P < .001 for interaction); early recurrence was associated with surgical resection (HR at 0-3 months, 5.94; 95% CI, 1.72-20.45), whereas late recurrence was associated with SRS (HR at >9 months, 2.77; 95% CI, 1.08-7.12). In adjusted models, increasing tumor size was associated with increased risk of local recurrence (HR, 1.03; 95% CI, 1.00-1.06), whereas the presence of macroscopic tumor outside the brain was associated with decreased risk of local recurrence (HR, 0.63; 95% CI, 0.40-0.99). Cumulative incidences of distant neurologic progression or deaths due to any cause are listed in eFigures 2 to 3 in Supplement 1. Sensitivity analysis considering time 0 as completion of local therapy yielded consistent results in direction and significance for risk of local recurrence according to SRS vs surgical resection (eTable 2 in Supplement 1).
Figure. Unadjusted Cumulative Incidence of Local Recurrence of Treated Brain Metastases.
Incidence is stratified according to local therapy and receipt of whole-brain radiotherapy (WBRT). SRS indicates stereotactic radiosurgery.
Table 2. Adjusted Competing Risk Regression Model for Factors Associated With Local Recurrence of Previously Treated Brain Metastasesa.
| Variable | HR (95% CI) | P Value |
|---|---|---|
| Surgical resection vs SRSb | 1.15 (0.72-1.83) | .56 |
| Time of recurrence, moc | ||
| 0-3 | 5.94 (1.72-20.45) | .005 |
| 3-6 | 1.37 (0.64-2.90) | .47 |
| 6-9 | 0.75 (0.28-2.00) | .56 |
| ≥9 | 0.36 (0.14-0.93) | .04 |
| Diameter of lesion | 1.03 (1.00-1.06) | .03 |
| Baseline neurologic status | ||
| No neurologic deficit | 1 [Reference] | NA |
| Some deficit, useful work | 1.31 (0.83-2.06) | .25 |
| Moderate impairment | 0.98 (0.53-1.79) | .94 |
| Metastasis site | ||
| Temporal | 1 [Reference] | NA |
| Frontal | 1.40 (0.71-2.76) | .33 |
| Occipital | 1.60 (0.76-3.40) | .22 |
| Parietal | 1.01 (0.48-2.12) | .98 |
| Posterior fossa | 0.95 (0.43-2.11) | .89 |
| Other | 0.24 (0.03-2.04) | .19 |
| Macroscopic tumor outside brain | ||
| Absent | 1 [Reference] | NA |
| Present | 0.63 (0.40-0.99) | .04 |
| No. of brain metastases | ||
| 1 | 1 [Reference] | NA |
| 2 | 0.78 (0.38-1.59) | .49 |
Abbreviations: HR, hazard ratio; NA, not applicable; SRS, stereotactic radiosurgery.
Model is stratified according to receipt of whole-brain radiotherapy.
Represents overall hazard of surgical resection vs SRS.
Evaluates surgical resection vs SRS according to period.
Discussion
The principal finding of our exploratory analysis of EORTC 22952-26001 is similar overall local control of brain metastases treated with SRS compared with surgical resection after adjustment for tumor size, metastasis site and number, neurologic status, and the presence of extracranial disease. Early local control favored SRS, although the advantage diminished in magnitude over time with more late recurrences observed in the SRS group.
Our findings are comparable to those of a randomized clinical trial demonstrating no significant differences in local control at 1 year between surgical resection and WBRT (82%) vs SRS (96.8%; P = .06).5 This trial was limited in power, accruing only 64 of 242 planned patients. In contrast, an institutional series of 75 patients with brain metastasis6 reported superior local control and overall survival after surgical resection compared with matched-paired patients receiving SRS. Another institutional series of patients with brain metastases from melanoma7 found improved local control for postoperative SRS compared with SRS alone.
In unadjusted analyses, local recurrence approached 50% by 9 months after surgical resection without adjuvant WBRT. Postoperative cavity SRS has been investigated as a means to redress poor local control after surgical resection in the absence of WBRT.8,9,10 Alliance N107C compared postoperative SRS with WBRT after surgical resection; overall survival was comparable and cognitive-deterioration–free survival was superior for postoperative SRS (HR, 0.47; 95% CI, 0.35-0.63; P < .001).9 However challenges associated with postoperative SRS include target delineation,11 leptomeningeal dissemination,12 and radionecrosis13 and have spurred interest in preoperative SRS. Initial reports11,14 suggest favorable control and low incidence of leptomeningeal disease and symptomatic radionecrosis. Prospective trials of preoperative SRS are currently accruing (NCT03163368, NCT01891318, NCT03368625). For larger resection cavities, postoperative fractionated radiotherapy for involved fields could represent an alternative option, because it allows effective doses while sparing uninvolved brain.15
Limitations
Limitations of our study arise from post hoc analysis. Although tumor size was accounted for in adjusted models, those treated with surgery had larger median tumor sizes, and increasing tumor size was associated with local recurrence. Other unmeasured confounders are likely derived from patient selection for local therapy outside of our adjusted models. Matching was not feasible due to the small sample size. The strength of this analysis lies within the high-quality data abstracted from an international phase 3 trial with scheduled brain surveillance.
Conclusions
Our exploratory analysis of the EORTC 22952-26001 trial found similar local control of treated brain metastases with SRS compared with surgical resection. Although early local control was improved in the SRS group, the risk of local recurrence increased with time for SRS relative to surgical resection. Prospective controlled trials are warranted to direct the optimal local approach for patients with brain metastases and to define whether any population may benefit from escalation in local therapy.
eFigure 1. CONSORT Diagram for Patient Selection
eFigure 2. Cumulative Incidences of Distant Neurological Progression (Development of Brain Metastases at New Sites) According to SRS and Surgical Resection
eFigure 3. Cumulative Incidences of Death Due to Any Cause According to SRS and Surgical Resection
eTable 1. Unadjusted Cumulative Incidence of Brain Metastasis Local Recurrence According to Local Therapy
eTable 2. Adjusted Competing Risk Regression Model for Factors Associated With Local Recurrence of Previously Treated Brain Metastases Using Time 0 as Completion of Local Therapy
References
- 1.Johnson JD, Young B. Demographics of brain metastasis. Neurosurg Clin N Am. 1996;7(3):337-344. doi: 10.1016/S1042-3680(18)30365-6 [DOI] [PubMed] [Google Scholar]
- 2.Patchell RA, Tibbs PA, Walsh JW, et al. . A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990;322(8):494-500. doi: 10.1056/NEJM199002223220802 [DOI] [PubMed] [Google Scholar]
- 3.Andrews DW, Scott CB, Sperduto PW, et al. . Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363(9422):1665-1672. doi: 10.1016/S0140-6736(04)16250-8 [DOI] [PubMed] [Google Scholar]
- 4.Kocher M, Soffietti R, Abacioglu U, et al. . Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J Clin Oncol. 2011;29(2):134-141. doi: 10.1200/JCO.2010.30.1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muacevic A, Wowra B, Siefert A, Tonn J-C, Steiger H-J, Kreth FW. Microsurgery plus whole brain irradiation versus gamma knife surgery alone for treatment of single metastases to the brain: a randomized controlled multicentre phase III trial. J Neurooncol. 2008;87(3):299-307. doi: 10.1007/s11060-007-9510-4 [DOI] [PubMed] [Google Scholar]
- 6.Bindal AK, Bindal RK, Hess KR, et al. . Surgery versus radiosurgery in the treatment of brain metastasis. J Neurosurg. 1996;84(5):748-754. doi: 10.3171/jns.1996.84.5.0748 [DOI] [PubMed] [Google Scholar]
- 7.Minniti G, Paolini S, D’Andrea G, et al. . Outcomes of postoperative stereotactic radiosurgery to the resection cavity versus stereotactic radiosurgery alone for melanoma brain metastases. J Neurooncol. 2017;132(3):455-462. doi: 10.1007/s11060-017-2394-z [DOI] [PubMed] [Google Scholar]
- 8.Soltys SG, Adler JR, Lipani JD, et al. . Stereotactic radiosurgery of the postoperative resection cavity for brain metastases. Int J Radiat Oncol Biol Phys. 2008;70(1):187-193. doi: 10.1016/j.ijrobp.2007.06.068 [DOI] [PubMed] [Google Scholar]
- 9.Brown PD, Ballman KV, Cerhan JH, et al. . Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC·3): a multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017;18(8):1049-1060. doi: 10.1016/S1470-2045(17)30441-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mahajan A, Ahmed S, McAleer MF, et al. . Post-operative stereotactic radiosurgery versus observation for completely resected brain metastases: a single-centre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017;18(8):1040-1048. doi: 10.1016/S1470-2045(17)30414-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Asher AL, Burri SH, Wiggins WF, et al. . A new treatment paradigm: neoadjuvant radiosurgery before surgical resection of brain metastases with analysis of local tumor recurrence. Int J Radiat Oncol Biol Phys. 2014;88(4):899-906. doi: 10.1016/j.ijrobp.2013.12.013 [DOI] [PubMed] [Google Scholar]
- 12.Atalar B, Modlin LA, Choi CYH, et al. . Risk of leptomeningeal disease in patients treated with stereotactic radiosurgery targeting the postoperative resection cavity for brain metastases. Int J Radiat Oncol Biol Phys. 2013;87(4):713-718. doi: 10.1016/j.ijrobp.2013.07.034 [DOI] [PubMed] [Google Scholar]
- 13.Prabhu RS, Press RH, Patel KR, et al. . Single-fraction stereotactic radiosurgery (SRS) alone versus surgical resection and SRS for large brain metastases: a multi-institutional analysis. Int J Radiat Oncol Biol Phys. 2017;99(2):459-467. doi: 10.1016/j.ijrobp.2017.04.006 [DOI] [PubMed] [Google Scholar]
- 14.Patel KR, Burri SH, Boselli D, et al. . Comparing pre-operative stereotactic radiosurgery (SRS) to post-operative whole brain radiation therapy (WBRT) for resectable brain metastases: a multi-institutional analysis. J Neurooncol. 2017;131(3):611-618. doi: 10.1007/s11060-016-2334-3 [DOI] [PubMed] [Google Scholar]
- 15.Shin SM, Vatner RE, Tam M, et al. . Resection followed by involved-field fractionated radiotherapy in the management of single brain metastasis. Front Oncol. 2015;5:206. doi: 10.3389/fonc.2015.00206 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. CONSORT Diagram for Patient Selection
eFigure 2. Cumulative Incidences of Distant Neurological Progression (Development of Brain Metastases at New Sites) According to SRS and Surgical Resection
eFigure 3. Cumulative Incidences of Death Due to Any Cause According to SRS and Surgical Resection
eTable 1. Unadjusted Cumulative Incidence of Brain Metastasis Local Recurrence According to Local Therapy
eTable 2. Adjusted Competing Risk Regression Model for Factors Associated With Local Recurrence of Previously Treated Brain Metastases Using Time 0 as Completion of Local Therapy