Key Points
Question
Are there determinants of importance for the persistence of atopic dermatitis at age 13 years?
Findings
In the Copenhagen Prospective Study on Asthma in Childhood 2000 birth cohort study, known genetic atopic dermatitis risk variants, paternal asthma and atopic dermatitis, high social circumstances, diagnostic minor criteria of Hanifin and Rajka, and disease severity at onset were significantly associated with persistent atopic dermatitis.
Meaning
The findings of this study suggest that genetic profile is one of the factors most often associated with persistent atopic dermatitis at age 13 years, and that the likelihood of persistence can be evaluated at the onset of atopic dermatitis using existing clinical tools.
Abstract
Importance
Knowledge about factors associated with persistence of atopic dermatitis (AD) during childhood is sparse.
Objective
To explore heritable, environmental, and clinical factors associated with persistent AD based on 13 years’ follow-up of an at-risk birth cohort.
Design, Setting, and Participants
In the Copenhagen Prospective Study on Asthma in Childhood 2000 (COPSAC2000) clinical birth cohort study, 411 children born to mothers with asthma were followed up until the age of 13 years at a clinical research unit in Copenhagen, Denmark, from August 1998 to June 2015. Atopic dermatitis was diagnosed prospectively during close clinical follow-up according to the criteria of Hanifin and Rajka. Data were gathered on parental history, social circumstances, and environmental factors through parent interviews. The cohort was followed up with biannual visits to the clinic until the age of 7 years and were seen again at age 13 years. Data were analyzed from August 2015 to January 2018.
Main Outcomes and Measures
Atopic dermatitis was diagnosed using Hanifin and Rajka major and minor criteria, and severity was determined by Scoring Atopic Dermatitis (SCORAD) index, with possible scores from 0 to 83, with higher scores indicating more severe AD.
Results
Of the 411 children in the cohort, 203 (49.4%) were male and 186 (45.3%) were diagnosed with AD before the age of 13 years; 40 of 166 children (24.1%) had persistent AD at the age of 13 years, and 126 (76.0%) experienced remission. Factors associated with persistent AD to age 13 years included heritability, environmental exposures, asthma and allergic sensitization, clinical presentation at the time of diagnosis, the composition of Hanifin and Rajka diagnostic minor criteria, and AD severity according to SCORAD. A higher AD genetic risk score was associated with an increased the risk for persistent AD (multivariable odds ratio [OR], 1.8; 95% CI, 1.1-2.9; P = .02), together with paternal asthma (multivariable OR, 3.7; 95% CI, 1.2-11.5; P = .02); paternal AD (multivariable OR, 6.2; 95% CI, 1.17-23.2; P = .007), and higher social circumstances (multivariable OR, 1.6; 95% CI, 1.0-2.5; P = .05). Particular clinical presentations at time of diagnosis were also associated with specific minor criteria of Hanifin and Rajka (Dennie-Morgan and anterior neck folds, white dermographism, intolerance to wool, itching when sweating, tendency to skin infection, food intolerance, and food allergy) (OR, 2.6; 95% CI, 1.1-6.2; P = .03) as well as increased severity at diagnosis (OR, 1.1; 95% CI, 1.0-1.1; P = .007).
Conclusions and Relevance
In a birth cohort of children at risk for asthma who received close clinical follow-up to age 13 years, known genetic AD risk variants, paternal asthma and AD, high social circumstances, diagnostic minor criteria, and disease severity at onset were associated with persistent AD at age 13 years. These findings may be applied in clinical practice to evaluate the likely disease course for individual patients.
This clinical birth cohort study evaluates the association of genetic, environmental, clinical, and social factors with the persistence of atopic dermatitis at age 13 years among children born to parents with asthma.
Introduction
Atopic dermatitis (AD) is a chronic relapsing inflammatory skin disease that most often begins in infancy1; between 1990 and 2010, it affected approximately 1 in 5 children in developed countries.2,3 Children with AD comprise a heterogeneous group with different disease courses, age at onset, clinical manifestations, severity, duration, and risk of comorbidity.4,5 Although most children with AD outgrow their disease, it is unclear how many experience persistence of symptoms into adulthood, and little is known about determinants of persistent AD.6
Known heritable AD risk factors include parental asthma, allergy, and AD (which almost doubles the child’s AD risk if present in both parents7) as well as concurrent asthma, wheezing, and allergic sensitization in the child.8,9,10,11 Furthermore, filaggrin gene (FLG) mutations are nonfunctional mutations that increase the risk of AD 2- to 3-fold.12,13,14,15 Other genetic risk loci have been identified but are of less importance.16 Suspected environmental risk factors for AD development include birth during winter,17,18 exposure to hard domestic water,19 air pollution, low ambient humidity,20,21 longer duration of breastfeeding,22 and maternal alcohol intake during pregnancy.23 However, exposure to a dog in the household near the time of birth has been found to protect against development of AD.24
There is an unmet need for an improved understanding of factors associated with a persistent disease course throughout childhood. This would be of clinical importance to patients, parents, and clinicians and could guide treatment.
In the present study, we examined the fluctuations in AD diagnosis throughout childhood and aimed to identify risk factors of persistent AD in a cohort of children followed up prospectively from birth to age 13 years.
Methods
Study Population
The Copenhagen Prospective Studies on Asthma in Childhood 2000 (COPSAC2000) is a single-center clinical cohort study of 411 children born to mothers with physician-diagnosed asthma. The cohort was followed up from August 1998 to June 2015, and data were analyzed from August 2015 to January 2018. Details on recruitment and characteristics of the cohort have been described previously.25,26,27 In brief, mothers were recruited during pregnancy and their children at 1 month of age. The children were seen in the research unit every 6 months for scheduled visits as well as on acute onset of airway or skin symptoms until the age of 7 years. The most recent follow-up visit was performed at age 13 years. Between the ages of 7 and 13 years, children were seen if they experienced any skin symptoms. Data validation and quality control followed the guidelines for good clinical practice, including the Danish Code of Conduct for Research Integrity, the European Union’s Directive on Good Clinical Practice, the International Conference on Harmonisation’s good clinical practice guidelines, the Danish Act on Processing of Personal Data, and the practice of the Danish Data Inspectorate. The study was performed in accordance with the Declaration of Helsinki28 and approved by the Copenhagen Ethics Committee and the Danish Data Protection Agency.25 Oral and written informed consent were obtained from parents or guardians before enrollment.
At every clinical visit, a full physical examination was performed by a physician with training in dermatology, and the families were interviewed using structured questions with closed response categories focusing on airway and skin symptoms, use of medication and health care, lifestyle factors, and domestic environmental exposures. The physicians at the COPSAC clinic were solely responsible for diagnosis and treatment of all respiratory, allergy, and skin-related symptoms according to standard operating procedures.1
Atopic Dermatitis Diagnosis
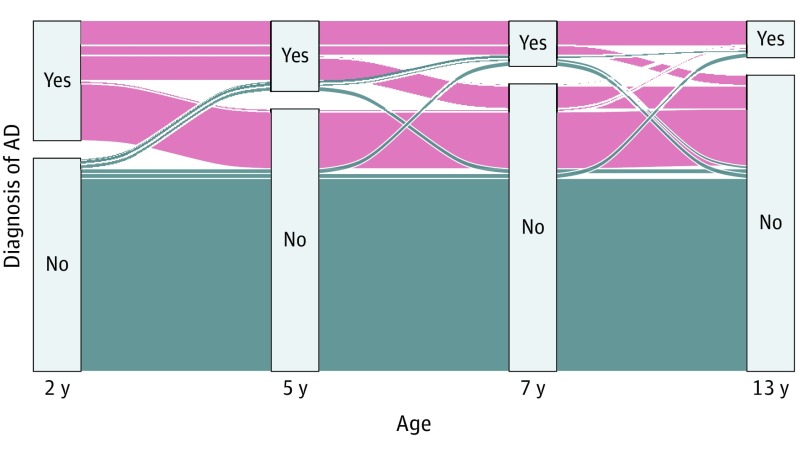
Atopic dermatitis was diagnosed prospectively until age 13 years according to the criteria of Hanifin and Rajka and with a clinical assessment.29 A diagnosis of AD required the presence of 3 of 4 major criteria (pruritus, typical morphologic features and distribution, chronic dermatitis, and personal or family history of atopy) and at least 3 of 23 minor criteria (early age at onset, xerosis, ichthyosis, keratosis pilaris, palmar hyperlinearity, hand or foot dermatitis, nipple eczema, cheilitis, perifollicular accentuation, pityriasis alba, anterior neck folds, itching when sweating, intolerance to wool, positive result on type 1 skin test, elevated serum IgE level, food intolerance, tendency to skin infection, facial pallor, white dermographism, conjunctivitis, Dennie-Morgan fold, orbital darkening, and course influenced by environmental factors, excluding keratoconus and anterior subcapsular cataracts. The severity of AD was scored at disease onset using the SCORAD (Scoring Atopic Dermatitis) index30; scores can range from 0 to 83 points (excluding the subjective components pruritus and sleeplessness from the modified SCORAD index, from which the subjective components of pruritus and sleeplessness have been excluded), with a higher score indicating more severe AD. Duration of AD was defined as days with ongoing AD diagnosis from 0 to 13 years of age. Exclusion criteria were incomplete follow-up from birth to 13 years of age and being older than 10 years at AD onset. Remission of AD was defined as a 1-year period without clinical symptoms and without use of topical anti-inflammatory treatment. A child with an AD diagnosis was classified as having persistent AD if the disease was ongoing at 13 years of age. Transient AD was defined as remission at 13 years of age. A child’s individual course from 0 to 13 years according to possible AD diagnosis at cross-sectional time points (2, 5, 7, and 13 years) was illustrated by an alluvial diagram that included only children with full follow-up.
Determinants of Atopic Dermatitis
Heritability
Genotyping for common loss-of-function mutations in FLG (OMIM 135940), R501X, 2282del4, R2447X, and S3247X was performed as previously described.12 An FLG mutation carrier was defined as an individual having at least 1 gene mutation. An AD genetic risk score was constructed based on genetic variants that were associated with AD in a large meta–genome-wide association study31 as well as common FLG mutations (eTable 1 in the Supplement). The number of risk alleles for each child was calculated and weighted according to the child’s odds ratios (OR) and was z-score transformed. Information about parental physician-diagnosed asthma, allergy, and AD was obtained in the clinic by personal interviews.
Environmental Exposures
Midwives collected cord blood by needle puncture from the umbilical cord vein, and plasma 25-hydroxyvitamin D levels were analyzed as previously detailed.32,33 The vitamin D level in cord blood was categorized as deficient (<20 ng/mL), insufficient (20-30 ng/mL), or sufficient (>30 ng/mL).34 (To convert vitamin D levels to nanomoles per liter, multiply by 2.496.)
Information about maternal smoking and antibiotic use during pregnancy, parity, duration of exclusive breastfeeding, child sex, gestational age, season of birth, mode of delivery, birth weight, head circumference at birth, as well as presence of a dog and cat in the home at birth was collected prospectively at scheduled visits.
“Social circumstances” included household income, maternal age, and maternal level of education when the child was aged 2 years and was analyzed in a principal components analysis (PCA). The first component explained 52% of the variance in the data and was used as a composite indicator of social circumstances.
Asthma and Allergy
Asthma or recurrent wheeze before age 3 years was diagnosed prospectively in the COPSAC research unit according to a strict predefined and previously validated quantitative algorithm based on daily symptoms captured prospectively in a diary.26
Allergic sensitization was measured at 6 and 18 months of age and defined as any skin prick test wheal larger than 2 mm (ALK-Abello) or specific serum IgE level of 0.35 kU/mL or greater using the ImmunoCAP Phadiatop Infant test (Pharmaca Diagnostics AB).6,35 (To convert the IgE level to milligrams per liter, multiply by 0.001.) Serum IgE levels were measured against 8 inhalant allergens (dog, cat, horse, birch, timothy grass, mugwort, Dermatophagoides pteronyssinus, and molds) and 9 food allergens (milk, egg, wheat flour, rye flour, oatmeal, cod, soybean, potato, and peanut).
Statistical Analysis
The differences between children with persistent and transient AD at age 13 years were analyzed by χ2 test, unpaired, 2-tailed t test, and logistic and linear regression. Thereafter, all significant determinants were included in a multivariable model. A PCA was used to identify common systematic variation in the Hanifin and Rajka minor criteria at diagnosis. Variables with no variance were omitted. Differences in the Hanifin and Rajka minor criteria patterns between transient and persistent AD were tested using a nonparametric Adonis PERMANOVA model from the R package “vegan,” version 2.3-5 (R Foundation for Statistical Computing) with euclidean distances. Analysis of severity by modified SCORAD at AD onset was analyzed with linear regression models adjusted for age at AD onset. A linear regression model was used to analyze the association between the AD genetic risk score, PC1 (first principal component) from the Hanifin and Rajka PCA, modified SCORAD score, and the duration of AD.
Results were reported with 95% CIs, and a significance level of P < .05 was used. Missing observations were treated as missing data. Data processing was conducted using SAS, version 9.3 for Windows (SAS Institute Inc) and R, version 3.3.0 (R Foundation for Statistical Computing).
Results
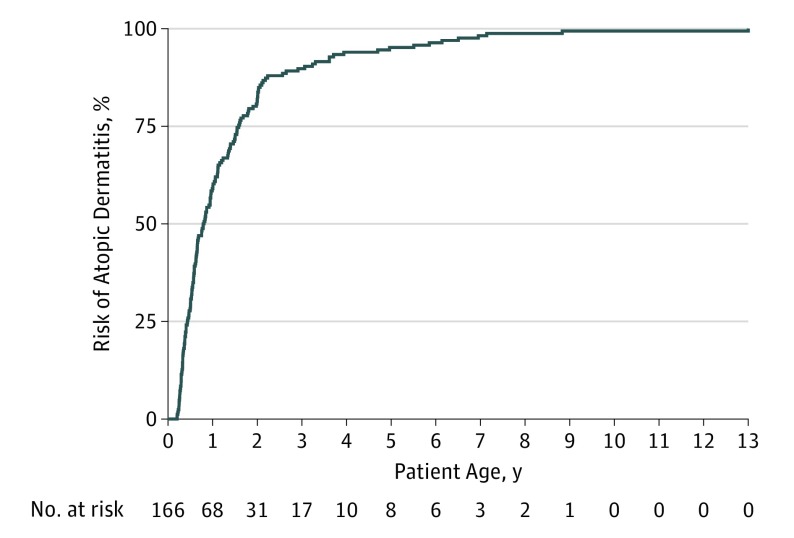
Age and race/ethnicity were homogeneous for the COPSAC2000 cohort; of the 411 children, 203 (49.4%) were male. Of the children in the cohort, 186 (45.3%) experienced AD from birth to age 13 years (Figure 1). Sixteen children were excluded owing to incomplete follow-up, and 4 owing to onset of AD after age 10 years, leaving 166 children with AD in the final study group of children (eFigure 1 in the Supplement). The 166 children with AD were characterized by having more mothers with AD and fathers with asthma and allergic rhinitis. Furthermore, fewer mothers used antibiotic medications during pregnancy in the AD group (eTable 2 in the Supplement). By age 13 years, 40 of the 166 children with AD (24.1%) had an ongoing diagnosis and were classified as having persistent AD, whereas 126 (76.0%) were in remission (transient).
Figure 1. Kaplan-Meier Curve Showing Development of Atopic Dermatitis From Birth to 13 Years of Age in a Cohort of 166 Children.
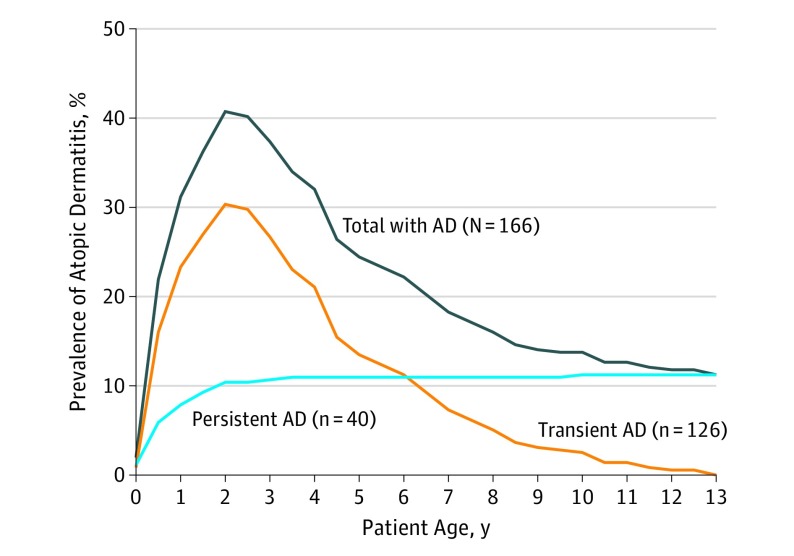
Figure 2 illustrates the prevalence of AD across the first 13 years of life for children with AD in the cohort. The prevalence peaked at age 2 years, when 159 (40.7%) of the children had AD, and declined gradually to age 13 years, when 40 (11.2%) still had a diagnosis of AD. The individual child’s AD course over this period was illustrated in an alluvial diagram showing the fluctuation in diagnosis between time points (Figure 3). Descriptive statistics for all determinants analyzed for association with persistent AD are shown in the Table and detailed below.
Figure 2. Prevalence of Children With Atopic Dermatitis (AD) From 0 to 13 Years Among All Children in the Copenhagen Prospective Study on Asthma in Childhood 2000 Study (Total) and According to Ongoing Diagnosis at Age 13 Years (Transient/Persistent).
Figure 3. Changes in Atopic Dermatitis (AD) Diagnosis Over Time in 411 Children With AD in the Copenhagen Prospective Study on Asthma in Childhood 2000 Cohort.
The alluvial diagram visualizes each child’s individual AD course according to diagnosis at different cross-sectional time points. The blue areas represent the children who did not develop AD.
Table. Determinants of Persistent AD at Age 13 Years in 166 Children Followed Up From Birth Who Received a Diagnosis of AD Before Age 10 Years.
| Characteristic | No. (%) | OR (95%CI) | P Value | ||
|---|---|---|---|---|---|
| All With AD (N = 166) | Persistent AD (n = 40) | Transient AD (n = 126) | |||
| Heritability | |||||
| White race | 161 (96.9) | 40 (100.0) | 121 (96.0) | NA | NA |
| Filaggrin gene mutations | 28 (17.0) | 11 (29.0) | 17 (13.5) | 2.6 (1.1-6.2) | .03 |
| AD genetic risk score, mean (SD) | 0.12 (0.98) | 0.47 (1.07) | 0.02 (0.94) | 1.6 (1.1-2.4) | .03 |
| Parental disease | |||||
| Maternal asthma | 166 (100) | 40 (100) | 126 (100) | NA | NA |
| Maternal AD | 82 (56.6) | 22 (66.7) | 60 (53.6) | 1.7(0.8-3.9) | .18 |
| Maternal allergic rhinitis | 121 (76.6) | 27 (77.1) | 94 (76.4) | 1.0 (0.4-2.5) | .93 |
| Paternal asthma | 31 (18.8) | 15 (37.5) | 16 (12.8) | 4.1 (1.8-9.4) | <.001 |
| Paternal AD | 18 (13.3) | 11 (30.6) | 7 (7.0) | 5.8 (2.0-17.0) | <.001 |
| Paternal allergic rhinitis | 55 (37.9) | 18 (47.4) | 37 (34.6) | 1.7 (0.8-3.6) | .16 |
| Environmental exposures | |||||
| Male sex | 85 (51.2) | 21 (52.5) | 64 (50.8) | 0.9 (0.5-1.9) | .85 |
| Nulliparous mother | 97 (61.0) | 19 (51.4) | 78 (63.9) | 0.6 (0.3-1.3) | .17 |
| Any smoking during pregnancy | 34 (20.5) | 8 (20.0) | 26 (20.6) | 1.0 (0.4-2.5) | .93 |
| Any antibiotic use during pregnancy | 41 (24.7) | 9 (22.5) | 32 (25.4) | 1.0 (0.4-2.2) | .96 |
| Cord blood vitamin D level, median (IQR), ng/mL | 102 (75-132) | 105 (67-150) | 102 (77-132) | 0.9 (0.4-2.0) | .78 |
| Season of birth | |||||
| Spring/summer | 71 (42.7) | 15 (37.5) | 56 (44.4) | 1.3 (0.6-2.7) | .44 |
| Gestational age, mean (SD), wk | 39.8 (1.6) | 39.5 (1.7) | 39.9 (1.6) | 0.8 (0.7-1.0) | .10 |
| Head circumference at birth, mean (SD), mm | 351 (16) | 349 (15) | 352 (17) | 1.0 (0.97-1.01) | .30 |
| Birth weight, mean (SD), kg | 3.5 (0.6) | 3.4 (0.5) | 3.5 (0.5) | 0.6 (0.3-1.1) | .11 |
| Cesarean delivery | 36 (21.7) | 7 (17.5) | 29 (23.0) | 1.4 (0.6-3.5) | .46 |
| Social circumstances, mean (SD)a | 0.05 (1.0) | 0.4 (1.1) | −0.06 (0.98) | 1.6 (1.1-2.2) | .02 |
| Any older children in the home | 66 (40.5) | 18 (48.7) | 48 (38.1) | 1.5 (0.7-3.2) | .27 |
| Duration of solely breastfeeding, median (IQR), d | 122 (92-168) | 125 (89-175) | 122 (92-167) | 1.0 (0.99-1.1) | .86 |
| Domestic dog exposure at birth | 22 (13.4) | 3 (7.5) | 19 (15.3) | 0.5 (0.1-1.6) | .21 |
| Domestic cat exposure at birth | 25 (15.2) | 7 (17.5) | 18 (14.5) | 1.3 (0.5-3.3) | .65 |
| Asthma and allergy | |||||
| Asthma at 0-3 y | 25 (15.0) | 8 (20.0) | 17 (13.5) | 1.6 (0.6-4.0) | .32 |
| Any sensitization at 6 or 18 mo | 39 (30.7) | 10 (32.3) | 29 (30.2) | 1.1 (0.5-2.6) | .83 |
Abbreviations: AD, atopic dermatitis; IQR, interquartile range; NA, not applicable; and OR, odds ratio.
SI conversion factor: To convert vitamin D levels to nanomoles per liter, multiply by 2.496.
Social circumstances represent information about maternal age, maternal educational level, and household income at 2 years of age.
Heritability
Paternal asthma was present in 15 of 40 children (37.5%) with persistent AD compared with 16 of 125 (12.8%) with transient AD (OR, 4.1; 95% CI, 1.8-9.4; P < .001). Eleven of the children (30.6%) with persistent AD had fathers with an AD diagnosis compared with 7 (7.0%) with transient AD (OR, 5.8; 95% CI, 2.0-17.0; P < .001). Maternal asthma, AD, and allergic rhinitis and paternal allergic rhinitis were not significantly associated with persistent AD.
Eleven of the 38 children (28.9%) with persistent AD had FLG mutations compared with 17 of 126 children (13.5%) with transient AD (OR, 2.6; 95% CI, 1.1-6.2; P = .03). Furthermore, a higher AD genetic risk score was associated with increased risk of persistent AD (OR, 1.6; 95% CI, 1.1-2.4; P = .03). Children with persistent AD had a mean (SD) AD genetic risk score of 0.47 (1.07) compared with 0.02 (0.94) for children with transient AD.
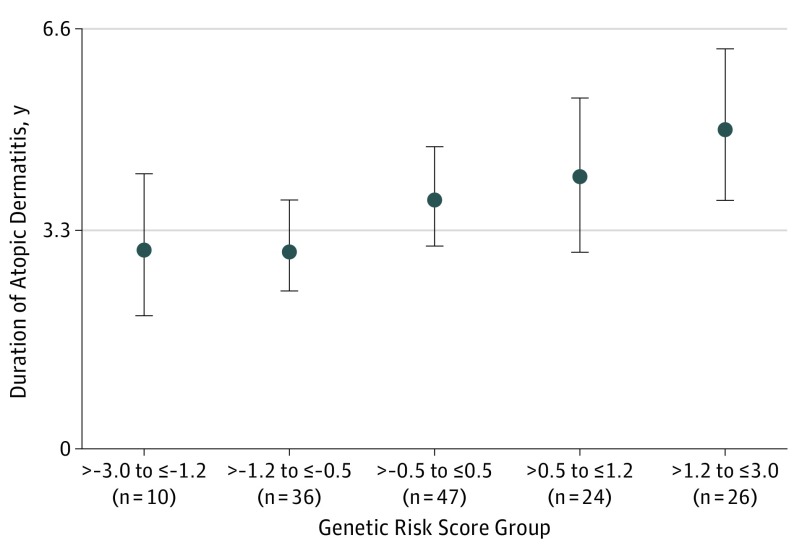
A positive dose-dependent linear association was found between the AD genetic risk score and duration of AD (Figure 4), with an increase of 270 days for each additional step in the AD genetic risk standard score (95% CI, 105-436; P = .002).
Figure 4. Genetic Risk Score, Including Common FLG Mutations, and Duration of Atopic Dermatitis in Years.
Increasing genetic risk score is associated with increased atopic dermatitis duration. Data markers represent median duration; error bars, 95% CIs.
Environmental Exposures
A higher level of social circumstances score (household income, maternal age, and maternal level of education) at age 2 years was associated with persistent AD (OR, 1.6; 95% CI, 1.1-2.2; P = .02). Sex, parity, and maternal smoking as well as antibiotic use during pregnancy, cord blood vitamin D levels, season of birth, gestational age, head circumference, weight at birth, mode of delivery, domestic exposure to a dog or cat around birth, having older children in the home, and days of exclusive breastfeeding were not significantly associated with persistent AD (Table).
Asthma and Allergy
No associations were found between asthma or persistent wheeze and persistent AD until 3 years of age (OR, 1.6; 95% CI, 0.6-4.0; P = .30) or allergic sensitization at 6 and 18 months (OR, 1.1; 95% CI, 0.5-2.6; P = .80) and persistent AD.
Multivariable Analysis
Heritable and environmental determinants significantly associated with the persistence of AD were analyzed in a multivariable model to examine covariance. In this combined analysis, we found that a higher AD genetic risk score was significantly associated with persistent AD (multivariable OR, 1.8; 95% CI, 1.1-2.9; P = .02). Paternal asthma and AD were also associated with persistent AD: paternal asthma (multivariable OR, 3.7; 95% CI, 1.2-11.5; P = .02) and paternal AD (multivariable OR, 6.2; 95% CI, 1.2-23.2; P = .007). Social circumstances were also associated with persistent AD (multivariable OR, 1.6; 95% CI, 1.0-2.5; P = .05).
Clinical Presentation at AD Onset
A PCA analysis of Hanifin and Rajka’s minor criteria registered at AD onset showed that PC1 explained 18% and PC2 explained 15% of the variation in the original variables. There was a significant overall shift in PCA scores between the persistent and transient AD groups (F = 2.0; R2 = 0.0; P = .003). This shift was most prominent in PC1, where increased scores among persistent AD for several features, including Dennie-Morgan folds, anterior neck folds, white dermographism, intolerance to wool, itching when sweating, a tendency to skin infection, food intolerance, and food allergy, were contributing factors (eFigure 2 in the Supplement).
The mean (SD) severity score at AD onset as measured by modified SCORAD in the COPSAC clinic was 18 (9.4) among all children with AD. The modified SCORAD mean (SD) score was higher for children with persistent (22 [8.6]) vs transient (17 [9.3]) AD (OR, 1.1; 95% CI, 1.0-1.1; P = .007). Duration of AD was increased by 27 days per 1-U increase in modified SCORAD score at the first visit (95% CI, 7-46; P < .001).
Discussion
Primary Findings
Atopic dermatitis genetic risk score, including common FLG mutations, was the only significant nonclinical risk factor for AD persistence.9,36 Furthermore, paternal asthma and AD as well as high social circumstances were risk factors for AD persistence at age 13 years in this at-risk cohort followed up prospectively from birth until 13 years of age. The clinical presentation at diagnosis, including selected Hanifin and Rajka minor criteria, and increased AD severity were also associated with AD persistence, whereas early-life wheezing and allergic sensitization were not associated with AD persistence. These findings suggest that heritability combined with AD characteristics at diagnosis may be used for personalized disease courseestimation.
Interpretation
We found that 24.1% of children diagnosed with AD during childhood had persistent disease at age 13 years, which is in line with a recent meta-analysis that included 45 studies showing that 20% of children with AD had persistent disease after 8 years of age.6 Additional longitudinal studies have shown genetic variance associated with persistent AD through childhood.9 We showed that most children had disease onset in the first 2 years of life, with a smaller fraction diagnosed after this age. We described the prevalence of AD according to age: the highest prevalence occurred at 2 years of age and declined gradually throughout childhood. We further illustrated the fluctuations of AD over the first 13 years of life, documenting that the majority of children with persistent AD at age 13 years had already received a diagnosis at age 2 years. Genetics, summarized by an AD genetic risk score, was the strongest nonclinical risk factor for persistent AD at age 13 years in a dose-dependent manner with the duration of AD.36,37,38,39 Twin studies have suggested that heritability of AD is approximately 80% to 90%.40 However, the genetic risk score explained approximately 8% of the difference between a persistent vs transient AD course from age 0 to 13 years, in line with previous AD genome-wide association study analyses that were able to explain approximately 15% of variance in the risk of developing AD.31 This finding suggests that both onset and duration of AD are contributed to by a large number of genetic variants with small effect size.
Our study suggests associations between paternal asthma and AD and persistence of childhood AD, whereas we observed no significant associations with maternal disease. This difference between maternal and paternal heritage might be explained by the at-risk nature of the cohort. It was an inclusion criterion that all mothers had asthma, and they were therefore also much more likely to have AD and allergic rhinitis, whereas there were no inclusion criteria involving paternal disease. Previously, AD has been reported to be a disease of children from high socioeconomic groups.41,42,43 Our results corroborate these findings and show that children with AD from families with higher social circumstances are at greater risk of experiencing persistent AD.
An increased prevalence of asthma and allergy among individuals with AD is often described as the “atopic march.”44,45 However asthma and persistent wheeze and allergic sensitization in early childhood were not significant risk factors for persistent AD at 13 years of age. This finding is supported by a study examining individual development of AD, asthma, and rhinitis in 9801 children.46 In that study, 3% of the examined children followed the “atopic march.” In fact, we proposed that the atopic march is restricted to a specific endotype of children with AD, but that it does not describe the natural development of AD, asthma, and sensitization in childhood.11
We examined the clinical presentation of children with AD at first diagnosis and found that the validated diagnostic minor criteria of Hanifin and Rajka29,30 were useful to estimate the likelihood of AD persistence at age 13 years. In particular, the minor criteria (Dennie-Morgan folds, anterior neck folds, white dermographism, intolerance to wool, itching when sweating, tendency to skin infection, food intolerance, and food allergy) contributed to this association. Furthermore, we found that high SCORAD at diagnosis was a risk factor for persistent AD. Both of these clinical AD factors were also associated with duration of AD. These, in combination with genetic assessments, might thus be useful tools for personalized disease course estimation.
Strengths and Limitations
A major advantage of the study is that all AD diagnoses were made longitudinally at the COPSAC clinic according to standard operating procedures.25 Furthermore, all children were seen in the COPSAC clinic at acute and scheduled visits for skin-related symptoms, which minimized the risk of parental recall bias. This meticulous prospective follow-up is a significant strength that enabled accurate assessment of AD onset and remission. Limitations of the study were that all mothers in the project had a history of asthma and that the findings require validation in an unselected population.
Conclusions
The findings of this study suggest that an AD genetic risk score, selected minor criteria of Hanifin and Rajka, and AD severity at diagnosis are risk factors for persistence of AD through childhood. These findings could be used in clinical practice for personalized disease course estimation.
eTable 1. Gene Variants Comprising the AD Gene Risk Score and Their Respective Beta Coefficient and Effect Allele
eTable 2. Baseline Characteristics of the AD Study Group at 13 Years Compared to the Remaining Cohort (AD Controls)
eFigure 1. Flowchart of the Atopic Dermatitis Study Population
eFigure 2. Loading Plot: PCA Comparison of Minor HR Criteria at Diagnosis of Children With Atopic Dermatitis
References
- 1.Halkjaer LB, Loland L, Buchvald FF, et al. Development of atopic dermatitis during the first 3 years of life: the Copenhagen Prospective Study on Asthma in Childhood cohort study in high-risk children. Arch Dermatol. 2006;142(5):561-566. doi: 10.1001/archderm.142.5.561 [DOI] [PubMed] [Google Scholar]
- 2.Rajka G. Natural history and clinical manifestations of atopic dermatitis. Clin Rev Allergy. 1986;4(1):3-26. doi: 10.1007/BF02991185 [DOI] [PubMed] [Google Scholar]
- 3.Deckers IAG, McLean S, Linssen S, Mommers M, van Schayck CP, Sheikh A. Investigating international time trends in the incidence and prevalence of atopic eczema 1990-2010: a systematic review of epidemiological studies. PLoS One. 2012;7(7):e39803. doi: 10.1371/journal.pone.0039803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deleuran M, Vestergaard C. Clinical heterogeneity and differential diagnosis of atopic dermatitis. Br J Dermatol. 2014;170(suppl 1):2-6. doi: 10.1111/bjd.12933 [DOI] [PubMed] [Google Scholar]
- 5.Ballardini N, Kull I, Lind T, et al. Development and comorbidity of eczema, asthma and rhinitis to age 12: data from the BAMSE birth cohort. Allergy. 2012;67(4):537-544. doi: 10.1111/j.1398-9995.2012.02786.x [DOI] [PubMed] [Google Scholar]
- 6.Kim JP, Chao LX, Simpson EL, Silverberg JI. Persistence of atopic dermatitis (AD): a systematic review and meta-analysis. J Am Acad Dermatol. 2016;75(4):681-687.e11. doi: 10.1016/j.jaad.2016.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Böhme M, Wickman M, Lennart Nordvall S, Svartengren M, Wahlgren CF. Family history and risk of atopic dermatitis in children up to 4 years. Clin Exp Allergy. 2003;33(9):1226-1231. doi: 10.1046/j.1365-2222.2003.01749.x [DOI] [PubMed] [Google Scholar]
- 8.Peters AS, Kellberger J, Vogelberg C, et al. Prediction of the incidence, recurrence, and persistence of atopic dermatitis in adolescence: a prospective cohort study. J Allergy Clin Immunol. 2010;126(3):590-595.e1-3. [DOI] [PubMed] [Google Scholar]
- 9.Margolis DJ, Kim B, Apter AJ, et al. Thymic stromal lymphopoietin variation, filaggrin loss of function, and the persistence of atopic dermatitis. JAMA Dermatol. 2014;150(3):254-259. doi: 10.1001/jamadermatol.2013.7954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garrett JP-D, Apter AJ, Hoffstad O, Spergel JM, Margolis DJ. Asthma and frequency of wheeze: risk factors for the persistence of atopic dermatitis in children. Ann Allergy Asthma Immunol. 2013;110(3):146-149. doi: 10.1016/j.anai.2012.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schoos A-MM, Chawes BL, Rasmussen MA, Bloch J, Bønnelykke K, Bisgaard H. Atopic endotype in childhood. J Allergy Clin Immunol. 2016;137(3):844-851.e4. doi: 10.1016/j.jaci.2015.10.004 [DOI] [PubMed] [Google Scholar]
- 12.Palmer CNA, Irvine AD, Terron-Kwiatkowski A, et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet. 2006;38(4):441-446. doi: 10.1038/ng1767 [DOI] [PubMed] [Google Scholar]
- 13.Bisgaard H, Halkjaer LB, Hinge R, et al. Risk analysis of early childhood eczema. J Allergy Clin Immunol. 2009;123(6):1355-1360.e5. doi: 10.1016/j.jaci.2009.03.046 [DOI] [PubMed] [Google Scholar]
- 14.Bønnelykke K, Pipper CB, Tavendale R, Palmer CNA, Bisgaard H. Filaggrin gene variants and atopic diseases in early childhood assessed longitudinally from birth. Pediatr Allergy Immunol. 2010;21(6):954-961. doi: 10.1111/j.1399-3038.2010.01073.x [DOI] [PubMed] [Google Scholar]
- 15.Carson CG, Rasmussen MA, Thyssen JP, Menné T, Bisgaard H. Clinical presentation of atopic dermatitis by filaggrin gene mutation status during the first 7 years of life in a prospective cohort study. PLoS One. 2012;7(11):e48678. doi: 10.1371/journal.pone.0048678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paternoster L, Standl M, Chen C-M, et al. ; Australian Asthma Genetics Consortium (AAGC); Genetics of Overweight Young Adults (GOYA) Consortium; EArly Genetics & Lifecourse Epidemiology (EAGLE) Consortium . Meta-analysis of genome-wide association studies identifies three new risk loci for atopic dermatitis. Nat Genet. 2011;44(2):187-192. doi: 10.1038/ng.1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kusunoki T, Asai K, Harazaki M, Korematsu S, Hosoi S. Month of birth and prevalence of atopic dermatitis in schoolchildren: dry skin in early infancy as a possible etiologic factor. J Allergy Clin Immunol. 1999;103(6):1148-1152. doi: 10.1016/S0091-6749(99)70191-0 [DOI] [PubMed] [Google Scholar]
- 18.Kusunoki T, Morimoto T, Sakuma M, et al. Effect of eczema on the association between season of birth and food allergy in Japanese children. Pediatr Int. 2013;55(1):7-10. doi: 10.1111/j.1442-200X.2012.03725.x [DOI] [PubMed] [Google Scholar]
- 19.Perkin MR, Craven J, Logan K, et al. ; Enquiring About Tolerance Study Team . Association between domestic water hardness, chlorine, and atopic dermatitis risk in early life: a population-based cross-sectional study. J Allergy Clin Immunol. 2016;138(2):509-516. doi: 10.1016/j.jaci.2016.03.031 [DOI] [PubMed] [Google Scholar]
- 20.Silverberg JI, Hanifin J, Simpson EL. Climatic factors are associated with childhood eczema prevalence in the United States. J Invest Dermatol. 2013;133(7):1752-1759. doi: 10.1038/jid.2013.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Engebretsen KA, Johansen JD, Kezic S, Linneberg A, Thyssen JP. The effect of environmental humidity and temperature on skin barrier function and dermatitis. J Eur Acad Dermatol Venereol. 2016;30(2):223-249. doi: 10.1111/jdv.13301 [DOI] [PubMed] [Google Scholar]
- 22.Giwercman C, Halkjaer LB, Jensen SM, Bønnelykke K, Lauritzen L, Bisgaard H. Increased risk of eczema but reduced risk of early wheezy disorder from exclusive breast-feeding in high-risk infants. J Allergy Clin Immunol. 2010;125(4):866-871. doi: 10.1016/j.jaci.2010.01.026 [DOI] [PubMed] [Google Scholar]
- 23.Carson CG, Halkjaer LB, Jensen SM, Bisgaard H. Alcohol intake in pregnancy increases the child’s risk of atopic dermatitis: the COPSAC prospective birth cohort study of a high risk population. PLoS One. 2012;7(8):e42710. doi: 10.1371/journal.pone.0042710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thorsteinsdottir S, Thyssen JP, Stokholm J, Vissing NH, Waage J, Bisgaard H. Domestic dog exposure at birth reduces the incidence of atopic dermatitis. Allergy. 2016;71(12):1736-1744. doi: 10.1111/all.12980 [DOI] [PubMed] [Google Scholar]
- 25.Bisgaard H. The Copenhagen Prospective Study on Asthma in Childhood (COPSAC): design, rationale, and baseline data from a longitudinal birth cohort study. Ann Allergy Asthma Immunol. 2004;93(4):381-389. doi: 10.1016/S1081-1206(10)61398-1 [DOI] [PubMed] [Google Scholar]
- 26.Bisgaard H, Hermansen MN, Loland L, Halkjaer LB, Buchvald F. Intermittent inhaled corticosteroids in infants with episodic wheezing. N Engl J Med. 2006;354(19):1998-2005. doi: 10.1056/NEJMoa054692 [DOI] [PubMed] [Google Scholar]
- 27.Bisgaard H, Hermansen MN, Buchvald F, et al. Childhood asthma after bacterial colonization of the airway in neonates. N Engl J Med. 2007;357(15):1487-1495. doi: 10.1056/NEJMoa052632 [DOI] [PubMed] [Google Scholar]
- 28.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 29.Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol. 1980;60(92)(suppl):44-47. doi: 10.2340/00015555924447 [DOI] [Google Scholar]
- 30.Severity scoring of atopic dermatitis: the SCORAD index: consensus report of the European Task Force on Atopic Dermatitis. Dermatology. 1993;186(1):23-31. doi: 10.1159/000247298 [DOI] [PubMed] [Google Scholar]
- 31.Paternoster L, Standl M, Waage J, et al. ; Australian Asthma Genetics Consortium (AAGC) . Multi-ancestry genome-wide association study of 21,000 cases and 95,000 controls identifies new risk loci for atopic dermatitis. Nat Genet. 2015;47(12):1449-1456. doi: 10.1038/ng.3424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maunsell Z, Wright DJ, Rainbow SJ. Routine isotope-dilution liquid chromatography-tandem mass spectrometry assay for simultaneous measurement of the 25-hydroxy metabolites of vitamins D2 and D3. Clin Chem. 2005;51(9):1683-1690. doi: 10.1373/clinchem.2005.052936 [DOI] [PubMed] [Google Scholar]
- 33.Højskov CS, Heickendorff L, Møller HJ. High-throughput liquid-liquid extraction and LCMSMS assay for determination of circulating 25(OH) vitamin D3 and D2 in the routine clinical laboratory. Clin Chim Acta. 2010;411(1-2):114-116. doi: 10.1016/j.cca.2009.10.010 [DOI] [PubMed] [Google Scholar]
- 34.Chawes BL, Bønnelykke K, Jensen PF, Schoos A-MM, Heickendorff L, Bisgaard H. Cord blood 25(OH)-vitamin D deficiency and childhood asthma, allergy and eczema: the COPSAC2000 birth cohort study. PLoS One. 2014;9(6):e99856. doi: 10.1371/journal.pone.0099856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Illi S, von Mutius E, Lau S, et al. ; Multicenter Allergy Study Group . The natural course of atopic dermatitis from birth to age 7 years and the association with asthma. J Allergy Clin Immunol. 2004;113(5):925-931. doi: 10.1016/j.jaci.2004.01.778 [DOI] [PubMed] [Google Scholar]
- 36.Barker JNWN, Palmer CNA, Zhao Y, et al. Null mutations in the filaggrin gene (FLG) determine major susceptibility to early-onset atopic dermatitis that persists into adulthood. J Invest Dermatol. 2007;127(3):564-567. doi: 10.1038/sj.jid.5700587 [DOI] [PubMed] [Google Scholar]
- 37.Kjellman NI. Atopic disease in seven-year-old children: incidence in relation to family history. Acta Paediatr Scand. 1977;66(4):465-471. doi: 10.1111/j.1651-2227.1977.tb07928.x [DOI] [PubMed] [Google Scholar]
- 38.Hoffjan S, Epplen JT. The genetics of atopic dermatitis: recent findings and future options. J Mol Med (Berl). 2005;83(9):682-692. doi: 10.1007/s00109-005-0672-2 [DOI] [PubMed] [Google Scholar]
- 39.Henderson J, Northstone K, Lee SP, et al. The burden of disease associated with filaggrin mutations: a population-based, longitudinal birth cohort study. J Allergy Clin Immunol. 2008;121(4):872-877.e9. doi: 10.1016/j.jaci.2008.01.026 [DOI] [PubMed] [Google Scholar]
- 40.Bataille V, Lens M, Spector TD. The use of the twin model to investigate the genetics and epigenetics of skin diseases with genomic, transcriptomic and methylation data. J Eur Acad Dermatol Venereol. 2012;26(9):1067-1073. doi: 10.1111/j.1468-3083.2011.04444.x [DOI] [PubMed] [Google Scholar]
- 41.Taylor-Robinson DC, Williams H, Pearce A, Law C, Hope S. Do early-life exposures explain why more advantaged children get eczema? findings from the U.K. Millennium Cohort Study. Br J Dermatol. 2016;174(3):569-578. doi: 10.1111/bjd.14310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Williams HC, Strachan DP, Hay RJ. Childhood eczema: disease of the advantaged? BMJ. 1994;308(6937):1132-1135. doi: 10.1136/bmj.308.6937.1132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Uphoff E, Cabieses B, Pinart M, Valdés M, Antó JM, Wright J. A systematic review of socioeconomic position in relation to asthma and allergic diseases. Eur Respir J. 2015;46(2):364-374. doi: 10.1183/09031936.00114514 [DOI] [PubMed] [Google Scholar]
- 44.Spergel JM, Paller AS. Atopic dermatitis and the atopic march. J Allergy Clin Immunol. 2003;112(6)(suppl):S118-S127. doi: 10.1016/j.jaci.2003.09.033 [DOI] [PubMed] [Google Scholar]
- 45.Shaker M. New insights into the allergic march. Curr Opin Pediatr. 2014;26(4):516-520. doi: 10.1097/MOP.0000000000000120 [DOI] [PubMed] [Google Scholar]
- 46.Belgrave DCM, Granell R, Simpson A, et al. Developmental profiles of eczema, wheeze, and rhinitis: two population-based birth cohort studies. PLoS Med. 2014;11(10):e1001748. doi: 10.1371/journal.pmed.1001748 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Gene Variants Comprising the AD Gene Risk Score and Their Respective Beta Coefficient and Effect Allele
eTable 2. Baseline Characteristics of the AD Study Group at 13 Years Compared to the Remaining Cohort (AD Controls)
eFigure 1. Flowchart of the Atopic Dermatitis Study Population
eFigure 2. Loading Plot: PCA Comparison of Minor HR Criteria at Diagnosis of Children With Atopic Dermatitis