This study examines the association of adjuvant radiation therapy vs surgery alone with survival in patients with advanced cutaneous squamous cell carcinoma and assesses which patients benefit the most from addition of adjuvant therapy to surgical treatment.
Key Points
Question
Is there an association between adjuvant radiation therapy and survival in patients with advanced cutaneous squamous cell carcinoma, and which patients benefit the most from addition of adjuvant therapy to surgical treatment?
Findings
In this multi-institutional study of 349 patients with advanced cutaneous squamous cell carcinoma, adjuvant radiation therapy was associated with improved disease-free survival and overall survival in patients with perineural invasion and regional adenopathy.
Meaning
The findings suggest that patients with perineural invasion and regional disease benefit the most from addition of adjuvant radiation therapy to surgical treatment.
Abstract
Importance
Cutaneous squamous cell carcinoma (CSCC) is one of the most common malignant tumors worldwide. There is conflicting evidence regarding the indications for and benefits of adjuvant radiation therapy for advanced CSCC tumors of the head and neck.
Objective
To assess indications for adjuvant radiation therapy in patients with CSCC.
Design, Setting, and Participants
Retrospective analysis of 349 patients with head and neck CSCC treated with primary resection with or without adjuvant radiation therapy at 2 tertiary referral centers from January 1, 2008, to June 30, 2016.
Main Outcomes and Measures
Data were compared between treatment groups with a χ2 analysis. Disease-free survival (DFS) and overall survival (OS) were analyzed using a Kaplan-Meier survival analysis with log-rank test and a Cox proportional hazards multivariate regression.
Results
A total of 349 patients had tumors that met the inclusion criteria (mean [SD] age, 70 [12] years; age range, 32-94 years; 302 [86.5%] male), and 191 (54.7%) received adjuvant radiation therapy. The 5-year Kaplan-Meier estimates were 59.4% for DFS and 47.4% for OS. Patients with larger, regionally metastatic, poorly differentiated tumors with perineural invasion (PNI) and younger immunosuppressed patients were more likely to receive adjuvant radiation therapy. On Cox proportional hazards multivariate regression, patients with periorbital tumors (hazard ratio [HR], 2.48; 95% CI, 1.00-6.16), PNI (HR, 1.90; 95% CI, 1.12-3.19), or N2 or greater nodal disease (HR, 2.16; 95% CI, 1.13-4.16) had lower DFS. Immunosuppressed patients (HR, 2.17; 95% CI, 1.12-4.17) and those with N2 or greater nodal disease (HR, 2.43; 95% CI, 1.42-4.17) had lower OS. Adjuvant radiation therapy was associated with improved OS for the entire cohort (HR, 0.59; 95% CI, 0.38-0.90). In a subset analysis of tumors with PNI, adjuvant radiation therapy was associated with improved DFS (HR, 0.47; 95% CI, 0.23-0.93) and OS (HR, 0.44; 95% CI, 0.24-0.86). Adjuvant radiation therapy was also associated with improved DFS (HR, 0.36; 95% CI, 0.15-0.84) and OS (HR, 0.30; 95% CI, 0.15-0.61) in patients with regional disease.
Conclusions and Relevance
Among patients with advanced CSCC, receipt of adjuvant radiation therapy was associated with improved survival in those with PNI and regional disease.
Introduction
Nonmelanoma skin cancer is the most common malignant tumor in the United States, 20% of which are cutaneous squamous cell carcinomas (CSCCs).1,2 Roughly 80% of these CSCCs occur on the head and neck, and their incidence has been increasing.3 In general, cure rates exceed 90% with early-stage disease4,5,6; however, patients with locally advanced or regionally metastatic disease have higher rates of recurrence, with some reported 5-year overall survival (OS) estimates less than 50%.7,8,9,10
Optimal treatment of advanced CSCC remains controversial because there are only a few large retrospective series that describe clinical outcomes, and no randomized clinical trials are available to guide decision making. Dermatologic series for all CSCCs have demonstrated that tumor differentiation, diameter, depth of invasion, and perineural invasion (PNI) are indicative of recurrence and poor survival.7,9,11,12,13,14,15,16 In advanced disease of the head and neck, factors such as immunosuppression, parotid and cervical nodal disease, and metastatic node size have also been associated with poor outcomes.8,10,17,18,19,20 After high-risk features have been identified, the question of which patients benefit from adjuvant therapy remains. Historically, recurrent tumors or tumors with PNI and increased diameter have been treated with postoperative radiation therapy.21 There is variability in the literature, however, regarding the efficacy of adjuvant therapy, with some studies22,23,24,25,26 noting improved locoregional control and OS and others reporting no difference in either. Given the ill-defined guidelines and inconsistent data in the literature on which factors are indications for adjuvant therapy, our objective was to compare outcomes between patients who underwent surgery alone with those who received adjuvant therapy and to assess whether adjuvant therapy was associated with disease-free survival (DFS) or OS.
Methods
Patient data were collected by querying the electronic medical records of patients seen at the University of California, Davis, and Washington University School of Medicine, St Louis, Missouri, for International Classification of Diseases, Ninth Revision (ICD-9) codes and identifying patients with CSCCs of the head and neck treated from January 1, 2008, to June 30, 2016. All patients undergoing primary surgery with or without adjuvant radiation therapy with curative intent for CSCCs were included. Patients were excluded if they had distant metastases at presentation, were treated with palliative intent, had no residual cancer found on excision, or had less than 3 months of follow-up. This study was approved by the institutional review boards of the University of California, Davis, and Washington University School of Medicine, and the need for informed consent was waived by both institutions because of the retrospective nature of data collection. All data were deidentified.
We collected patient data (age, sex, immunologic status, and history of radiation therapy) and tumor characteristics (primary site, diameter, margin status, lymphovascular invasion, PNI, presence of regional nodal disease, histologic differentiation, adjuvant radiation therapy, and whether tumors were recurrent at presentation). Depth of invasion was rarely described on pathology reports; therefore, these data were not collected. The age variable was categorized into 2 groups: younger than 70 years and 70 years or older. The diameter variable was grouped into smaller than 2 cm, 2 to 4 cm, and larger than 4 cm given the American Joint Committee on Cancer threshold of a 2-cm diameter as a high-risk feature.11 Histologic differentiation was grouped as poorly differentiated and well to moderately differentiated. Collected outcome data included recurrence, date of recurrence, location of recurrence, date of last follow-up, and date of death. When date of death was not available in the electronic medical record, the Social Security Death Index was queried.
Surgical resection of primary tumors was performed with the goal of achieving negative margins through wide local excision and included radical and craniofacial resection when appropriate. Patients with positive margins at the dura deemed to be unresectable were excluded from the analysis. Decision to perform a parotidectomy and/or cervical lymphadenectomy was made by the primary surgeon (P.P., R.S.J., M.G.M., D.G.F., or A.F.B.) for each case, with patients undergoing therapeutic lymphadenectomy for clinically evident disease or elective lymphadenectomy when deemed at high risk for nodal failure. High-risk patients were generally defined as patients with recurrent tumors, those with tumors larger than 2 cm, immunocompromised patients, or those with PNI; however, no strict cutoff was used.4,17,23 Adjuvant radiation therapy was delivered at the discretion of the radiation oncologists (K.N.B.N., S.R.).
Statistical analyses were performed using SPSS statistical software, version 25.0 (SPSS Inc). Five-year estimates of DFS and OS were made using the Kaplan-Meier method. Patient and tumor characteristics were compared between treatment groups with a χ2 analysis. The associations of patient and tumor characteristics and adjuvant therapy with survival were examined with univariate and multivariate Cox proportional hazards regressions. A subset analysis was then performed to determine which patients with high-risk features had improved survival with adjuvant therapy. Kaplan-Meier log-rank test was used to determine 1-sided P values. P < .05 was considered to be statistically significant.
Results
A total of 349 patients met the inclusion criteria (mean [SD] age, 70 [12] years; age range, 32-94 years; median age, 72 years; 302 [86.5%] male). Only 33 patients (9.5%) were immunocompromised because of maintenance of a transplanted organ or chronic lymphocytic leukemia. Most tumors were recurrent (204 [58.5%]), and many patients had PNI (136 [39.0%]), extracapsular tumor extension (67 [19.2%]), poorly differentiated histologic features (85 [24.4%]), or regional disease at presentation (129 [37.0%]). Patient and tumor characteristics are summarized in Table 1.
Table 1. Tumor Characteristics.
| Characteristic | No. (%) of Patients (N = 349) |
|---|---|
| Sex | |
| Male | 302 (86.5) |
| Female | 47 (13.5) |
| Immunosuppressed | 33 (9.5) |
| Recurrent disease | 204 (58.5) |
| Primary site | 69 (19.8) |
| Ear | 49 (14.0) |
| Periorbital | 13 (3.7) |
| Lip | 30 (8.6) |
| Nose | 23 (6.6) |
| Scalp | 54 (15.5) |
| Temple | 27 (7.7) |
| Unknown | 22 (6.3) |
| Diameter, cm | |
| <2 | 73 (20.9) |
| 2-4 | 102 (29.2) |
| >4 | 77 (22.1) |
| Positive margins | 34 (19.2) |
| LVI | 47 (13.5) |
| PNI | 136 (39.0) |
| ECE | 67 (19.2) |
| Poorly differentiated | 85 (24.4) |
| Regional disease | 129 (37.0) |
| Postoperative radiation therapy | 191 (54.7) |
Abbreviations: ECE, extracapsular tumor extension; LVI, lymphovascular invasion; PNI, perineural invasion.
The mean (SD) follow-up time was 37 (55) months. There were 97 documented recurrences, and 64 (66%) of all recurrences were local. The 5-year Kaplan-Meier survival estimates were 59.4% for DFS and 47.4% for OS.
A total of 176 patients (50.4%) received adjuvant radiation therapy, and another 15 (4.3%) received adjuvant chemoradiation therapy. Patients with larger, regionally metastatic, poorly differentiated tumors with PNI or younger immunosuppressed patients were more likely to receive adjuvant radiation therapy than were other patients. These results are summarized in Table 2. Patients with extracapsular tumor extension or positive margins were more likely to receive adjuvant chemoradiation therapy.
Table 2. Patient and Tumor Characteristics.
| Characteristic | No. (%) of Patients | |
|---|---|---|
| Surgery Alone | Surgery and Radiation Therapy | |
| Age, y | ||
| ≥70 | 61 (70.7) | 99 (89.3) |
| <70 | 88 (78.3) | 89 (98.7) |
| Recurrent disease | 78 (38.6) | 118 (58.4) |
| Periorbital, cm | 5 (38.5) | 8 (61.5) |
| <2 | 42 (31.7) | 28 (38.3) |
| 2-4 | 50 (43.9) | 47 (53.1) |
| >4 | 36 (33.1) | 37 (40.0) |
| PNI | 40 (59.3) | 91 (71.7) |
| Poorly differentiated | 28 (37.6) | 55 (45.4) |
| Regional disease | 31 (53.4) | 87 (64.6) |
| Immunosuppression | 8 (33.3) | 24 (72.7) |
Abbreviation: PNI, perineural invasion.
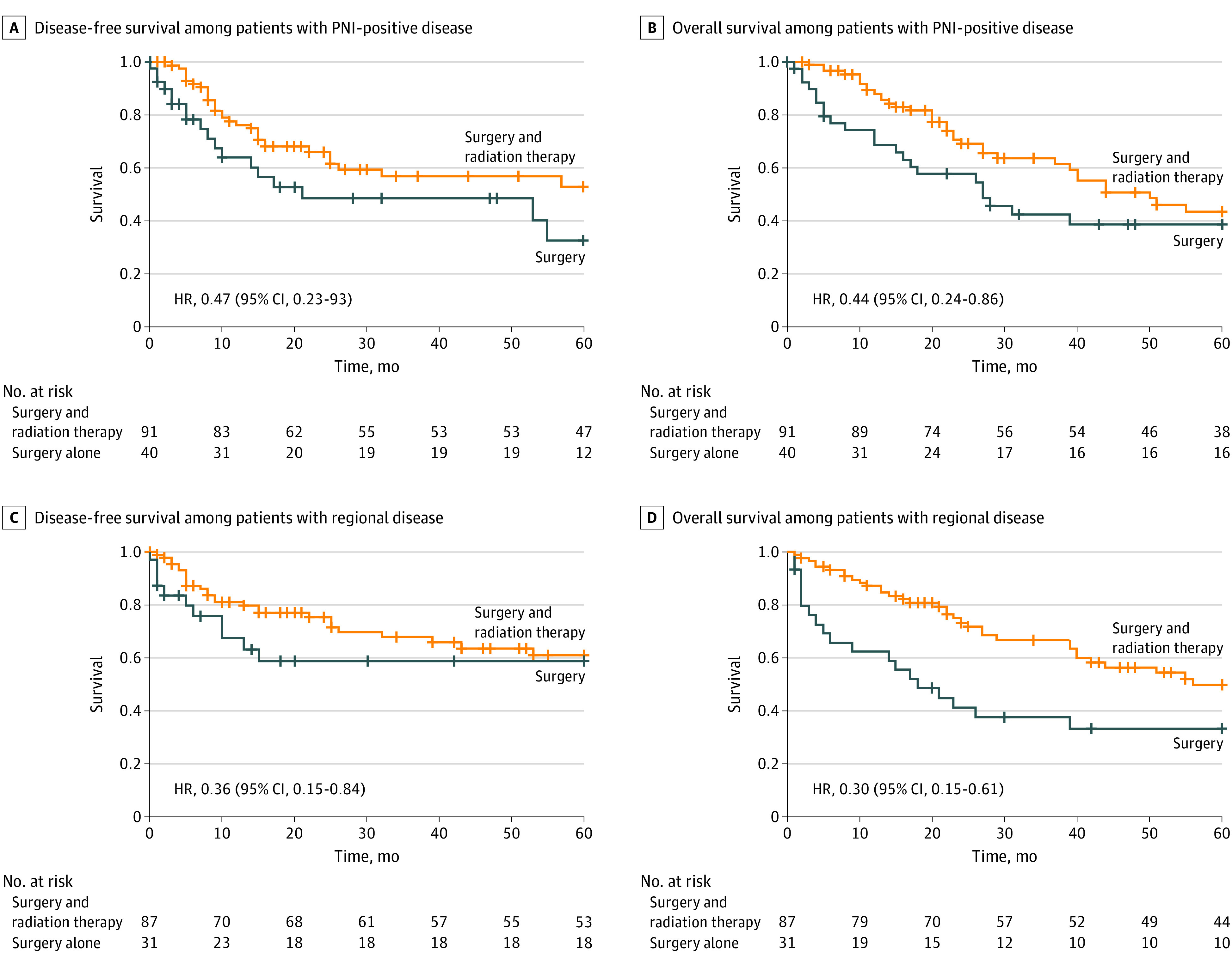
When patient and tumor data were controlled for with Cox proportional hazards multivariate regression, patients with periorbital tumors (hazard ratio [HR], 2.48; 95% CI, 1.00-6.16), PNI (HR, 1.90; 95% CI, 1.12-3.19), or N2 or greater nodal disease (HR, 2.16; 95% CI, 1.13-4.16) had worse DFS than patients with other tumor locations. Immunosuppressed patients (HR, 2.17; 95% CI, 1.12-4.17) and those with N2 or greater nodal disease (HR, 2.43; 95% CI, 1.42-4.17) had worse OS. On univariate analysis, adjuvant therapy was not associated with improvement in DFS or OS (Figure 1). On multivariate analysis, however, adjuvant radiation therapy was associated with improved OS in the entire cohort (HR, 0.59; 95% CI, 0.38-0.90) (Table 3). A subset analysis was then performed for patients with PNI and regional disease, controlling for all other tumor characteristics given the association of these factors with survival. In a subset analysis of tumors with PNI, adjuvant therapy was associated with improved DFS (HR, 0.47; 95% CI, 0.23-0.93) and OS (HR, 0.44; 95% CI, 0.24-0.86). Adjuvant therapy was also associated with improved DFS (HR, 0.36; 95% CI, 0.15-0.84) and OS (HR, 0.30; 95% CI, 0.15-0.61) in patients with regional disease (Figure 2). There was no association between adjuvant radiation therapy and DFS and OS for T3/T4 or poorly differentiated tumors.
Figure 1. Comparison of Surgery vs Surgery Plus Adjuvant Radiation Therapy for the Entire Cohort.
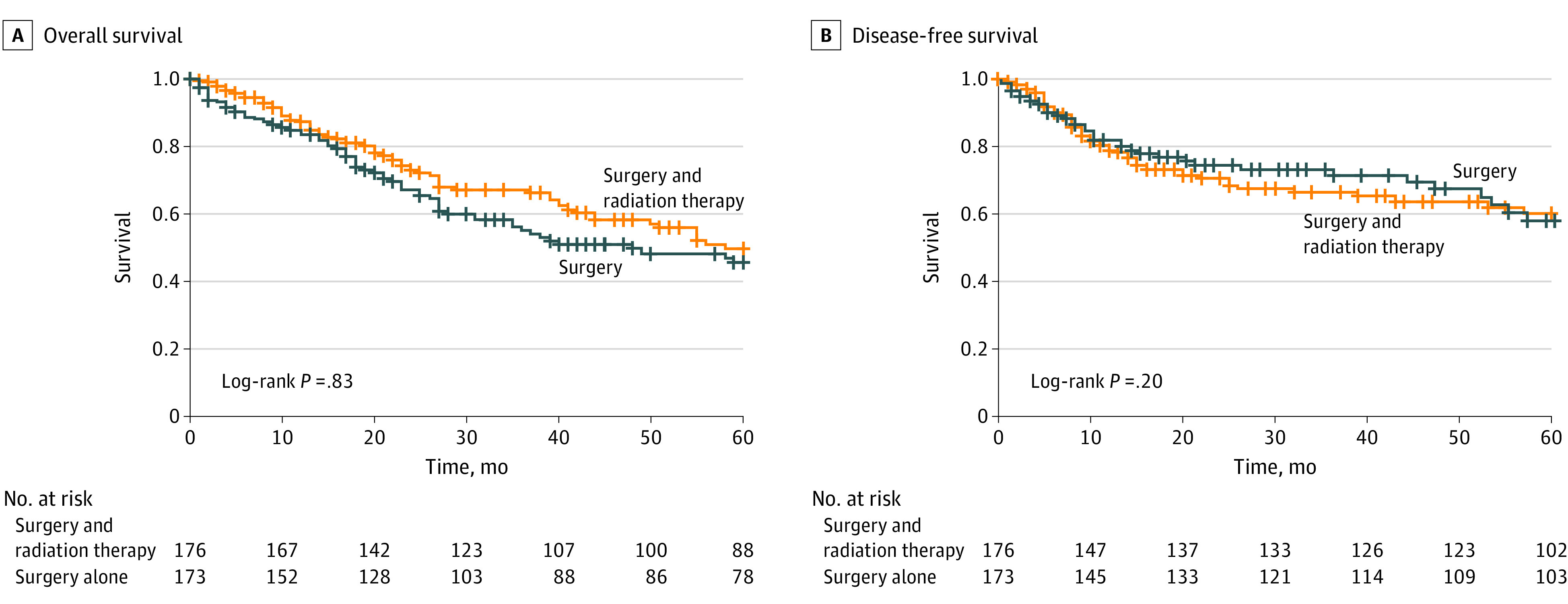
Table 3. Cox Proportional Hazards Regression Multivariate Analysisa.
| Factor | HR (95% CI) | |
|---|---|---|
| DFS | OS | |
| Age ≥70 y | 1.50 (0.92-2.45) | 1.80 (1.20-2.67) |
| Immunosuppressed | 0.89 (0.32-2.52) | 2.17 (1.12-4.17) |
| Periorbital primary | 2.48 (1.00-6.16) | 1.47 (0.62-3.52) |
| PNI-positive disease | 1.90 (1.12-3.19) | 1.32 (0.87-2.00) |
| N2 or greater disease | 2.16 (1.13-4.16) | 2.43 (1.42-4.17) |
| Postoperative radiation therapy | 0.67 (0.40-1.15) | 0.59 (0.38-0.90) |
Abbreviations: DFS, disease-free survival; HR, hazard ratio; OS, overall survival; PNI, perineural invasion.
Results were controlled for recurrent tumor, lymphovascular invasion, differentiation, and T stage. Reference groups were age younger than 70 years, other primary site, and N0 disease.
Figure 2. Subset Analysis of Patients With Perineural Invasion (PNI) and Regional Disease .
Discussion
Although recently published data have improved our knowledge of factors associated with recurrence and survival for advanced-stage CSCC of the head and neck, the evidence behind treatment recommendations for adjuvant radiation therapy remains limited.5,11,17 Given the increasing incidence of CSCC and the high morbidity and mortality of this disease, more data are needed to help guide adjuvant treatment recommendations to improve survival outcomes.2,7,8,9,10,18,19 Our study included 349 patients with advanced CSCCs of the head and neck, 54.7% of whom received adjuvant radiation therapy. To our knowledge, this is the largest series to date specifically examining the role of adjuvant radiation therapy in treatment of advanced CSCC of the head and neck.
In the present series, patients with tumors with PNI, increased diameter, poor differentiation, or regionally metastatic disease were more likely to undergo adjuvant therapy. Although these findings have been described as high-risk features for CSCC, only PNI and regional disease are currently recognized indications for adjuvant therapy.22,23,24 When controlling for patient and tumor characteristics, adjuvant radiation therapy was associated with a survival benefit in the entire cohort. Adjuvant radiation and chemoradiation therapies have been associated with improved outcomes in a number of studies of patients with advanced head and neck CSCCs, although the exact role of chemoradiation therapy is unclear.9,18,19,21,27
On subset analysis of the 136 tumors with PNI, adjuvant radiation therapy was associated with improved DFS and OS. Despite acceptance of PNI as an indication for adjuvant therapy, the evidence for this recommendation is limited. Jambusaria-Pahlajani et al28 published a systematic review of dermatologic series in 2009 that found no benefit to adjuvant radiation therapy after surgery for tumors with PNI. In one of the few series that addressed PNI in advanced head and neck CSCC, Warren et al26 found a DFS of 62% after treatment with surgery and adjuvant radiation therapy in tumors with PNI, similar to our DFS rate. They also found a disease-specific survival rate of 75% and overall survival rate of 64%. They did not, however, provide a direct comparison to patients who received surgery alone. Balamucki et al29 determined that clinical PNI was a poor prognostic indicator for local control and found a DFS benefit with the addition of radiation therapy. Our data suggest that patients with PNI in particular benefit from the addition of adjuvant radiation.
On subset analysis of the 123 tumors with regional disease, receipt of adjuvant radiation therapy was associated with improved DFS and OS compared with treatment with surgery alone. Veness et al21 studied 167 patients with CSCC metastatic to the lymph nodes and/or parotid and found that those treated with surgery had survival outcomes inferior only to those of patients treated with surgery and adjuvant radiation. On the basis of their study, they recommend adjuvant radiation therapy for those with metastatic CSCC to provide the best chance of locoregional control. In their study however, less than one-third of patients received adjuvant therapy. Similarly, Ch’ng et al,19 Givi et al,9 and Pfister et al12 found that patients with head and neck CSCC and regional disease who received adjuvant radiation therapy had a significant survival advantage.
Limitations
We acknowledge that the retrospective nature of this study and the inherent selection bias for patients who received adjuvant radiation therapy may limit the conclusions that can be drawn from these results. Of importance, some of the patients in this series completed adjuvant radiation treatment at outside institutions, and we cannot account for variations in administration. Additional studies evaluating the specific role of radiation dose and field design for tumor control would be of great value. Additional prospective studies are needed to determine the efficacy of adjuvant radiation therapy in patients with advanced CSCC of the head and neck and specifically which patients may derive the most benefit.
Conclusions
In our series of surgically treated advanced CSCC of the head and neck, adjuvant radiation therapy was more commonly given to patients with PNI, increased tumor diameter, poor differentiation, or regionally metastatic disease. In patients with PNI and regional disease, adjuvant radiation therapy was associated with improved survival compared with surgery alone.
References
- 1.Rogers HW, Weinstock MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146(3):283-287. doi: 10.1001/archdermatol.2010.19 [DOI] [PubMed] [Google Scholar]
- 2.Christenson LJ, Borrowman TA, Vachon CM, et al. Incidence of basal cell and squamous cell carcinomas in a population younger than 40 years. JAMA. 2005;294(6):681-690. doi: 10.1001/jama.294.6.681 [DOI] [PubMed] [Google Scholar]
- 3.Terra JB, Gaster MB, Halmos GB, et al. Local control of 151 head and neck cutaneous squamous cell carcinoma after radiation therapy: a retrospective study on efficacy and prognostic factors. Clin Otolaryngol. 2017;42(4):851-855. doi: 10.1111/coa.12707 [DOI] [PubMed] [Google Scholar]
- 4.Schmults CD, Karia PS, Carter JB, Han J, Qureshi AA. Factors predictive of recurrence and death from cutaneous squamous cell carcinoma: a 10-year, single-institution cohort study. JAMA Dermatol. 2013;149(5):541-547. doi: 10.1001/jamadermatol.2013.2139 [DOI] [PubMed] [Google Scholar]
- 5.Jambusaria-Pahlajani A, Kanetsky PA, Karia PS, et al. Evaluation of AJCC tumor staging for cutaneous squamous cell carcinoma and a proposed alternative tumor staging system. JAMA Dermatol. 2013;149(4):402-410. doi: 10.1001/jamadermatol.2013.2456 [DOI] [PubMed] [Google Scholar]
- 6.Estall V, Allen A, Webb A, Bressel M, McCormack C, Spillane J. Outcomes following management of squamous cell carcinoma of the scalp: a retrospective series of 235 patients treated at the Peter MacCallum Cancer Centre. Australas J Dermatol. 2017;58(4):e207-e215. doi: 10.1111/ajd.12520 [DOI] [PubMed] [Google Scholar]
- 7.Clayman GL, Lee JJ, Holsinger FC, et al. Mortality risk from squamous cell skin cancer. J Clin Oncol. 2005;23(4):759-765. doi: 10.1200/JCO.2005.02.155 [DOI] [PubMed] [Google Scholar]
- 8.Sweeny L, Zimmerman T, Carroll WR, Schmalbach CE, Day KE, Rosenthal EL. Head and neck cutaneous squamous cell carcinoma requiring parotidectomy: prognostic indicators and treatment selection. Otolaryngol Head Neck Surg. 2014;150(4):610-617. doi: 10.1177/0194599814520686 [DOI] [PubMed] [Google Scholar]
- 9.Givi B, Andersen PE, Diggs BS, Wax MK, Gross ND. Outcome of patients treated surgically for lymph node metastases from cutaneous squamous cell carcinoma of the head and neck. Head Neck. 2011;33(7):999-1004. doi: 10.1002/hed.21574 [DOI] [PubMed] [Google Scholar]
- 10.McDowell LJ, Tan TJ, Bressel M, et al. Outcomes of cutaneous squamous cell carcinoma of the head and neck with parotid metastases. J Med Imaging Radiat Oncol. 2016;60(5):668-676. doi: 10.1111/1754-9485.12484 [DOI] [PubMed] [Google Scholar]
- 11.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trottie A III, eds. AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer; 2009. [Google Scholar]
- 12.Pfister DG, Spencer S, Brizel DM, et al. Head and neck cancers, version 1.2015. J Natl Compr Canc Netw. 2015;13(7):847-855. doi: 10.6004/jnccn.2015.0102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goepfert H, Dichtel WJ, Medina JE, Lindberg RD, Luna MD. Perineural invasion in squamous cell skin carcinoma of the head and neck. Am J Surg. 1984;148(4):542-547. doi: 10.1016/0002-9610(84)90385-4 [DOI] [PubMed] [Google Scholar]
- 14.Moore BA, Weber RS, Prieto V, et al. Lymph node metastases from cutaneous squamous cell carcinoma of the head and neck. Laryngoscope. 2005;115(9):1561-1567. doi: 10.1097/01.mlg.0000173202.56739.9f [DOI] [PubMed] [Google Scholar]
- 15.Kyrgidis A, Tzellos TG, Kechagias N, et al. Cutaneous squamous cell carcinoma (SCC) of the head and neck: risk factors of overall and recurrence-free survival. Eur J Cancer. 2010;46(9):1563-1572. doi: 10.1016/j.ejca.2010.02.046 [DOI] [PubMed] [Google Scholar]
- 16.Haisma MS, Plaat BEC, Bijl HP, et al. Multivariate analysis of potential risk factors for lymph node metastasis in patients with cutaneous squamous cell carcinoma of the head and neck. J Am Acad Dermatol. 2016;75(4):722-730. doi: 10.1016/j.jaad.2016.06.010 [DOI] [PubMed] [Google Scholar]
- 17.Harris BN, Bayoumi A, Rao S, Moore MG, Farwell DG, Bewley AF. Factors associated with recurrence and regional adenopathy for head and neck cutaneous squamous cell carcinoma. Otolaryngol Head Neck Surg. 2017;156(5):863-869. doi: 10.1177/0194599817697053 [DOI] [PubMed] [Google Scholar]
- 18.Dean NR, Sweeny L, Magnuson JS, et al. Outcomes of recurrent head and neck cutaneous squamous cell carcinoma. J Skin Cancer. 2011;2011:972497. doi: 10.1155/2011/972497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ch’ng S, Maitra A, Allison RS, et al. Parotid and cervical nodal status predict prognosis for patients with head and neck metastatic cutaneous squamous cell carcinoma. J Surg Oncol. 2008;98(2):101-105. doi: 10.1002/jso.21092 [DOI] [PubMed] [Google Scholar]
- 20.O’Brien CJ, McNeil EB, McMahon JD, Pathak I, Lauer CS, Jackson MA. Significance of clinical stage, extent of surgery, and pathologic findings in metastatic cutaneous squamous carcinoma of the parotid gland. Head Neck. 2002;24(5):417-422. doi: 10.1002/hed.10063 [DOI] [PubMed] [Google Scholar]
- 21.Veness MJ, Morgan GJ, Palme CE, Gebski V. Surgery and adjuvant radiotherapy in patients with cutaneous head and neck squamous cell carcinoma metastatic to lymph nodes: combined treatment should be considered best practice. Laryngoscope. 2005;115(5):870-875. doi: 10.1097/01.MLG.0000158349.64337.ED [DOI] [PubMed] [Google Scholar]
- 22.Tanvetyanon T, Padhya T, McCaffrey J, et al. Postoperative concurrent chemotherapy and radiotherapy for high-risk cutaneous squamous cell carcinoma of the head and neck. Head Neck. 2015;37(6):840-845. doi: 10.1002/hed.23684 [DOI] [PubMed] [Google Scholar]
- 23.Fu T, Aasi SZ, Hollmig ST. Management of high-risk squamous cell carcinoma of the skin. Curr Treat Options Oncol. 2016;17(7):34. doi: 10.1007/s11864-016-0408-2 [DOI] [PubMed] [Google Scholar]
- 24.Koyfman SA, Joshi N, Vidimos A. Adjuvant radiotherapy in high-risk cutaneous squamous cell cancer of the head and neck in immunosuppressed patients. JAAD Case Rep. 2015;1(6):S5-S7. doi: 10.1016/j.jdcr.2015.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu SM, Lien WW. Concurrent radiation therapy with cetuximab or platinum-based chemotherapy for locally advanced cutaneous squamous cell carcinoma of the head and neck. Am J Clin Oncol. 2018;41(1):95-99. doi: 10.1097/COC.0000000000000228 [DOI] [PubMed] [Google Scholar]
- 26.Warren TA, Panizza B, Porceddu SV, et al. Outcomes after surgery and postoperative radiotherapy for perineural spread of head and neck cutaneous squamous cell carcinoma. Head Neck. 2016;38(6):824-831. doi: 10.1002/hed.23982 [DOI] [PubMed] [Google Scholar]
- 27.Porceddu SV, Bressel M, Poulsen MG, et al. Postoperative concurrent chemoradiotherapy versus postoperative radiotherapy in high-risk cutaneous squamous cell carcinoma of the head and neck: the randomized phase III TROG 05.01 Trial. J Clin Oncol. 2018;36(13):1275-1283. doi: 10.1200/JCO.2017.77.0941 [DOI] [PubMed] [Google Scholar]
- 28.Jambusaria-Pahlajani A, Miller CJ, Quon H, Smith N, Klein RQ, Schmults CD. Surgical monotherapy versus surgery plus adjuvant radiotherapy in high-risk cutaneous squamous cell carcinoma: a systematic review of outcomes. Dermatol Surg. 2009;35(4):574-585. doi: 10.1111/j.1524-4725.2009.01095.x [DOI] [PubMed] [Google Scholar]
- 29.Balamucki CJ, Mancuso AA, Amdur RJ, et al. Skin carcinoma of the head and neck with perineural invasion. Am J Otolaryngol. 2012;33(4):447-454. doi: 10.1016/j.amjoto.2011.11.004 [DOI] [PubMed] [Google Scholar]


