Key Points
Question
Does paracetamol (acetaminophen) combined with ibuprofen reduce postoperative morphine usage relative to the use of each drug alone in patients undergoing total hip arthroplasty (THA), and does ibuprofen increase the incidence of serious adverse events (SAEs)?
Findings
In this randomized clinical trial that included 556 patients who underwent THA, morphine usage in the first 24 hours was statistically significantly lower for the combination of paracetamol 1000 mg and ibuprofen 400 mg than for either alone; however, the combined medications did not meet the prespecified threshold for clinically important postoperative morphine reduction (10 mg) compared with ibuprofen alone. The percentage of patients with SAEs for those in any of the ibuprofen groups vs paracetamol alone was 15% vs 11%, which was not statistically significant.
Meaning
Although the combined use of paracetamol and ibuprofen reduced immediate postoperative morphine consumption compared with paracetamol alone in patients undergoing THA, ibuprofen alone resulted in comparable pain control without increasing SAEs, suggesting that ibuprofen alone may be a reasonable option.
Abstract
Importance
Multimodal postoperative analgesia is widely used but lacks evidence of benefit.
Objective
Investigate beneficial and harmful effects of 4 nonopioid analgesics regimens.
Design, Setting, and Participants
Randomized, blinded, placebo-controlled, 4-group trial in 6 Danish hospitals with 90-day follow-up that included 556 patients undergoing total hip arthroplasty (THA) from December 2015 to October 2017. Final date of follow-up was January 1, 2018.
Interventions
Participants were randomized to receive paracetamol (acetaminophen) 1000 mg plus ibuprofen 400 mg (n = 136; PCM + IBU), paracetamol 1000 mg plus matched placebo (n = 142; PCM), ibuprofen 400 mg plus matched placebo (n = 141; IBU), or half-strength paracetamol 500 mg plus ibuprofen 200 mg (n = 140; HS–PCM + IBU) orally every 6 hours for 24 hours postoperatively, starting 1 hour before surgery.
Main Outcomes and Measures
Two co–primary outcomes: 24-hour morphine consumption using patient-controlled analgesia in pairwise comparisons between the 4 groups (multiplicity-adjusted thresholds for statistical significance, P < .0042; minimal clinically important difference, 10 mg), and proportion of patients with 1 or more serious adverse events (SAEs) within 90 days (multiplicity-adjusted thresholds for statistical significance, P < .025).
Results
Among 559 randomized participants (mean age, 67 years; 277 [50%] women), 556 (99.5%) completed the trial and were included in the analysis. Median 24-hour morphine consumption was 20 mg (99.6% CI, 0-148) in the PCM + IBU group, 36 mg (99.6% CI, 0-166) for PCM alone, 26 mg (99.6% CI, 2-139) for IBU alone, and 28 mg (99.6% CI, 2-145) for HS–PCM + IBU. The median difference in morphine consumption between the PCM + IBU group vs PCM alone was 16 mg (99.6% CI, 6.5 to 24; P < .001); for the PCM-alone group vs HS–PCM + IBU, 8 mg (99.6% CI, −1 to 14; P = .001); and for the PCM + IBU group vs IBU alone, 6 mg (99.6% CI, −2 to 16; P = .002). The difference in morphine consumption was not statistically significant for the PCM + IBU group vs HS–PCM + IBU (8 mg [99.6% CI, −2 to 16]; P = .005) or for the PCM-alone group vs IBU alone (10 mg [99.6% CI, −2 to 16]; P = .004) after adjustment for multiple comparisons and 2 co–primary outcomes. There was no significant difference between the IBU-alone group vs HS–PCM + IBU (2 mg [99.6% CI, −10 to 7]; P = .81). The proportion of patients with SAEs in groups receiving IBU was 15%, and in the PCM-alone group, was 11%. The relative risk of SAE was 1.44 (97.5% CI, 0.79 to 2.64; P = .18).
Conclusions and Relevance
Among patients undergoing THA, paracetamol plus ibuprofen significantly reduced morphine consumption compared with paracetamol alone in the first 24 hours after surgery; there was no statistically significant increase in SAEs in the pooled groups receiving ibuprofen alone vs with paracetamol alone. However, the combination did not result in a clinically important improvement over ibuprofen alone, suggesting that ibuprofen alone may be a reasonable option for early postoperative oral analgesia.
Trial Registration
ClinicalTrials.gov Identifier: NCT02571361
This randomized clinical trial compares the effects of combination paracetamol (acetaminophen) and ibuprofen at full vs half strength vs either drug alone on 24-hour patient-controlled morphine consumption and 90-day serious adverse events after total hip arthroplasty (THA).
Introduction
Multimodal analgesia is the leading principle for management of acute postoperative pain.1 One form of multimodal analgesia combines nonopioid analgesics to reduce postoperative pain and opioid usage. Recent guidelines recommend combinations of at least paracetamol (acetaminophen) and a nonsteroidal anti-inflammatory drug (NSAID) for most types of surgeries.2
The analgesic effects of both paracetamol and an NSAID in postoperative pain are well established when the individual drugs are compared with placebo.3,4,5 Although paracetamol and an NSAID are used routinely,2 there is little high-quality evidence for an additive or synergistic analgesic effect when they are combined.6,7,8
In the perioperative period, the safety of NSAIDs, including the combination with paracetamol, is largely unknown.7 The rate of perioperative adverse events, such as cardiovascular events, gastrointestinal complications, and renal failure, is substantial,9,10 and it is important that analgesic medications do not exacerbate these events. NSAIDs have been linked to adverse events in other settings,11,12 however, this link has not been investigated in the perioperative period.
The aim of the PANSAID (Paracetamol and NSAID in combination) trial was to investigate the analgesic (morphine sparing) and harmful effects of 4 multimodal analgesic regimens with paracetamol, ibuprofen, or both in combination after total hip arthroplasty (THA).13 The 2 co–primary outcomes were 24-hour morphine consumption using patient-controlled analgesia (PCA) and proportion of patients with 1 or more serious adverse events (SAEs) within 90 days after surgery (defined as SAE according to the International Conference on Harmonisation-Good Clinical Practice [ICH-GCP] guidelines14 but without prolongation of hospitalization). The hypotheses were as follows: (1) the combination of paracetamol and ibuprofen would lead to lower opioid consumption compared with each drug alone; (2) the combination of lower doses of paracetamol and ibuprofen would lead to opioid consumption comparable to or lower than higher doses of each drug alone; and (3) ibuprofen would increase the rate of SAEs.
Methods
Trial Oversight and Population
This trial was a multicenter, randomized, blinded trial in patients having planned primary THA investigating the use of paracetamol, ibuprofen, and combinations of both drugs. The trial protocol13 and the statistical analysis plan15 appear in Supplement 1. The trial was conducted in accordance with the Declaration of Helsinki and monitored by the Good Clinical Practice Units at Odense and Copenhagen University Hospitals. Ethical approval was granted by the Biomedical Research Ethics Committee of Region Zealand (SJ-462). All participants provided written informed consent prior to enrollment. The trial protocol adhered to the SPIRIT statement,16 and the reporting of the trial adhered to the CONSORT statement.17
The trial was conducted at 6 hospitals in Denmark (5 public and 1 private), ranging from smaller regional hospitals to large university hospitals. All patients scheduled for elective, primary, unilateral THA were screened for participation. Key exclusion criteria were daily use of opioids (however, patients using tramadol or codeine were not excluded) and contraindications to ibuprofen or paracetamol. A complete list of inclusion and exclusion criteria is provided in Supplement 2.
Interventions, Randomization, and Blinding
All patients received one of the following interventions: paracetamol 1000 mg plus ibuprofen 400 mg, paracetamol 1000 mg plus matching placebo, ibuprofen 400 mg plus matching placebo, or half-strength paracetamol 500 mg plus ibuprofen 200 mg. The trial medication was given orally starting 1 hour before surgery and given every 6 hours for 24 hours postoperatively for a total of 4 doses of the medication on the first postoperative day.
Patients were randomized by a web-based central allocation service provided by the Copenhagen Trial Unit, Denmark, to 1 of 4 groups in a 1:1:1:1 ratio, using a computer-generated randomized sequence with varying unknown block sizes (either 4 or 8) and stratification for site. The randomization code could only be broken by calling a 24-hour telephone service provided by the Copenhagen Trial Unit.
The trial medication (paracetamol, ibuprofen, and placebo) was packed and masked by the Pharmacy of the Capital Region, Herlev, Denmark. A dose of trial medication consisted of 3 identical opaque capsules. Patients, staff, investigators, outcome assessors, and the statistician were blinded to the intervention. Based on the masked results, abstracts were written and agreed upon by the trial steering committee (April 6, 2018) before revealing the identity of the groups (Supplement 2).
Trial Procedures
Technical aspects of the surgery were left to the surgeon’s discretion. The patients had either cementless or cemented components inserted. Patients received spinal (preferred) or general anesthesia. For spinal anesthesia, bupivacaine plain (10-15 mg) was used, combined with continuous propofol infusion if sedation was needed. For general anesthesia, propofol and remifentanil were preferred, and at the end of surgery, intravenous sufentanil (0.3 μg/kg) was administered.
All participants had PCA morphine (morphine 1 mg/mL, no background infusion, bolus 2 mg, lock-out 10 minutes) for 24 hours postoperatively. According to the protocol, additional boluses of 2-mg morphine on patient request were allowed during the first postoperative hour. These additional dosages were added to the total PCA morphine consumption for the primary outcome. If any other opioid was administered during the first 24 hours postoperatively (due to mistake, malfunction of PCA pump, or other such problem), this was converted to morphine equivalents and added to the PCA morphine.
No pain medication (including peripheral regional anesthesia) other than the trial medication and the PCA morphine was allowed. Patients usually treated with gabapentinoids, glucocorticoids, selective serotonin reuptake inhibitors, tramadol, or codeine continued these medications during the intervention period (0-24 hours).
Trial Outcomes
The trial had 2 co–primary outcomes: total morphine consumption for the first 24 hours postoperatively and proportion of patients with 1 or more modified SAEs from the surgery to 90 days postoperatively.
The outcome of proportion of modified SAEs was defined as SAE according to ICH-GCP guidelines14 (defined as any untoward medical occurrence that results in death; is life-threatening; requires hospitalization or prolongation of hospitalization; or results in significant or persistent disability or incapacity, birth defects, or a medical intervention to prevent 1 of the before-mentioned outcomes (excluding prolongation of hospitalization because these could not be adjudicated because of differences in length of stay). Data regarding postoperative hospitalization were collected from Danish National Patient Registry, and vital status was collected from the Danish Civil Registration System. All patients were interviewed by phone at 90 days postoperatively to investigate if there had been any events requiring medical intervention since surgery.18 To investigate harm of ibuprofen, patients in the 3 groups randomized to receive ibuprofen were compared with patients in the paracetamol-alone group for the modified SAE outcome. We prespecified a sensitivity analysis excluding patients who used NSAIDs in the follow-up period from the paracetamol-alone group, thus comparing patients taking an NSAID at some point from surgery to follow-up with patients who did not take an NSAID at all.
The secondary outcome was pain (indicated by the patient) using the visual analog scale (VAS; score range, 0 mm [no pain] to 100 mm [worst imaginable pain]) during 30° flexion of the hip and at rest at 6 and 24 hours, and adverse events from 0 to 24 hours. The exploratory outcomes were level of nausea, sedation, dizziness (none, mild, moderate, and severe; patients indicated their own level); vomiting (number of vomiting episodes); use of antiemetic (ondansetron, milligrams); blood loss during the surgical procedure (milliliters); and days alive and outside hospital within 90 days postrandomization.
Statistical Analysis
To maintain an overall familywise error rate of less than .05, the threshold for type I error rate was adjusted for each of the 2 co–primary outcomes to .025 (2-sided). Furthermore, the threshold for type I error rate was adjusted for the pairwise comparisons between the 4 groups (6 comparisons) to .025/6 = .0042 for the co–primary outcome of morphine consumption.13,15,19
Because there was no prior literature clearly identifying what a minimal clinically important difference (MCID) would be for the reduction of morphine use after surgery, we established the MCID based on our clinical experience. Observation from Næstved Hospital revealed that patients undergoing THA and receiving only paracetamol postoperatively, would use approximately 30 to 35 mg of morphine in the first 24 hours. Studies had shown that opioid use could be reduced by approximately 30% by the use of multimodal analgesia, resulting in an estimation of a minimal clinically important difference of approximately 10 mg over 24 hours.33 We therefore chose a predefined MCID of 10 mg morphine consumption between the compared groups.20 With a standard deviation of 20 mg and a power of 0.90, an enrollment of 556 patients was needed. For the co–primary outcome of the proportion of modified SAEs, a power of 0.80 was attained to detect an increase from 10% to 21%.
Analyses were performed by an independent statistician (J.C.J.) by means of dummy group assignments. The primary analyses were by the intention-to-treat principle and the primary analysis population was composed of randomized patients who underwent THA surgery. The primary analysis of the co–primary outcome of morphine consumption was pairwise comparisons between groups using the Van Elteren test, due to nonnormally distributed data.21 For the primary analysis of the co–primary outcome of proportion of modified SAEs, generalized estimating equations were used with the stratification variable (ie, site)22 as a cluster variable to estimate relative risk (RR) (method selected because of its ability to handle correlated data and few events per site23).
Secondary analyses included adjusted analyses (for sex, age, prior use of NSAIDs, and prior use of paracetamol) and analyses based on the strictly per-protocol population. All analyses were stratified for site. For the primary and secondary outcomes, site and variables used in the adjusted analyses were tested for interaction with intervention groups (by adding an interaction term in the generalized estimating equation model using the STATA 15 command XTGEE).24 Analyses would have used multiple imputation if missing data exceeded 5% and the Littles test was significant to account for missing data, and if used, it constituted the main analysis.25 All statistical tests were predefined and published15 before randomization of the last participant. Two sites with few included patients were merged for statistical analyses (ie, Holbæk and Nykøbing Falster Hospitals).
The statistical analysis plan specified the dichotomization of nausea, sedation, and dizziness to none or mild vs moderate or severe, but during data analysis, there were numerous problems with few events (perfect prediction and collinearity), and consequently, nausea, sedation, and dizziness were dichotomized post hoc to none vs mild/moderate/severe.
Data are presented as means with standard deviations for normally distributed data and medians with 99.6% CIs for nonnormally distributed data. The level of significance and corresponding confidence interval were .0042 and 99.6% for the co-primary outcome of morphine consumption, .025 and 97.5% for the co-primary outcome of modified SAEs, .0083 and 99.2% for the secondary outcomes, and 0.05 and 95% for the exploratory outcomes. All analyses were performed using STATA 15/MP (StataCorp).
Results
From December 2015 to October 2017, a total of 559 participants were enrolled in this trial. Following randomization, surgery was canceled for 3 participants; thus, 556 patients were included in the primary analysis population (Figure 1). The demographic, surgical, and anesthesia characteristics (Table 1) were comparable between groups. Multiple imputation was not used for any outcome because there were few missing data. The final date of follow-up was January 1, 2018.
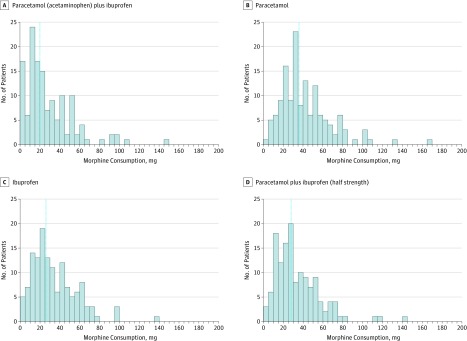
Figure 1. Patient Flow Comparing the Combination of Paracetamol (Acetaminophen) and Ibuprofen vs Either Alone on Patient-Controlled Morphine Consumption in the First 24 Hours After Total Hip Arthroplasty (THA).
aBody mass index was calculated as weight in kilograms divided by height in meters squared.
bAccording to Danish law, fertile women must be using (hormonal) contraception and have a negative pregnancy test if they are to be included in any trial. In this trial, some women were fertile but not using contraceptives and therefore did not meet the inclusion criteria.
cThe trial had 2 co–primary outcomes: total morphine comsumption for the first 24 hours postoperatively and proportion of patients with 1 or more modified serious adverse events (SAEs) from surgery to 90 days postoperatively.
Table 1. Patient Characteristics in Comparison of the Combination of Paracetamol (Acetaminophen) and Ibuprofen vs Either Alone on Patient-Controlled Morphine Consumption Following Total Hip Arthroplastya.
| Paracetamol Plus Ibuprofen n=136b |
Paracetamol Plus Placebo n=142b |
Ibuprofen Plus Placebo n=139b |
Paracetamol Plus Ibuprofen (Half Strength) n=139b |
|
|---|---|---|---|---|
| Age, mean (SD), y | 67 (10) | 67 (10) | 67 (11) | 66 (10) |
| Men | 68 (50) | 76 (54) | 72 (52) | 63 (45) |
| Women | 68 (50) | 66 (46) | 67 (48) | 76 (55) |
| ASA score | ||||
| 1, Healthy | 34 (25) | 44 (31) | 44 (32) | 43 (31) |
| 2, Mild systemic disease | 87 (64) | 84 (59) | 80 (57) | 84 (60) |
| 3, Severe systemic disease | 15 (11) | 14 (10) | 15 (11) | 12 (9) |
| Height, mean (SD), cm | 172 (9) | 172 (8) | 172 (9) | 171 (9) |
| Weight, mean (SD), kg | 83 (16) | 82 (15) | 80 (15) | 81 (16) |
| BMI, mean (SD) | 27.7 (4.3) | 27.4 (4.1) | 26.8 (3.9) | 27.6 (4.7) |
| Prior use of paracetamol | ||||
| No use | 53 (39) | 52 (37) | 51 (37) | 51 (37) |
| As needed | 29 (21) | 24 (17) | 24 (17) | 22 (16) |
| Daily use | 54 (40) | 66 (46) | 64 (46) | 66 (47) |
| Prior use of NSAID | ||||
| No use | 72 (53) | 77 (54) | 74 (53) | 72 (52) |
| As needed | 21 (15) | 18 (13) | 16 (12) | 21 (15) |
| Daily use | 43 (32) | 47 (33) | 49 (35) | 46 (33) |
| Prior use of codeine | ||||
| No use | 134 (98) | 140 (99) | 136 (98) | 137 (98) |
| As needed | 1 (1) | 0 | 1 (1) | 1 (1) |
| Daily use | 1 (1) | 1 (1) | 2 (1) | 1 (1) |
| Prior use of tramadol | ||||
| No use | 121 (89) | 127 (90) | 125 (90) | 122 (88) |
| As needed | 5 (4) | 6 (4) | 6 (4) | 8 (6) |
| Daily use | 10 (7) | 9 (6) | 8 (6) | 9 (6) |
| Duration of surgery, mean (SD), min | 54 (19)c | 51 (14)c | 53 (18) | 53 (15) |
| Type of surgery | ||||
| No cement | 122 (90) | 129 (91) | 127 (92) | 122 (88) |
| Cement | 2 (1) | 3 (2) | 2 (1) | 4 (3) |
| Hybrid | 12 (9) | 10 (7) | 10 (7) | 13 (9) |
| Anesthesia | ||||
| Spinal with sedation | 65 (48) | 70 (49) | 66 (48) | 69 (50) |
| Spinal | 39 (29) | 42 (30) | 43 (31) | 27 (19) |
| General | 29 (21) | 20 (14) | 24 (17) | 38 (27) |
| Conversion of spinal to general | 3 (2) | 10 (7) | 6 (4) | 5 (4) |
| Bupivacaine, SA, mean (SD), mg | 12 (2) | 12 (2) | 12 (2) | 12 (2) |
| Sufentanil used if general anesthesia, mean (SD), μg | 24 (7) | 20 (9) | 21 (8) | 21 (8) |
Abbreviations: ASA, American Society of Anesthesiologists; BMI, body mass index; NSAID, nonsteroidal anti-inflammatory drug; SA, spinal anesthesia.
Data are reported as No. (%) unless otherwise indicated.
Acetaminophen is the US adopted name for paracetamol. Paracetamol was given at 1000 mg to patients in the paractamol plus ibuprofen group and in the paracetamol plus placebo group, and patients received 500 mg in the half-strength group. Ibuprofen was given at 400 mg to patients in the paracetamol plus ibuprofen group and in the ibuprofen plus placebo group, and patients received 200 mg in the half-strength group.
Duration of surgery was calculated based on 135 patients for the paracetamol plus ibuprofen group and on 141 patients for the paracetamol plus placebo group due to missing data on this variable.
Primary Outcomes
Median 24-hour morphine consumption was 20 mg (99.6% CI, 0-148) in the paracetamol plus ibuprofen group, 36 mg (99.6% CI, 0-166) for paracetamol alone, 26 mg (99.6% CI, 2-139) for ibuprofen alone, and 28 mg (99.6% CI, 2-145) for half-strength paracetamol plus ibuprofen (Figure 2). In the pairwise comparisons (Table 2), the median difference was 16 mg (99.6% CI, 6.5 to 24; P < .001) between the paracetamol plus ibuprofen group and the paracetamol-alone group. For all other pairwise comparisons, none of the median differences were above the MCID (Table 2). The difference was 8 mg (99.6% CI, −1 to 14; P = .001) between the paracetamol plus placebo group and the half-strength paracetamol plus ibuprofen group, and 6 mg (99.6% CI, −2 to 16; P = .002) between the paracetamol plus ibuprofen group and ibuprofen-alone group. The differences between the paracetamol plus ibuprofen group and the half-strength paracetamol plus ibuprofen group (8 mg [99.6% CI, −2 to 16]; P = .005) and paracetamol-alone group and the ibuprofen-alone group (10 mg [99.6% CI, −2 to 16]; P = .004) were not statistically significant when adjusted for multiple comparisons and 2 co–primary outcomes. There was no significant difference between the ibuprofen-alone group and the half-strength paracetamol plus ibuprofen group (2 mg [99.6% CI, −10 to 7]; P = .81). For the comparison between the paracetamol plus ibuprofen group and the half-strength paracetamol plus ibuprofen group, we found a qualitative and statistically significant interaction between intervention and site (eTables 1 and 2 in Supplement 2).
Figure 2. Distribution of Morphine Consumption by Study Group, 24 Hours Postoperatively.
Paracetamol was given at 1000 mg to patients in the paractamol plus ibuprofen group and in the paracetamol plus placebo group, and patients received 500 mg in the half-strength group. Ibuprofen was given at 400 mg to patients in the paracetamol plus ibuprofen group and in the ibuprofen plus placebo group, and patients received 200 mg in the half-strength group. Blue dotted lines indicate median level of consumption.
Table 2. Between-Group Comparisons of 24-Hour Morphine Consumption.
| Paracetamol Plus Ibuprofena | Paracetamol Plus Placeboa | Ibuprofen Plus Placeboa | Paracetamol Plus Ibuprofen (Half Strength)a | |
|---|---|---|---|---|
| 24-Hour morphine consumption, median (99.6% CI), mg | 20 (0-148) | 36 (0-166) | 26 (2-139) | 28 (2-145) |
| Difference | ||||
| Compared with paracetamol plus ibuprofen, median (99.6% CI), mgb | NA | −16 (−24 to −6.5) | −6 (−16 to 2) | −8 (−16 to 2) |
| P valuec | <.001 | .002 | .005 | |
| Compared with paracetamol plus placebo, median (99.6% CI), mgb | NA | NA | 10 (−2 to 16) | 8 (−1 to 14) |
| P valuec | .004d | .001 | ||
| Compared with ibuprofen plus placebo, median (99.6% CI), mgb | NA | NA | NA | −2 (−7 to 10) |
| P valuec | .81 |
Abbreviation: NA, not applicable.
Acetaminophen is the US adopted name for paracetamol. Paracetamol was given at 1000 mg to patients in the paractamol plus ibuprofen group and in the paracetamol plus placebo group, and patients received 500 mg in the half-strength group. Ibuprofen was given at 400 mg to patients in the paracetamol plus ibuprofen group and in the ibuprofen plus placebo group, and patients received 200 mg in the half-strength group.
CIs were calculated by bootstrapping the median difference (percentile based).
P values were calculated using Van Elteren test. Bootstapped CIs may, in rare events, include zero, even when the P value is below the level of statistical significance due to specific distribution of the data.
This P value=.0044 is above the prespecified threshold (level of significance) of P=.0042.
The overall proportion of patients with 1 or more SAE was 14% (97.5% CI, 11% to 18%). The proportion in patients randomized to the ibuprofen-alone group was 15% (97.5% CI, 12% to 20%), and it was 11% (97.5% CI, 6% to 18%) in the paracetamol-alone group. The corresponding RR was 1.44 (97.5% CI, 0.79 to 2.64; P = .18). A sensitivity analysis excluding patients using NSAIDs in the follow-up period (eTable 3 in Supplement 2) from the paracetamol-alone group showed a lower RR for patients using NSAIDs compared with paracetamol alone (RR, 0.71 [95% CI, 0.34 to 1.50]; P = .37). An overview of the types of SAEs is provided online (eTable 4 in Supplement 2). In a post hoc analysis, there was no interaction between the collated groups (paracetamol plus ibuprofen, ibuprofen alone, and half-strength paracetamol plus ibuprofen) and the risk of SAEs (P = .43).
Secondary and Exploratory Outcomes
At 6 hours, the only statistically significant difference in pain scores (Table 3) was between the parecetamol plus ibuprofen group and the paracetamol-alone group at rest (8 mm [99.2% CI, 0 to 15]; P = .005). At 24 hours, the paracetamol plus ibuprofen group had lower pain scores than the paracetamol-alone group (11 mm [99.2% CI, 3 to 19]; P < .001) and the half-strength paracetamol plus ibuprofen group (8 mm [99.2% CI, 0 to 16]; P = .005) during mobilization. At 24 hours, the paracetamol plus ibuprofen group had lower pain scores at rest than all other groups compared with the paracetamol-alone group (11 mm [99.2% CI, 5 to 17]; P < .001), the ibuprofen-alone group (8 mm [99.2% CI, 2 to 13]; P < .001), and the half-strength paracetamol plus ibuprofen group (6 mm [99.2% CI, 0 to 11]; P = .004). The proportions of patients with 1 or more adverse events were 15% (99.2% CI, 8% to 25%) in the paracetamol plus ibuprofen group, 16% (99.2% CI, 10% to 26%) in the paracetamol-only group, 16% (99.2% CI, 9% to 26%) in the ibuprofen-alone group , and 14% (99.2%, CI 8% to 24%) in the half-strength paracetamol plus ibuprofen group. There were no significant differences in adverse events in any pairwise comparison (Table 3; eTable 5 in Supplement 2).
Table 3. Secondary Outcomes in Comparison of the Combination of Paracetamol (Acetaminophen) and Ibuprofen vs Either Alone on Patient-Controlled Morphine Consumption Following Total Hip Arthroplasty.
| Paracetamol Plus Ibuprofena | Paracetamol Plus Placeboa | Ibuprofen Plus Placeboa | Paracetamol Plus Ibuprofen (Half Strength)a | |
|---|---|---|---|---|
| Pain scores, with mobilization at 6 h, mean (99.2% CI ), mm | 45 (39 to 51) | 52 (47 to 58) | 50 (44 to 55) | 53 (48 to 58) |
| Difference, mean (99.2% CI ), mm | ||||
| Compared with paracetamol plus ibuprofenb | −7 (−15 to 1) | −4 (−12 to 4) | −8 (−15 to 0) | |
| P value | .03 | .17 | .01 | |
| Compared with paracetamol plus placebob | 3 (−5 to 11) | −1 (−8 to 7) | ||
| P value | .34 | .86 | ||
| Compared with ibuprofen plus placebob | −3 (−11 to 4) | |||
| P value | .23 | |||
| Pain scores, at rest at 6 h, mean (99.2% CI), mm | 32 (27 to 37) | 39 (34 to 44) | 37 (32 to 42) | 36 (31 to 41) |
| Difference, mean (99.2% CI ), mm | ||||
| Compared with paracetamol plus ibuprofenb | −8 (−15 to 0) | −5 (−12 to 2) | −5 (−11 to 2) | |
| P value | .005c | .05 | .08 | |
| Compared with paracetamol plus placebob | 2 (−5 to 10) | 3 (−4 to 10) | ||
| P value | .38 | .25 | ||
| Compared with ibuprofen plus placebob | 1 (−6 to 8) | |||
| P value | .81 | |||
| Pain scores with mobilization at 24 h, mean (99.2% CI), mm | 37 (32 to 43) | 49 (43 to 54) | 45 (39 to 51) | 46 (40 to 51) |
| Difference, mean (99.2% CI ), mm | ||||
| Compared with paracetamol plus ibuprofenb | −11 (−19 to −3) | −8 (−15 to 0) | −8 (−16 to −0) | |
| P value | <.001c | .009 | .005c | |
| Compared with paracetamol plus placebob | 4 (−4 to 12) | 3 (−5 to 11) | ||
| P value | .24 | .32 | ||
| Compared with ibuprofen plus placebob | −1 (−8 to 7) | |||
| P value | .86 | |||
| Pain scores at rest at 24 h, mean (99.2% CI), mm | 13 (10 to 17) | 24 (19 to 29) | 21 (16 to 26) | 19 (15 to 23) |
| Difference, mean (99.2% CI ), mm | ||||
| Compared with paracetamol plus ibuprofenb | −11 (−17 to -5) | −8 (−13 to −2) | −6 (−11 to −0) | |
| P value | <.001c | .001c | .004c | |
| Compared with paracetamol plus placebob | 3 (−4 to 10) | 5 (−2 to 11) | ||
| P value | .21 | .04 | ||
| Compared with ibuprofen plus placebob | 2 (−4 to 8) | |||
| P value | .46 | |||
| Adverse events in the first 24 h (99.2% CI), % | 15 (8 to 25) | 16 (10 to 26) | 16 (9 to 26) | 14 (8 to 24) |
| Mean difference (RR) [99.2% CI], % | ||||
| Compared with paracetamol plus ibuprofenb | −1 (0.91) [0.43 to 1.91] | −1 (0.93) [0.44 to 1.98] | 1 (1.02) [0.47 to 2.22] | |
| P value | .73 | .80 | .94 | |
| Compared with paracetamol plus placebob | 0 (1.02) [0.50 to 2.11] | 2 (1.13) [0.53 to 2.37] | ||
| P value | .93 | .67 | ||
| Compared with ibuprofen plus placebob | 2 (1.1) [0.52 to 2.34] | |||
| P value | .74 |
Abbreviations: NA, not applicable; RR, relative risk; VAS, visual analogue scale.
Acetaminophen is the US adopted name for paracetamol. Paracetamol was given at 1000 mg to patients in the paractamol plus ibuprofen group and in the paracetamol plus placebo group, and patients received 500 mg in the half-strength group. Ibuprofen was given at 400 mg to patients in the paracetamol plus ibuprofen group and in the ibuprofen plus placebo group, and patients received 200 mg in the half-strength group.
Indicates VAS scores (range, 0 mm [no pain] to 100 mm [worst imaginable pain]) during 30° flexion of the hip and at rest at 6 and 24 hours, and adverse events from 0 to 24 hours (level of significance=.0083).
Indicates a statistically significant P value. For the secondary outcomes a generalized estimating equation (GEE) was used, which gives both CIs and P values. In this case, the interpretation of CIs is as usual.
Key exploratory findings were reduced risk of nausea at 24 hours for group paracetamol plus ibuprofen group compared with all other groups (paracetamol-alone group [RR, 0.53 {95% CI, 0.31 to 0.90}; P = .02]; ibuprofen-alone group [RR, 0.56 {95% CI, 0.32 to 0.96}; P = .04]; and the half-strength paracetamol plus ibuprofen group [RR, 0.45 {95% CI: 0.27 to 0.76}; P = .003]), and reduced risk of dizziness at 6 hours for the paracetamol plus ibuprofen group compared with the paracetamol-alone group (RR, 0.56 [95% CI, 0.34 to 0.93]; P = .02) and compared with the half-strength paracetamol plus ibuprofen group (RR, 0.58 [95% CI, 0.35 to 0.98]; P = .04). Key findings from the secondary analyses were that the differences in morphine consumption between the paracetamol plus ibuprofen group and the ibuprofen-alone group were not statistically significant in adjusted analyses and analyses in the per-protocol population, contrary to the main unadjusted analysis in the primary analysis population. All exploratory outcomes and results from secondary analyses are found online (eTables 6 to 13 in Supplement 2).
Discussion
This trial demonstrated that a combination of paracetamol 1000 mg and ibuprofen 400 mg resulted in a clinically relevant reduction in morphine consumption compared with paracetamol 1000 mg alone on the first postoperative day after THA. For all other comparisons, the differences in morphine consumption were less than the predefined minimal clinically relevant difference of 10 mg (the difference in morphine consumption between the paracetamol [1000 mg] plus ibuprofen [400 mg] group vs the ibuprofen [400 mg]–alone group was 6 mg). Further, the trial showed a substantial proportion of patients with 1 or more SAEs within 90 days after surgery; however, there was no statically significant difference in patients randomized to receuve ibuprofen compared with patients randomized to receive paracetamol only.
These findings (from a multicenter trial with few exclusions due to logistic reasons) support the principle of multimodal analgesia with paracetamol plus ibuprofen compared with paracetamol alone for the first postoperative day. However, compared with ibuprofen alone, the morphine-sparing effect was below the prespecified threshold for clinically important postoperative morphine reduction. In light of the current opioid crisis,26 using the lowest possible amount of opioid is important.27 The combination of paracetamol 1000 mg and ibuprofen 400 mg did not only result in the lowest morphine consumption, but it also resulted in lower pain scores at rest at 24 hours and lower risk of nausea at 24 hours postoperatively across all comparisons.
A recent network meta-analysis investigating all nonopioid analgesics for major surgery28 indicated similar results regarding morphine-sparing effects as this trial, but the network meta-analysis only included very few trials with direct comparisons of the combination of paracetamol and an NSAID vs paracetamol alone, and an NSAID alone. The authors of the network meta-analysis found no association with opioid-associated adverse effects or SAEs, and both trials with low, high, and unclear risk of bias were included in their analyses.28 In the most recent Cochrane review on established pain in dental surgery29 the analgesic efficacy (numbers needed to treat to achieve minimum 50% pain reduction) of combining paracetamol 1000 mg and ibuprofen 400 mg seemed similar to the combination of paracetamol 500 mg and ibuprofen 200 mg. The results from this trial could not replicate this finding.
Although the proportion of patients with 1 or more SAEs was not statistically significantly higher in patients randomized to the ibuprofen-alone group compared with patients randomized to the paracetamol-alone group, there were numerically more SAEs, especially when considering “medical SAEs,” in patients randomized to ibuprofen. To our knowledge, this is the first trial to include systematic 90-day follow-up for safety through national registries and telephone interviews as a primary outcome in this research area, in which reporting of harm in perioperative pain trials is often inadequate.7,30 Equal focus on benefits and harms is recommended to be common practice of clinical trials,31,32 and further trials focusing on safety of NSAIDs in the perioperative period are urgently needed.
Limitations
This trial has several limitations. First, the intervention period was only 24 hours, and a prolonged intervention period could have been more appropriate as treatment with paracetamol plus ibuprofen seldom is used for only 24 hours in a clinical context.
Second, there were fewer patients having spinal anesthesia in the half-strength paracetamol plus ibuprofen group, which might have influenced morphine consumption and pain levels.
Third, no specific analgesics were recommended to patients in the follow-up period, and only a few patients did not use NSAIDs at all (eTable 3 in Supplement 2). This makes attribution of the SAEs to ibuprofen difficult; however, there were no significant differences in NSAID use in the follow-up period between groups, and the sensitivity analysis excluding patients using NSAIDs in the follow-up period from the paracetamol-only group did not alter the conclusion from the main analysis.
Fourth, the power estimation of the co–primary outcome of SAE (80% power to detect or discard an increase from 10% to 21%) was based on limited data on postoperative incidence of SAEs as previous studies have not used the ICH-GCP definition of SAE and rigorous follow-up by registry data and telephone interview. Hence, the anticipated intervention effect (an increase from 10% to 21%) might be too large, and consequently, this trial may not have adequate power to assess differences in SAEs.
Fifth, the power of the null test for interaction between the collated groups receiving ibuprofen and SAEs was not assessed a priori, thus, it is not certain that it is reasonable to combine these groups.
Conclusions
Among patients undergoing total hip arthroplasty, paracetamol plus ibuprofen significantly reduced morphine consumption compared with paracetamol alone in the first 24 hours after surgery; there was no statistically significant increase in serious adverse events in the pooled groups receiving ibuprofen alone vs with paracetamol alone. However, the combination did not result in a clinically important improvement over ibuprofen alone, suggesting that ibuprofen alone may be a reasonable option for early postoperative oral analgesia.
Trial Protocol
eAppendix 1. Complete List of Inclusion and Exclusion Criteria
eAppendix 2. Abstracts Written Before Breaking the Randomization Code
eAppendix 3. Breaking the Blind
eAppendix 4. Preparing of the Trial Database
eTable 1. Interaction Between Site and Intervention for Pairwise Comparisons of Primary and Secondary Outcomes
eTable 2. Interaction Between Site and Intervention for the Primary Outcomes of Patients With 1 or More Modified SAEs
eTable 3. Patients Taking NSAIDs in the Follow-up Period
eTable 4. Types of SAEs
eTable 5 Types of Adverse Events
eTable 6. Exploratory Outcomes
eTable 7 Strictly Per-Protocol Population
eTable 8. Withdrawn Because of Opioid-Associated Adverse Effects or Insufficient Pain Treatment
eTable 9. Analyses Adjusted for Age, Sex, Prior Use of Paracetamol, and Prior Use of NSAIDs for the Co–Primary Outcome of Morphine Consumption With 95% CIs
eTable 10. Analyses on the Strictly Per-Protocol Population for the Co–Primary Outcome of Morphine Consumption
eTable 11. Adjusted Analyses of Secondary Outcomes
eTable 12. Analyses on the Strictly Per-Protocol Population of Secondary Outcomes
eTable 13. Analyses on the Strictly Per-Protocol Population of Exploratory Outcomes
Data Sharing Statement
References
- 1.Wick EC, Grant MC, Wu CL. Postoperative multimodal analgesia pain management with nonopioid analgesics and techniques: a review. JAMA Surg. 2017;152(7):691-697. doi: 10.1001/jamasurg.2017.0898 [DOI] [PubMed] [Google Scholar]
- 2.Wu CL, Raja SN. Treatment of acute postoperative pain. Lancet. 2011;377(9784):2215-2225. doi: 10.1016/S0140-6736(11)60245-6 [DOI] [PubMed] [Google Scholar]
- 3.Maund E, McDaid C, Rice S, Wright K, Jenkins B, Woolacott N. Paracetamol and selective and non-selective non-steroidal anti-inflammatory drugs for the reduction in morphine-related side-effects after major surgery: a systematic review. Br J Anaesth. 2011;106(3):292-297. doi: 10.1093/bja/aeq406 [DOI] [PubMed] [Google Scholar]
- 4.De Oliveira GS Jr, Castro-Alves LJ, McCarthy RJ. Single-dose systemic acetaminophen to prevent postoperative pain: a meta-analysis of randomized controlled trials. Clin J Pain. 2015;31(1):86-93. doi: 10.1097/AJP.0000000000000081 [DOI] [PubMed] [Google Scholar]
- 5.McNicol ED, Ferguson MC, Haroutounian S, Carr DB, Schumann R. Single dose intravenous paracetamol or intravenous propacetamol for postoperative pain. Cochrane Database Syst Rev. 2016;(5):CD007126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dahl JB, Nielsen RV, Wetterslev J, et al. ; Scandinavian Postoperative Pain Alliance (ScaPAlli) . Post-operative analgesic effects of paracetamol, NSAIDs, glucocorticoids, gabapentinoids and their combinations: a topical review. Acta Anaesthesiol Scand. 2014;58(10):1165-1181. doi: 10.1111/aas.12382 [DOI] [PubMed] [Google Scholar]
- 7.Mathiesen O, Wetterslev J, Kontinen VK, et al. ; Scandinavian Postoperative Pain Alliance (ScaPAlli) . Adverse effects of perioperative paracetamol, NSAIDs, glucocorticoids, gabapentinoids and their combinations: a topical review. Acta Anaesthesiol Scand. 2014;58(10):1182-1198. doi: 10.1111/aas.12380 [DOI] [PubMed] [Google Scholar]
- 8.Rawal N. Current issues in postoperative pain management. Eur J Anaesthesiol. 2016;33(3):160-171. doi: 10.1097/EJA.0000000000000366 [DOI] [PubMed] [Google Scholar]
- 9.Hansen MS, Petersen EE, Dahl JB, Wetterslev J. Post-operative serious adverse events in a mixed surgical population—a retrospective register study. Acta Anaesthesiol Scand. 2016;60(9):1209-1221. doi: 10.1111/aas.12762 [DOI] [PubMed] [Google Scholar]
- 10.Vascular Events In Noncardiac Surgery Patients Cohort Evaluation (VISION) Study Investigators; Devereaux PJ, Chan MT, Alonso-Coello P, et al. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA. 2012;307(21):2295-2304. doi: 10.1001/jama.2012.5502 [DOI] [PubMed] [Google Scholar]
- 11.Coxib and traditional NSAID Trialists’ (CNT) Collaboration; Bhala N, Emberson J, Merhi A, et al. Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trials. Lancet. 2013;382(9894):769-779. doi: 10.1016/S0140-6736(13)60900-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bally M, Dendukuri N, Rich B, et al. Risk of acute myocardial infarction with NSAIDs in real world use: bayesian meta-analysis of individual patient data. BMJ. 2017;357:j1909. doi: 10.1136/bmj.j1909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thybo KH, Hägi-Pedersen D, Wetterslev J, et al. PANSAID—paracetamol and NSAID in combination: study protocol for a randomised trial. Trials. 2017;18(1):11. doi: 10.1186/s13063-016-1749-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.International Conference on Harmonisation Expert Working Group International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use: ICH harmonised tripartite guideline. Guideline for Good Clinical Practice E6 (R1). June 10. 1996. https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R1_Guideline.pdf. Accessed January 19, 2019.
- 15.Thybo KH, Jakobsen JC, Hägi-Pedersen D, et al. PANSAID-paracetamol and NSAID in combination: detailed statistical analysis plan for a randomised, blinded, parallel, four-group multicentre clinical trial. Trials. 2017;18(1):465. doi: 10.1186/s13063-017-2203-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan AW, Tetzlaff JM, Altman DG, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med. 2013;158(3):200-207. doi: 10.7326/0003-4819-158-3-201302050-00583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schulz KF, Altman DG, Moher D; CONSORT Group . CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c332. doi: 10.1136/bmj.c332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.International Conference on Harmonisation Expert Working Group International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use: ICH harmonised tripartite guideline. Clinical safety data management: definitions and standards for expedited reporting E2A. October 27, 1994. https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E2A/Step4/E2A_Guideline.pdf. Accessed May 17, 2018.
- 19.Cao J, Zhang S. Multiple comparison procedures. JAMA. 2014;312(5):543-544. doi: 10.1001/jama.2014.9440 [DOI] [PubMed] [Google Scholar]
- 20.McGlothlin AE, Lewis RJ. Minimal clinically important difference: defining what really matters to patients. JAMA. 2014;312(13):1342-1343. doi: 10.1001/jama.2014.13128 [DOI] [PubMed] [Google Scholar]
- 21.Qu Y, Zhao YD, Rahardja D. Wilcoxon-Mann-Whitney test: stratify or not? J Biopharm Stat. 2008;18(6):1103-1111. doi: 10.1080/10543400802369103 [DOI] [PubMed] [Google Scholar]
- 22.Kahan BC, Morris TP. Improper analysis of trials randomised using stratified blocks or minimisation. Stat Med. 2012;31(4):328-340. doi: 10.1002/sim.4431 [DOI] [PubMed] [Google Scholar]
- 23.Kahan BC, Harhay MO. Many multicenter trials had few events per center, requiring analysis via random-effects models or GEEs. J Clin Epidemiol. 2015;68(12):1504-1511. doi: 10.1016/j.jclinepi.2015.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.STATA Longitudinal-Data/Panal-Data Reference Manual. http://www.stata.com/bookstore/longitudinal-panel-data-reference-manual/. Accessed January 19, 2019.
- 25.Jakobsen JC, Gluud C, Wetterslev J, Winkel P. When and how should multiple imputation be used for handling missing data in randomised clinical trials—a practical guide with flowcharts. BMC Med Res Methodol. 2017;17(1):162. doi: 10.1186/s12874-017-0442-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gomes T, Tadrous M, Mamdani MM, Paterson JM, Juurlink DN. The burden of opioid-related mortality in the United States. JAMA Netw Open. 2018;1(2):e180217. doi: 10.1001/jamanetworkopen.2018.0217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson SP, Chung KC, Zhong L, et al. Risk of prolonged opioid use among opioid-naïve patients following common hand surgery procedures. J Hand Surg Am. 2016;41(10):947-957.e3. doi: 10.1016/j.jhsa.2016.07.113 [DOI] [PubMed] [Google Scholar]
- 28.Martinez V, Beloeil H, Marret E, Fletcher D, Ravaud P, Trinquart L. Non-opioid analgesics in adults after major surgery: systematic review with network meta-analysis of randomized trials. Br J Anaesth. 2017;118(1):22-31. doi: 10.1093/bja/aew391 [DOI] [PubMed] [Google Scholar]
- 29.Derry CJ, Derry S, Moore RA. Single dose oral ibuprofen plus paracetamol (acetaminophen) for acute postoperative pain. Cochrane Database Syst Rev. 2013;6(6):CD010210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fabritius ML, Mathiesen O, Wetterslev J, Dahl JB. Post-operative analgesia: focus has been on benefit—are we forgetting the harm? Acta Anaesthesiol Scand. 2016;60(7):839-841. doi: 10.1111/aas.12729 [DOI] [PubMed] [Google Scholar]
- 31.Cuervo LG, Clarke M. Balancing benefits and harms in health care. BMJ. 2003;327(7406):65-66. doi: 10.1136/bmj.327.7406.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Connor D, Green S, Higgins JPT. Chapter 5. Defining the review question and developing criteria for including studies. In: Higgins JPT, Green S, eds. Cochrane Handbook of Systematic Reviews of Intervention. Version 5.1.0 [updated March 2011] London, England: Cochrane Collaboration;2011. [Google Scholar]
- 33.Ong CK, Seymour RA, Lirk P, Merry AF. Combining paracetamol (acetaminophen) with nonsteroidal antiinflammatory drugs: a qualitative systematic review of analgesic efficacy for acute postoperative pain. Anesth Analg. 2010;110(4):1170-1179. doi: 10.1213/ANE.0b013e3181cf9281 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eAppendix 1. Complete List of Inclusion and Exclusion Criteria
eAppendix 2. Abstracts Written Before Breaking the Randomization Code
eAppendix 3. Breaking the Blind
eAppendix 4. Preparing of the Trial Database
eTable 1. Interaction Between Site and Intervention for Pairwise Comparisons of Primary and Secondary Outcomes
eTable 2. Interaction Between Site and Intervention for the Primary Outcomes of Patients With 1 or More Modified SAEs
eTable 3. Patients Taking NSAIDs in the Follow-up Period
eTable 4. Types of SAEs
eTable 5 Types of Adverse Events
eTable 6. Exploratory Outcomes
eTable 7 Strictly Per-Protocol Population
eTable 8. Withdrawn Because of Opioid-Associated Adverse Effects or Insufficient Pain Treatment
eTable 9. Analyses Adjusted for Age, Sex, Prior Use of Paracetamol, and Prior Use of NSAIDs for the Co–Primary Outcome of Morphine Consumption With 95% CIs
eTable 10. Analyses on the Strictly Per-Protocol Population for the Co–Primary Outcome of Morphine Consumption
eTable 11. Adjusted Analyses of Secondary Outcomes
eTable 12. Analyses on the Strictly Per-Protocol Population of Secondary Outcomes
eTable 13. Analyses on the Strictly Per-Protocol Population of Exploratory Outcomes
Data Sharing Statement