This randomized clinical trial assesses whether intranasal ketamine is noninferior to intranasal fentanyl for reducing pain in children with traumatized limb injuries treated in emergency departments.
Key Points
Question
Is intranasal ketamine (1.5 mg/kg) noninferior to intranasal fentanyl (2 µg/kg) for reducing moderate to severe pain in children aged 8 to 17 years with traumatic extremity injuries?
Findings
In this noninferiority randomized clinical trial of 90 children presenting to emergency departments with pain due to traumatic limb injury, intranasal ketamine was noninferior to intranasal fentanyl for pain relief, with mean reductions in visual analog scale pain scores of 30.6 mm and 31.9 mm, respectively, at 30 minutes after intervention.
Meaning
Ketamine provides effective analgesia that is not inferior to fentanyl, although participants who received ketamine had an increase in adverse events that were minor and transient; intranasal ketamine may be an appropriate alternative to opioids for pain associated with acute extremity injuries.
Abstract
Importance
Timely analgesia is critical for children with injuries presenting to the emergency department, yet pain control efforts are often inadequate. Intranasal administration of pain medications provides rapid analgesia with minimal discomfort. Opioids are historically used for significant pain from traumatic injuries but have concerning adverse effects. Intranasal ketamine may provide an effective alternative.
Objective
To determine whether intranasal ketamine is noninferior to intranasal fentanyl for pain reduction in children presenting with acute extremity injuries.
Design, Setting, and Participants
The Pain Reduction With Intranasal Medications for Extremity Injuries (PRIME) trial was a double-blind, randomized, active-control, noninferiority trial in a pediatric, tertiary, level 1 trauma center. Participants were children aged 8 to 17 years presenting to the emergency department with moderate to severe pain due to traumatic limb injuries between March 2016 and February 2017. Analyses were intention to treat and began in May 2017.
Interventions
Intranasal ketamine (1.5 mg/kg) or intranasal fentanyl (2 µg/kg).
Main Outcomes and Measures
The primary outcome was reduction in visual analog scale pain score 30 minutes after intervention. The noninferiority margin for this outcome was 10.
Results
Of 90 children enrolled, 45 (50%) were allocated to ketamine (mean [SD] age, 11.8 [2.6] years; 26 boys [59%]) and 45 (50%) to fentanyl (mean [SD] age, 12.2 [2.3] years; 31 boys [74%]). Thirty minutes after medication, the mean visual analog scale reduction was 30.6 mm (95% CI, 25.4-35.8) for ketamine and 31.9 mm (95% CI, 26.6-37.2) for fentanyl. Ketamine was noninferior to fentanyl for pain reduction based on a 1-sided test of group difference less than the noninferiority margin, as the CIs crossed 0 but did not cross the prespecified noninferiority margin (difference in mean pain reduction between groups, 1.3; 90% CI, −6.2 to 8.7). The risk of adverse events was higher in the ketamine group (relative risk, 2.5; 95% CI, 1.5-4.0), but all events were minor and transient. Rescue analgesia was similar between groups (relative risk, 0.89; 95% CI, 0.5-1.6).
Conclusions and Relevance
Ketamine provides effective analgesia that is noninferior to fentanyl, although participants who received ketamine had an increase in adverse events that were minor and transient. Intranasal ketamine may be an appropriate alternative to intranasal fentanyl for pain associated with acute extremity injuries. Ketamine should be considered for pediatric pain management in the emergency setting, especially when opioids are associated with increased risk.
Trial Registration
ClinicalTrials.gov Identifier: NCT02778880
Introduction
Inadequate pain control in the emergency department (ED) is a major public health concern, as emphasized by a recent Institute of Medicine report.1 Despite increased awareness, pain continues to be underdiagnosed and undertreated, particularly in children.2 When children do receive pain medication, they often encounter long delays in administration,3 in part owing to the time required to obtain intravenous access. The intranasal administration route is a more efficient alternative for delivering analgesia4 and is gaining popularity owing to its rapid onset of action, minimal discomfort, and relative simplicity.
Opioids are the most commonly used class of analgesics for children presenting with severe pain due to traumatic injuries5 but have several concerning adverse effects (AEs), including increased risk of serious AEs requiring interventions when given before procedural sedation.6 Patients also may have a genetic predisposition to diminished opioid sensitivity7 or an opioid allergy. Therefore, ideal dosing to adequately control severe pain yet avoid medication-related AEs is difficult to ascertain,8 which may lead clinicians to seek nonopioid alternatives.
As a dissociative anesthetic, ketamine is used to facilitate painful procedures in the pediatric ED.9 At subdissociative doses, ketamine is emerging as an alternative medication for treating pain10,11,12 in adults13,14,15,16,17,18,19,20 and children.21,22,23,24 Two studies of intranasal ketamine for treatment of acute injury-related pain in the pediatric ED setting exist. The PICHFORK trial22 compared intranasal ketamine (1 mg/kg) and intranasal fentanyl (1.5 µg/kg) and reported similar and clinically meaningful pain reduction at 30 minutes. Reynolds et al24 compared the same medications, dosing, and routes and reported similar pain reduction at 20 minutes with frequent AEs. Limitations of these studies include conservative dosing, routine coadministration of either ibuprofen or acetaminophen, and merging of pain scales limiting the precision of the pain reduction estimate.
The objective of the Pain Reduction With Intranasal Medications for Extremity Injuries (PRIME) trial was to demonstrate that intranasal subdissociative ketamine is noninferior to intranasal fentanyl for treatment of acute pain associated with traumatic limb injuries in children presenting to the ED without routinely administered ibuprofen/acetaminophen.
Methods
Study Design and Participants
This was a prospective, double-blind, randomized, noninferiority clinical trial of intranasal ketamine compared with intranasal fentanyl in a tertiary care children’s hospital ED from March 2016 to February 2017. The full trial protocol is available in Supplement 1. Analyses began May 2017. The study sample was identified through an established triage process identifying children with an acute painful extremity injury. Inclusion criteria were (1) age 8 to 17 years, (2) presence of acute extremity injury, (3) visual analog scale (VAS) score higher than 35 mm (moderate to severe pain),25 and (4) legal guardian presence. Exclusion criteria included (1) significant head, chest, abdomen or spine injury, (2) Glasgow Coma Scale less than 15 or inability to report a VAS score, (3) nasal trauma or aberrant nasal anatomy, (4) active epistaxis, (5) ketamine or fentanyl allergy, (6) history of psychosis, (7) opioid administration prior to arrival, (8) non-English speaking, (9) in police custody, and (10) postmenarchal girls without a negative pregnancy test. This study was approved by the Cincinnati Children’s Hospital Medical Center institutional review board and regulated by the US Food and Drug Administration (NCT02778880). Legal guardians provided written informed consent prior to enrollment. Patients provided assent if they were 12 years or older and had a VAS score less than 60.25
Randomization and Allocation Concealment
Randomization was achieved using computer-generated, randomly varied blocks of 6 and 8 using a 1:1 ratio within blocks. Allocation was concealed using sequentially numbered, sealed envelopes, and blinding after randomization was ensured by using syringes with identical volume, color, and odor.
Study Procedures, Interventions, Dose Preparation, and Dispensing
Patients were randomized to receive either ketamine (1.5 mg/kg) or fentanyl (2 µg/kg). Undiluted study medication, 50 mg/mL of ketamine hydrochloride (Mylan Institutional LLC) or 50 µg/mL fentanyl citrate (West-Ward Pharmaceuticals), was drawn into a 3-mL syringe prepared by the Investigational Drug Services pharmacy. Emergency department and study staff, the patient, and family members were all blinded to the intervention.
Syringes were individually stored with a deidentified, weight-based dose administration reference (eFigure 1 in Supplement 2). Drug volumes were prespecified according to weight ranges for each study medication and rounded to the nearest 0.1 mL. With the maximum deliverable intranasal volume of 2 mL,26 the maximum study drug doses were 100 mg of ketamine and 100 µg of fentanyl. Each syringe contained an additional 0.1 mL of medication representing the atomizer priming volume. Doses were administered in alternating 0.5 mL aliquots between nares via an intranasal mucosal atomization device (Wolfe-Tory Medical, Inc).
Data were gathered from study forms and electronic medical records. Video monitoring of patients provided continuous vital sign data during the first 15 minutes after study drug administration. All outcomes were collected at baseline (before study drug administration) and at 15, 30, and 60 minutes after study intervention. Video review allowed vital signs to be collected every minute for the first 15 minutes. To assess blinding, study staff were asked to guess at the 30-minute assessment which medication had been received. Enrolled patients were monitored for AEs (graded by the Common Terminology Criteria for Adverse Events, version 4.027) and abnormal vital signs for 120 minutes after study medication administration. Demographic and physical examination information was collected. Patients were contacted 30 days later to determine if additional AEs or unanticipated health care visits had occurred. All AEs related to study medication were followed to resolution.
Outcome Measures
The primary outcome was the difference in pain reduction between groups 30 minutes after treatment, as measured by the VAS, a sensitive and validated tool for children 8 years or older in which the child places a perpendicular mark along a 100-mm horizontal line anchored with “no pain” at 1 end and “worst possible pain” at the other.28,29 The distance from the “no pain” anchor to the mark is measured in millimeters, providing a score of 0 to 100 (eFigure 2 in Supplement 2). Secondary outcomes included sedation level as measured by the University of Michigan Sedation Scale (eTable 1 in Supplement 2),30 capnometry values, AEs, need for rescue analgesia, and change in vital signs using the Pediatric Advanced Life Support guideline to define normal vital signs.31
Statistical Analysis
The primary outcome was the difference in mean pain score reduction from baseline to 30 minutes after the study intervention between the ketamine and fentanyl groups. Sample size was determined using a noninferiority test for the difference between 2 means. Given that the literature suggests a minimum clinically significant difference in VAS pain score in children of 10 mm to 13 mm,32,33,34 we chose a noninferiority margin of 10. Using data from the PICHFORK trial,22 we assumed a true difference between means of 5. Using the interquartile range data from the PICHFORK trial22 and assuming normality for the pain scores, we estimated the SDs to be 29.63 and 22.22 for the rating reduction at 30 minutes for the fentanyl and ketamine groups, respectively. Therefore, with α = .05 and β = .20 (80% power), the sample size required to detect this difference was estimated to be 39 patients in each group, for a total of 78 patients. We enrolled 90 patients, anticipating that not all patients would be fully evaluable.
Baseline and demographic characteristics were summarized using means and SDs for continuous variables that were normally distributed, medians for nonnormally distributed variables, and proportions for categorical variables. The 2 study groups were compared using the t test for normally distributed data, Mann-Whitney U test for nonparametric data, and χ2 or Fisher exact test to evaluate proportions in dichotomous outcomes. For the primary outcome, a 1-sided 2-sample t test was performed while taking into consideration the noninferiority margin to test whether pain reduction with ketamine was noninferior to fentanyl. Risk differences with 95% CIs were used to compare dichotomous outcomes such as rescue analgesia and AEs. P values less than .05 were considered statistically significant regarding the primary outcome. All statistical analyses were conducted using SAS 9.4 (SAS Institute Inc).
Results
Characteristics of Study Participants
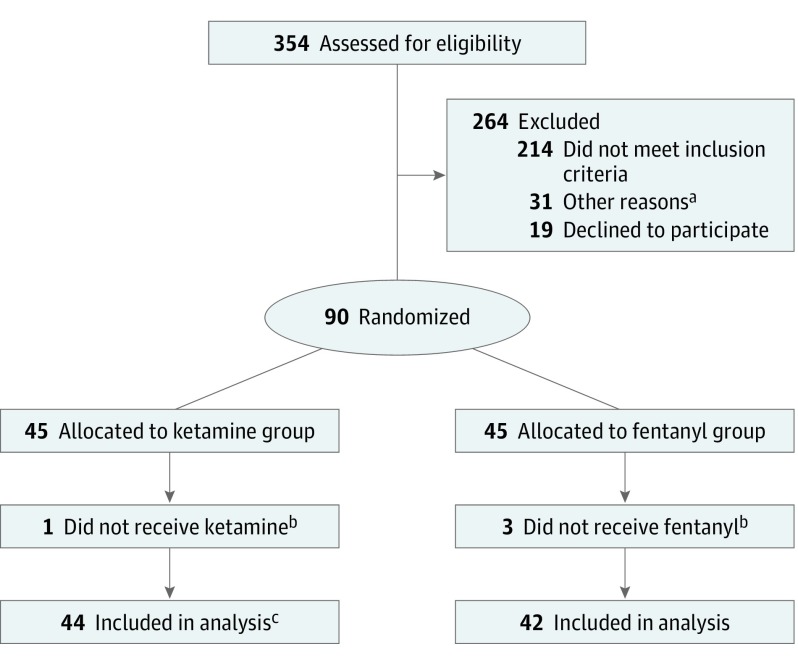
During the study period, 354 children were assessed for eligibility, of which 264 were excluded (Figure 1). Ninety patients were enrolled and randomized. Four patients were withdrawn before medication administration. Eighty-six patients were included in this analysis. Fifteen patients were unable to be contacted at the 30-day telephone call (7 patients in the ketamine group and 8 patients in the fentanyl group). Demographic, baseline, and injury characteristics of randomized patients are depicted in Table 1. Baseline pain scores between groups were similar.
Figure 1. CONSORT Flow Diagram.
aOther reasons: research coordinator was not present, patient was not in proper location, study enrollment was on hold for regulatory purposes, and clinician preference.
bReasons for withdrawal: inability to provide urine for pregnancy test, parental preference changed, clinician preference, and unavailability of medication.
cFor 1 patient, only secondary outcome data were reported owing to missing baseline visual analog scale score. Therefore, 43 had primary outcome measures available.
Table 1. Baseline Characteristics.
| Variable | No. (%) | |
|---|---|---|
| Ketamine (n = 44) | Fentanyl (n = 42) | |
| Age, mean (SD), y | 11.8 (2.6) | 12.2 (2.3) |
| Male | 26 (59) | 31 (74) |
| Weight, mean (SD), kg | 45.8 (14.4) | 50.8 (22.8) |
| Non-Hispanic | 44 (100) | 40 (95) |
| Race | ||
| White | 30 (68) | 29 (69) |
| Black | 11 (25) | 10 (24) |
| Other | 3 (7) | 3 (7) |
| Mechanism of injury | ||
| Sports related/recreational | 30 (68) | 26 (62) |
| Motor vehicle crash | 1 (2) | 2 (5) |
| Fall | 10 (23) | 13 (31) |
| Othera | 3 (7) | 1 (2) |
| Time of injury prior to arrival, median (IQR), min | 43.0 (27.0-63.0) | 51.5 (30.0-85.0) |
| Extremityb | ||
| Upper | 33 (75) | 28 (67) |
| Lower | 13 (30) | 15 (36) |
| Diagnosisc | ||
| Fracture | 39 (85) | 34 (81) |
| Dislocation | 4 (9) | 2 (5) |
| Sprain/strain | 1 (2) | 5 (12) |
| Otherd | 2 (4) | 1 (2) |
| Reduction required | 19 (43) | 23 (55) |
| Sedation required | 19 (43) | 20 (48) |
| Analgesic prior to arrival | ||
| Ibuprofen | 4 (9) | 4 (10) |
| Acetaminophen | 1 (2) | 2 (5) |
| Naproxen | 0 | 1 (2) |
| Initial VAS score, mean (SD), mm | 74.7 (15.3) | 72.0 (18.6) |
| Baseline, mean (SD) | ||
| Heart rate, bpm | 90.8 (15.2) | 88.6 (14.8) |
| Respiratory rate, breaths per min | 22.1 (6.6) | 23.0 (7.4) |
| SBP, mm Hg | 126.5 (19.1) | 128.9 (19.6) |
| DBP, mm Hg | 78.9 (13.6) | 73.5 (12.9) |
| Oxygen saturation, % | 99.8 (0.6) | 99.6 (0.6) |
| EtCO2, mm Hg | 36.6 (6.4) | 38.0 (4.4) |
| Time to study medication from arrival, mean (SD), min | 26.6 (9.9) | 26.1 (10.3) |
| Volume of study medication administered, median (IQR), mL | 1.3 (1.1-1.6) | 1.8 (1.3-2.0) |
| Dose of study medication administered, median (IQR) | 1.5 (1.5-1.5)e | 1.9 (1.7-1.9)f |
Abbreviations: bpm, beats per minute; DBP, diastolic blood pressure; EtCO2, end tidal carbon dioxide; IQR, interquartile range; SBP, systolic blood pressure; VAS, visual analog scale.
Bicycle crash (1), pedestrian vs car (2), and punched wall (1).
Percentages do not add up to 100 because 3 patients had more than 1 extremity involved.
Two patients had both a fracture and a dislocation.
Contusion (2) and hematoma (1).
Measured in milligram per killigram.
Measured in microgram per kilogram.
Main Results
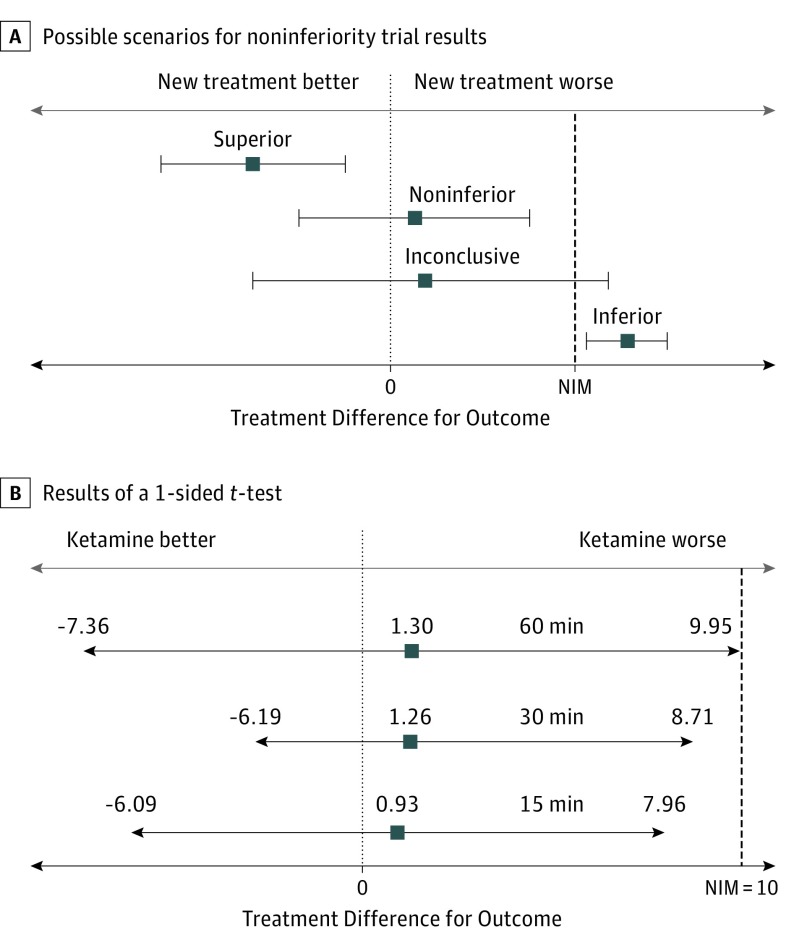
Table 2 and Figure 235 summarize results from noninferiority testing for pain score differences. One patient in the ketamine group did not have a baseline pain score documented and 4 patients withdrew, resulting in 43 patients randomized to the ketamine group and 42 patients randomized to the fentanyl group included in the primary outcome analysis. Both groups experienced significant pain reduction at 15, 30, and 60 minutes with similar pain reduction between groups at each time. Pain reduction was sustained over the first 60 minutes for both groups. Ketamine was noninferior to fentanyl with regard to the primary outcome of pain reduction 30 minutes after study medication administration, as the CIs crossed 0 but did not cross the prespecified noninferiority margin (Figure 2).
Table 2. Pain Reduction at 15, 30, and 60 Minutes.
| Time After Study Medication | Mean Difference (95% CI)a |
|---|---|
| 15 min | |
| Ketamine (n = 42)b | −24.4 (−29.3 to −19.4) |
| Fentanyl (n = 42) | −25.3 (−30.3 to −20.3) |
| 30 min | |
| Ketamine (n = 43)b | −30.6 (−35.8 to −25.4) |
| Fentanyl (n = 42) | −31.9 (−37.2 to −26.6) |
| 60 min | |
| Ketamine (n = 43)b | −27.7 (−33.8 to −21.6) |
| Fentanyl (n = 42) | −29.0 (−35.1 to −22.8) |
Mean difference in visual analog scale scores compared with baseline.
Patients with missing values were not included in the analysis.
Figure 2. Difference Between Fentanyl and Ketamine Groups in Mean Pain Reduction and Possible Treatment Differences for Noninferiority Trials.
Results of a 1-sided t test demonstrating pain reduction with ketamine is noninferior to pain reduction with fentanyl. Lower tail P values were calculated to test whether ketamine is noninferior to fentanyl with a noninferiority margin (NIM) of 10. Noninferiority is indicated by CIs that cross 0 but do not cross the NIM and by P < .05. Ketamine is not superior to fentanyl at any time because all CIs cross 0. Reprtined with permission from JAMA.35
There was no significant difference in highest achieved sedation scores between the groups (eTable 2 in Supplement 2). Neither group experienced a University of Michigan Sedation Scale score higher than 2. No differences were seen with respect to vital signs between groups or in the change in vital signs at each assessment time (eTable 3 in Supplement 2). No vital sign abnormalities required intervention. No patient experienced hypotension31 or an oxygen saturation lower than 95%. Mean capnometry values were similar between groups at each assessment point and without statistically significant difference between assessments. Based on video review, 9 patients (20.9%) in the ketamine group and 2 patients (4.8%) in the fentanyl group experienced a decrease in capnometry value 10 mm Hg or more during the 15 minutes immediately following study medication administration. All of these lasted less than 30 seconds and were self-limited.
Forty-seven of 86 patients (54.7%) experienced 63 AEs (Table 3). Fifty-one AEs were patient reported, and 12 were discovered on video review. All AEs were minor (Common Terminology Criteria for Adverse Events, version 4.0, grade 1 or 2) and transient. The risk of AE was greater in the ketamine group (relative risk, 2.5; 95% CI, 1.5-4.0) with 34 of 44 patients (77%) experiencing at least 1 AE, while 13 of 42 patients (31%) in the fentanyl group experienced at least 1 AE. There was no significant difference in the number of AEs between groups at each assessment point, except for the 15-minute assessment (Table 3).
Table 3. Adverse Events by Treatment Group.
| Symptoms | Adverse Events, No. | |||
|---|---|---|---|---|
| Total (N = 63)a | At 15 min (n = 35) | |||
| Ketamine | Fentanyl | Ketamine | Fentanyl | |
| Dizziness | 9 | 0 | 7 | 0 |
| Dysphoria/dissociation | 1 | 0 | 1 | 0 |
| Unpleasant taste | 9 | 2 | 1 | 1 |
| Drowsiness | 21 | 10 | 17 | 4 |
| Nausea/vomiting | 3 | 0 | 0 | 0 |
| Itchiness | 1 | 0 | 0 | 0 |
| Vision changes | 2 | 0 | 1 | 0 |
| Headache | 0 | 1 | 0 | 0 |
| Rash | 0 | 1 | 0 | 1 |
| Light-headedness | 2 | 0 | 2 | 0 |
| Nystagmus | 1b | 0 | 0 | 0 |
| Total | 49 | 14 | 29 | 6 |
For the ketamine group, 34 patients reported 49 adverse events. For the fentanyl group, 13 patients reported 14 adverse events.
Discovered during 30-day telephone call but occurred during initial emergency department visit. There were no problems pertaining to behavior or sleep reported and no other adverse events reported at the 30-day follow-up calls.
The need for rescue analgesia at 15, 30, and 60 minutes did not differ significantly between groups with only 20 patients (23%) requiring additional analgesia, 11 in the ketamine group and 9 in the fentanyl group (relative risk, 0.89; 95% CI, 0.5-1.6) (eTable 4 in Supplement 2).
With regard to the blinding assessment, study medication was guessed correctly in 54 patients (63%). Observers guessed correctly 52% of the time for patients receiving ketamine (n = 23) and 74% for patients receiving fentanyl (n = 31).
Discussion
In this randomized, double-blind, active-control, noninferiority trial, intranasal ketamine (1.5 mg/kg) provided similar and noninferior pain relief when compared with intranasal fentanyl (2 µg/kg) for traumatic extremity injuries in children. Both medications produced clinically meaningful pain reduction within 15 minutes, which was sustained for 60 minutes without requiring additional analgesia in the majority of patients.
We sought to determine whether improved pain control could be achieved by using higher intranasal doses of both ketamine and fentanyl than previously reported and to address limitations of other ED pediatric studies by evaluating pain relief in the absence of routinely administered ibuprofen/acetaminophen through use of a single, validated pediatric pain scale. Surprisingly, the higher doses of ketamine (1.5 mg/kg vs 1 mg/kg in prior studies) and fentanyl (2 µg/kg vs 1.5 µg/kg in prior studies) in this study did not achieve superior pain relief (mean reductions of 31 mm and 32 mm at 30 minutes for ketamine and fentanyl, respectively) compared with the PICHFORK study22 (median reductions of 45 mm and 40 mm at 30 minutes for ketamine and fentanyl, respectively) and the study by Reynolds et al24 (mean reductions of 44 mm and 35 mm at 20 minutes for ketamine and fentanyl, respectively). The reason for this is likely 2-fold. First, in the PICHFORK study,22 patients received 10-mg/kg ibuprofen, and in the study of Reynolds et al,24 patients received either 10-mg/kg ibuprofen or 15-mg/kg acetaminophen. Multiple studies have demonstrated the comparative effectiveness of nonnarcotic and narcotic analgesics.36,37,38,39,40,41 The contribution of ibuprofen/acetaminophen to overall pain reduction in the PICHFORK study22 and the study by Reynolds et al24 is likely to be important, but the precise effect cannot be discerned. Second, both studies combined pain scales (Faces Pain Scale-Revised and VAS) in reporting pain reduction outcomes. The 6-point Faces Pain Scale-Revised was converted to a corresponding 0-20-40-60-80-100 scale. The effect of averaging this ordinal scale with the continuous VAS likely inflates pain reduction estimates, as is demonstrated in reported outcomes of subsets of PICHFORK22 patients. Patients reporting only VAS scores (with ages identical to our study) had similar results (median reductions of 35 mm and 33 mm at 30 minutes for ketamine and fentanyl, respectively). As only 11% of the ketamine group and 16% of the fentanyl group received either ibuprofen/naproxen or acetaminophen prior to arrival in our study and owing to the use of a single validated pain scale, our estimates of pain reduction are more likely to represent the isolated effect of ketamine and fentanyl more accurately than prior studies.
Similar to the PICHFORK study22 and the study by Reynolds et al,24 we found AEs to be common, minor, and transient with both medications. Approximately half of AEs occurred within the first 15 minutes after administration. Despite using higher doses of both study medications and video review for data collection in the initial 15 minutes after medication administration, our patients experienced fewer AEs overall (63 total AEs in 47 of 86 patients [54.7%] in the present study, 91 AEs in 43 of 73 children [58.9%] in the PICHFORK study,22 and 170 AEs in 66 of 82 patients [80.5%] in the study by Reynolds et al24). Drowsiness, dizziness, and unpleasant taste were the most common AEs in all 3 trials. Similar to prior studies, about 75% of AEs were in the ketamine group. One patient in the ketamine group experienced dysphoria/dissociation and had received an additional dose of intravenous ketamine (0.53 mg/kg) for rescue analgesia within 15 minutes of study drug administration. The reason for the decreased overall number of AEs in the present study is not entirely clear. We had a similar proportion of patients receive rescue analgesia (the present study, 23%; PICHFORK,22 23%; Reynolds et al,24 29% received additional study drug and 18% received rescue opioids). Reynolds et al24 specifically queried patients regarding each individual AE, which may, in part, explain their increased frequency of AEs.
With regard to sedation, our distribution of University of Michigan Sedation Scale scores over time mirrored those of the PICHFORK study22 with no patient having a score higher than 2. Although ketamine patients had higher sedation scores overall, this was not clinically meaningful as these patients were still easily aroused with verbal command or light tactile stimulation.30 Patients were also monitored through video review for hypopneic hypoventilation, a decrease in tidal volume without a change in respiratory rate, which is common in children undergoing ketamine procedural sedation.42 A decrease in exhaled end tidal carbon dioxide level persisting 30 seconds or longer often occurs just prior to a decrease in oxygen saturation. Eleven patients experienced a decrease in end tidal carbon dioxide of 10 mm Hg or more (9 patients in the ketamine group and 2 in the fentanyl group). These decreases were brief (<30 seconds) and accompanied by a change in respiratory rate but no change in oxygen saturation. Therefore, we suspect that none of the study patients experienced true hypopneic hypoventilation, but additional investigation would be warranted.
Clinicians may choose ketamine in lieu of opioids for multiple reasons. Ketamine is useful in children who have known AEs with opioids, have developed opioid tolerance as a result of chronic painful conditions, have poor opioid sensitivity owing to genetic predisposition,7,43 or in trauma patients with the potential for hypotension.44 Given that intranasal ketamine is noninferior to intranasal fentanyl with respect to pain reduction, a key advantage of intranasal ketamine is the avoidance of opioids in children before administration of procedural sedation. Adverse effects during sedation have been associated with the administration of prior opioid administration; thus, avoiding presedation opioids may decrease sedation recovery time45 and the risk of serious AEs during sedation,6 but further exploration is warranted.
Ketamine is widely available (although a 50 mg/mL concentration is needed for the intranasal route) and monitoring is similar to opioids. However, ketamine should be avoided in patients younger than 3 months or with known allergy.
Limitations
There are several potential limitations. We enrolled a convenience sample and do not have data on missed eligible patients to compare with our study sample. This sampling method increased feasibility of study completion but may limit generalizability. However, our results are similar to the existing ED pediatric studies investigating these intranasal medications.22,24 Four patients were withdrawn before medication administration making an intention-to-treat analysis impossible, although data from these patients would be unlikely to affect the overall results. To maintain identical volumes in all syringes for blinding purposes, the maximum dose for fentanyl was 100 µg and for ketamine, 100 mg. Therefore, only patients weighing 50 kg or less could receive the full intended dose of fentanyl while patients weighing up to 65 kg could receive the full intended dose of ketamine. However, there were no statistical differences in pain relief for those underdosed compared with those adequately dosed within and between study groups (data not shown). Our choice of a noninferiority margin of 10 was conservative and based on clinical reasoning and literature regarding the definition of clinically significant pain reduction, which has notable variation.28,34,46 Without a placebo group, we were unable to determine if splinting or child life specialist interventions contributed to pain reduction. However, it is unlikely that these covariates had a significant influence on the results owing to successful randomization. Finally, the type and dose of rescue analgesia was at the discretion of the primary clinician. However, there were similar occurrences of rescue analgesia between the 2 groups, so this was unlikely to have contributed to differences in pain scores or AEs.
Conclusions
Intranasal subdissociative ketamine provides effective analgesia that is not inferior to intranasal fentanyl for pain associated with acute extremity injuries in children. Ketamine was associated with more AEs, but all were mild and transient. Ketamine should be considered for pediatric pain management in the emergency setting, especially when opioids are contraindicated or associated with increased risk, such as prior to procedural sedation.
Trial Protocol
eTable 1. University of Michigan Sedation Scale
eTable 2. Highest Achieved UMSS Score
eTable 3. Vital Signs
eTable 4. Rescue Analgesia
eFigure 1. Weight-Based Dose Administration Reference
eFigure 2. Visual Analog Scale
Data Sharing Statement
References
- 1.Institute of Medicine Committee on Advancing Pain Research Care, and Education Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: National Academies Press; 2011. [PubMed] [Google Scholar]
- 2.Rupp T, Delaney KA. Inadequate analgesia in emergency medicine. Ann Emerg Med. 2004;43(4):494-503. doi: 10.1016/j.annemergmed.2003.11.019 [DOI] [PubMed] [Google Scholar]
- 3.Boccio E, Wie B, Pasternak S, Salvador-Kelly A, Ward MF, D’Amore J. The relationship between patient age and pain management of acute long-bone fracture in the ED. Am J Emerg Med. 2014;32(12):1516-1519. doi: 10.1016/j.ajem.2014.09.025 [DOI] [PubMed] [Google Scholar]
- 4.Del Pizzo J, Callahan JM. Intranasal medications in pediatric emergency medicine. Pediatr Emerg Care. 2014;30(7):496-501. doi: 10.1097/PEC.0000000000000171 [DOI] [PubMed] [Google Scholar]
- 5.Dong L, Donaldson A, Metzger R, Keenan H. Analgesic administration in the emergency department for children requiring hospitalization for long-bone fracture. Pediatr Emerg Care. 2012;28(2):109-114. [DOI] [PubMed] [Google Scholar]
- 6.Bhatt M, Johnson DW, Chan J, et al. ; Sedation Safety Study Group of Pediatric Emergency Research Canada (PERC) . Risk factors for adverse events in emergency department procedural sedation for children. JAMA Pediatr. 2017;171(10):957-964. doi: 10.1001/jamapediatrics.2017.2135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nagashima M, Katoh R, Sato Y, Tagami M, Kasai S, Ikeda K. Is there genetic polymorphism evidence for individual human sensitivity to opiates? Curr Pain Headache Rep. 2007;11(2):115-123. doi: 10.1007/s11916-007-0008-8 [DOI] [PubMed] [Google Scholar]
- 8.Bijur PE, Kenny MK, Gallagher EJ. Intravenous morphine at 0.1 mg/kg is not effective for controlling severe acute pain in the majority of patients. Ann Emerg Med. 2005;46(4):362-367. doi: 10.1016/j.annemergmed.2005.03.010 [DOI] [PubMed] [Google Scholar]
- 9.Green SM, Roback MG, Kennedy RM, Krauss B. Clinical practice guideline for emergency department ketamine dissociative sedation: 2011 update. Ann Emerg Med. 2011;57(5):449-461. doi: 10.1016/j.annemergmed.2010.11.030 [DOI] [PubMed] [Google Scholar]
- 10.Hirota K, Lambert DG. Ketamine: new uses for an old drug? Br J Anaesth. 2011;107(2):123-126. doi: 10.1093/bja/aer221 [DOI] [PubMed] [Google Scholar]
- 11.Craven R. Ketamine. Anaesthesia. 2007;62(Suppl 1):48-53. doi: 10.1111/j.1365-2044.2007.05298.x [DOI] [PubMed] [Google Scholar]
- 12.Visser E, Schug SA. The role of ketamine in pain management. Biomed Pharmacother. 2006;60(7):341-348. [DOI] [PubMed] [Google Scholar]
- 13.Shimonovich S, Gigi R, Shapira A, et al. Intranasal ketamine for acute traumatic pain in the emergency department: a prospective, randomized clinical trial of efficacy and safety. BMC Emerg Med. 2016;16(1):43. doi: 10.1186/s12873-016-0107-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shrestha R, Pant S, Shrestha A, Batajoo KH, Thapa R, Vaidya S. Intranasal ketamine for the treatment of patients with acute pain in the emergency department. World J Emerg Med. 2016;7(1):19-24. doi: 10.5847/wjem.j.1920-8642.2016.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yeaman F, Meek R, Egerton-Warburton D, Rosengarten P, Graudins A. Sub-dissociative-dose intranasal ketamine for moderate to severe pain in adult emergency department patients. Emerg Med Australas. 2014;26(3):237-242. doi: 10.1111/1742-6723.12173 [DOI] [PubMed] [Google Scholar]
- 16.Andolfatto G, Willman E, Joo D, et al. Intranasal ketamine for analgesia in the emergency department: a prospective observational series. Acad Emerg Med. 2013;20(10):1050-1054. doi: 10.1111/acem.12229 [DOI] [PubMed] [Google Scholar]
- 17.Beik N, Sylvester K, Rocchio M, Stone MB. Evaluation of the use of ketamine for acute pain in the emergency department at a tertiary academic medical center. Pharmacol Pharm. 2016;7:19-24. doi: 10.4236/pp.2016.71003 [DOI] [Google Scholar]
- 18.Miller JP, Schauer SG, Ganem VJ, Bebarta VS. Low-dose ketamine vs morphine for acute pain in the ED: a randomized controlled trial. Am J Emerg Med. 2015;33(3):402-408. doi: 10.1016/j.ajem.2014.12.058 [DOI] [PubMed] [Google Scholar]
- 19.Motov S, Rockoff B, Cohen V, et al. Intravenous subdissociative-dose ketamine vs morphine for analgesia in the emergency department: a randomized controlled trial. Ann Emerg Med. 2015;66(3):222-229. doi: 10.1016/j.annemergmed.2015.03.004 [DOI] [PubMed] [Google Scholar]
- 20.Bowers KJ, McAllister KB, Ray M, Heitz C. Ketamine as an adjunct to opioids for acute pain in the emergency department: a randomized controlled trial. Acad Emerg Med. 2017;24(6):676-685. doi: 10.1111/acem.13172 [DOI] [PubMed] [Google Scholar]
- 21.Bredmose PP, Grier G, Davies GE, Lockey DJ. Pre-hospital use of ketamine in paediatric trauma. Acta Anaesthesiol Scand. 2009;53(4):543-545. doi: 10.1111/j.1399-6576.2008.01852.x [DOI] [PubMed] [Google Scholar]
- 22.Graudins A, Meek R, Egerton-Warburton D, Oakley E, Seith R. The PICHFORK (Pain in Children Fentanyl or Ketamine) trial: a randomized controlled trial comparing intranasal ketamine and fentanyl for the relief of moderate to severe pain in children with limb injuries. Ann Emerg Med. 2015;65(3):248-254. doi: 10.1016/j.annemergmed.2014.09.024 [DOI] [PubMed] [Google Scholar]
- 23.Yeaman F, Oakley E, Meek R, Graudins A. Sub-dissociative dose intranasal ketamine for limb injury pain in children in the emergency department: a pilot study. Emerg Med Australas. 2013;25(2):161-167. doi: 10.1111/1742-6723.12059 [DOI] [PubMed] [Google Scholar]
- 24.Reynolds SL, Bryant KK, Studnek JR, et al. Randomized controlled feasibility trial of intranasal ketamine compared to intranasal fentanyl for analgesia in children with suspected extremity fractures. Acad Emerg Med. 2017;24(12):1430-1440. doi: 10.1111/acem.13313 [DOI] [PubMed] [Google Scholar]
- 25.Hirschfeld G, Zernikow B. Cut points for mild, moderate, and severe pain on the VAS for children and adolescents: what can be learned from 10 million ANOVAs? Pain. 2013;154(12):2626-2632. doi: 10.1016/j.pain.2013.05.048 [DOI] [PubMed] [Google Scholar]
- 26.Pediatric & Neonatal Lexi-Drugs. Lexi-Comp, Inc. https://online.lexi.com/lco/action/home. Accessed January 1, 2016.
- 27.Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. https://evs.nci.nih.gov/ftp1/CTCAE/About.html. Accessed May 29, 2018.
- 28.Bailey B, Gravel J, Daoust R. Reliability of the visual analog scale in children with acute pain in the emergency department. Pain. 2012;153(4):839-842. doi: 10.1016/j.pain.2012.01.006 [DOI] [PubMed] [Google Scholar]
- 29.von Baeyer CL. Children's self-reports of pain intensity: scale selection, limitations and interpretation. Pain Res Manag. 2006;11(3):157-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malviya S, Voepel-Lewis T, Tait AR, Merkel S, Tremper K, Naughton N. Depth of sedation in children undergoing computed tomography: validity and reliability of the University of Michigan Sedation Scale (UMSS). Br J Anaesth. 2002;88(2):241-245. doi: 10.1093/bja/88.2.241 [DOI] [PubMed] [Google Scholar]
- 31.American Heart Association PALS Provider Manual eBook. 1st ed. Dallas, TX: American Heart Association Inc; 2016. [Google Scholar]
- 32.Bijur PE, Silver W, Gallagher EJ. Reliability of the visual analog scale for measurement of acute pain. Acad Emerg Med. 2001;8(12):1153-1157. [DOI] [PubMed] [Google Scholar]
- 33.Todd KH, Funk KG, Funk JP, Bonacci R. Clinical significance of reported changes in pain severity. Ann Emerg Med. 1996;27(4):485-489. doi: 10.1016/S0196-0644(96)70238-X [DOI] [PubMed] [Google Scholar]
- 34.Powell CV, Kelly AM, Williams A. Determining the minimum clinically significant difference in visual analog pain score for children. Ann Emerg Med. 2001;37(1):28-31. doi: 10.1067/mem.2001.111517 [DOI] [PubMed] [Google Scholar]
- 35.Piaggio G, Elbourne DR, Pocock SJ, Evans SJ, Altman DG; CONSORT Group . Reporting of noninferiority and equivalence randomized trials: extension of the CONSORT 2010 statement. JAMA. 2012;308(24):2594-2604. doi: 10.1001/jama.2012.87802 [DOI] [PubMed] [Google Scholar]
- 36.Clark E, Plint AC, Correll R, Gaboury I, Passi B. A randomized, controlled trial of acetaminophen, ibuprofen, and codeine for acute pain relief in children with musculoskeletal trauma. Pediatrics. 2007;119(3):460-467. doi: 10.1542/peds.2006-1347 [DOI] [PubMed] [Google Scholar]
- 37.Koller DM, Myers AB, Lorenz D, Godambe SA. Effectiveness of oxycodone, ibuprofen, or the combination in the initial management of orthopedic injury-related pain in children. Pediatr Emerg Care. 2007;23(9):627-633. doi: 10.1097/PEC.0b013e31814a6a39 [DOI] [PubMed] [Google Scholar]
- 38.Drendel AL, Gorelick MH, Weisman SJ, Lyon R, Brousseau DC, Kim MK. A randomized clinical trial of ibuprofen versus acetaminophen with codeine for acute pediatric arm fracture pain. Ann Emerg Med. 2009;54(4):553-560. doi: 10.1016/j.annemergmed.2009.06.005 [DOI] [PubMed] [Google Scholar]
- 39.Poonai N, Bhullar G, Lin K, et al. Oral administration of morphine versus ibuprofen to manage postfracture pain in children: a randomized trial. CMAJ. 2014;186(18):1358-1363. doi: 10.1503/cmaj.140907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Le May S, Gouin S, Fortin C, Messier A, Robert MA, Julien M. Efficacy of an ibuprofen/codeine combination for pain management in children presenting to the emergency department with a limb injury: a pilot study. J Emerg Med. 2013;44(2):536-542. doi: 10.1016/j.jemermed.2012.06.027 [DOI] [PubMed] [Google Scholar]
- 41.Friday JH, Kanegaye JT, McCaslin I, Zheng A, Harley JR. Ibuprofen provides analgesia equivalent to acetaminophen-codeine in the treatment of acute pain in children with extremity injuries: a randomized clinical trial. Acad Emerg Med. 2009;16(8):711-716. doi: 10.1111/j.1553-2712.2009.00471.x [DOI] [PubMed] [Google Scholar]
- 42.Langhan ML, Chen L, Marshall C, Santucci KA. Detection of hypoventilation by capnography and its association with hypoxia in children undergoing sedation with ketamine. Pediatr Emerg Care. 2011;27(5):394-397. doi: 10.1097/PEC.0b013e318217b538 [DOI] [PubMed] [Google Scholar]
- 43.Ikeda K, Ide S, Han W, Hayashida M, Uhl GR, Sora I. How individual sensitivity to opiates can be predicted by gene analyses. Trends Pharmacol Sci. 2005;26(6):311-317. doi: 10.1016/j.tips.2005.04.001 [DOI] [PubMed] [Google Scholar]
- 44.Tran KP, Nguyen Q, Truong XN, et al. A comparison of ketamine and morphine analgesia in prehospital trauma care: a cluster randomized clinical trial in rural Quang Tri province, Vietnam. Prehosp Emerg Care. 2014;18(2):257-264. doi: 10.3109/10903127.2013.851307 [DOI] [PubMed] [Google Scholar]
- 45.Losek JD, Reid S. Effects of initial pain treatment on sedation recovery time in pediatric emergency care. Pediatr Emerg Care. 2006;22(2):100-103. doi: 10.1097/01.pec.0000199566.10006.96 [DOI] [PubMed] [Google Scholar]
- 46.Gallagher EJ, Liebman M, Bijur PE. Prospective validation of clinically important changes in pain severity measured on a visual analog scale. Ann Emerg Med. 2001;38(6):633-638. doi: 10.1067/mem.2001.118863 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. University of Michigan Sedation Scale
eTable 2. Highest Achieved UMSS Score
eTable 3. Vital Signs
eTable 4. Rescue Analgesia
eFigure 1. Weight-Based Dose Administration Reference
eFigure 2. Visual Analog Scale
Data Sharing Statement