Key Points
Questions
Are laws that allow schools and child care centers to maintain 2 twin-pack units of non–patient-designated epinephrine autoinjectors to treat allergic reactions cost-effective?
Findings
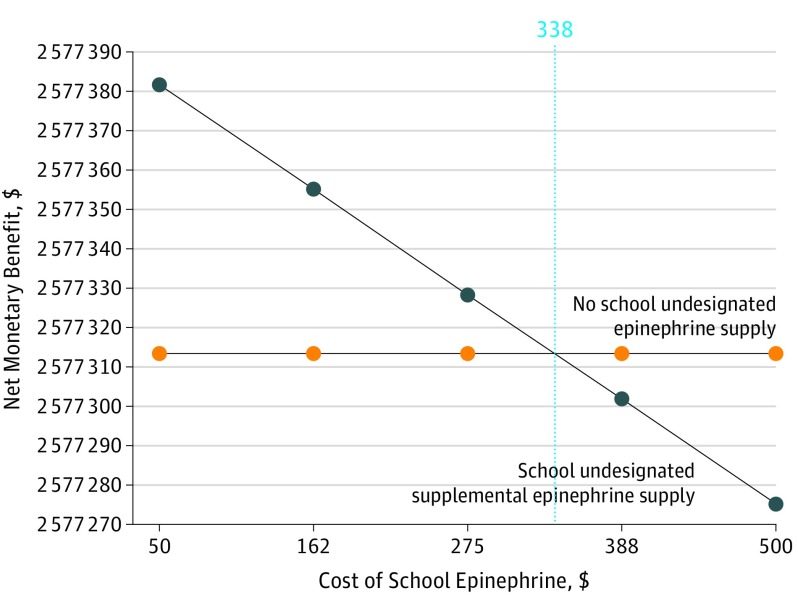
Based on Markov simulations of the Chicago Public Schools system (371 382 students), the current policy (schools maintain 2 undesignated stock twin-pack units that supplement additionally provided student-designated units) is cost-effective when total school epinephrine acquisition expenses do not exceed $338 per school per year. However, a universal (stock only) model, without requiring student-designated units, provides superior value.
Meaning
This simulation suggests a value-based price for stock epinephrine under the current policy, with high cost savings if a universal model is adopted.
This analysis uses Markov simulations of the Chicago Public Schools system to define value-based strategies and cost-effectiveness of undesignated school stock epinephrine programs based on analysis of children with peanut allergies.
Abstract
Importance
Children experiencing anaphylaxis at school may lack access to a personal epinephrine device, prompting recent legislation permitting undesignated (eg, non–student specific) stock epinephrine autoinjector units at school. However, epinephrine device costs vary, and the cost-effectiveness of undesignated school stock epinephrine is uncharacterized to date.
Objective
To define value-based strategies for undesignated school stock epinephrine programs.
Design, Setting, and Participants
Markov simulations of the Chicago Public Schools system were used over extended time horizons to model 2 school stock epinephrine autoinjector policies to provide access for at-risk students. The dates of the data used in the analysis were September 2017 to June 2018 (the 2017-2018 school year).
Main Outcomes and Measures
This study compared the following 3 strategies: no school undesignated epinephrine supply, school undesignated supplemental epinephrine supply (supplemental model), and school undesignated universal epinephrine supply (universal model). The base-case model assumed a 10-fold reduced fatality risk with having undesignated stock epinephrine units available vs not having undesignated stock epinephrine units available. Costs of school stock epinephrine units available for acquisition by schools were evaluated from a societal perspective. Quality-adjusted life-years (QALYs) and total epinephrine acquisition expenses were calculated.
Results
Based on Markov simulations of the Chicago Public Schools system (371 382 students), the cost was $107 816 (95% CI, $107 382-$108 250) for no school undesignated epinephrine supply compared with $108 160 (95% CI, $107 725-$108 595) for the supplemental model and $100 397 (95% CI, $99 979-$100 815) for the universal model. Undesignated stock epinephrine improved outcomes, with 26.869 (95% CI, 26.841-26.897) QALYs accrued as the model concluded compared with 26.867 (95% CI, 26.839-26.896) QALYs for the strategy without undesignated stock epinephrine. When comparing supplemental model stock epinephrine to the strategy without undesignated devices, the incremental cost-effectiveness ratio was high at $268 811 per QALY in the base-case simulation. However, the cost of the supplemental model fell below $100 000 per QALY when the annual undesignated epinephrine acquisition costs did not exceed $338 per school (compared with stock epinephrine unavailability). The universal model dominated all others and was associated with significant cost savings ($7419 per student at risk who would otherwise be prescribed an individual school epinephrine supply).
Conclusions and Relevance
Undesignated school stock epinephrine is cost-effective at device acquisition costs not exceeding $338 per school per year, although a universal model vs a supplemental model is associated with superior health and economic outcomes.
Introduction
Legislation to either permit or mandate school-based stock epinephrine programs has emerged to provide timely access to epinephrine for children with known anaphylaxis risk and for children without documented risk given data that as many as 25% of first-time anaphylactic reactions occur in school (inclusive of both adults and children at the school).1 Because food allergy is estimated to affect up to 8% of US children2 (approximately 5.9 million children younger than 18 years and 3.3 million children aged 6 to 17 years) and because not all allergic children have a personal epinephrine device available at school at all times (although this is recommended), these programs can enable access to an epinephrine device and facilitate appropriate anaphylaxis management in the school setting.3 In a study of the Chicago Public Schools system voluntary stock epinephrine policy and outcomes, DeSantiago-Cardenas and colleagues4 reported that 38 stock autoinjectors were administered in the first year of the program. Students received 92.1% (35 of 38) of the epinephrine administrations, while 7.9% (3 of 38) of the epinephrine units were administered to school staff. More than half of all doses were administered for reported first-time anaphylactic reactions, including 21 of 38 cases for reactions attributable to food, 2 of 38 cases for venom, 2 of 38 cases for an inhalant allergen, and 13 of 38 cases for undocumented triggers.4
Recent controversies have emerged around the cost escalation of epinephrine autoinjectors, and ongoing and future cost inflation could pose a barrier to epinephrine access in some settings, as could interruption to supply (experienced for Auvi-Q [Kaléo] and more recently for EpiPen [Mylan N.V.]).5,6,7,8 Evidence suggests that significant variability exists in epinephrine autoinjector cost in relation to brand, insurance coverage, and discount coupon availability.9,10 Because wide variation in epinephrine autoinjector device costs exists, we undertook Markov simulations of the Chicago Public Schools system to further characterize the value-based costing of undesignated school supplies of emergency epinephrine and to explore an optimal stock epinephrine policy. The objective of this study was to define value-based strategies for undesignated school stock epinephrine programs.
Methods
Study Design
Computer-based mathematical microsimulations (TreeAge Pro 2018; TreeAge Software, Inc) were used to evaluate hypothetical cohorts of children with peanut allergy from kindergarten through high school graduation, with a continued horizon to age 80 years (eFigure in the Supplement). Markov models are well suited to health care simulations and were used because they can account for the annual costs and recurrent risks of peanut exposure and reactions, allowing transitions between different health states. The dates of the data used in the analysis were September 2017 to June 2018 (the 2017-2018 school year). According to the criteria of the Colorado Multiple Institutional Review Board, this study was exempt from institutional review board review.
Undesignated School-Based Epinephrine Strategies Compared
We compared different approaches to undesignated school-based epinephrine policies. Because many children who experience anaphylaxis in the school setting are treated with school devices, we chose to compare supplemental and universal stocking practices. The study compared the following 3 strategies: (1) no school undesignated epinephrine supply, in which the only devices available are personal autoinjectors provided by students with an allergic condition; (2) school undesignated supplemental epinephrine supply, in addition to student-supplied devices (supplemental model); and (3) school undesignated universal epinephrine supply, in which school-based devices supplant the need for student-supplied devices (universal model). In the supplemental model, in addition to the school receiving an annual supply of 2 twin packs of undesignated stock epinephrine, children with peanut allergy attending school also received an additional annual prescription of an epinephrine twin pack dedicated for exclusive use at school (which is not available for at-home use and requires that an extra unit be dispensed as part of the prescription) per the current policy11,12,13; after high school graduation, they received an annual prescription for a single twin pack (because colleges and universities do not have stock policies and because this is the typical management for peanut-allergic adults). The current practice in the 49 states with stock epinephrine law is the supplemental model; before the first stock legislation in 2011, no stock epinephrine was available, and allergic individuals provided their own units for school in every state. We are unaware of any state or school district using the universal model. Because individual epinephrine prescriptions are often not available or carried when needed for anaphylaxis, it was assumed that having undesignated stock epinephrine units available would be associated with a 10-fold overall risk reduction in fatality among school-age children compared with not having undesignated stock epinephrine units available.14,15 This assumption is based on prior publications,14,15 is intended to be deliberately conservative, and represents an estimate of a plausible range given that no study to date has defined the fatality risk reduction attributable to having epinephrine available (or could ethically do so). Home epinephrine autoinjector twin packs were prescribed to every allergic child in all strategies compared because this is the standard of care independent of any stock epinephrine strategy modeled. We assumed that all schools assigned to each strategy compared adopted their assigned approach to stocking epinephrine.
Assumptions
Base-case assumptions and probabilities were derived from similar prior cost-effectiveness analyses of peanut-allergic children (Table 1).10,16,18,19,20,22,23,24,25,26,27 In all strategies, children who received epinephrine at school were transported to and seen in an emergency department for further evaluation and management per current guideline recommendations.12,28,29,30 The incidence of pediatric food allergy fatality was modeled at 3.25 cases per 1 million at-risk person-years for school-age children without school-based epinephrine and at 3.25 cases per 10 million at-risk person-years for school-age children with access to undesignated school-based epinephrine.17 We modeled a high rate of children at risk for anaphylaxis (prevalence rate, 8%; range, 5%-11%) to account for other children without peanut allergy who may also experience anaphylaxis in the school setting.2,11,12,31 Costs of school stock epinephrine units available for acquisition by schools were evaluated from a societal perspective among this population, and it was assumed that all students would have the stock epinephrine device administered for treatment of a reaction occurring at school, irrespective of that student also having supplied his or her own personally designated unit at school (to achieve model parsimony). For these calculations, 2 epinephrine twin packs costing $715 (95% CI, $685-$743) per package were procured annually for each school (total cost, $1430; 95% CI, $1370-$1486 per individual school per year)10,32 based on the Chicago Public Schools system (646 schools, 371 382 students, 30 622 school-based employees, and school day length between 7 and 7.5 hours for a minimum of 176 calendar days per year per state law).21,33 This school system has been previously characterized for epinephrine use.4,29 At an anaphylaxis incidence of 0.20 (95% CI, 0.09-0.43) cases per 100 person-years, this translates to approximately 29 (95% CI, 13-62) potential cases per year for the district, assuming a food-allergic child as the most likely anaphylaxis occurrence in school.17 We evaluated peanut-allergic children for simulation purposes given a focus on peanut allergy at school for other policy-related issues, such as school or classroom–wide bans.11,34 Costs and quality-adjusted life-years (QALYs) were discounted equally at 3% per annum.23
Table 1. Simulation Model Inputs.
| Variable | Model Reference | Source |
|---|---|---|
| US life table | National Vital Statistics Reports, April 2017 | Arias et al,16 2017 |
| Incidence of food allergy fatality | 5 to 19 y: 3.25 (95% CI, 1.73 to 6.10) cases per 1 million at-risk person-years (sensitivity, 3.25 to 33) | Umasunthar et al,17 2013 |
| ≥20 y: 1.81 (95% CI, 0.94 to 3.45) cases per 1 million at-risk person-years (sensitivity, 1.81 to 18.1) | ||
| Probabilities, % | ||
| Unintentional peanut exposure if allergic | 11.7 Per year (sensitivity, 5 to 50) | Vander Leek et al,18 2000 |
| Moderate to severe reaction after peanut exposure | 52 (sensitivity, 10 to 70) | Vander Leek et al,18 2000 |
| Hospitalization after severe food-allergic reaction | 0.18 Per year after allergic reaction (sensitivity, 0.16 to 0.20) | Shaker,19 2017 |
| Outpatient visits for food allergy after allergic reaction | 20.4 Per year after allergic reaction (sensitivity, 20.0 to 21.0) | Shaker,19 2017 |
| Spontaneous peanut tolerance | 1.1 Per year (age range, 5 to 20 y) (sensitivity, 0.5 to 2) | Skolnick et al,20 2001 |
| Students at risk for anaphylaxis | 8 (range, 5 to 11) | Gupta et al,22011 |
| Costs, 2018 US $ | ||
| Personal epinephrine autoinjector (annual cost per twin pack) | 715 (95% CI, 685 to 743) | Shaker et al,10 2017 |
| Undesignated school epinephrine | 1430 Per school (shared by total No. of children at risk in each school) | Shaker et al,10 2017; Gupta et al,2 2011; Chicago Public Schools21 |
| Hospitalization | 5899 (95% CI, 5732 to 6066) | Patel et al,22 2011; Bureau of Labor Statistics23 |
| ED visit | 691 (95% CI, 689 to 693) | Patel et al,22 2011; Bureau of Labor Statistics23 |
| Outpatient visit for food-allergic reactions | 235 (sensitivity, 225 to 245) | Patel et al,22 2011; Bureau of Labor Statistics23 |
| Ambulance runs for allergic reactions | 573 (sensitivity, 512 to 632) | Patel et al,22 2011; Bureau of Labor Statistics23 |
| Pediatrician visits (mean incremental annual cost for food allergy diagnosis) | 100 (sensitivity, 94 to 105) | Bureau of Labor Statistics23; Gupta et al,24 2013 |
| Allergist visits for food allergy (mean incremental annual cost for food allergy diagnosis) | 149 (sensitivity, 143 to 155) | Bureau of Labor Statistics23; Gupta et al,24 2013 |
| Nutritionist visits for food allergy (per year) | 17 (sensitivity, 15 to 18) | Bureau of Labor Statistics23; Gupta et al,24 2013 |
| Alternative health care professional visits for food allergy (per year) | 25 (sensitivity, 22 to 27) | Bureau of Labor Statistics23; Gupta et al,24 2013 |
| Incremental annual grocery costs (living with food allergy) | 310 (sensitivity, 290 to 330) | Bureau of Labor Statistics23; Gupta et al,24 2013 |
| Job-related opportunity costs from food allergy (per year) | 2597 (sensitivity, 0 to 2697) | Bureau of Labor Statistics23; Gupta et al,24 2013 |
| Additional Assumptions | ||
| Fatality reduction from undesignated school epinephrine | 10-Fold (sensitivity, 10-fold to 100-fold) | NA |
| Start age, y | 5 (sensitivity, 3 to 7) | NA |
| Annual discount rate | 0.03 (sensitivity, 0 to 0.03) | Bureau of Labor Statistics23 |
| Negative health state consequence for food allergy | −0.09 (sensitivity, −0.02 to −.11) | Mittman et al,25 1999; Carroll and Downs,26 2009 |
Abbreviations: ED, emergency department; NA, not applicable (meaning that this was an assumption we made and there is no source).
Results
In the base-case simulation, the cost was $107 816 (95% CI, $107 382-$108 250) for no school undesignated epinephrine supply compared with $108 160 (95% CI, $107 725-$108 595) for the supplemental model and $100 397 (95% CI, $99 979-$100 815) for the universal model. Health outcomes were better with undesignated stock epinephrine, with 26.869 (95% CI, 26.841-26.897) QALYs accrued as the model concluded compared with 26.867 (95% CI, 26.839-26.896) QALYs for the strategy without undesignated stock epinephrine.
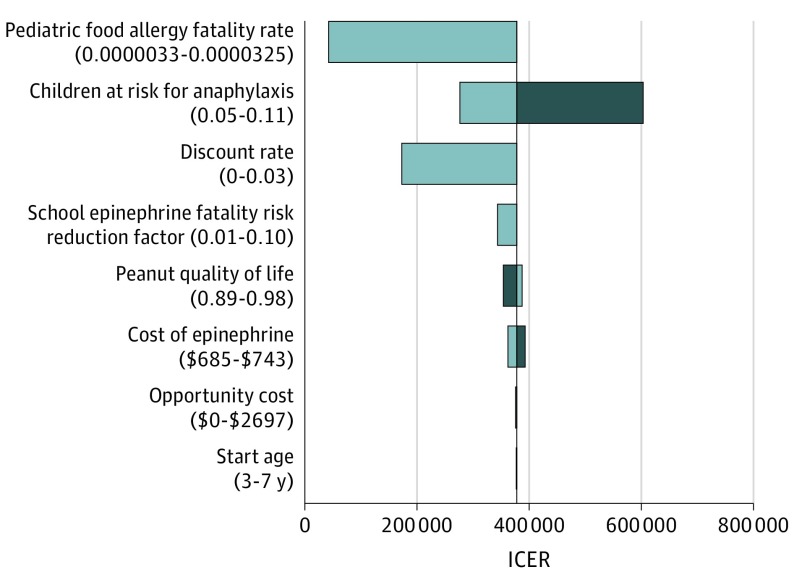
Under base-case assumptions, the supplemental model was not cost-effective compared with no school undesignated epinephrine supply (base-case incremental cost-effectiveness ratio, $268 811 per QALY) (Table 2). However, in sensitivity analyses for this comparison, if the annual undesignated epinephrine acquisition costs to the school did not exceed $338, the supplemental model was a cost-effective public health policy, assuming a willingness-to-pay threshold of $100 000 per QALY (Figure 1).27 Assuming a lower willingness-to-pay threshold of $50 000 per QALY, then the value-based annual undesignated epinephrine acquisition ceiling costs fell to $162. In addition to epinephrine acquisition costs, the base-case model was also sensitive to fatality rates from anaphylaxis. At a pediatric fatality incidence of 1.37 cases per 100 000 person-years, school stock epinephrine cost did not exceed $100 000 per QALY (Figure 2). Additional deterministic sensitivity analyses showed consistent findings across all ranges.
Table 2. Comparison of Alternative Potential Stock Epinephrine Strategies.
| Strategy | Cost (95% CI), $ | Effectiveness (95% CI), QALYs | ICER, $ |
|---|---|---|---|
| No school undesignated epinephrine supply | 107 816 (107 382-108 250) | 26.867 (26.839-26.896) | NA |
| School undesignated supplemental epinephrine supply (supplemental model) | 108 160 (107 725-108 595) | 26.869 (26.841-26.897) | 268 811 per QALYa |
| School undesignated universal epinephrine supply (universal model) | 100 397 (99 979-100 815) | Dominated |
Abbreviations: ICER, incremental cost-effectiveness ratio; NA, not applicable; QALY, quality-adjusted life-year.
Supplemental model vs no school undesignated epinephrine supply.
Figure 1. Sensitivity Analysis on Cost of School Epinephrine, Assuming a Willingness-to-Pay Threshold of $100 000 per Quality-Adjusted Life-Year.
School undesignated supplemental epinephrine supply is cost-effective when the annual school epinephrine expenditures do not exceed $338 per school (dashed line). The vertical dashed line indicates the intersection of the 2 colored lines, representing the device price threshold at or below which the current policy is cost-effective.
Figure 2. Tornado Diagram Showing Deterministic Sensitivity Analyses of No School Undesignated Epinephrine Supply vs School Undesignated Supplemental Epinephrine Supply (Supplemental Model).
Gray bars show values above the base-case assumptions, while blue bars show values below the base-case assumptions. When the pediatric fatality incidence exceeds 1.37 cases per 100 000 person-years, the supplemental model is cost-effective at base-case model epinephrine school acquisition costs (willingness-to-pay, $100 000/quality-adjusted life-year). ICER indicates incremental cost-effectiveness ratio. The universal model dominated across all sensitivity ranges.
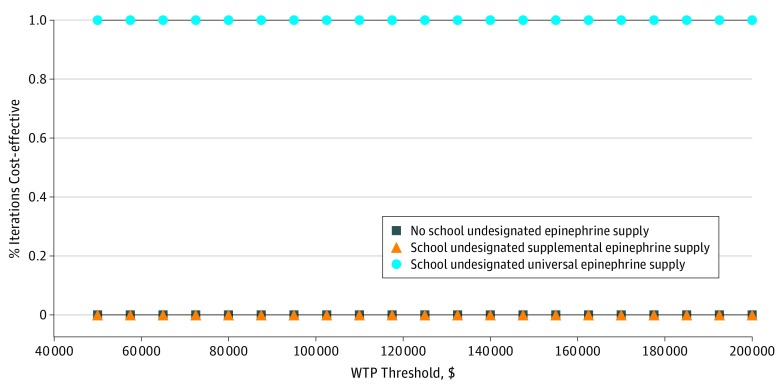
The universal model, where children were not prescribed additional epinephrine units for exclusive use at school but instead used the school stock supply if needed, improved health outcomes at lower costs. Over the universal model horizon, cost savings were $7419 per student at risk who would otherwise be prescribed an individual school epinephrine supply. Probabilistic sensitivity analyses (10 000 simulations) demonstrated the universal model epinephrine strategy to be optimal in 99.9% of iterations (Figure 3).
Figure 3. Cost-effectiveness Acceptability Curve.
Probabilistic sensitivity analyses performed on 10 000 iterations of Markov simulations compare 3 strategies: no school undesignated epinephrine supply, in which the only devices available are personal autoinjectors; school undesignated supplemental epinephrine supply, in addition to student-supplied devices (supplemental model); and school undesignated universal epinephrine supply, in which school-based devices supplant the need for student-supplied devices (universal model). WTP indicates willingness-to-pay.
Discussion
Access to epinephrine is a critical issue for children at risk for anaphylaxis; because many allergic children may potentially attend school without having an available personal epinephrine autoinjector, a stock supply of school autoinjectors can potentially be a lifesaving intervention.1,3,4 At a value-based annual total school epinephrine acquisition expense not exceeding $338 per school per year, the supplemental model (school undesignated supplemental epinephrine supply) is a cost-effective societal intervention compared with no school undesignated epinephrine supply. However, the universal model (school undesignated universal epinephrine supply) available for any student at school with known or unknown allergy risk—that replaces having the allergic child bring a dedicated autoinjector twin pack for the school year (an additional prescription on top of their twin pack prescribed for home)—dominated the base-case scenario in terms of producing superior health benefit and cost savings within the Chicago Public Schools system, with cost savings of $7419 per student at risk. Our aims of this analysis were to model under what assumptions the current stock epinephrine policy in the 49 US states that have such laws (schools stock undesignated units, while allergic children provide a dedicated unit) could be cost-effective and to investigate if an optimal policy could be devised that would maximize the benefits and minimize the costs.35 The universal model for stock epinephrine described herein shows great potential cost savings.
The current school epinephrine policy is inconsistent. All states except Hawaii have stock epinephrine legislation, but most states “opt in,” meaning that the law provides individual districts the option to allow schools to stock epinephrine, as opposed to mandating that all schools must do so.35 While inconsistencies in device prescription, the rate of opting in per district, and funding to obtain devices are occurring in opt-in states (even within mandated states), there are similar difficulties.36 Few studies exist to help understand the benefits of the legislation, the barriers to its effective implementation, and the areas in need of improvement. The exact number of devices used per year in schools with either type of stock epinephrine law—a state law mandating that schools and districts stock epinephrine or a law providing the option for schools and districts to stock this if they so choose (and the circumstances prompting their use)—is unknown, and there is no mechanism in place to date to track this nationally, although there are limited programs in states with mandated policies. Differences in epinephrine use policies (eg, who is treated with an undesignated device or a designated device) also may contribute to this uncertainty surrounding the outcomes that stock laws may achieve.36
In situations where both the school has an undesignated stock epinephrine unit and the student also provides his or her own (how the current laws are implemented), it is unclear which unit is actually to be used: this is not specified in either the state law or the student’s individual plan.12,35,36 To our knowledge, there are no previous studies that have explored the outcomes of the 2 scenarios that we modeled (supplemental vs universal). Moreover, the value provided by currently recommended epinephrine use strategies—such as to immediately inject epinephrine for known or suspected allergen ingestion in the absence of symptoms and to call 911 and seek emergency care as a default policy after epinephrine use (vs a “wait and see” course of action if symptoms immediately resolve after initial treatment)—may have questionable health and economic value.15 Both of these management paths also influence the health and economic benefits stock epinephrine can provide given that their outcomes are compounded in any analysis of stock policies because the schools are instructed to do both per current anaphylaxis management plans.
Supply-side economics also factor into these scenarios. Epinephrine device cost has become a significant issue, stemming from a recent voluntary withdrawal of one branded device for almost 18 months, during which time the price of another branded autoinjector rose steeply, creating potential hardship and possible issues of access for some patients.5,6,7 Worse, the devices are subject to production shortages on top of such withdrawals.8,37 Multiple device products are available, including 2 generic options (generic EpiPen [Mylan N.V.] and generic Adrenaclick [Lineage Therapeutics]) and 3 branded options (EpiPen [Mylan N.V.], Auvi-Q [Kaléo], and Adrenaclick [Amneal Pharmaceutical]), allowing competition on price and patient preference.38 However, these devices are not necessarily similar in their use, despite delivering equivalent amounts of intramuscular epinephrine, and may require additional training of school personnel. Out-of-pocket costs of these devices are heterogeneous, particularly in the setting of variable co-payments, deductibles, and coupon and rebate offers.10,32 A value-based acquisition cost not exceeding $338 per school per year for school-based stock epinephrine (2 twin packs) is well within a potential price range available for epinephrine devices. However, the premise of value-based pricing for a device viewed as lifesaving is novel, although epinephrine autoinjectors fit an ideal description of a drug potentially subject to such pricing, particularly in the school environment, where current funding for the provision of stock devices is unclear.39,40 The EpiPen4Schools41 program has supplied a large number of districts with free devices and is tracking multiple aspects of the use of these devices among participating schools. For the 2014-2015 school year (12 275 schools), there were 2191 reported anaphylaxis cases, and 89.8% occurred in students (54% from food triggers), with the highest rate among students of high school age (40.1%).41 Epinephrine was administered on school property for 63.7% of the reactions, and the stock epinephrine supply was used in 631 of 1267 (49.8%).41 While few other data suggest how such devices are being used, this evidence underscores the importance of school stock epinephrine.41 There are limited data from other stock programs or states with stock epinephrine laws.34
Limitations
This study has limitations and is based on multiple assumptions. The fatality risk differential between having and not having stock epinephrine is unknown, and we estimated this to conservatively be 10-fold, which we have published previously.15 We also assumed the current price for a single twin-pack epinephrine device, which may be at a historical high level and could be subject to pricing and availability volatility.32 However, an advantage of simulations is that multiple sensitivity analyses can be conducted to create more robust models: we investigated other risk assumptions and sensitivity of the device acquisition price to find a common ground for both to show where stock epinephrine could be cost-effective. That said, data inputs for actual rates of use are limited, particularly in large school systems. In our model, we used the Chicago Public Schools system given its size and diverse sociodemographics, and it is one of the only large districts with available epinephrine use reporting statistics.4,29 Therefore, the Chicago Public Schools system was a good example for modeling in this simulation, although it may not generalize to other school districts. Fatality rates were obtained from the limited published data available,17,42 but they are difficult to track and estimate in the setting of food allergy and could also differ based on the source used. Overall, fatality from a food allergen or anaphylaxis is a rare event, but rates similar to ours have been used in other published analyses.17,42 While the costs of emergency medical services activation and ambulance transport were included in these simulations in the present study, the cost-effectiveness of emergency medical services use for children with resolved allergic reactions has been questioned.14 In additional sensitivity analyses that we performed where school-based epinephrine was used as an alternative to children each bringing their own patient-specific device to school, there were cost savings; however, an obstacle to such an approach includes providing anaphylaxis preparedness to children during school transportation (particularly for those riding a school bus). While the universal model approach described herein is cost saving, there may be advantages to having students also provide their own designated individual epinephrine units, which could enable patient-specific devices to be available in transit to and from school (eg, on the school bus), provided that the child can self-carry these and the devices do not remain at school (eg, for use only at school). Because epinephrine is first-line management for anaphylaxis, it should be within reach for treatment of at-risk children. However, entity legislation covering related but off-campus school activities does not presently exist.
Conclusions
We show that a model of school-based stock epinephrine policy (with each allergic student bringing his or her own twin-pack device) is cost-effective when the annual acquisition price for undesignated school epinephrine does not exceed $338 per school, assuming a 10-fold reduction in fatality associated with stock epinephrine. However, a universal model approach where schools stock epinephrine for use by all students—but individual students are not asked to provide their own device for exclusive use at school—is cost saving and dominated other strategies. Under the right assumptions, school-based stock epinephrine can be a cost-effective strategy, and implementation of these programs should be encouraged and supported.
eFigure. Simulation Health States
References
- 1.McIntyre CL, Sheetz AH, Carroll CR, Young MC. Administration of epinephrine for life-threatening allergic reactions in school settings. Pediatrics. 2005;116(5):1134-1140. doi: 10.1542/peds.2004-1475 [DOI] [PubMed] [Google Scholar]
- 2.Gupta RS, Springston EE, Warrier MR, et al. . The prevalence, severity, and distribution of childhood food allergy in the United States. Pediatrics. 2011;128(1):e9-e17. doi: 10.1542/peds.2011-0204 [DOI] [PubMed] [Google Scholar]
- 3.Zadikoff EH, Whyte SA, Desantiago-Cardenas L, Harvey-Gintoft B, Gupta RS. The development and implementation of the Chicago Public Schools emergency EpiPen policy. J Sch Health. 2014;84(5):342-347. doi: 10.1111/josh.12147 [DOI] [PubMed] [Google Scholar]
- 4.DeSantiago-Cardenas L, Rivkina V, Whyte SA, Harvey-Gintoft BC, Bunning BJ, Gupta RS. Emergency epinephrine use for food allergy reactions in Chicago Public Schools. Am J Prev Med. 2015;48(2):170-173. doi: 10.1016/j.amepre.2014.09.005 [DOI] [PubMed] [Google Scholar]
- 5.CBS News Mylan CEO on EpiPen drug price controversy: “I get the outrage.” https://www.cbsnews.com/news/epipen-price-hike-controversy-mylan-ceo-heather-bresch-speaks-out/. Published January 27, 2017. Accessed May 25, 2018.
- 6.Mangan D. Mylan hit with racketeering suit over big price hikes of EpiPen. https://www.cnbc.com/2017/04/03/mylan-hit-with-racketeering-suit-over-big-price-hikes-of-epipen.html. Published April 3, 2007. Accessed May 25, 2018.
- 7.Duhigg C. Outcry over EpiPen prices hasn’t made them lower. New York Times https://www.nytimes.com/2017/06/04/business/angry-about-epipen-prices-executive-dont-care-much.html. Published June 4, 2017. Accessed May 25, 2018.
- 8.Scutti S. “Short-term” EpiPen shortage, anticipates FDA. https://edition.cnn.com/2018/05/09/health/epipen-fda-drug-shortages-list-bn/index.html. Published May 9, 2018. Accessed May 25, 2018.
- 9.Shaker M, Kanaoka T, Murray RGP, Toy D, Shaker S, Drew A. A survey of caregiver perspectives on emergency epinephrine autoinjector sharing. J Allergy Clin Immunol Pract. 2018;6(5):1792-1795. doi: 10.1016/j.jaip.2018.02.017 [DOI] [PubMed] [Google Scholar]
- 10.Shaker M, Bean K, Verdi M. Economic evaluation of epinephrine auto-injectors for peanut allergy. Ann Allergy Asthma Immunol. 2017;119(2):160-163. doi: 10.1016/j.anai.2017.05.020 [DOI] [PubMed] [Google Scholar]
- 11.Oria MP, Stallings VA, eds. Finding a Path to Safety in Food Allergy: Assessment of the Global Burden, Causes, Prevention, Management, and Public Policy. Washington, DC: National Academies Press; 2016. [PubMed] [Google Scholar]
- 12.Wang J, Sicherer SH. Section on Allergy and Immunology . Guidance on completing a written allergy and anaphylaxis emergency plan. Pediatrics. 2017;139(3):e20164005. doi: 10.1542/peds.2016-4005 [DOI] [PubMed] [Google Scholar]
- 13.Song TT, Brown D, Karjalainen M, Lehnigk U, Lieberman P. Value of a second dose of epinephrine during anaphylaxis: a patient/caregiver survey. J Allergy Clin Immunol Pract. 2018;6(5):1559-1567. doi: 10.1016/j.jaip.2018.01.019 [DOI] [PubMed] [Google Scholar]
- 14.Shaker M, Kanaoka T, Feenan L, Greenhawt M. An economic evaluation of immediate versus non-immediate activation of emergency medical services after epinephrine use for peanut-induced anaphylaxis [published online July 16, 2018]. Ann Allergy Asthma Immunol. doi: 10.1016/j.anai.2018.06.035 [DOI] [PubMed] [Google Scholar]
- 15.Shaker M, Greenhawt M. The health and economic outcomes of peanut allergy management practices [published online May 8, 2018]. J Allergy Clin Immunol Pract. doi: 10.1016/j.jaip.2018.04.036 [DOI] [PubMed] [Google Scholar]
- 16.Arias E, Heron M, Xu J. United States life tables, 2013. Natl Vital Stat Rep. 2017;66(3):1-64. [PubMed] [Google Scholar]
- 17.Umasunthar T, Leonardi-Bee J, Hodes M, et al. . Incidence of fatal food anaphylaxis in people with food allergy: a systematic review and meta-analysis. Clin Exp Allergy. 2013;43(12):1333-1341. doi: 10.1111/cea.12211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vander Leek TK, Liu AH, Stefanski K, Blacker B, Bock SA. The natural history of peanut allergy in young children and its association with serum peanut-specific IgE. J Pediatr. 2000;137(6):749-755. doi: 10.1067/mpd.2000.109376 [DOI] [PubMed] [Google Scholar]
- 19.Shaker MS. An economic analysis of a peanut oral immunotherapy study in children. J Allergy Clin Immunol Pract. 2017;5(6):1707-1716. doi: 10.1016/j.jaip.2017.04.016 [DOI] [PubMed] [Google Scholar]
- 20.Skolnick HS, Conover-Walker MK, Koerner CB, Sampson HA, Burks W, Wood RA. The natural history of peanut allergy. J Allergy Clin Immunol. 2001;107(2):367-374. doi: 10.1067/mai.2001.112129 [DOI] [PubMed] [Google Scholar]
- 21.Chicago Public Schools. School progress reports. https://www.cps.edu/SCHOOLDATA/Pages/SchoolProgressReports.aspx. Accessed September 17, 2018.
- 22.Patel DA, Holdford DA, Edwards E, Carroll NV. Estimating the economic burden of food-induced allergic reactions and anaphylaxis in the United States. J Allergy Clin Immunol. 2011;128(1):110-115.e5. doi: 10.1016/j.jaci.2011.03.013 [DOI] [PubMed] [Google Scholar]
- 23.Bureau of Labor Statistics, United States Department of Labor http://www.bls.gov. Accessed September 28, 2017.
- 24.Gupta R, Holdford D, Bilaver L, Dyer A, Holl JL, Meltzer D. The economic impact of childhood food allergy in the United States. JAMA Pediatr. 2013;167(11):1026-1031. doi: 10.1001/jamapediatrics.2013.2376 [DOI] [PubMed] [Google Scholar]
- 25.Mittmann N, Trakas K, Risebrough N, Liu BA. Utility scores for chronic conditions in a community-dwelling population. Pharmacoeconomics. 1999;15(4):369-376. doi: 10.2165/00019053-199915040-00004 [DOI] [PubMed] [Google Scholar]
- 26.Carroll AE, Downs SM. Improving decision analyses: parent preferences (utility values) for pediatric health outcomes. J Pediatr. 2009;155(1):21-25, 25.e1-25.e5. doi: 10.1016/j.jpeds.2009.01.040 [DOI] [PubMed] [Google Scholar]
- 27.Winkelmayer WC, Weinstein MC, Mittleman MA, Glynn RJ, Pliskin JS. Health economic evaluations: the special case of end-stage renal disease treatment. Med Decis Making. 2002;22(5):417-430. doi: 10.1177/027298902320556118 [DOI] [PubMed] [Google Scholar]
- 28.Simons FE, Ebisawa M, Sanchez-Borges M, et al. . 2015 update of the evidence base: World Allergy Organization anaphylaxis guidelines. World Allergy Organ J. 2015;8(1):32. doi: 10.1186/s40413-015-0080-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lieberman P, Nicklas RA, Randolph C, et al. . Anaphylaxis: a practice parameter update 2015. Ann Allergy Asthma Immunol. 2015;115(5):341-384. doi: 10.1016/j.anai.2015.07.019 [DOI] [PubMed] [Google Scholar]
- 30.Sampson HA, Aceves S, Bock SA, et al. ; Joint Task Force on Practice Parameters; Practice Parameter Workgroup . Food allergy: a practice parameter update-2014. J Allergy Clin Immunol. 2014;134(5):1016-25.e43. doi: 10.1016/j.jaci.2014.05.013 [DOI] [PubMed] [Google Scholar]
- 31.Young MC, Muñoz-Furlong A, Sicherer SH. Management of food allergies in schools: a perspective for allergists. J Allergy Clin Immunol. 2009;124(2):175-182. doi: 10.1016/j.jaci.2009.04.004 [DOI] [PubMed] [Google Scholar]
- 32.Epi-Pen supply information. https://www.epipen.com/about-epipen-and-generic/supply-information. Accessed June 3, 2018.
- 33.City of Chicago SD 299. Total school days. https://www.illinoisreportcard.com/district.aspx?source=environment&source2=numberschooldays&Districtid=15016299025. Accessed September 17, 2018.
- 34.Bartnikas LM, Huffaker MF, Sheehan WJ, et al. . Impact of school peanut-free policies on epinephrine administration. J Allergy Clin Immunol. 2017;140(2):465-473. doi: 10.1016/j.jaci.2017.01.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Allergy & Asthma Network School stock epinephrine laws. http://www.allergyasthmanetwork.org/advocacy/current-issues/stock-epinephrine/. Accessed April 2018.
- 36.Greenhawt M, Wallace D, Sublett JW, et al. . Current trends in food allergy–induced anaphylaxis management at school. Ann Allergy Asthma Immunol. 2018;121(2):174-178. doi: 10.1016/j.anai.2018.04.015 [DOI] [PubMed] [Google Scholar]
- 37.Barry F. Sanofi says injectable recall could cost company $110m. https://www.inpharmatechnologist.com/Article/2015/11/02/Sanofi-says-injectable-recall-could-cost-company-110m. Accessed June 3, 2018.
- 38.Camargo CA Jr, Guana A, Wang S, Simons FE. Auvi-Q versus EpiPen: preferences of adults, caregivers, and children. J Allergy Clin Immunol Pract. 2013;1(3):266-72.e1, 3. doi: 10.1016/j.jaip.2013.02.004 [DOI] [PubMed] [Google Scholar]
- 39.Kaltenboeck A, Bach PB. Value-based pricing for drugs: theme and variations. JAMA. 2018;319(21):2165-2166. doi: 10.1001/jama.2018.4871 [DOI] [PubMed] [Google Scholar]
- 40.Bach PB, Pearson SD. Payer and policy maker steps to support value-based pricing for drugs. JAMA. 2015;314(23):2503-2504. doi: 10.1001/jama.2015.16843 [DOI] [PubMed] [Google Scholar]
- 41.White MV, Hogue SL, Bennett ME, et al. . EpiPen4Schools pilot survey: occurrence of anaphylaxis, triggers, and epinephrine administration in a U.S. school setting. Allergy Asthma Proc. 2015;36(4):306-312. doi: 10.2500/aap.2015.36.3859 [DOI] [PubMed] [Google Scholar]
- 42.Turner PJ, Jerschow E, Umasunthar T, Lin R, Campbell DE, Boyle RJ. Fatal anaphylaxis: mortality rate and risk factors. J Allergy Clin Immunol Pract. 2017;5(5):1169-1178. doi: 10.1016/j.jaip.2017.06.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Simulation Health States