Key Points
Question
What is the effect of a nurse-led preventive, complex psychological intervention delivered to critically ill patients and initiated in the intensive care unit on posttraumatic stress disorder (PTSD) symptom severity?
Findings
In this cluster-randomized clinical trial, involving 1458 patients, there was no significant difference in patient-reported PTSD symptom severity at 6 months (adjusted difference of −0.03 on the PTSD Symptom Scale; score range, 0-51, with higher scores indicating greater symptom severity; the minimal clinically important difference was considered to be 4.2 points).
Meaning
A nurse-led preventive, complex psychological intervention delivered to critically ill patients and initiated in the intensive care unit did not significantly reduce patient-reported PTSD symptom severity at 6 months.
Abstract
Importance
A meta-analysis of outcomes during the 6 months after intensive care unit (ICU) discharge indicate a prevalence for clinically important posttraumatic stress disorder (PTSD) symptoms of 25%.
Objective
To determine whether a nurse-led preventive, complex psychological intervention, initiated in the ICU, reduces patient-reported PTSD symptom severity at 6 months.
Design, Setting, and Participants
A multicenter, parallel-group, cluster-randomized clinical trial with integrated economic and process evaluations conducted in 24 ICUs in the United Kingdom. Participants were critically ill patients who regained mental capacity following receipt of level 3 (intensive) care. A total of 2961 eligible patients were identified from September 2015 to January 2017. A total of 2048 were approached for participation in the ICU, of which 1458 provided informed consent. Follow-up was completed December 2017.
Interventions
Twenty four ICUs were randomized 1:1 to the intervention or control group. Intervention ICUs (n = 12; 669 participants) delivered usual care during a baseline period followed by an intervention period. The preventive, complex psychological intervention comprised promotion of a therapeutic ICU environment plus 3 stress support sessions and a relaxation and recovery program delivered by trained ICU nurses to high-risk (acutely stressed) patients. Control ICUs (n = 12; 789 participants) delivered usual care in both baseline and intervention periods.
Main Outcomes and Measures
The primary clinical outcome was PTSD symptom severity among survivors at 6 months measured using the PTSD Symptom Scale–Self-Report questionnaire (score range, 0-51, with higher scores indicating greater symptom severity; the minimal clinically important difference was considered to be 4.2 points).
Results
Among 1458 enrolled patients (mean [SD] age, 58 [16] years; 599 women [41%]), 1353 (93%) completed the study and were included in the final analysis. At 6 months, the mean PTSD Symptom Scale–Self-Report questionnaire score in intervention ICUs was 11.8 (baseline period) compared with 11.5 (intervention period) (difference, −0.40 [95% CI, −2.46 to 1.67]) and in control ICUs, 10.1 (baseline period) compared with 10.2 (intervention period) (difference, 0.06 [95% CI, −1.74 to 1.85]) between periods. There was no significant difference in PTSD symptom severity at 6 months (treatment effect estimate [difference in differences] of −0.03 [95% CI, −2.58 to 2.52]; P = .98).
Conclusions and Relevance
Among critically ill patients in the ICU, a nurse-led preventive, complex psychological intervention did not significantly reduce patient-reported PTSD symptom severity at 6 months. These findings do not support the use of this psychological intervention.
Trial Registration
This cluster-randomized clinical trial compares the effect of a nurse-led intervention intended to alleviate acute stress and memories of frightening intensive care unit (ICU) experiences vs usual care on patient-reported posttraumatic stress disorder (PTSD) symptom severity 6 months after ICU discharge.
Introduction
Among patients admitted to the intensive care unit (ICU), a meta-analysis of outcomes for survivors, during the first 6 months after ICU discharge, indicated a pooled prevalence for clinically important posttraumatic stress disorder (PTSD) symptoms of 25%.1 Acute stress while in the ICU2,3 and early memories of frightening ICU experiences (eg, hallucinations, paranoid delusions, and nightmares)1 have been identified as independent risk factors for longer-term psychological morbidity, including PTSD symptom severity. Evidence suggests that addressing these risk factors early, commencing while in the ICU, might prevent longer-term consequences4 and that addressing such factors after discharge may be too late.5 Research on ICU patients with trauma indicated that fewer experienced PTSD symptoms if they were seen by clinical psychologists while in the ICU.6
Cognitive behavioral therapy (CBT) techniques have been found to be effective in reducing symptoms of stress in patients with mental or physical illness,7 mitigating hallucinations and paranoid delusions in mental health settings,8 and in reducing PTSD symptoms.9 Studies have evaluated CBT techniques as effective when delivered by nonexperts (including nurses) to patients in physical and mental health settings.10,11
A UK National Institute for Health and Care Excellence evidence update9 on PTSD suggested that an early (immediate), brief (3 sessions), trauma-focused CBT intervention “may reduce the development of subsequent trauma symptoms more than no such intervention” and called for further research. It was hypothesized that a preventive, complex psychological intervention initiated in the ICU would reduce development of patient-reported PTSD symptom severity at 6 months. Acknowledging that few National Health Service ICUs have regular access to psychologists and evidence indicating that nonexperts can be trained to deliver effective psychological interventions, it was decided to train ICU-selected nurses to deliver a preventive, complex psychological intervention. Following intervention feasibility testing and refinement,12 its clinical and cost-effectiveness were evaluated in a cluster-randomized clinical trial.13
Methods
Study Design and Oversight
The South Central–Oxford B research ethics committee approved the protocol, which is available in Supplement 1. All participants provided written informed consent.
The POPPI (Psychological Outcomes Following a Nurse-Led Preventive Psychological Intervention for Critically Ill Patients) trial was a multicenter, parallel-group, cluster-randomized clinical trial, with integrated economic and process evaluations.13 Randomization was at the ICU (cluster) level because it would not be possible to restrict parts of the preventive, complex psychological intervention to individual patients.
The UK National Institute for Health Research convened an independently chaired trial steering committee and independent data monitoring and ethics committee. The Intensive Care National Audit & Research Centre (ICNARC) Clinical Trials Unit managed the trial.
Sites and Patients
The trial was conducted in 24 adult general ICUs. ICUs were eligible if they were active participants in the ICNARC Case Mix Programme, able to adhere to randomization and deliver the intervention, and could demonstrate potential to recruit to target. Stand-alone high-dependency units and specialist critical care units were excluded, along with ICUs offering formal psychological support (ICU diary use was permitted as not deemed an early intervention). Adult patients (≥18 years of age) were eligible if they met the inclusion criteria, which included at least 48 hours in the ICU, receipt of level 3 (intensive) care (eg, advanced respiratory monitoring and support or monitoring and support for ≥2 organ systems) during that time, between a score of +1 and −1 on the Richmond Agitation Sedation Scale,14 Glasgow Coma Scale score of 15,15 English-speaking, and able to communicate orally. Patients were approached to participate while in the ICU. State anxiety, using the State Trait Anxiety Inventory (6-item version, STAI-6),16 and health-related quality of life (HrQoL) using a visual analogue scale, were assessed at time of consent (see eMethods in Supplement 2).
Randomization and Treatment Groups
Using a stepped rollout, ICUs were allocated geographically, into 3 steps of 8, opening at 2-month intervals. ICUs in each step were randomized 1:1 to the intervention (n = 4) or control (n = 4) group in the second month of the baseline period. Allocation was conducted using a restricted randomization approach to ensure balance of hospital teaching status and size of the ICU.17 The recruitment period was 17 months (eFigure 1 in Supplement 2).
Intervention
The preventive, complex psychological intervention was designed to alleviate acute stress and memories of frightening ICU experiences such as hallucinations, paranoid delusions, and nightmares. It comprised promotion of a therapeutic environment (via online training) and stress support for high-risk patients delivered by trained ICU nurses. Building on existing evidence and drawing on theories and techniques of CBT for reducing stress, trauma, hallucinations, and paranoid delusions adapted for use with ICU patients, the content and training courses and materials for delivery of the complex intervention were informed, developed, and refined by the lead adult ICU health psychologist (D.M.W.), supported by 2 senior psychologists (C.R.B. and J. Weinman), with oversight from an expert psychology advisory group, independently chaired by a senior clinical psychologist (Daniel Freeman, PhD, DClinPsy, University of Oxford, Oxford, United Kingdom). The expert psychology advisory group also included expertise in clinical education, ICU nursing, and lived experience from 3 former ICU patients.
Intervention ICUs delivered usual care during the baseline period (months 1-5) followed by a 1-month transition period (month 6). At the start of the transition period, all patient-facing ICU staff were provided access to the online training and ICU nurses, self-selected by ICUs, attended a 3-day training course (led by D.M.W.) on the content and delivery of the stress support. Following practical experience in their ICUs, at the end of the transition period, the trained ICU nurses were assessed for competency.
During the intervention period (month 7 onwards), ICU staff were encouraged to engage in activities to promote a therapeutic environment. In parallel, patients who had consented were assessed for acute stress using the Intensive care Psychological Assessment Tool (IPAT).18 Patients scoring 7 points or more (high risk) were offered 3 one-to-one stress support sessions. These incorporated CBT approaches such as emotional expression, normalization, psychoeducation, cognitive reappraisal, and “homework” tasks. Sessions were intended to last approximately 30 minutes each, be delivered by the same trained ICU nurse, and be completed within a week in hospital. Patients were reassessed with the STAI-6 after completion of the third session. Between sessions, patients could use the relaxation and recovery program (incorporating coping strategies, such as relaxing music and meditation exercises, and patient recovery stories) on a tablet computer. A DVD with similar content and a self-help booklet, with a codesigned personal action plan, were provided to patients for use after hospital discharge (eFigure 2 in Supplement 2).
Debriefing and support were provided to the trained ICU nurses (eMethods in Supplement 2). An independent, integrated process evaluation was conducted (eMethods in Supplement 2). Control ICUs delivered usual care throughout.
Outcome Measures
The primary clinical effectiveness outcome was mean patient-reported PTSD symptom severity, measured using the PTSD Symptom Scale–Self-Report (PSS-SR) questionnaire,19 among survivors at 6 months. Scores on the PSS-SR questionnaire range from 0 to 51, with higher scores indicating greater symptom severity (see eTables 1 and 2 and eFigure 3 in Supplement 2 for prior psychometric evaluation). To our knowledge, no studies have been conducted to establish a minimal clinically important difference; a value of 4.2 points was established from feasibility studies and baseline trial data (see the Statistical Analysis section). The cost-effectiveness analysis reported incremental costs, including those of adopting the intervention, quality-adjusted life-years, and incremental net monetary benefit of the intervention at 6 months (eTables 3 to 4 in Supplement 2).
Secondary outcomes were days alive and free from sedation to day 30; duration of ICU stay; and, among survivors at 6 months, PSS-SR questionnaire threshold for prediction of current or future PTSD (>18 points),20 anxiety and depression (measured using the Hospital Anxiety and Depression Scale [HADS])21, and HrQoL (measured using the European Quality of Life–5 Dimensions 5-level questionnaire).22 HADS comprises 2 subscales (anxiety and depression); subscale scores range from 0 to 21, with higher scores indicating worse severity and a value of 8 points considered the threshold for likely anxiety or depression. The European Quality of Life–5 Dimensions 5-level questionnaire utility scale ranges from −0.285 to 1, with lower scores indicating worse HrQoL, anchored at 0 (death) and 1 (perfect health).23 For details of all outcomes, see eMethods in Supplement 2.
Statistical Analysis
The original design was for 24 ICUs to recruit for 11 months, consisting of a 5-month baseline, a 1-month transition, and a 5-month intervention period. The proposed design was reviewed following completion of feasibility studies,12 retaining 90% power with a 5% type I error rate, and using the method of Hussey and Hughes24 for a general, multiperiod, cluster-randomized clinical trial, under the following assumptions based on data from the feasibility studies: a mean (SD) PSS-SR score of 6 (7.5) points; an estimated intracluster correlation of 0.138, based on an assumption of 0.5 for the between-ICU coefficient of variation; a detectable treatment effect of a reduction of 2.9 points on the PSS-SR questionnaire (based on a difference equivalent to the reliable change index for the PSS-SR score of 8.6 points calculated from feasibility study data using the method of Jacobson and Truax25 being observed among 40% of patients assessed as acutely stressed and receiving stress support sessions and assuming 16% declined the intervention); and an estimated harmonic mean of the number of patients completing follow-up of 22 per ICU during each 5-month period (anticipating mortality of 10% and loss to follow-up of 20% at 6 months).
The expected total number of patients to be recruited was 1914. The pretrial power calculation was updated during the baseline period. Using available data, the mean (SD) PSS-SR score in the control group at 6 months was estimated as 10.3 (10.8) and an intracluster correlation of 0.087. Based on a minimal clinically important difference of 4.2 points (retaining the same effect size as a multiple of the SD) and a harmonic mean of 16.5 patients per ICU completing follow-up, a minimum of 1378 patients were needed to achieve 85% power. Recruitment was therefore extended in the intervention period to ensure this minimum sample size was achieved.
Patients were analyzed according to their randomization group and all analyses were prespecified.26 Multiple imputation was used to address missing baseline, outcome, and resource use data under the assumption that responses were missing at random, conditional on the observed data (eTable 5 in Supplement 2).27,28 The final analyses were conducted using Stata/SE version 14.2 (StataCorp LP). Multiple imputation was performed in R version 3.3.2 (The R Foundation for Statistical Computing; Vienna, Austria). Sensitivity analyses assumed data were (1) missing completely at random (using complete case data) and (2) missing not at random (using expert elicitation) (eMethods in Supplement 2). A P value of less than .05 indicated statistical significance. All tests were 2-sided, with no adjustment for multiple comparisons (secondary analyses should therefore be interpreted as exploratory). Continuous variables are reported as mean and SD or median and interquartile range. Categorical variables are reported as proportions. Patients recruited during the 1-month transition period were excluded from the primary analysis.
We used generalized linear mixed models, with identity link at the individual patient level (patients nested within ICUs within treatment group/time periods), to compare differences in the clinical primary outcome. The primary effect estimate is the interaction between treatment group and time period. Secondary analyses of the primary outcome included estimation of the adherence-adjusted causal effect of the stress support sessions. Analyses of secondary outcomes were performed using generalized linear mixed models with identity link for continuous and logit link for binary outcomes. Analyses were adjusted for age, sex, race/ethnicity (extracted from the patient record, coded using standard National Health Service categories), deprivation, preexisting anxiety/depression, planned admission following elective surgery, and ICNARC Physiology Score.29 These factors were selected a priori from a systematic review of risk factors for PTSD after critical care.30
We conducted prespecified subgroup analyses by testing interactions between the effect of the intervention and age, sex, socioeconomic status,31 duration of delirium, STAI-6 score16 (strongly correlated with IPAT18), surgical status, predicted PSS-SR score,32 and degree of intervention implementation (assessing dose, reach, and fidelity) in intervention ICUs (eTable 6 in Supplement 2).
Results
Sites and Participants
All 24 ICUs opened to recruitment between September 2015 and January 2016. When compared with nonparticipating ICUs, participating ICUs, while larger (beds and admissions), were representative of teaching status and geography (eTable 7 in Supplement 2). ICU diary use was reported at 9 intervention and 7 control ICUs. All 38 ICU nurses (3 from each intervention ICU and 2 replacements) were trained and assessed as competent.
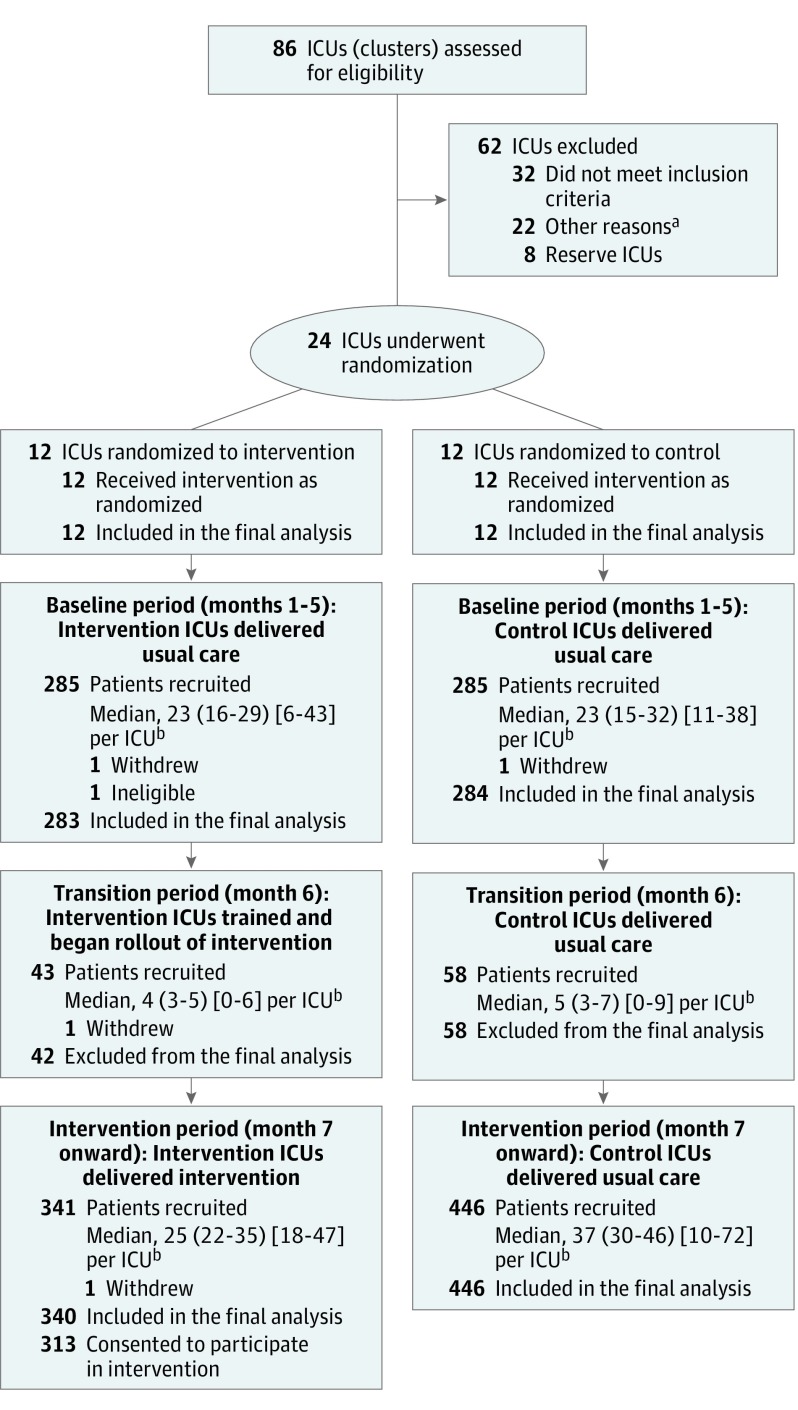
Of 2961 potentially eligible patients, 2048 were approached for informed consent and 1458 consented and were recruited. Five withdrew consent, resulting in 1453 (100 recruited during the transition period were excluded from the primary analyses, as planned). Similar eligibility rates were observed across treatment group and time period, but refusal of consent was more frequent at intervention ICUs during the intervention period (eTable 8 in Supplement 2). Figure 1 shows the CONSORT flow diagram33 of ICUs and patients through the trial. Participants were well matched across treatment group and time period and representative of the target ICU population (Table 1; eTables 9-12 in Supplement 2).
Figure 1. Recruitment, Randomization, and Assessment of Intensive Care Units (ICUs) and Patients.
aOther reasons included poor performance in previous multicenter randomized clinical trials, low evidence of ICU-wide enthusiasm and engagement, already delivering early psychological support, and had planned to increase psychological support during the trial period.
bMedian number (interquartile range) [range] of patients per ICU are shown.
Table 1. Characteristics of Patients in the POPPI Trial.
| Characteristic | Intervention ICUs, No. (%) | Control ICUs, No. (%) | ||
|---|---|---|---|---|
| Baseline Period | Intervention Period | Baseline Period | Intervention Period | |
| No. of patients | 283 | 340 | 284 | 446 |
| Age, mean (SD), y | 59.5 (16.0) | 60.4 (15.0) | 57.2 (16.2) | 57.2 (15.6) |
| Sex | ||||
| Male | 168 (59.4) | 187 (55.0) | 179 (63.0) | 268 (60.1) |
| Female | 115 (40.6) | 153 (45.0) | 105 (37.0) | 178 (39.9) |
| Race/ethnicitya | ||||
| White | 254 (89.8) | 320 (94.1) | 264 (93.0) | 406 (91.0) |
| Mixed | 0 (0.0) | 1 (0.3) | 1 (0.4) | 2 (0.4) |
| Asian | 4 (1.4) | 1 (0.3) | 3 (1.1) | 6 (1.3) |
| Black | 7 (2.5) | 3 (0.9) | 1 (0.4) | 2 (0.4) |
| Other | 8 (2.8) | 2 (0.6) | 0 (0.0) | 4 (0.9) |
| Not stated | 10 (3.5) | 13 (3.8) | 15 (5.3) | 26 (5.8) |
| Quintile of Index of Multiple Deprivation 2015b | ||||
| 1 (Least deprived) | 41 (14.5) | 57 (16.9) | 57 (20.1) | 95 (21.3) |
| 2 | 46 (16.3) | 74 (21.9) | 65 (22.9) | 107 (24.0) |
| 3 | 56 (19.8) | 76 (22.5) | 52 (18.3) | 73 (16.4) |
| 4 | 71 (25.1) | 73 (21.6) | 57 (20.1) | 88 (19.8) |
| 5 (Most deprived) | 69 (24.4) | 58 (17.2) | 53 (18.7) | 82 (18.4) |
| Documented preexisting anxiety/depressionc | ||||
| Anxiety | 3 (1.1) | 12 (3.5) | 4 (1.4) | 9 (2.0) |
| Depression | 19 (6.7) | 32 (9.4) | 19 (6.7) | 33 (7.4) |
| Both | 17 (6.0) | 21 (6.2) | 8 (2.8) | 13 (2.9) |
| Elective surgical admission | 17 (6.0) | 20 (5.9) | 24 (8.5) | 37 (8.3) |
| Selected ICU admission diagnoses | ||||
| Pneumonia | 41 (14.5) | 60 (17.6) | 41 (14.4) | 53 (11.9) |
| Trauma | 26 (9.2) | 26 (7.6) | 23 (8.1) | 30 (6.7) |
| Tumor or malignancy | 14 (4.9) | 22 (6.5) | 23 (8.1) | 32 (7.2) |
| Coma or seizures | 11 (3.9) | 15 (4.4) | 21 (7.4) | 37 (8.3) |
| ICNARC Physiology Score, mean (SD)d | 21.1 (7.0) | 21.0 (7.6) | 21.2 (7.1) | 21.4 (7.2) |
| APACHE II score, mean (SD)e | 16.9 (6.5) | 17.7 (6.4) | 16.7 (5.8) | 16.9 (6.2) |
| CAM-ICU–positive (delirium) duration in ICU prior to consent, median (IQR), df | 1 (0-2) | 1 (0-2) | 1 (0-3) | 2 (1-3) |
| Assessed, No. | 162 | 147 | 113 | 180 |
| Time from ICU admission to consent, median (IQR), d | 7 (4-13) | 9 (5-15) | 7 (4-12) | 8 (5-14) |
| Last NEWS prior to consent, mean (SD)g | 3.2 (2.2) | 2.8 (2.1) | 3.1 (2.4) | 2.8 (2.4) |
| STAI-6 score at time of consent, median (IQR)h | 43 (33-57) | 43 (30-55) | 43 (30-53) | 43 (33-50) |
| HrQoL (health thermometer score) at time of consent, median (IQR)i | 50 (35-70) | 50 (30-70) | 50 (40-70) | 50 (40-70) |
Abbreviations: APACHE, Acute Physiology and Chronic Health Evaluation; CAM-ICU, Confusion Assessment Method for the Intensive Care Unit; HrQoL, health-related quality of life; ICNARC, Intensive Care National Audit & Research Centre; ICU, intensive care unit; IQR, interquartile range; NEWS, National Early Warning Score; STAI-6, State Trait Anxiety Inventory.
Information on race/ethnicity was collected as part of the Case Mix Programme data set and was ascertained by review of medical records. This field was collected to help describe the demographics of the patient population and assess the representativeness of the sample.
Higher values indicate greater deprivation.
Documented preexisting anxiety/depression was ascertained by review of medical records.
Scores on the ICNARC Physiology Score range from 0 to 100 (higher scores indicate greater severity of illness). The score was calculated using physiology readings from the first 24 hours following ICU admission.
Scores on the APACHE II range from 0 to 71 (higher scores indicate greater severity of illness). The score was calculated using physiology readings from the first 24 hours following ICU admission. A score of 17 on day 1 corresponds to hospital mortality of around 18%.
Not all patients were assessed for delirium prior to consent due to either being ventilated and sedated (therefore not assessable) or the CAM-ICU not being routinely used or documented in the medical records.
Scores on the NEWS range from 0 to 20 (higher scores indicate greater severity of illness) and calculated from the last physiology readings before consent.
Scores on the 6-item STAI range from 20 to 80 (higher scores indicate greater anxiety). Patients with a mean of 43 on the STAI-6 are above the cut point for clinically significant symptoms of state (“at this moment”) anxiety. STAI-6 was self-reported by patients at the time of consent.
HrQoL scores range from 0 (“the worst health you can imagine”) to 100 (“the best health you can imagine”). HrQoL was self-reported by patients at the time of consent.
Delivery of the Intervention
By the end of the transition period, 971 of 1669 ICU staff members had completed the online training, equating to a median percentage of staff completing online training of 58% (interquartile range, 49% to 69%), with all ICUs achieving 80% (prespecified minimum target) by intervention period month 3 (eFigure 4 in Supplement 2). Some ICUs reinforced the online training with education. Initiatives observed during the process evaluation included optimization of sleep (through sleep packs, night-time lighting, and clustering care); reduction of noise (through soft-close bins and minimization of alarm and telephone noise); improved patient orientation (through clocks, staff-patient interaction, and whiteboards); and increased family involvement. Some ICUs reported difficulties both in changing long-standing practices and being restricted by the environmental limitations of the unit.
Of the 340 patients recruited at intervention ICUs during the intervention period, 27 consented solely to questionnaire follow-up, leaving 313 who consented to assessment with the IPAT and subsequent stress support sessions (where applicable). All 313 patients were assessed with the IPAT and, of these, 199 (63.6%) scored 7 or more and were eligible to receive the stress support sessions: 127 (63.8%) received 3, 33 (16.6%) received 2, 21 (10.6%) received 1, and 18 (9.0%) received none (eFigure 5 in Supplement 2). Subsequent sessions were not delivered to 49 patients due to discharge from hospital, with only 14 declining further sessions (eTable 13 in Supplement 2). The first stress support session was delivered in the ICU for 72 patients (39.8%) (eTable 14 in Supplement 2). Of the 181 patients who had at least 1 session, 170 (93.9%) accepted a tablet computer and, of these, 130 (76.5%) reported using the relaxation and recovery program. Medical interventions received in the ICU are summarized in eTable 15 in Supplement 2.
No changes in psychological support were observed at control ICUs during the trial.
Patient Follow-up
At follow-up, 978 patients (79.3%) who survived to 6 months completed questionnaires (range, 78.4% to 79.9% across treatment group and time period), with no difference in response rates or characteristics of responders between group or period (eTables 16 and 17 in Supplement 2).
Effectiveness
There was no significant difference in the primary outcome. At 6 months, the mean PSS-SR score among survivors recruited in intervention ICUs was 11.8 in the baseline compared with 11.5 in the intervention period. In control ICUs, the mean PSS-SR score was 10.1 in the baseline compared with 10.2 in the intervention period. After adjustment, this corresponded to a primary treatment effect (interaction between treatment group and time period) estimate of −0.03 (95% CI, −2.58 to 2.52; P = .98) (Table 2). Among patients receiving at least 2 stress support sessions, the adherence-adjusted causal effect of the intervention on mean PSS-SR score was −0.18 (95% CI, −5.50 to 5.14; P = .95).
Table 2. Primary and Secondary Outcomes.
| Intervention ICUs | Control ICUs | Difference in Differencea | P Value | ICC (95% CI) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline Period, Mean (95% CI) | Intervention Period, Mean (95% CI) | Difference (95% CI) | Baseline Period, Mean (95% CI) | Intervention Period, Mean (95% CI) | Difference (95% CI) | ||||
| Primary Outcome at 6 mob | |||||||||
| No. of patients | 245 | 314 | 259 | 415 | |||||
| PSS-SR symptom severity scorec | 11.8 (10.3 to 13.3) | 11.5 (10.0 to 12.9) | −0.40 (−2.46 to 1.67) | 10.1 (8.7 to 11.6) | 10.2 (9.1 to 11.3) | 0.06 (−1.74 to 1.85) | −0.03 (−2.58 to 2.52) | .98 | 0.01 (0.00 to 0.40) |
| Secondary Outcomes | |||||||||
| Short-term | |||||||||
| No. of patients | 283 | 340 | 284 | 446 | |||||
| Days alive and free from sedation to day 30 | 23.0 (22.1 to 23.9) | 23.3 (22.4 to 24.1) | 0.24 (−0.98 to 1.47) | 24.3 (23.6 to 25.0) | 24.0 (23.4 to 24.7) | −0.30 (−1.29 to 0.69) | 0.47 (−1.03 to 1.96) | .54 | 0.00 (0.00 to 0.94) |
| Duration of ICU stay, d | 14.0 (12.1 to 15.8) | 14.6 (12.7 to 16.4) | 0.61 (−2.02 to 3.23) | 12.2 (10.6 to 13.8) | 13.5 (12.2 to 14.8) | 1.31 (−0.79 to 3.41) | −0.28 (−3.45 to 2.88) | .86 | 0.00 (0.00 to 0.00) |
| At 6 mob | |||||||||
| No. of patients | 245 | 314 | 259 | 415 | |||||
| PSS-SR >18 pointsc | 23.9 (18.1 to 29.7)d | 24.1 (18.8 to 29.4)d | 1.01 (0.66 to 1.56)e | 19.8 (14.5 to 25.3)d | 17.6 (13.6 to 22.0)d | 0.87 (0.56 to 1.35)e | 1.32 (0.66 to 2.67)f | .43 | 0.00 (0.00 to 1.00) |
| HADS anxiety scoreg | 6.9 (6.2 to 7.6) | 6.3 (5.7 to 7.0) | −0.60 (−1.57 to 0.37) | 5.9 (5.3 to 6.7) | 5.7 (5.2 to 6.2) | −0.26 (−1.13 to 0.62) | −0.24 (−1.50 to 1.01) | .70 | 0.01 (0.00 to 0.50) |
| HADS depression scoreg | 6.0 (5.3 to 6.7) | 5.8 (5.1 to 6.4) | −0.28 (−1.21 to 0.65) | 5.3 (4.7 to 6.0) | 5.3 (4.8 to 5.8) | 0.01 (−0.82 to 0.84) | −0.22 (−1.40 to 0.95) | .71 | 0.00 (0.00 to 1.00) |
| EQ-5D-5L utility scoreh | 0.66 (0.62 to 0.70) | 0.67 (0.63 to 0.71) | 0.01 (−0.05 to 0.06) | 0.70 (0.66 to 0.74) | 0.69 (0.66 to 0.72) | −0.01 (−0.06 to 0.04) | 0.01 (−0.06 to 0.08) | .85 | 0.02 (0.01 to 0.07) |
Abbreviations: EQ-5D-5L, European Quality of Life–5 Dimensions 5-level questionnaire; HADS, Hospital Anxiety and Depression Scale; ICC, intracluster correlation; ICU, intensive care unit; ICNARC, Intensive Care National Audit & Research Centre; MCID, minimal clinically important difference; PSS-SR, PTSD Symptom Scale–Self-Report.
Adjusted difference in means (95% CI), unless otherwise indicated. Adjusted for age, sex, race/ethnicity, deprivation, preexisting anxiety/depression, planned admission following elective surgery, and ICNARC Physiology Score. As a post hoc secondary analysis of the primary outcome (clinical effectiveness), the model was refitted including an additional site-level covariate of the natural logarithm of the standardized mortality ratio (ratio of observed to predicted hospital deaths from the ICNARCH-2015 risk prediction model)29 for the period from April 2014 to March 2015. Adjusting for site-level standardized mortality ratio had minimal effect, reporting a treatment effect estimate of −0.14 (95% CI, −2.66 to 2.38; P = .91).
Reported for patients alive at 6 months after applying multiple imputation to handle missing data.
PSS-SR scale is from 0 to 51 (higher scores indicate greater posttraumatic stress symptoms). A value of greater than 18 points is considered the threshold for prediction of likely current or future PTSD. The MCID was considered to be 4.2 points based on observing an improvement equal to the reliable change index among patients receiving stress support sessions.
Percentage (95% CI).
Odds ratio (95% CI).
Adjusted odds ratio (95% CI).
HADS comprises 2 subscales (anxiety and depression), both range from 0 to 21 (higher scores indicate worse severity). A value of 8 points is considered the threshold for likely anxiety or depression; the percentages of patients meeting this threshold across the groups were 40.1%, 38.6%, 35.4%, and 33.7% for anxiety and 36.7%, 33.1%, 29.9%, and 31.3% for depression for intervention ICUs in the baseline and intervention periods and control ICUs in the baseline and intervention periods, respectively. Among survivors of acute respiratory failure, an MCID of 2.0 to 2.5 has been suggested for the anxiety subscale and 1.9 to 2.3 for the depression subscale.35
EQ-5D-5L utility scale ranges from −0.285 to 1 (lower scores indicate worse health-related quality of life). The scale is anchored at 0 (death) and 1 (perfect health). Health utilities were assigned using the EQ-5D-5L value set for England.23 Among patients with chronic respiratory disease, an MCID of around 0.05 has been suggested34; no studies have been conducted to establish an MCID for patients recovering from critical illness.
Of patients surviving to 6 months, 21.2% had missing PSS-SR score data. Sensitivity analyses, using both complete case analysis and expert elicitation, had minimal effect on the primary outcome, reporting treatment effect estimates of −0.02 (95% CI, −2.52 to 2.47; P = .99) and, combining views of all experts, −0.59 (95% credible interval, −3.28 to 2.09). These results were robust to a range of individual expert opinions (eResults, eFigures 6 and 7, and eTables 18 and 19 in Supplement 2).
There were no significant differences between groups in any of the secondary outcomes, either within hospital or at 6 months (Table 2). The proportion of patients with a PSS-SR score of more than 18 points at 6 months in intervention ICUs was 23.9% in the baseline period and 24.1% in the intervention period, compared with 19.8% and 17.6%, respectively, in control ICUs. After adjustment, this resulted in an odds ratio of 1.32 (95% CI, 0.66 to 2.67; P = .43). The mean HADS anxiety score at 6 months in intervention ICUs was 6.9 in the baseline period and 6.3 in the intervention period compared with 5.9 and 5.7, respectively, in control ICUs, resulting in an adjusted difference in difference of −0.24 (95% CI −1.50 to 1.01; P = .70). The mean HADS depression score at 6 months in intervention ICUs was 6.0 in the baseline period and 5.8 in the intervention period compared with 5.3 and 5.3, respectively, in control ICUs, resulting in an adjusted difference in difference of −0.22 (95% CI, −1.40 to 0.95; P = .71).
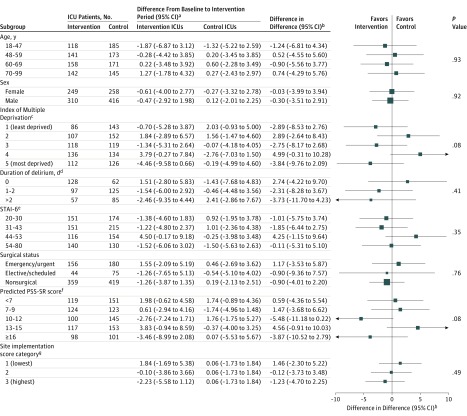
There was no significant interaction between treatment effect and time period on mean PSS-SR scores at 6 months in any of the prespecified subgroups (Figure 2).
Figure 2. Subgroup Analyses of the Primary Clinical Effectiveness Outcome.
ICU indicates intensive care unit; PSS-SR, PTSD Symptom Scale–Self-Report, and STAI-6, State Trait Anxiety Inventory.
aMean (95% CI) of the difference between baseline and intervention periods in mean PSS-SR score. The PSS-SR scale is from 0 to 51 (higher scores indicate greater posttraumatic stress symptoms). A value of 18 points is considered the threshold for prediction of likely current or future PTSD. The minimal clinically important difference was considered to be 4.2 points based on observing an improvement equal to the reliable change index among patients receiving stress support sessions.
bAdjusted for age, sex, race/ethnicity, deprivation, preexisting anxiety/depression, planned admission following elective surgery, and Intensive Care National Audit & Research Centre Physiology Score.
cThe Index of Multiple Deprivation 2015 is reported by quintiles (higher values indicate greater deprivation).
dDuration of delirium is reported as the number of days on which patients were assessed as positive for delirium on the Confusion Assessment Method for the ICU.
eScores on the STAI-6 range from 20 to 80 (higher scores indicate greater anxiety). STAI-6 was self-reported by participants at the time of consent.
fPredicted PSS-SR (heterogeneity of treatment effect) is reported by quintiles from a prediction model for the primary outcome derived using data from patients receiving usual care and adjusted for a priori important covariates (age, sex, socioeconomic status, duration of delirium, STAI-6, and surgical status).
gSite implementation score encompasses dose, fidelity, and reach and is derived from data collected as part of the independent process evaluation (see eMethods and eTable 6 in Supplement 2 for more information). In the intervention ICUs, there were 170, 174, and 215 patients in low, medium, and high score categories, respectively. In the control ICUs, there were 674 patients receiving usual care.
The incremental net monetary benefit comparing the preventive, complex psychological intervention vs usual care was positive (£835 [US $1188]), but the statistical uncertainty surrounding this result was substantial (95% CI, −£4322 to £5992 [US −$6148 to $8523]) (see eResults, eTables 20-26, and eFigures 8-14 in Supplement 2 for full results of the cost-effectiveness analysis).
Post Hoc Analysis
For those patients who received 3 stress support sessions and had data (91%), post hoc analysis observed a reduction in the mean STAI-6 score from 49.3 at the time of consent to 40.3 at the end of their third session (eTable 27 in Supplement 2).
Discussion
This trial found that implementation of a nurse-led preventive, complex psychological intervention, initiated in the ICU, did not significantly reduce PTSD symptom severity at 6 months compared with usual care. There were no statistically significant differences in any of the prespecified subgroups (including degree of implementation of the intervention) or in any of the secondary outcomes (including anxiety, depression, or HrQoL). Levels of PTSD symptom severity were in line with previously reported prevalence in this patient group.
This multicenter, cluster-randomized evaluation of a preventive, complex psychological intervention was delivered alongside routine care in a setting representative of the proposed target population. The complex intervention was informed, developed, and refined according to Medical Research Council guidance by experts with considerable experience in psychology and adult ICUs.12,36 Evidence and theories of CBT for stress, trauma, hallucinations, paranoid delusions, and nightmares—adapted for use with ICU patients—were used. It was designed to be delivered early to reduce development of longer-term psychological morbidity by alleviating acute stress and frightening ICU memories both by improving the ICU environment and providing stress support direct to high-risk patients. It was pragmatic, from a resource perspective, training ICU-selected nurses to deliver it.
There are several reasons why the preventive, complex psychological intervention may not have worked. First, although a recent systematic review has suggested that addressing risk factors early, initiating it in the ICU, may be beneficial,37 the timing of the stress support sessions may have been too early. Perhaps patients are still too ill and fatigued to focus on (and remember) the therapeutic messages taught and/or to make best use of the relaxation and recovery program. In addition, the sessions were based on therapeutic techniques deemed appropriate for patients in early recovery (ie, those potentially still enduring the traumatic experience), precluding the use of more challenging techniques such as exposure to traumatic memories (one element of trauma-focused CBT), and the latter may be required to reduce PTSD symptom severity.4
Second, 3 sessions were not delivered to all patients. Even though more than 80% of patients received at least 2 stress support sessions, approximately one-third of patients did not receive session 3. For those patients who received 3 sessions, a reduction in reported state anxiety was observed. Given the main reason for not receiving session 3 was discharge from hospital, an intervention that follows patients into the community may be required.
Third, ICU nurses, as nonexperts, may have struggled to deliver the sessions as intended. Findings from the process evaluation indicated that, even though the ICU nurses felt fully equipped to deliver sessions after training, they found it difficult when dealing with patients with complex needs (ie, keeping them on track with the session content). This might have been enhanced by more extensive training and greater supervision or, potentially, such interventions need to be delivered by experts.
Fourth, although there was high uptake (>80% of ICU staff) for the online training intended to promote a therapeutic environment in the ICU, difficulties in changing long-standing practices or with environmental limitations in some ICUs may have impeded translation into practice. Other methods of knowledge translation and implementation may be needed to make substantive changes to the ICU environment.
Fifth, while the intervention aimed to reduce PTSD symptom severity by alleviating acute stress and memories of frightening ICU experiences, other psychological interventions—for example, to prevent delirium and agitation early in the ICU stay or to alleviate anxiety and/or depression—may warrant investigation. Further exploratory analyses of the data could inform future developments aimed at reducing post-ICU psychological morbidity.
Limitations
This trial has several limitations. First, it was challenging to deliver, potentially due to the type of intervention (eg, recruitment rate reduced in intervention ICUs during the intervention period), as patients reported associating the intervention with a stigma of mental illness. Second, it was not possible to blind patients or staff due to the nature of the intervention, but contamination was avoided by using a cluster design, with geographically diverse ICUs and a short recruitment period. Third, there was loss to follow-up for the patient-reported primary outcome (accounted for in the power calculation) but no difference in responders’ characteristics between treatment group and time period. Fourth, the trial design mandated that the initial approach for consent was in the ICU, as it was assumed that patients would regain mental capacity prior to discharge and this did not always occur. Fifth, audio-recording of a sample of stress support sessions was planned to assess fidelity. In practice, and likely due to the nature of the interaction, the nurses reported that patients found this aspect of the trial intrusive and felt uncomfortable approaching them for this. Future studies may consider using more detailed ethnographic methods to assess fidelity.
Conclusions
Among critically ill patients in the ICU, a nurse-led preventive, complex psychological intervention did not significantly reduce patient-reported PTSD symptom severity at 6 months. These findings do not support the use of this psychological intervention.
Trial Protocol
eMethods
eResults
eFigure 1. POPPI Cluster-RCT Schedule
eFigure 2. POPPI Cluster-RCT Patient Flow
eFigure 3. Histogram of PSS-SR Scores (n=62) From the POPPI RCT Processes and Procedures Study
eFigure 4. Monthly POPPI Online Training Uptake at Each Intervention ICU
eFigure 5. Number of Stress Support Sessions Received by Patients
eFigure 6. Sensitivity Analysis That Reports the Primary Treatment Effect Estimate at Six-Months According to Alternative Missing Not at Random Assumptions Compared to the Primary and Complete Case Analyses
eFigure 7. Sensitivity Analysis That Reports the Treatment Effect on Health-Related Quality of Life Score at Six Months According to Alternative Missing Not at Random Assumptions Compared to the Primary and Complete Case Analyses
eFigure 8. Mean Cost and QALY Differences at Six Months; Distribution for the Preventive, Complex Psychological Intervention Versus Usual Care
eFigure 9. Kaplan-Meier Survival Curves
eFigure 10. Sensitivity Analysis That Reports the Incremental Net Benefit (at £20,000 per QALY) Within Six Months Post-recruitment According to Alternative Missing Not at Random Assumptions Compared to the Primary and Complete Case Analyses
eFigure 11. Cost-effectiveness Acceptability Curve – At Six-Months
eFigure 12. Subgroup Analyses for Incremental Net Benefit at Six Months at £20,000 per QALY
eFigure 13. Sensitivity Analyses for the Cost-effectiveness Analysis at Six Months
eFigure 14. Cost-effectiveness Acceptability Curve – Lifetime Extrapolation
eTable 1. PSS-SR Item Responses (n=62) From the POPPI RCT Processes and Procedures Study
eTable 2. PSS-SR Total Score (n=62) From the POPPI RCT Processes and Procedures Study
eTable 3. Resource Use Associated With the Preventive, Complex Psychological Intervention
eTable 4. Unit Costs in GB Pounds (£)
eTable 5. Variables Considered for Multiple Imputation and Form of Imputation Model
eTable 6. Criteria for Component Adherence Scoring From Process Evaluation
eTable 7. Representativeness of Participating ICUs, n/N (%)
eTable 8. Screening and Recruitment by Treatment Group and Period
eTable 9. Patient Characteristics – Demographics
eTable 10. Patient Characteristics – at ICU Admission
eTable 11. Patient Characteristics – at Time of Consent
eTable 12. Nesting of POPPI Patients in Case Mix Programme Data
eTable 13. Reasons for Not Receiving Stress Support Sessions
eTable 14. Stress Support Session Delivery Locations
eTable 15. Medical Interventions Received in the ICU by Treatment Group and Time Period
eTable 16. Patient Follow-up by Treatment Group and Time Period
eTable 17. Response Rate by Patient Characteristics
eTable 18. Summary of Elicited PSS-SR Scores Across All Usable Experts (n=29)
eTable 19. Summary of Elicited EQ-5D-5L Scores Across All Usable Experts (n=30)
eTable 20. Cost-effectiveness Outcomes – at Six Months
eTable 21. Costs (£) up to Six Months
eTable 22. Mean (SD) Resource Use up to Six Months
eTable 23. Mean (SD) Resource Use From Health Services Questionnaire Between Hospital Discharge and Six Months
eTable 24. EuroQol 5-Dimensions, Mortality, and Quality-Adjusted Life Years up to Six Months
eTable 25. Alternative Assumptions for Cost-effectiveness Sensitivity Analysis
eTable 26. Lifetime Total Costs (£), Lifetime Quality-Adjusted Life Years, and Lifetime Incremental Net Benefit
eTable 27. Comparison of Baseline and Post-Stress Support Session Three STAI-6a Scores for Patients Completing Both Assessments
eReferences
Data Sharing Statement
Section Editor: Derek C. Angus, MD, MPH, Associate Editor, JAMA (angusdc@upmc.edu).
References
- 1.Parker AM, Sricharoenchai T, Raparla S, Schneck KW, Bienvenu OJ, Needham DM. Posttraumatic stress disorder in critical illness survivors: a metaanalysis. Crit Care Med. 2015;43(5):1121-1129. doi: 10.1097/CCM.0000000000000882 [DOI] [PubMed] [Google Scholar]
- 2.Wade DM, Howell DC, Weinman JA, et al. Investigating risk factors for psychological morbidity three months after intensive care: a prospective cohort study. Crit Care. 2012;16(5):R192. doi: 10.1186/cc11677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davydow DS, Zatzick D, Hough CL, Katon WJ. In-hospital acute stress symptoms are associated with impairment in cognition 1 year after intensive care unit admission. Ann Am Thorac Soc. 2013;10(5):450-457. doi: 10.1513/AnnalsATS.201303-060OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wade DF, Moon Z, Windgassen SS, Harrison AM, Morris L, Weinman JA. Non-pharmacological interventions to reduce ICU-related psychological distress: a systematic review. Minerva Anestesiol. 2016;82(4):465-478. [PubMed] [Google Scholar]
- 5.Jensen JF, Egerod I, Bestle MH, et al. A recovery program to improve quality of life, sense of coherence and psychological health in ICU survivors: a multicenter randomized controlled trial, the RAPIT study. Intensive Care Med. 2016;42(11):1733-1743. doi: 10.1007/s00134-016-4522-1 [DOI] [PubMed] [Google Scholar]
- 6.Peris A, Bonizzoli M, Iozzelli D, et al. Early intra-intensive care unit psychological intervention promotes recovery from post traumatic stress disorders, anxiety and depression symptoms in critically ill patients [published correction appears in Crit Care. 2011;15(2):418]. Crit Care. 2011;15(1):R41. doi: 10.1186/cc10003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beck AT, Dozois DJ. Cognitive therapy: current status and future directions. Annu Rev Med. 2011;62:397-409. doi: 10.1146/annurev-med-052209-100032 [DOI] [PubMed] [Google Scholar]
- 8.Bighelli I, Salanti G, Huhn M, et al. Psychological interventions to reduce positive symptoms in schizophrenia: systematic review and network meta-analysis. World Psychiatry. 2018;17(3):316-329. doi: 10.1002/wps.20577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Institute for Health and Care Excellence Post-traumatic stress disorder (PTSD): evidence update December 2013. https://arms.evidence.nhs.uk/resources/hub/1031525/attachment. Accessed January 16, 2019.
- 10.Tyrer P, Cooper S, Salkovskis P, et al. Clinical and cost-effectiveness of cognitive behaviour therapy for health anxiety in medical patients: a multicentre randomised controlled trial. Lancet. 2014;383(9913):219-225. doi: 10.1016/S0140-6736(13)61905-4 [DOI] [PubMed] [Google Scholar]
- 11.Turkington D, Kingdon D, Rathod S, Hammond K, Pelton J, Mehta R. Outcomes of an effectiveness trial of cognitive-behavioural intervention by mental health nurses in schizophrenia. Br J Psychiatry. 2006;189:36-40. doi: 10.1192/bjp.bp.105.010884 [DOI] [PubMed] [Google Scholar]
- 12.Wade D, Als N, Bell V, et al. ; POPPI investigators . Providing psychological support to people in intensive care: development and feasibility study of a nurse-led intervention to prevent acute stress and long-term morbidity. BMJ Open. 2018;8(7):e021083. doi: 10.1136/bmjopen-2017-021083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Richards-Belle A, Mouncey PR, Wade D, et al. ; POPPI Trial Investigators . Psychological Outcomes following a nurse-led Preventative Psychological Intervention for critically ill patients (POPPI): protocol for a cluster-randomised clinical trial of a complex intervention. BMJ Open. 2018;8(2):e020908. doi: 10.1136/bmjopen-2017-020908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sessler CN, Gosnell MS, Grap MJ, et al. The Richmond Agitation–Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166(10):1338-1344. doi: 10.1164/rccm.2107138 [DOI] [PubMed] [Google Scholar]
- 15.Teasdale G, Jennett B. Assessment of coma and impaired consciousness: a practical scale. Lancet. 1974;2(7872):81-84. doi: 10.1016/S0140-6736(74)91639-0 [DOI] [PubMed] [Google Scholar]
- 16.Marteau TM, Bekker H. The development of a six-item short-form of the state scale of the Spielberger State-Trait Anxiety Inventory (STAI). Br J Clin Psychol. 1992;31(pt 3):301-306. doi: 10.1111/j.2044-8260.1992.tb00997.x [DOI] [PubMed] [Google Scholar]
- 17.Carter BR, Hood K. Balance algorithm for cluster randomized trials. BMC Med Res Methodol. 2008;8:65. doi: 10.1186/1471-2288-8-65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wade DM, Hankins M, Smyth DA, et al. Detecting acute distress and risk of future psychological morbidity in critically ill patients: validation of the intensive care psychological assessment tool. Crit Care. 2014;18(5):519. doi: 10.1186/s13054-014-0519-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foa EB, Cashman L, Jaycox L, et al. The validation of a self-report measure of posttraumatic stress disorder: the Posttraumatic Diagnostic Scale. Psychol Assess. 1997;9:445-451. doi: 10.1037/1040-3590.9.4.445 [DOI] [Google Scholar]
- 20.Ehring T, Kleim B, Clark DM, Foa EB, Ehlers A. Screening for posttraumatic stress disorder: what combination of symptoms predicts best? J Nerv Ment Dis. 2007;195(12):1004-1012. doi: 10.1097/NMD.0b013e31815c1999 [DOI] [PubMed] [Google Scholar]
- 21.Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67(6):361-370. doi: 10.1111/j.1600-0447.1983.tb09716.x [DOI] [PubMed] [Google Scholar]
- 22.Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20(10):1727-1736. doi: 10.1007/s11136-011-9903-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Devlin NJ, Shah KK, Feng Y, Mulhern B, van Hout B. Valuing health-related quality of life: An EQ-5D-5L value set for England. Health Econ. 2018;27(1):7-22. doi: 10.1002/hec.3564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hussey MA, Hughes JP. Design and analysis of stepped wedge cluster randomized trials. Contemp Clin Trials. 2007;28(2):182-191. doi: 10.1016/j.cct.2006.05.007 [DOI] [PubMed] [Google Scholar]
- 25.Jacobson NS, Truax P. Clinical significance: a statistical approach to defining meaningful change in psychotherapy research. J Consult Clin Psychol. 1991;59(1):12-19. doi: 10.1037/0022-006X.59.1.12 [DOI] [PubMed] [Google Scholar]
- 26.Wulff J, Sadique Z, Grieve R, et al. Psychological outcomes following a nurse-led preventative psychological intervention for critically ill patients trial: statistical and health economic analysis plan. J Intensive Care Soc. 2018;19(4):281-286. doi: 10.1177/1751143718755016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York, NY: J Wiley & Sons; 1987. doi: 10.1002/9780470316696 [DOI] [Google Scholar]
- 28.Carpenter JR, Kenward MG. Multiple Imputation and Its Application. Chichester, England: Wiley; 2013. doi: 10.1002/9781119942283 [DOI] [Google Scholar]
- 29.Ferrando-Vivas P, Jones A, Rowan KM, Harrison DA. Development and validation of the new ICNARC model for prediction of acute hospital mortality in adult critical care. J Crit Care. 2017;38:335-339. doi: 10.1016/j.jcrc.2016.11.031 [DOI] [PubMed] [Google Scholar]
- 30.Wade D, Hardy R, Howell D, Mythen M. Identifying clinical and acute psychological risk factors for PTSD after critical care: a systematic review. Minerva Anestesiol. 2013;79(8):944-963. [PubMed] [Google Scholar]
- 31.Ministry of Housing, Communities & Local Government English indices of deprivation 2015. https://www.gov.uk/government/statistics/english-indices-of-deprivation-2015. Published September 30, 2015. Accessed July 25, 2018.
- 32.Iwashyna TJ, Burke JF, Sussman JB, Prescott HC, Hayward RA, Angus DC. Implications of heterogeneity of treatment effect for reporting and analysis of randomized trials in critical care. Am J Respir Crit Care Med. 2015;192(9):1045-1051. doi: 10.1164/rccm.201411-2125CP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Campbell MK, Piaggio G, Elbourne DR, Altman DG; CONSORT Group . Consort 2010 statement: extension to cluster randomised trials. BMJ. 2012;345:e5661. doi: 10.1136/bmj.e5661 [DOI] [PubMed] [Google Scholar]
- 34.Nolan CM, Longworth L, Lord J, et al. The EQ-5D-5L health status questionnaire in COPD: validity, responsiveness and minimum important difference. Thorax. 2016;71(6):493-500. doi: 10.1136/thoraxjnl-2015-207782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chan KS, Aronson Friedman L, Bienvenu OJ, et al. Distribution-based estimates of minimal important difference for Hospital Anxiety and Depression Scale and Impact of Event Scale-Revised in survivors of acute respiratory failure. Gen Hosp Psychiatry. 2016;42:32-35. doi: 10.1016/j.genhosppsych.2016.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M; Medical Research Council Guidance . Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ. 2008;337:a1655. doi: 10.1136/bmj.a1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roberts MB, Glaspey LJ, Mazzarelli A, et al. Early Interventions for the prevention of posttraumatic stress symptoms in survivors of critical illness: a qualitative systematic review. Crit Care Med. 2018;46(8):1328-1333. doi: 10.1097/CCM.0000000000003222 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eMethods
eResults
eFigure 1. POPPI Cluster-RCT Schedule
eFigure 2. POPPI Cluster-RCT Patient Flow
eFigure 3. Histogram of PSS-SR Scores (n=62) From the POPPI RCT Processes and Procedures Study
eFigure 4. Monthly POPPI Online Training Uptake at Each Intervention ICU
eFigure 5. Number of Stress Support Sessions Received by Patients
eFigure 6. Sensitivity Analysis That Reports the Primary Treatment Effect Estimate at Six-Months According to Alternative Missing Not at Random Assumptions Compared to the Primary and Complete Case Analyses
eFigure 7. Sensitivity Analysis That Reports the Treatment Effect on Health-Related Quality of Life Score at Six Months According to Alternative Missing Not at Random Assumptions Compared to the Primary and Complete Case Analyses
eFigure 8. Mean Cost and QALY Differences at Six Months; Distribution for the Preventive, Complex Psychological Intervention Versus Usual Care
eFigure 9. Kaplan-Meier Survival Curves
eFigure 10. Sensitivity Analysis That Reports the Incremental Net Benefit (at £20,000 per QALY) Within Six Months Post-recruitment According to Alternative Missing Not at Random Assumptions Compared to the Primary and Complete Case Analyses
eFigure 11. Cost-effectiveness Acceptability Curve – At Six-Months
eFigure 12. Subgroup Analyses for Incremental Net Benefit at Six Months at £20,000 per QALY
eFigure 13. Sensitivity Analyses for the Cost-effectiveness Analysis at Six Months
eFigure 14. Cost-effectiveness Acceptability Curve – Lifetime Extrapolation
eTable 1. PSS-SR Item Responses (n=62) From the POPPI RCT Processes and Procedures Study
eTable 2. PSS-SR Total Score (n=62) From the POPPI RCT Processes and Procedures Study
eTable 3. Resource Use Associated With the Preventive, Complex Psychological Intervention
eTable 4. Unit Costs in GB Pounds (£)
eTable 5. Variables Considered for Multiple Imputation and Form of Imputation Model
eTable 6. Criteria for Component Adherence Scoring From Process Evaluation
eTable 7. Representativeness of Participating ICUs, n/N (%)
eTable 8. Screening and Recruitment by Treatment Group and Period
eTable 9. Patient Characteristics – Demographics
eTable 10. Patient Characteristics – at ICU Admission
eTable 11. Patient Characteristics – at Time of Consent
eTable 12. Nesting of POPPI Patients in Case Mix Programme Data
eTable 13. Reasons for Not Receiving Stress Support Sessions
eTable 14. Stress Support Session Delivery Locations
eTable 15. Medical Interventions Received in the ICU by Treatment Group and Time Period
eTable 16. Patient Follow-up by Treatment Group and Time Period
eTable 17. Response Rate by Patient Characteristics
eTable 18. Summary of Elicited PSS-SR Scores Across All Usable Experts (n=29)
eTable 19. Summary of Elicited EQ-5D-5L Scores Across All Usable Experts (n=30)
eTable 20. Cost-effectiveness Outcomes – at Six Months
eTable 21. Costs (£) up to Six Months
eTable 22. Mean (SD) Resource Use up to Six Months
eTable 23. Mean (SD) Resource Use From Health Services Questionnaire Between Hospital Discharge and Six Months
eTable 24. EuroQol 5-Dimensions, Mortality, and Quality-Adjusted Life Years up to Six Months
eTable 25. Alternative Assumptions for Cost-effectiveness Sensitivity Analysis
eTable 26. Lifetime Total Costs (£), Lifetime Quality-Adjusted Life Years, and Lifetime Incremental Net Benefit
eTable 27. Comparison of Baseline and Post-Stress Support Session Three STAI-6a Scores for Patients Completing Both Assessments
eReferences
Data Sharing Statement