Abstract
This before-and-after cohort analysis assesses whether implementing a recommendation of antenatal magnesium use at an institutional level in 2009 resulted in fewer subsequent cases of moderate or severe cerebral palsy.
Cerebral palsy (CP) is the leading cause of long-term childhood disability with lifetime societal and economic costs.1 Without a cure for CP, efforts have focused on prevention and on improving function. International guidelines for early detection and intervention for CP were recently published.2 Our institution implemented these recommendations in 2015, earlier than most clinical efforts worldwide. After publication of the Beneficial Effects of Antenatal Magnesium Sulfate (BEAM) clinical trial,3 The American College of Obstetricians and Gynecologists advised implementing institutional policies for magnesium sulfate administration before anticipated early preterm birth to reduce risk of CP.4 Although BEAM did not show a reduction in the primary outcome (composite of stillbirth, infant death, and moderate or severe CP), rates of moderate or severe CP were reduced among survivors.3 If effective, implementing this recommendation at an institutional level in 2009 should have resulted in fewer subsequent cases of moderate or severe CP at our institution.
Methods
We conducted a before-and-after cohort analysis5 at The Ohio State University Wexner Medical Center and Nationwide Children’s Hospital in Columbus, Ohio, identifying all children born from 2002 to 2014 who received a diagnosis of CP and were delivered before 32 weeks’ gestation. The fetal neuroprotection protocol implemented in 2009 matched BEAM criteria but also included preeclampsia to reflect current clinical practice. The primary outcome was CP severity assessed by the Gross Motor Function Classification System (GMFCS).6 Secondary outcomes were CP severity assessed using the Manual Ability Classification System (MACS) and the Communication Function Classification System (CFCS). The Ohio State University Wexner Medical Center and Nationwide Children’s Hospital institutional review boards approved this study and waived the need for obtaining informed patient consent owing to the retrospective nature of the study. Data analysis was conducted from June 2016 to April 2018 using SigmaPlot, version 12.5 (Systat), and Stata, version 13.0 (StataCorp). A 2-sided P < .05 was considered statistically significant. Multiple logistic regression analyses controlled for magnesium administration duration, chorioamnionitis, spontaneous or iatrogenic preterm birth, bronchopulmonary dysplasia, and age at diagnosis.
Results
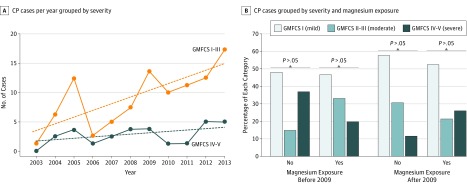
Of 3736 eligible deliveries, 110 infants had CP, of which 57 (52%) received antenatal magnesium sulfate, 63 (57%) were male, and 81 (74%) were non-Hispanic white. After 2008, more mothers of infants with CP received magnesium than before 2008 (15 of 42 [36%] vs 42 of 68 [62%], P = .008). More infants with CP were diagnosed over time (r = 0.71, P = .01) owing to increased diagnosis of mild or moderate (r = 0.72, P = .01, Figure, A) but not severe (r = 0.41, P = .20; Table) CP. Infants exposed to magnesium after protocol implementation did not have reduced severity of CP as assessed by GMFCS (Figure, B, P = .42), MACS (P = .38), or CFCS (P = .40), and exclusion of magnesium administration for treatment of preeclampsia did not change this result. The CP severity decreased after 2008 (adjusted odds ratio, 0.363; 95% CI, 0.02-0.91; P = .03) independent of magnesium administration. Children born after 2008 were younger at CP diagnosis (21 vs 27 months, adjusted odds ratio, 0.96; 95% CI, 0.91-0.98; P = .002), especially those born after 2012 (17 vs 32 months, adjusted OR, 0.90; 95% CI, 0.85-0.96; P < .001).
Figure. Severity of Cerebral Palsy in Infants Born Before or After Implementation of Magnesium Sulfate Administration for Neuroprotection.
A, Cerebral palsy (CP) cases grouped as mild or moderate (I-III) vs severe (IV-V) using the Gross Motor Function Classification System (GMFCS). Dashed lines indicate the slope of each regression. B, Percentage of CP cases before and after magnesium administration protocol implementation (2009). Data were analyzed by use of the χ2 test, and there was no significant difference between the indicated groups.
Table. Demographic and Clinical Characteristics of 110 Infants.
| Variable | Magnesium Administration | P Value | |||
|---|---|---|---|---|---|
| 2002-2008 | 2009-2014 | ||||
| No (n = 27) | Yes (n = 15) | No (n = 26) | Yes (n = 42) | ||
| Maternal and neonatal variables | |||||
| Multiple gestationa | 9 (33) | 6 (40) | 10 (39) | 13 (31) | .89 |
| Clinical chorioamnionitisa | 10 (37) | 0 (0) | 2 (8) | 6 (14) | .003 |
| PPROMa | 13 (48) | 4 (27) | 8 (31) | 21 (50) | .23 |
| Magnesium exposure duration, hb | 0 | 25 (13-59)c | 0 | 28 (4-52)c | .001 |
| Gestational age at delivery, wkb | 25 (24-28) | 26 (23-29) | 27 (24-29) | 25 (24-28) | .64 |
| Infant male sexa | 19 (70) | 7 (47) | 13 (50) | 24 (57) | .37 |
| Infant nonwhite race/ethnicitya | 6 (22) | 2 (13) | 9 (35) | 12 (29) | .46 |
| Birth weight, gb | 769 (602-1160) | 745 (567-879) | 722 (543-999) | 838 (609-1046) | .39 |
| Intraventricular hemorrhagea | 15 (56) | 6 (40) | 16 (62) | 25 (60) | .41 |
| Bronchopulmonary dysplasiaa | 15 (56) | 10 (67) | 19 (73) | 36 (86) | .049 |
| Infant neurological outcomed | |||||
| Age at CP diagnosis, mob | 29 (22-41) | 26 (20-33) | 23 (14-34) | 21 (15-26) | .03 |
| GMFCS levela | |||||
| I | 13 (48) | 7 (47) | 15 (58) | 22 (52) | .42 |
| II | 1 (4) | 2 (13) | 5 (19) | 8 (19) | |
| III | 3 (11) | 3 (20) | 3 (12) | 1 (2) | |
| IV | 4 (15) | 1 (7) | 1 (4) | 6 (14) | |
| V | 6 (22) | 2 (13) | 2 (8) | 5 (12) | |
Abbreviations: CP, cerebral palsy; GMFCS, Gross Motor Function Classification System; PPROM, preterm premature rupture of membranes.
Data presented as number (percentage) and analyzed using the multinomial χ2 test.
Data presented as median (interquartile range) and analyzed using the Kruskal-Wallis analysis of variance on ranks values.
P < .05 compared with infants who were not exposed to magnesium during the same period.
Assessed by investigators (B.S., B.K., and I.B.) unaware of antenatal magnesium exposure.
Discussion
Our CP follow-up program enabled us to perform the largest analysis to date of antenatal magnesium exposure of preterm infants, to our knowledge. Similar to national trends, we observed adherence to magnesium administration. Our finding of decreased CP severity over time concurs with the findings of large CP registries. However, reduction in CP severity could not be attributed to magnesium. Earlier diagnosis of CP among children born after 2012 at our institution may be associated with advances in neonatal intensive care (eg, avoidance of postnatal steroids and systematic use of caffeine), but may also be due to implementation of guidelines for evidence-based early recognition of CP. Earlier diagnosis, especially before 2 years of age, may facilitate access to interventions that improve functional outcomes. Our finding may be unique to the current setting, where studies of early CP intervention starting at 6 months of age have been ongoing since 2013. A limitation to the generalizability of our findings may be that 3 clinical trials of early CP motor interventions have been ongoing since 2013.
Administration of magnesium is a resource-intensive intervention that is not without risks. Given our findings, the role of magnesium for fetal neuroprotection may need reevaluation at the national level. Physicians should evaluate the long-term impact of clinical interventions, a mandate not unique to magnesium trials for prevention of CP. Many industries have developed lean thinking tools, such as the plan-do-check-adjust cycle, that can drive evaluation of health care recommendations adopted following large randomized trials.
References
- 1.Novak I, Morgan C, Adde L, et al. . Early, accurate diagnosis and early intervention in cerebral palsy: advances in diagnosis and treatment. JAMA Pediatr. 2017;171(9):897-907. doi: 10.1001/jamapediatrics.2017.1689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cerebral Palsy Foundation. Early Detection: web log post. http://yourcpf.org/early-recognition/. Accessed August 15, 2018.
- 3.Rouse DJ, Hirtz DG, Thom E, et al. ; Eunice Kennedy Shriver NICHD Maternal-Fetal Medicine Units Network . A randomized, controlled trial of magnesium sulfate for the prevention of cerebral palsy. N Engl J Med. 2008;359(9):895-905. doi: 10.1056/NEJMoa0801187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American College of Obstetricians and Gynecologists Committee on Obstetric Practice; Society for Maternal-Fetal Medicine . Committee Opinion No. 455: magnesium sulfate before anticipated preterm birth for neuroprotection. Obstet Gynecol. 2010;115(3):669-671. doi: 10.1097/AOG.0b013e3181d4ffa5 [DOI] [PubMed] [Google Scholar]
- 5.Thiese MS. Observational and interventional study design types; an overview. Biochem Med (Zagreb). 2014;24(2):199-210. doi: 10.11613/BM.2014.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol. 1997;39(4):214-223. doi: 10.1111/j.1469-8749.1997.tb07414.x [DOI] [PubMed] [Google Scholar]