Key Points
Question
Does the use of a resuscitation strategy targeting normalization of capillary refill time, compared with a strategy targeting serum lactate levels, reduce mortality among patients with septic shock?
Findings
In this randomized clinical trial of 424 patients with early septic shock, 28-day mortality was 34.9% in the peripheral perfusion–targeted resuscitation group compared with 43.4% in the lactate level–targeted resuscitation group, a difference that did not reach statistical significance.
Meaning
These findings do not support the use of a peripheral perfusion–targeted resuscitation strategy in patients with septic shock.
Abstract
Importance
Abnormal peripheral perfusion after septic shock resuscitation has been associated with organ dysfunction and mortality. The potential role of the clinical assessment of peripheral perfusion as a target during resuscitation in early septic shock has not been established.
Objective
To determine if a peripheral perfusion–targeted resuscitation during early septic shock in adults is more effective than a lactate level–targeted resuscitation for reducing mortality.
Design, Setting, and Participants
Multicenter, randomized trial conducted at 28 intensive care units in 5 countries. Four-hundred twenty-four patients with septic shock were included between March 2017 and March 2018. The last date of follow-up was June 12, 2018.
Interventions
Patients were randomized to a step-by-step resuscitation protocol aimed at either normalizing capillary refill time (n = 212) or normalizing or decreasing lactate levels at rates greater than 20% per 2 hours (n = 212), during an 8-hour intervention period.
Main Outcomes and Measures
The primary outcome was all-cause mortality at 28 days. Secondary outcomes were organ dysfunction at 72 hours after randomization, as assessed by Sequential Organ Failure Assessment (SOFA) score (range, 0 [best] to 24 [worst]); death within 90 days; mechanical ventilation–, renal replacement therapy–, and vasopressor-free days within 28 days; intensive care unit and hospital length of stay.
Results
Among 424 patients randomized (mean age, 63 years; 226 [53%] women), 416 (98%) completed the trial. By day 28, 74 patients (34.9%) in the peripheral perfusion group and 92 patients (43.4%) in the lactate group had died (hazard ratio, 0.75 [95% CI, 0.55 to 1.02]; P = .06; risk difference, −8.5% [95% CI, −18.2% to 1.2%]). Peripheral perfusion–targeted resuscitation was associated with less organ dysfunction at 72 hours (mean SOFA score, 5.6 [SD, 4.3] vs 6.6 [SD, 4.7]; mean difference, −1.00 [95% CI, −1.97 to −0.02]; P = .045). There were no significant differences in the other 6 secondary outcomes. No protocol-related serious adverse reactions were confirmed.
Conclusions and Relevance
Among patients with septic shock, a resuscitation strategy targeting normalization of capillary refill time, compared with a strategy targeting serum lactate levels, did not reduce all-cause 28-day mortality.
Trial Registration
ClinicalTrials.gov Identifier: NCT03078712
This randomized trial compares the effects of resuscitation strategies targeting normalization of capillary refill time vs normalization or decrease of serum lactate levels on 28-day mortality among patients with septic shock.
Introduction
Early resuscitation is a key factor to limit progression to multiple organ dysfunction and death in patients with septic shock.1,2 Shock is characterized by increased serum lactate levels and signs of tissue hypoperfusion including abnormal peripheral perfusion.2
Considering the strong relationship between hyperlactatemia, lactate kinetics, and mortality,3 and the results of a recent study,4 the Surviving Sepsis Campaign, proposes to guide hemodynamic resuscitation by repeated measurement of blood lactate levels every 2 to 4 hours until normalization.5 However, persistent hyperlactatemia may be related to causes other than tissue hypoperfusion,6 lactate kinetics is relatively slow even in survivors,3,7 and measurements of lactate levels may not be universally available. Therefore, the exploration of alternative resuscitation targets is an important research priority in sepsis.8
Observational studies have shown that persistent abnormal peripheral perfusion after resuscitation is associated with organ failure9 and mortality.10 Capillary refill time (CRT) is an easy-to-use, resource-independent method to assess peripheral perfusion.11,12 CRT has been shown to rapidly respond to resuscitation,7,13 and its assessment might be effectively used to allow adjustments of therapy.14
Consequently, a multicenter randomized clinical trial comparing peripheral perfusion–targeted resuscitation to lactate level–targeted resuscitation in patients with early septic shock was conducted, hypothesizing that resuscitation guided by peripheral perfusion would be associated with improved outcomes.
Methods
Study Design and Oversight
The ANDROMEDA-SHOCK randomized clinical trial was conducted at 28 hospitals in 5 countries (Argentina, Chile, Colombia, Ecuador, Uruguay). The institutional review board at each site approved the study. Written informed consent was obtained from all patients or surrogates. The protocol and statistical analysis plan have been previously published15,16 and are available in Supplement 1. The trial was logistically supported by the Pontificia Universidad Católica of Chile.
The members of the steering committee designed the trial and analyzed the data. The data and safety monitoring committee oversaw the trial. The steering committee vouches for the accuracy of the data and adherence to the study protocol.
Patient Selection and Randomization
Consecutive adult patients (≥18 years) with septic shock admitted to the intensive care unit (ICU) were considered eligible. Septic shock was defined as suspected or confirmed infection, plus hyperlactatemia (≥2.0 mmol/L) and requirements of vasopressors to maintain a mean arterial pressure (MAP) of 65 mm Hg or higher after an intravenous fluid load of at least 20 mL/kg over 60 minutes.17 Patients were recruited within 4 hours after fulfilling criteria. Exclusion criteria included bleeding, severe acute respiratory distress syndrome, and do-not-resuscitate status (Supplement 1).
Eligible patients were randomly allocated to peripheral perfusion–targeted resuscitation (peripheral perfusion) or lactate level–targeted resuscitation (lactate) groups. A randomization sequence by permuted blocks of 8 with an allocation of 1:1 was generated by a computer program. Allocation concealment was maintained by means of central randomization. Investigators called a representative of the study coordinating center, who was available via a dedicated telephone line. Group allocation was only disclosed after the information was centrally checked and recorded.
Study Interventions
The intervention period was 8 hours. Before starting the study, all centers were trained to assess capillary refill time with a standardized technique.15 Briefly, CRT was measured by applying firm pressure to the ventral surface of the right index finger distal phalanx with a glass microscope slide. The pressure was increased until the skin was blank and then maintained for 10 seconds. The time for return of the normal skin color was registered with a chronometer, and a refill time greater than 3 seconds was defined as abnormal.
For assessment of fluid responsiveness,18 each center used their standard technique when feasible and an additional algorithm was provided for difficult cases15 (Supplement 1).
Lactate level was assessed every 2 hours.5 CRT was evaluated every 30 minutes, because of its faster rate of recovery,7,19 until normalization and then every hour during the intervention period.
The goal for the peripheral perfusion group was to normalize CRT, whereas the goal for the lactate group was to normalize or to decrease lactate levels by 20% every 2 hours.
After initial fluid resuscitation and norepinephrine to maintain a MAP of 65 mm Hg or higher, both groups were managed with an identical sequential protocolized approach to resuscitation (eFigure 1 in Supplement 2).1,2
The first step was assessment of fluid responsiveness,18 followed by fluid challenges with 500 mL of crystalloids every 30 minutes in fluid responders until the goal was achieved as assessed at intervals depending on the allocated group, a central venous pressure safety limit was reached,20 or the patient became fluid unresponsive, whichever came first. In patients in whom fluid responsiveness could not be determined, fluid resuscitation was continued until the goal was met or a safety limit was reached.
In patients with chronic hypertension,1,2,21 if the previous interventions did not meet the goals, a vasopressor test was conducted, transiently increasing norepinephrine dose until reaching a MAP of 80 to 85 mm Hg, followed by a reassessment of CRT or lactate level after 1 or 2 hours, respectively. If the goal was met, this MAP level was maintained throughout the intervention period. Otherwise, norepinephrine was decreased to the previous dose and the patient moved to the next step, similar to patients without chronic hypertension with persistent hypoperfusion.
The third step consisted of an inodilator test with low-dose dobutamine or milrinone (depending on local protocols) if the target was still not reached.1 Patients were again reassessed after 1 or 2 hours in the peripheral perfusion and lactate groups, respectively. If the end points were still not met or a safety issue arose,15 the inodilator was discontinued.
A distinctive characteristic of the protocol was that when required, higher MAP targets or inodilators were introduced as a test, meaning that the effect was reassessed after a short period and the interventions maintained only in responders.
Investigators were recommended to follow Surviving Sepsis Campaign guidelines for background, refractory shock, and source control management.1 Other monitoring and interventions during and after the intervention period could be used in both groups at the discretion of the attending physicians.
Resuscitation, Perfusion, and Hemodynamic Variables
Data were collected on several perfusion and hemodynamic variables, listed in eTable 2 in Supplement 2 during the first 72 hours after randomization.
Outcome Measures
The primary outcome was all-cause mortality at 28 days. Secondary prespecified outcomes were death within 90 days; organ dysfunction during the first 72 hours after randomization (assessed by Sequential Organ Failure Assessment [SOFA] score, with higher scores indicating a greater severity of organ dysfunction in critically ill patients),22 mechanical ventilation–free days within 28 days; renal replacement therapy–free days within 28 days; vasopressor-free days within 28 days; and ICU and hospital length of stay. Patients who died were assigned zero free days. Mechanical ventilation–free days, renal replacement–free days, or vasopressor-free days within 28 days were defined as the number of days alive and without use of the specific supportive therapy from randomization to day 28. Tertiary prespecified exploratory outcomes were resuscitation fluids during the intervention period; total fluid balance within 8, 24, and 72 hours ; occurrence of intra-abdominal hypertension within 72 hours; use of renal replacement therapy within 28 days; and in-hospital mortality.16
A rigorous methodology was developed to reduce loss to follow-up. The true survival state at days 28 or 90 (either in or outside the hospital at that day) was determined. For patients still hospitalized at days 28 or 90, actual status was gathered from hospital registers. Hospital mortality was truncated at the date of the database lock (June 12, 2018). For patients discharged before the critical outcome dates, follow-ups were performed by telephone calls previously announced during the informed consent process or by consulting potential death status in the national civil register or the specific health system registry, depending on the country. Electronic reminders were sent to centers before the critical dates.
Data also were collected on cases of suspected unexpected serious adverse reactions, defined as any adverse event reported by study investigators for being unexpected, serious, and having a reasonable possibility of a causal relationship with the study procedures. These reports were analyzed by the study coordinating center together with local investigators, and its relationship with the study protocol was determined.
Statistical Analysis
We planned to enroll 420 patients. We calculated that with this sample size the study would have 90% power to detect a reduction in 28-day mortality from 45% in the lactate group to 30% in the peripheral perfusion group,4,10,11 at an α level of .05. Interim analyses after the inclusion of the first 100 and 300 patients were performed by the data and safety monitoring committee, which had no preestablished formal stopping rules. After both analyses the committee recommended to continue the trial without alterations.
We compared resuscitation, perfusion, and hemodynamic categorical variables between treatment groups with Fisher exact tests. For continuous variables, generalized linear mixed models with different distributions were used: Gaussian distribution was used for heart rate, central venous oxygen saturation, and systolic, diastolic, and mean arterial blood pressures; gamma distribution was used for norepinephrine dose, diuresis, lactate level, CRT, and central venous–arterial Pco2 gradient; binomial (logistic model) was used for norepinephrine use.
The treatment effect on the primary outcome was calculated with Cox proportional hazards, with adjustment for 5 prespecified baseline covariates: Acute Physiology and Chronic Health Evaluation (APACHE) II score,23 SOFA score,24 lactate level, CRT, and source of infection. The proportional hazards assumption was tested with the Grambsch and Therneau method.25 Results are reported as hazard ratio with 95% confidence interval and as Kaplan-Meier curves.
The effect on 90-day all-cause mortality was assessed with Cox proportional hazards model. Other binary secondary and tertiary outcomes were tested using Fisher exact tests. Treatment effect on mechanical ventilation–free days, renal replacement therapy–free days, and vasopressor-free days within 28 days was analyzed by zero-inflated negative binomial models. ICU and hospital length of stay and resuscitation fluids were assessed with generalized linear models with gamma distribution. Fluid balance was compared with linear regression. The treatment effect on organ dysfunction at 72 hours was evaluated with linear regression adjusting for baseline SOFA score. As a post hoc analysis, we compared SOFA values measured during the 72 hours (at 8, 24, 48, and 72 hours) between treatment groups using a mixed linear regression model, with adjustment for baseline SOFA score, considering time as a continuous variable, patient as random effect, and a treatment × time interaction term. Analyses of secondary or tertiary outcomes were not adjusted for covariates.
A prespecified sensitivity analysis was performed using a frailty Cox model with sites as random effects, assuming a gamma distribution, adjusted for the same covariates as in the main model—results were presented as marginal effects. Frailty models account for the possible heterogeneity of treatment effects across trial sites. Subgroup analyses, with Cox proportional hazards adjusted for the same covariates as in the main model, were conducted to assess interactions between treatment effect and the following prespecified baseline characteristics: lactate levels (>4.0 vs ≤4.0 mmol/L); APACHE II score (<25 vs ≥25); SOFA score (<10 vs ≥10); source of infection (confirmed vs unconfirmed); variation of lactate level between first measurement and baseline measurement (≥10% vs <10%).16 Several additional post hoc sensitivity analyses listed in the eMethods in Supplement 2, including per protocol analyses, were performed. Furthermore, in a post hoc analysis, we assessed whether treatment effect on the primary outcome might differ across sites using a Cox proportional hazards model adjusted for the same covariates as the main model and a treatment × site interaction term.
Patients were analyzed according to randomization group, except when indicated otherwise. All hypothesis tests were 2-sided, with a significance level of .05 and no adjustments for the interim analyses, multiple outcomes, or subgroup analyses. Therefore, analyses of secondary outcomes and other outcomes should be considered exploratory. Analyses were conducted using R version 3.4.1 (R Core Team).
Results
Patients
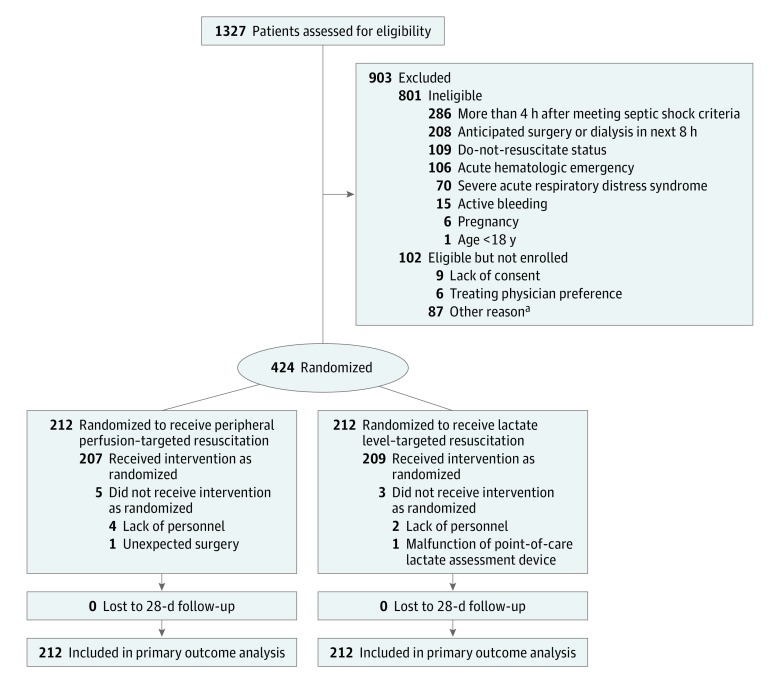
From March 2017 through March 2018, 1327 patients were assessed for eligibility (Figure 1). A total of 424 patients were enrolled (mean age, 63 years; 226 [53%] women), with 212 assigned to each group (Figure 1; eFigure 2 and eTable 1 in Supplement 2). Data for the primary and secondary outcomes were obtained for all patients. All patients were included in the intention-to-treat analysis for the primary outcome.
Figure 1. Flow of Participants Through the Study.
aAmong the 87 patients eligible but not enrolled because of other reasons, 55 were not enrolled because of logistic issues (lack of personnel, multiple simultaneous admissions) and 32 because of delay in transfer from the emergency department to the intensive care unit after meeting criteria and then losing the window of intervention.
Baseline patient characteristics were similar (Table 1). Seventy-one percent of the patients were admitted from the emergency department, 17% from wards, 7% from step-down units, and 5% directly from the operating room.
Table 1. Patient Characteristics at Baselinea.
| Characteristic | Peripheral Perfusion–Targeted Resuscitation (n = 212) |
Lactate Level–Targeted Resuscitation (n = 212) |
|---|---|---|
| Age, mean (SD), y | 62 (17) | 64 (17) |
| Sex, No. (%) | ||
| Men | 108 (50.9) | 90 (42.5) |
| Women | 104 (49.1) | 122 (57.5) |
| Charlson Comorbidity Index, median (IQR)b | 3 (1-5) | 3 (1-5) |
| APACHE II, mean (SD)c | 21.9 (8.0) | 22.0 (7.6) |
| SOFA, mean (SD)d | 9.7 (3.4) | 9.6 (3.5) |
| Chronic hypertension, No. (%) | 83 (39.2) | 93 (43.9) |
| Confirmed microbiology, No. (%) | 151 (71.2) | 153 (72.2) |
| Septic shock source, No. (%) | ||
| Intra-abdominal infection | 72 (34.0) | 77 (36.3) |
| Pneumonia | 70 (33.0) | 58 (27.4) |
| Urinary tract infection | 42 (19.8) | 45 (21.2) |
| Other sourcese | 18 (8.5) | 19 (9.0) |
| Unknown origin | 10 (4.7) | 13 (6.1) |
| Hemodynamic and perfusion-related variables | ||
| Heart rate, mean (SD), /min | 103 (24) | 104 (23) |
| Arterial blood pressure, mean (SD), mm Hg | 69 (14) | 68 (13) |
| Norepinephrine dose, median (IQR), µg/kg/min | 0.24 (0.11-0.40) | 0.20 (0.10-0.35) |
| Central venous pressure, No. | 199 | 194 |
| Median (IQR), mm Hg | 9 (6-13) | 9 (6-12) |
| Serum lactate, mean (SD), mmol/L | 4.6 (4.3) | 4.5 (2.5) |
| Central venous oxygen saturation, No. | 204 | 197 |
| Mean (SD) | 71 (13) | 71 (12) |
| Venous-arterial Pco2 gradient, No. | 203 | 195 |
| Median (IQR), mm Hg | 7 (5-10) | 7 (5-10) |
| Capillary refill time | ||
| Median (IQR), s | 5 (4-6) | 4 (3-6) |
| ≤3 s, No. (%) | 48 (22.6) | 60 (28.3) |
| Initial management data, median (IQR) | ||
| Time from matching entry criteria to randomization, h | 1.5 (0.0-3.0) | 1.3 (0.0-2.6) |
| Intravenous fluid loading per weight, mL/kgf | 25 (16-40) | 30 (20-43) |
| Time from diagnosis of septic shock to antibiotics, h | 2.0 (1.0-2.0) | 1.5 (1.0-2.0) |
Abbreviations: APACHE, Acute Physiology and Chronic Health Evaluation; IQR, interquartile range; SOFA, Sequential Organ Failure Assessment.
For variables with missing data, summary data are based on available cases.
Range, 0 to 33; higher scores indicate a greater burden of disease.
Range, 0 to 71; higher scores indicate greater severity of illness and risk of in-hospital death (eg, a score of 22 in a medical patient with sepsis predicts an in-hospital mortality of 45%).22
Range, 0 to 24; higher scores indicate a greater severity of organ dysfunction in critically ill patients and risk of in-hospital death (eg, a score of 10 predicts an in-hospital mortality of 50%).22
Other sources of infection were soft-tissue infection (n = 18), meningitis (n = 6), central line–associated bloodstream infection (n = 4), endocarditis (n = 2), mediastinitis (n = 2), herpes encephalitis (n = 1), subdural empyema (n = 1), pleural empyema (n = 1), septic arthritis (n = 1), and septic abortion (n = 1).
Total intravenous fluids include fluids administered during the interval between presentation to the emergency department and randomization.
Adherence to the Protocol
Lack of adherence was registered in 29 patients (13.7%) in the peripheral perfusion group and 23 (10.8%) in the lactate group (eTable 3 in Supplement 2). Most of these instances were protocol deviations (26 in the peripheral perfusion group and 20 in the lactate group), and 8 patients did not receive the assigned intervention. Protocol deviations were protocol mismanagement in 16 instances in each group, and early termination of assigned treatment for refractory shock in 8 instances in the peripheral perfusion group and 4 instances in the lactate group.
Resuscitation, Perfusion, and Hemodynamic Variables
Two-hundred forty-two patients (57%) were fluid responsive and 106 (25%) fluid unresponsive at baseline, without differences between groups. Fluid responsiveness could not be determined in 76 patients (18%). The most frequently used techniques were pulse pressure variation and passive leg raising with velocity time integral determination in 144 patients in each group. Evolution of fluid responsiveness during the intervention period is shown in eFigure 3 in Supplement 2.
Fewer patients in the peripheral perfusion group (28.8%) than in the lactate group (40.1%) required a vasopressor test (difference, −11.3% [95% CI, −20.8% to −1.9%]; P = .02) (eTable 4 in Supplement 2), although success was not different (44% vs 38%, respectively; P = .86). Sixty-six of the patients (15.6%) received inodilators, without any significant difference between groups.
When considering the whole group of 424 patients independently of fluid responsiveness status, lactate levels were significantly lower at 48 hours and 72 hours in the peripheral perfusion group than in the lactate group (mean difference, −0.36 mmol/L [95% CI, −0.62 to −0.09]; P = .01 in the peripheral perfusion group vs −0.34 mmol/L [95% CI, −0.57 to −0.10] in the lactate group; P < .01), although there were no statistically significant differences at 2, 4, 8, and 24 hours (eTable 2 in Supplement 2). CRT values were significantly lower at 4, 8, and 24 hours in the peripheral perfusion group compared with the lactate group (difference between medians, −0.45 seconds [95% CI, −0.78 to −0.12]; P = .01 at 4 hours, −0.55 [95% CI, −0.85 to −0.25]; P < .01 at 8 hours, −0.42 [95% CI, −0.71 to −0.13]; P < .01 at 24 hours), with no statistically significant differences at 2, 48, and 72 hours. Evolution of CRT and lactate levels exclusively in fluid unresponsive patients is shown in eTable 5 in Supplement 2.
Central venous oxygen saturation and central venous–arterial Pco2 gradients were not significantly different between groups (eTable 2 and eFigure 4 in Supplement 2). Other parameters are shown in eTable 2 in Supplement 2.
Primary Outcome
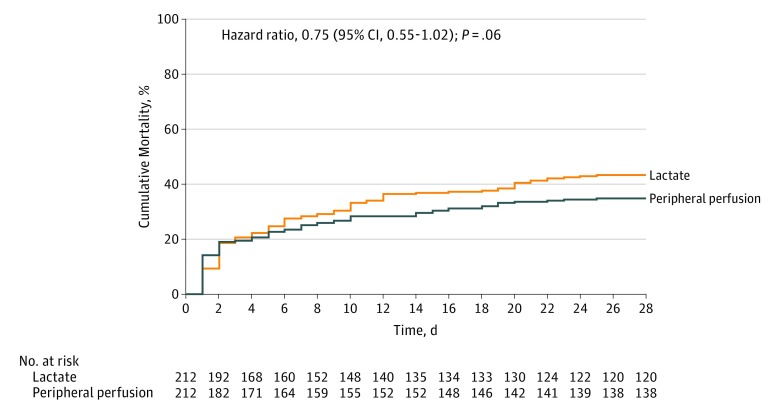
By day 28, a total of 74 patients (34.9%) in the peripheral perfusion group and 92 (43.4%) in the lactate group had died (hazard ratio, 0.75 [95% CI, 0.55 to 1.02]; P = .06; risk difference, −8.5% [95% CI, −18.2% to 1.2%]) (Table 2 and Figure 2). There was no evidence of violation of the proportional hazards assumption (Grambsch and Therneau test P = .07).
Table 2. Main Outcomes of the Study of Resuscitation Strategies in Septic Shock.
| Outcome | Peripheral Perfusion–Targeted Resuscitation (n = 212) |
Lactate Level–Targeted Resuscitation (n = 212) |
Unadjusted Absolute Difference (95% CI) | Adjusted Relative Measure (95% CI) | P Value |
|---|---|---|---|---|---|
| Primary Outcome | |||||
| Death within 28 d, No. (%) | 74 (34.9) | 92 (43.4) | −8.5 (−18.2 to 1.2)b | HR, 0.75 (0.55 to 1.02)a | .06a |
| Secondary Outcomes | |||||
| Death within 90 d, No. (%) | 87 (41.0) | 99 (46.7) | −5.7 (−15.6 to 4.2)b | HR, 0.82 (0.61 to 1.09)a | .17a |
| Mechanical ventilation–free days within 28 d, mean (SD)c | 14.6 (12.1) | 12.7 (12.2) | 1.9 (−0.6 to 4.3) | .14 | |
| Renal replacement therapy–free days within 28 d, mean (SD)c | 18.5 (12.1) | 16.9 (12.1) | 1.7 (−1.5 to 4.8) | .31 | |
| Vasopressor-free days within 28 d, mean (SD)c | 16.7 (12.0) | 15.1 (12.3) | 1.6 (−0.7 to 3.9) | .18 | |
| SOFA at 72 h, No.d | 165 | 166 | .045 | ||
| Mean (SD) | 5.6 (4.3) | 6.6 (4.7) | −1.00 (−1.97 to −0.02) | ||
| ICU length of stay, mean (SD), de | 9.1 (9.8) | 9.0 (9.6) | 0.1 (−1.7 to 2.0) | .91 | |
| Hospital length of stay, mean (SD), df | 22.9 (28.8) | 18.3 (19.0) | 4.6 (0.0 to 9.1) | .05 | |
| Exploratory Outcomes | |||||
| Amount of resuscitation fluids within the first 8 h, No. | 206 | 209 | |||
| Mean (SD), mL | 2359 (1344) | 2767 (1749) | −408 (−705 to −110) | .01 | |
| Total fluid balance, mLg | |||||
| Within 8 h, No. | 198 | 205 | |||
| Mean (SD) | 1587 (1388) | 1874 (1756) | −288 (−598 to 22.0) | .07 | |
| Within 24 h, No. | 176 | 185 | |||
| Mean (SD) | 2025 (2181) | 2343 (2336) | −318 (−785 to 149) | .18 | |
| Within 48 h, No. | 153 | 160 | |||
| Mean (SD) | 992 (1810) | 1224 (3336) | −233 (−831 to 366) | .45 | |
| Within 72 h, No. | 157 | 162 | |||
| Mean (SD) | 1389 (2809) | 1601 (3069) | −212 (−858 to 434) | .52 | |
| Intra-abdominal hypertension, No. of events/total (%)h | 75/119 (63.0) | 68/120 (56.7) | 6.4 (−6.9 to 19.6) | RR, 1.11 (0.90 to 1.37) | .36i |
| Use of renal replacement therapy, No. (%) | 30 (14.2) | 42 (19.8) | −5.7 (−13.3 to 1.9) | RR, 0.71 (0.47 to 1.10) | .15i |
| In-hospital mortality, No. (%) | 84 (39.6) | 97 (45.8) | −6.1 (−16.0 to 3.7) | RR, 0.87 (0.69 to 1.08) | .20i |
Abbreviations: HR, hazard ratio; ICU, intensive care unit; RR, risk ratio; SOFA, Sequential Organ Failure Assessment.
Hazard ratio (95% CI) and P value calculated with Cox proportional hazards model with adjustment for baseline values of Acute Physiology and Chronic Health Evaluation II score, SOFA score, lactate levels, capillary refill time, and source of infection.
Absolute difference (95% CI) calculated from Cox proportional hazard model without adjustment for covariates.
Treatment effects on mechanical ventilation, renal replacement therapy, and vasopressor use assessed with zero inflated negative binomial models.
Treatment effect calculated with linear regression adjusting for baseline SOFA score.
Treatment effects on ICU stay, hospital stay, and resuscitation fluids assessed with generalized linear models with gamma distribution.
Patients still in the hospital for 90 days or more after randomization were considered to be discharged alive at day 90.
Fluid balance within 8, 24, and 72 hours was calculated as all intravenous fluids minus urine output and gastrointestinal losses from randomization to the specified time point. Treatment effect on total fluid balance was calculated with linear regression.
Defined as an intra-abdominal pressure equal or higher than 12 mm Hg. Intra-abdominal pressure was measured via the bladder, with instillation of 25 mL of sterile saline at end-expiration in the complete supine position, with transducer zeroed at the level of the mid-axillary line.
From Fisher exact test.
Figure 2. Kaplan-Meier Estimates of Cumulative Mortality Within 28 Days Among Patients Treated With Peripheral Perfusion–Targeted Resuscitation vs Lactate Level–Targeted Resuscitation.
Hazard ratio, 95% confidence interval, and P value were calculated with a Cox proportional hazards model that included as covariates baseline Acute Physiology and Chronic Health Evaluation (APACHE) II score,23 Sequential Organ Failure Assessment (SOFA) score,24 lactate level, capillary refill time, and source of infection. Median follow-up for peripheral perfusion–targeted resuscitation was 28 days (interquartile range, 8-28 days) and for lactate level–targeted resuscitation was 28 days (interquartile range, 6-28 days).
Secondary and Tertiary Outcomes
There was significantly less organ dysfunction at 72 hours after randomization in the peripheral perfusion group (mean difference in SOFA score, −1.00 [95% CI, −1.97 to −0.02]; P = .045) (Table 2). There were no significant between-group differences in the other 6 secondary outcomes (Table 2).
Patients in the peripheral perfusion group received less resuscitation fluids within the first 8 hours (mean difference, −408 mL [95% CI, −705 to −110]; P = .01) (Table 2).
Twelve cases of suspected unexpected serious adverse reactions were reported by centers, without differences between groups, but none was considered as likely related to the study protocol.
Subgroup and Sensitivity Analyses
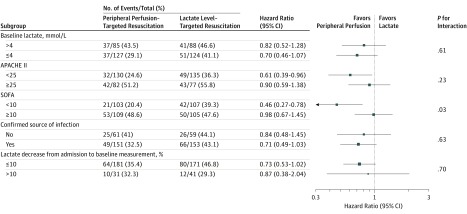
Results of the prespecified subgroup analysis are reported in Figure 3. Treatment effect on the primary outcome was heterogeneous according to baseline SOFA subgroups (P = .03 for interaction). Among patients with SOFA score less than 10, the hazard ratio for 28-day mortality was 0.46 (95% CI, 0.27 to 0.78), whereas among patients with SOFA score 10 or greater the hazard ratio was 0.98 (95% CI, 0.66 to 1.44). There were no significant differences in treatment effect in the other prespecified subgroup analyses. There was also no evidence of heterogeneity of treatment effects across sites (eFigure 5 in Supplement 2). Estimates of treatment effect on 28-day mortality from the prespecified sensitivity analysis (frailty Cox model) were similar to those from the main analysis.
Figure 3. Risk of Death Within 28 Days in the Prespecified Subgroups Among Patients Treated With Peripheral Perfusion–Targeted Resuscitation vs Lactate Level–Targeted Resuscitation.
The area of the square representing the hazard ratio is proportional to the number of events in each subgroup. Horizontal bars represent 95% CI. P values are for heterogeneity of treatment effect on the primary outcome in each subgroup. Hazard ratios and 95% CIs were calculated with Cox proportional hazards model adjusted for the baseline covariates Acute Physiology and Chronic Health Evaluation (APACHE) II score,23 Sequential Organ Failure Assessment (SOFA) score,24 lactate level, capillary refill time, and source of infection. P values were calculated with treatment × subgroup interaction terms. See Table 1 notes for APACHE II and SOFA definitions.
Post Hoc Analysis
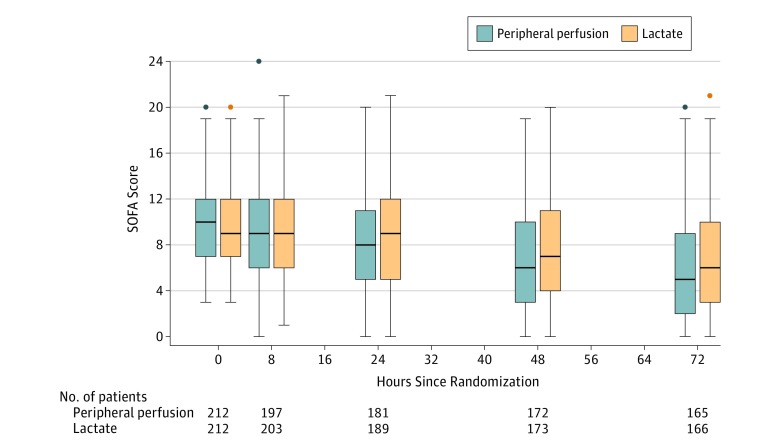
Estimates of treatment effect for several post hoc sensitivity analyses, including per protocol analyses, were also similar to the main analysis (eTable 6 in Supplement 2). There was no evidence of heterogeneity of treatment effects across sites (eFigure 5 in Supplement 2). In a post hoc analysis considering all SOFA measurements during the first 72 hours, there was significantly less organ dysfunction among patients in the peripheral perfusion group compared with the lactate group (mean difference, −1.16 [95% CI, −1.96 to −0.36]; P = .01) (Figure 4).
Figure 4. Organ Dysfunction During the First 72 Hours After Randomization Among Patients Treated With Peripheral Perfusion–Targeted Resuscitation vs Lactate Level–Targeted Resuscitation.
This post hoc analysis is a mixed linear regression considering patient as random effect and adjusting for baseline Sequential Organ Failure Assessment (SOFA) score. P < .001 for mean SOFA score treatment × time interaction within 72 hours. Organ dysfunction assessed by SOFA score23 at baseline and 8, 24, 48, and 72 hours after randomization. SOFA scores range from 0 to 24, with higher scores indicating a greater severity of organ dysfunction and risk of death.23 Box plots indicate 75th percentile (top edge of box), 25th percentile (bottom edge of box), and 50th percentile (bold horizontal line in box); whiskers indicate minimum and maximum values up to 1.5 times the interquartile range from the quartiles; circles indicate values that exceed 1.5 times the interquartile range.
Discussion
In this multicenter randomized clinical trial involving patients with early septic shock, a peripheral perfusion–targeted resuscitation strategy did not result in a significantly lower 28-day mortality when compared with a lactate level–targeted strategy.
The study protocol operationalized, in a stepwise fashion, interventions for septic shock resuscitation widely recommended by current guidelines1,5 or consensus/expert recommendations.2,26 The small but significant differences found in some protocol-related interventions suggest that targets were actively pursued according to the assigned group. The protocol was applied in a context of heterogeneous multinational ICUs with few registered violations, but its potential generalizability requires further studies.
Fluid administration was based on fluid responsiveness status during the intervention period15 and directed by repeated evaluation of the respective assigned targets. In this sense, peripheral perfusion–targeted resuscitation resulted in a small but significant difference in resuscitation fluids, which is consistent with recent observations14 and could merit further exploration in the context of increasing awareness of the risks of fluid overload. In contrast to previous studies,27 fluid responsiveness was determined in more than 80% of the patients. The participation of only highly committed centers with experience in assessment of fluid responsiveness might have contributed to this.
Increasing MAP to levels of 80 to 85 mm Hg in patients with chronic hypertension has been recommended by recent guidelines and expert opinions.1,2 This recommendation was operationalized in the vasopressor test when CRT or lactate targets were not reached. The increase in MAP resulted in the achievement of the respective resuscitation target in about 40% of patients in both study groups. These results could provide a basis for further exploring the use of higher MAP targets in patients with septic shock and a history of chronic hypertension.
Peripheral perfusion–targeted resuscitation was associated with beneficial effects on the secondary outcome of SOFA score at 72 hours and lower 28-day mortality in the predefined subgroup of patients with less severe organ dysfunction at baseline. These results are in line with those from some observational studies that showed that normalization of peripheral perfusion after initial resuscitation was associated with lower mortality and less organ dysfunction9,10,11 and from a pilot study that suggested that restriction of fluid resuscitation based on normal peripheral perfusion was associated with improvement in organ dysfunction.14 Because of the exploratory nature of such secondary outcomes and analyses, these findings should be confirmed by further research.
The use of CRT in clinical practice is not devoid of problems. CRT is dependent on age, sex, ambient temperature and light, and pressure applied during the maneuver—all factors that might influence results.28,29,30 Although no relationship between CRT and hypovolemia was found in older studies,31 more recent studies performed in critically ill patients, including those with septic shock, have shown clinically relevant associations with outcome.9,10,11,19,32 More importantly, CRT was used as a measure of tissue perfusion rather than a surrogate for macrohemodynamics.
The issue of interrater reliability is controversial.33 However, objective CRT measurements obtained by trained ICU physicians using a chronometer revealed good interrater reliability,11,12 contrasting with unreliable observations when CRT was subjectively measured.34 To reduce inaccuracies, a standardized procedure adopting a CRT of 3 seconds as normal was used according to recent clinical observations.11
Limitations
This study has several limitations. First, the nonblinded design might have introduced bias. However, a primary outcome not subject to observer bias was used. In addition, several measurements were taken to minimize potential cross-treatments, including intensive training, auditing, and periodic reinforcement of the study procedures. At the end, adherence and major violations were not different between groups (eTable 3 in Supplement 2). Second, the study may have been underpowered to exclude a clinically meaningful difference between groups. In fact, this is the first major interventional trial testing the potential role of CRT as a resuscitation target, and therefore no previous data to facilitate a power calculation were available. Thus, the estimated effect of peripheral perfusion–targeted resuscitation and the sample size calculation were based mainly on small observational studies and might have been subject to error. Third, interrater variability for CRT was not evaluated; nevertheless, personnel at all centers were thoroughly trained to assess CRT using a standardized technique. Fourth, randomization was not stratified by sites. Therefore, imbalances in the allocation to the treatment groups within sites may have occurred by chance. However, this may have a small or null effect on the effect estimates, since the results of the sensitivity analysis with the frailty Cox model, which consider within-site effects, were consistent with those from the main analysis. Fifth, while the protocol used might appear complex, it only operationalizes interventions widely recommended by current guidelines into a stepwise protocol. Sixth, since this was an ICU-based study, it does not provide information on how effective this approach might be in other contexts, such as wards or resource-limited settings.
Conclusions
Among patients with septic shock, a resuscitation strategy targeting normalization of capillary refill time, compared with a strategy targeting serum lactate levels, did not reduce all-cause 28-day mortality.
Study Protocol
eMethods
eFigure 1. Resuscitation Protocol During the Intervention Period
eFigure 2. Cumulative Recruitment per Month
eFigure 3. Evolution of Fluid Responsiveness State During the 8h Intervention Period
eFigure 4. Perfusion Variables From Baseline to 72 Hours in the Peripheral Perfusion and Lactate Groups
eFigure 5. Treatment Effect on 28-day Mortality Across Sites
eTable 1. Characteristics of Study Centers, Number of Patients Enrolled, and Rate of Enrollment
eTable 2. Hemodynamic and Perfusion Variables From Baseline to 72 Hours in the Peripheral and Lactate Groups
eTable 3. Protocol Violations and Deviations
eTable 4. Use of Vasopressor Test, Inodilator Test, and Adjunctive Drugs for Shock
eTable 5. Capillary Refill Time and Lactate levels at Each Time Point During the Intervention Period in Fluid Unresponsive Patients
eTable 6. Sensitivity Analyses of the Treatment Effect on the Primary Outcome
Data Sharing Statement
Section Editor: Derek C. Angus, MD, MPH, Associate Editor, JAMA (angusdc@upmc.edu).
References
- 1.Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: international guidelines for management of sepsis and septic shock: 2016. Crit Care Med. 2017;45(3):486-552. doi: 10.1097/CCM.0000000000002255 [DOI] [PubMed] [Google Scholar]
- 2.Cecconi M, De Backer D, Antonelli M, et al. ; Task Force of the European Society of Intensive Care Medicine . Consensus on circulatory shock and hemodynamic monitoring. Intensive Care Med. 2014;40(12):1795-1815. doi: 10.1007/s00134-014-3525-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vincent JL, Quintairos E Silva A, Couto L Jr, Taccone FS. The value of blood lactate kinetics in critically ill patients: a systematic review. Crit Care. 2016;20(1):257. doi: 10.1186/s13054-016-1403-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jansen TC, van Bommel J, Schoonderbeek FJ, et al. ; LACTATE Study Group . Early lactate-guided therapy in intensive care unit patients: a multicenter, open-label, randomized controlled trial. Am J Respir Crit Care Med. 2010;182(6):752-761. doi: 10.1164/rccm.200912-1918OC [DOI] [PubMed] [Google Scholar]
- 5.Levy MM, Evans LE, Rhodes A. The Surviving Sepsis Campaign bundle: 2018 update. Crit Care Med. 2018;46(6):997-1000. doi: 10.1097/CCM.0000000000003119 [DOI] [PubMed] [Google Scholar]
- 6.Garcia-Alvarez M, Marik P, Bellomo R. Sepsis-associated hyperlactatemia. Crit Care. 2014;18(5):503. doi: 10.1186/s13054-014-0503-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hernandez G, Luengo C, Bruhn A, et al. When to stop septic shock resuscitation: clues from a dynamic perfusion monitoring. Ann Intensive Care. 2014;4:30. doi: 10.1186/s13613-014-0030-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coopersmith CM, De Backer D, Deutschman CS, et al. Surviving Sepsis Campaign: research priorities for sepsis and septic shock. Crit Care Med. 2018;46(8):1334-1356. doi: 10.1097/CCM.0000000000003225 [DOI] [PubMed] [Google Scholar]
- 9.Lima A, Jansen TC, van Bommel J, Ince C, Bakker J. The prognostic value of the subjective assessment of peripheral perfusion in critically ill patients. Crit Care Med. 2009;37(3):934-938. doi: 10.1097/CCM.0b013e31819869db [DOI] [PubMed] [Google Scholar]
- 10.Lara B, Enberg L, Ortega M, et al. Capillary refill time during fluid resuscitation in patients with sepsis-related hyperlactatemia at the emergency department is related to mortality. PLoS One. 2017;12(11):e0188548. doi: 10.1371/journal.pone.0188548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ait-Oufella H, Bige N, Boelle PY, et al. Capillary refill time exploration during septic shock. Intensive Care Med. 2014;40(7):958-964. doi: 10.1007/s00134-014-3326-4 [DOI] [PubMed] [Google Scholar]
- 12.van Genderen ME, Paauwe J, de Jonge J, et al. Clinical assessment of peripheral perfusion to predict postoperative complications after major abdominal surgery early: a prospective observational study in adults. Crit Care. 2014;18(3):R114. doi: 10.1186/cc13905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lima A, van Genderen ME, van Bommel J, Klijn E, Jansem T, Bakker J. Nitroglycerin reverts clinical manifestations of poor peripheral perfusion in patients with circulatory shock. Crit Care. 2014;18(3):R126. doi: 10.1186/cc13932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Genderen ME, Engels N, van der Valk RJ, et al. Early peripheral perfusion–guided fluid therapy in patients with septic shock. Am J Respir Crit Care Med. 2015;191(4):477-480. doi: 10.1164/rccm.201408-1575LE [DOI] [PubMed] [Google Scholar]
- 15.Hernández G, Cavalcanti AB, Ospina-Tascón G, et al. ; ANDROMEDA-SHOCK Study Investigators . Early goal-directed therapy using a physiological holistic view: the ANDROMEDA-SHOCK—a randomized controlled trial. Ann Intensive Care. 2018;8(1):52. doi: 10.1186/s13613-018-0398-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hernández G, Cavalcanti AB, Ospina-Tascón G, et al. Statistical analysis plan for early goal-directed therapy using a physiological holistic view—the ANDROMEDA-SHOCK: a randomized controlled trial. Rev Bras Ter Intensiva. 2018;30(3):253-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shankar-Hari M, Phillips GS, Levy ML, et al. ; Sepsis Definitions Task Force . Developing a new definition and assessing new clinical criteria for septic shock: for the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):775-787. doi: 10.1001/jama.2016.0289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Monnet X, Marik PE, Teboul JL. Prediction of fluid responsiveness: an update. Ann Intensive Care. 2016;6(1):111. doi: 10.1186/s13613-016-0216-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hernandez G, Pedreros C, Veas E, et al. Evolution of peripheral vs metabolic perfusion parameters during septic shock resuscitation: a clinical-physiologic study. J Crit Care. 2012;27(3):283-288. doi: 10.1016/j.jcrc.2011.05.024 [DOI] [PubMed] [Google Scholar]
- 20.Pinsky MR, Kellum JA, Bellomo R. Central venous pressure is a stopping rule, not a target of fluid resuscitation. Crit Care Resusc. 2014;16(4):245-246. [PubMed] [Google Scholar]
- 21.Asfar P, Meziani F, Hamel JF, et al. ; SEPSISPAM Investigators . High versus low blood-pressure target in patients with septic shock. N Engl J Med. 2014;370(17):1583-1593. doi: 10.1056/NEJMoa1312173 [DOI] [PubMed] [Google Scholar]
- 22.Ferreira FL, Bota DP, Bross A, Mélot C, Vincent JL. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA. 2001;286(14):1754-1758. doi: 10.1001/jama.286.14.1754 [DOI] [PubMed] [Google Scholar]
- 23.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818-829. doi: 10.1097/00003246-198510000-00009 [DOI] [PubMed] [Google Scholar]
- 24.Vincent JL, Moreno R, Takala J, et al. ; Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine . The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med. 1996;22(7):707-710. doi: 10.1007/BF01709751 [DOI] [PubMed] [Google Scholar]
- 25.Grambsch P, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81(3):515-526. doi: 10.1093/biomet/81.3.515 [DOI] [Google Scholar]
- 26.Teboul JL, Saugel B, Cecconi M, et al. Less invasive hemodynamic monitoring in critically ill patients. Intensive Care Med. 2016;42(9):1350-1359. doi: 10.1007/s00134-016-4375-7 [DOI] [PubMed] [Google Scholar]
- 27.Cecconi M, Hofer C, Teboul JL, et al. ; FENICE Investigators; ESICM Trial Group . Fluid challenges in intensive care: the FENICE study: a global inception cohort study [published correction appears in Intensive Care Med. 2015;41(9):1737-1738]. Intensive Care Med. 2015;41(9):1529-1537. doi: 10.1007/s00134-015-3850-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anderson B, Kelly AM, Kerr D, Clooney M, Jolley D. Impact of patient and environmental factors on capillary refill time in adults. Am J Emerg Med. 2008;26(1):62-65. doi: 10.1016/j.ajem.2007.06.026 [DOI] [PubMed] [Google Scholar]
- 29.Brown LH, Prasad NH, Whitley TW. Adverse lighting condition effects on the assessment of capillary refill. Am J Emerg Med. 1994;12(1):46-47. doi: 10.1016/0735-6757(94)90196-1 [DOI] [PubMed] [Google Scholar]
- 30.Saavedra JM, Harris GD, Li S, Finberg L. Capillary refilling (skin turgor) in the assessment of dehydration. Am J Dis Child. 1991;145(3):296-298. [DOI] [PubMed] [Google Scholar]
- 31.Schriger DL, Baraff LJ. Capillary refill—is it a useful predictor of hypovolemic states? Ann Emerg Med. 1991;20(6):601-605. doi: 10.1016/S0196-0644(05)82375-3 [DOI] [PubMed] [Google Scholar]
- 32.van Genderen ME, Lima A, Akkerhuis M, Bakker J, van Bommel J. Persistent peripheral and microcirculatory perfusion alterations after out-of-hospital cardiac arrest are associated with poor survival. Crit Care Med. 2012;40(8):2287-2294. doi: 10.1097/CCM.0b013e31825333b2 [DOI] [PubMed] [Google Scholar]
- 33.Pickard A, Karlen W, Ansermino JM. Capillary refill time: is it still a useful clinical sign? Anesth Analg. 2011;113(1):120-123. doi: 10.1213/ANE.0b013e31821569f9 [DOI] [PubMed] [Google Scholar]
- 34.Alsma J, van Saase JLCM, Nanayakkara PWB, et al. ; FAMOUS Study Group . The power of flash mob research: conducting a nationwide observational clinical study on capillary refill time in a single day. Chest. 2017;151(5):1106-1113. doi: 10.1016/j.chest.2016.11.035 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Study Protocol
eMethods
eFigure 1. Resuscitation Protocol During the Intervention Period
eFigure 2. Cumulative Recruitment per Month
eFigure 3. Evolution of Fluid Responsiveness State During the 8h Intervention Period
eFigure 4. Perfusion Variables From Baseline to 72 Hours in the Peripheral Perfusion and Lactate Groups
eFigure 5. Treatment Effect on 28-day Mortality Across Sites
eTable 1. Characteristics of Study Centers, Number of Patients Enrolled, and Rate of Enrollment
eTable 2. Hemodynamic and Perfusion Variables From Baseline to 72 Hours in the Peripheral and Lactate Groups
eTable 3. Protocol Violations and Deviations
eTable 4. Use of Vasopressor Test, Inodilator Test, and Adjunctive Drugs for Shock
eTable 5. Capillary Refill Time and Lactate levels at Each Time Point During the Intervention Period in Fluid Unresponsive Patients
eTable 6. Sensitivity Analyses of the Treatment Effect on the Primary Outcome
Data Sharing Statement