Key Points
Question
Did the US Food and Drug Administration’s (FDA’s) Transmucosal Immediate-Release Fentanyl (TIRF) Risk Evaluation and Mitigation Strategy (REMS) meet its goal of preventing inappropriate use of TIRF products, such as prescribing to patients without opioid tolerance?
Findings
In this review of FDA documents from 2012 to 2017, surveys of 786 individuals 12 months after program inception indicated that 86.1% of pharmacists, 87.4% of prescribers, and 90.6% of patients correctly reported that TIRFs are contraindicated in opioid-nontolerant patients, yet claims-based analyses 60 months after program inception indicated that 34.6% to 55.4% of patients prescribed TIRFs were opioid-nontolerant.
Meaning
Pharmacist, prescriber, and patient surveys conducted as part of the TIRF REMS generally indicated adequate knowledge about the proper use of TIRFs, yet some survey items and claims-based analyses indicated substantial rates of inappropriate use.
Abstract
Importance
Transmucosal immediate-release fentanyls (TIRFs), indicated solely for breakthrough cancer pain in opioid-tolerant patients, are subject to a US Food and Drug Administration (FDA) Risk Evaluation and Mitigation Strategy (REMS) to prevent them from being prescribed inappropriately.
Objectives
To evaluate knowledge assessments of pharmacists, prescribers, and patients regarding appropriate TIRF use; to describe sponsor assessments, based on claims data, of whether the REMS program was meeting its goals; and to characterize how the FDA responded to REMS assessments.
Design, Setting, and Participants
Qualitative analysis of 4877 pages of FDA documents obtained through a Freedom of Information Act request, including 6 annual REMS assessment reports (2012-2017), FDA evaluations of these reports, and FDA-sponsor correspondence about safety issues.
Exposure
A REMS program to reduce the risk of adverse outcomes, including misuse, abuse, addiction, and overdose, arising from use of TIRFs.
Main Outcomes and Measures
(1) Knowledge assessments of pharmacists, prescribers, and patients; (2) survey and claims-based prescribing assessments; (3) FDA and TIRF sponsor communications; (4) modifications to the REMS program; and (5) disenrollment of noncompliant prescribers.
Results
Twelve months after initiation of the program, 24 of 302 pharmacists (7.9%), 35 of 302 prescribers (11.6%), and 5 of 192 patients (2.6%) incorrectly reported that TIRFs can be prescribed to opioid-nontolerant patients, with similar levels of misunderstanding maintained in the subsequent reports. At 60 months, product-specific analyses of claims data indicated that between 34.6% and 55.4% of patients prescribed TIRFs were opioid-nontolerant. In the 48-month survey, 106 of 310 prescribers (34.2%) reported prescribing TIRFs for opioid-tolerant patients with chronic, noncancer pain; at 60 months, 54 of 302 prescribers (18.4%) and 148 of 310 patients (47.7%) erroneously reported that TIRFs were FDA-approved for such use. Over the 60-month period examined, there were few substantive changes made to the REMS to address evidence of high rates of off-label TIRF use, and, although the REMS program had a noncompliance plan, there was no report of prescribers being disenrolled for inappropriate prescribing.
Conclusions and Relevance
In this review of FDA documents pertaining to the TIRF REMS, surveys of pharmacists, prescribers, and patients reflected generally high levels of knowledge regarding proper TIRF prescribing, yet some survey items as well as claims-based analyses indicated substantial rates of inappropriate TIRF use. Despite these findings, the FDA did not require substantive changes to the program.
This study uses FDA documents to describe transmucosal immediate-release fentanyl (TIRF) prescribing patterns after the agency’s 2011 REMS intended to reduce the rate of TIRF-related adverse outcomes, misuse, abuse, addiction, and overdose.
Introduction
More than 47 000 people in the United States died from opioid overdose in 2017,1 the highest in any year on record,2 and more than 2.1 million US residents were estimated to have fulfilled the criteria for an opioid use disorder within the past year.3
Since 2007, the US Food and Drug Administration (FDA) has used Risk Evaluation and Mitigation Strategies (REMS) to support the safe use of prescription medications.4 Because of their potency and potential for overdose if used inappropriately, on December 28, 2011, the FDA approved a highly restrictive REMS for all transmucosal immediate-release fentanyl (TIRF) products, a class of short-acting fentanyls delivered through sublingual and buccal tablets, lozenges, and sprays approved solely for the management of breakthrough cancer pain in opioid-tolerant patients.5
The overarching goal of the TIRF REMS program was to reduce the risk of adverse outcomes, misuse, abuse, addiction, and overdose arising from use of TIRFs, in part by preventing TIRFs from being prescribed to patients who were not already opioid tolerant and, secondarily, supporting judicious prescribing in appropriate patient populations, such as patients with the labeled indication of breakthrough cancer pain. Before dispensing, prescribing, or using TIRFs, pharmacists, prescribers, and patients were required to certify their understanding of the indications, appropriate use, and risks of TIRFs.6 The sponsors were required to provide formal assessments of the REMS to the FDA 6 and 12 months following the initiation of the program, and every 12 months thereafter. This study assessed how the FDA managed the REMS program to maximize appropriate TIRF prescribing and use, as well as how the program was modified over time to meet this goal.
Methods
The study was exempted from review by the Johns Hopkins Bloomberg School of Public Health Institutional Review Board.
Study Design and FOIA History
We performed a review of documents obtained from the FDA through a Freedom of Information Act (FOIA) request, including assessments conducted by TIRF sponsors as part of the consolidated REMS program, the responses of the FDA to these assessments, and other communications between sponsors and the FDA. Because this was a class-wide REMS, assessments were completed by an industry-wide consortium representing TIRF manufacturers, the TIRF REMS Industry Group (TRIG).
In January 2018, the FDA provided 3812 pages of materials related to the TIRF REMS program. Some documents were redacted based on exceptions for “confidential commercial information” [5 USC §552 (b)(4)] and/or intra-agency “deliberative process” [5 USC §552 (b)(5)]. These redactions were appealed by the authors, resulting in the successful acquisition of an additional 1065 pages of documents.
Coding and Data Extraction
Because the consolidated REMS program required sponsors to submit annual reports to the FDA, we first coded each document based on which REMS assessment it applied to (eg, 12-month, 24-month), as well as whether it was a sponsor assessment, an FDA evaluation of these assessments, or information regarding the REMS program. Because information was at a class-wide level, no analysis of individual TIRF products was possible. Our initial document classification was performed by 1 author (J.E.R.), although each document was reviewed by at least 2 independent analysts. Next, we extracted quantitative information provided by sponsors into data tables, noting whether the TRIG set numeric targets for quantitative outcomes.
Some data came from prescriber, pharmacist, and patient knowledge, attitudes, and behavior surveys. These surveys solicited responses among a subset of active REMS enrollees recruited by US mail, fax, and email, and were closed after 300 responses had been obtained from each group. Respondents received $125 (prescribers), $50 (pharmacists), or $25 to $50 (patients) compensation. The TRIG reported that the survey population was selected randomly, however, the precise sampling method was not clear from the documents reviewed. According to the REMS protocol, the target response size was “based on both practical and statistical considerations,” and survey respondents were excluded from recruitment for subsequent surveys. Although TIRF manufacturers performed surveys at each follow-up point, we report 12- and 60-month assessments herein for brevity; the response scores for specific questions in the 24-, 36-, and 48-month assessments generally fell between the scores for the same questions in the 12- and 60-month assessments.
We also extracted active safety surveillance data, such as an analysis of commercial claims (eAppendix in the Supplement). We received additional safety data from the American Association of Poison Control Centers (AAPCC), the FDA’s Adverse Event Reporting System (FAERS), and the Researched Abuse, Diversion and Addiction-Related Surveillance (RADARS) System. However, the AAPCC and FAERS are passive surveillance sources and the RADARS data were extensively redacted, thus, we did not include these data in our analysis.
In addition, we extracted information relevant to program noncompliance. This information included any instances of prescriber noncompliance and disenrollment from the REMS as a result of noncompliance.
Analysis
Our primary outcomes were results of knowledge, attitudes, and behavior surveys of prescribers, pharmacists, and patients; survey- and claims-based prescribing assessments; iterative communication between the FDA and TIRF manufacturers from March 2012, when the REMS was initiated, to December 2017, the date of the last FDA assessment we received; subsequent modifications to the REMS program; and detection and disenrollment of physicians prescribing TIRFs to opioid-nontolerant patients. All statistical analyses were performed or commissioned by other parties, including the TRIG and the FDA, and no new statistical analyses were performed by the authors. Reflecting the intent of the REMS, our qualitative assessment of communications and REMS modifications focused primarily on TIRF use among opioid-nontolerant patients, defined as patients who had not been receiving regular opioid therapy for at least 1 week, as determined using prescription claims data, and, secondarily, on TIRF use for noncancer pain. This latter use, while not contraindicated, is an off-label usage that runs contrary to the mandatory REMS training materials. We assessed off-label prescribing using prescribers’ self-reported practices in the surveys. Throughout this process, we used an inductive, or grounded theory, approach, in which data were used to inform hypothesis development and to generate further ideas on how to analyze and interpret the materials. Thus, during data extraction, synthesis, and analysis, authors met frequently to share perspectives and to develop consensus, through iterative review of the source documents and discussion of core themes, regarding the overall approach and substantive interpretation of the documents received.
Results
REMS Participants
At the time of the 60-month assessment report, 8151 prescribers, 42 665 pharmacies, and 42 164 patients were enrolled in the TIRF REMS program, representing a modest increase in participating pharmacies and more than a tripling of patients compared with the 12-month assessment. Products covered by the REMS are detailed in eTable 1 in the Supplement.
REMS Goals
Based on the information reviewed, no numerical benchmarks were established by the FDA to measure achievement of the REMS goal to reduce the risk of adverse safety outcomes; however, the FDA and the TRIG agreed that “a correct response rate of 65% or greater would be considered to represent adequate understanding of each concept or key risk message”6 for the knowledge surveys of pharmacists, prescribers, and patients, including questions about appropriate TIRF prescribing. The Table depicts the results of those cross-sectional surveys during the 12- and 60-month REMS assessments.
Table. Pharmacist, Prescriber, and Patient Knowledge, Attitudes, and Beliefs Regarding Transmucosal Immediate-Release Fentanyls (TIRFs)a .
| Correct Response, % (95% CI)b | ||
|---|---|---|
| 12-Month REMS Assessment | 60-Month REMS Assessment | |
| Pharmacist surveyc | n = 300 | n = 318 |
| According to TIRF labeling, patients with cancer who are opioid-tolerant are patients… | ||
| Taking around-the-clock opioid therapy for underlying cancer pain for 1 week or longer (true) | 87.4d | 95.6 (92.7-97.6) |
| With no known contraindications to fentanyl, but not currently taking around-the-clock opioids (false) | 84.4d | 82.1 (77.4-86.1) |
| TIRFs are contraindicated in opioid-nontolerant patients because life-threatening respiratory depression could occur at any dose. (true) | 86.1 (81.7-89.8) | 88.4 (84.3-91.7) |
| TIRFs may be used in opioid-nontolerant patients. (false) | 78.5 (73.4-83.0) | 87.4 (83.3-90.9) |
| For which of the following indications can TIRFs be prescribed to opioid-tolerant patients? | ||
| Acute or postoperative pain (false) | 78.1 (73.1-82.7) | 85.8 (81.5-89.5) |
| Headache or migraine pain (false) | 89.1 (85.0-92.4) | 94.3 (91.2-96.6) |
| Dental pain (false) | 94.7(91.5-96.9) | 96.2 (93.5-98.0) |
| Breakthrough pain from cancer (true) | 83.4 (78.8-87.5) | 91.8 (88.2-94.6) |
| Chronic, noncancer pain (false) | 29.8 | 50.9 (45.3-56.6) |
| Prescriber surveye | n = 294 | n = 302 |
| According to TIRF labeling, patients with cancer who are opioid-tolerant are those… | ||
| Taking around-the-clock opioids for cancer pain for 1 week or longer (true) | 89.7d | 94.9 (91.7–97.1) |
| Who have no known contraindications to fentanyl, but are not currently taking around-the-clock opioid therapy (false) | 83.1d | 92.5 (88.9–95.3) |
| TIRFs are contraindicated in opioid-nontolerant patients because life-threatening respiratory depression could occur at any dose. (true) | 87.4 (83.1-90.9) | 91.8 (88.1–94.7) |
| TIRFs may be used to treat opioid-nontolerant patients. (false) | 82.5 (77.7-86.6) | 88.4 (84.2-91.9) |
| For which of the following indications do you prescribe TIRFs to your opioid-tolerant patients?b | ||
| Acute or postoperative pain (no) | 86.4 (82.0-90.1) | 90.3 (86.5-93.4)c |
| Headache or migraine pain (no) | 86.8 (82.4-90.4) | 94.8 (91.8-97.0)f |
| Dental pain (no) | 96.0 (93.2-97.7) | 98.4 (96.3-99.5)f |
| Breakthrough pain from cancer (yes) | 95.4 (92.3-97.4) | 92.9 (89.5-95.5)f |
| Chronic, noncancer pain (no) | 54.3 | 64.8 (59.2-70.2)f |
| Patient surveyg | n = 192 | n = 310 |
| TIRF medicines can cause life-threatening breathing problems leading to death (true) | 90.1 (85.0-93.9) | 91.6 (88.0-94.4) |
| TIRF medicines should only be taken by opioid-tolerant patients (true) | 90.6 (85.6-94.3) | 89.4 (85.4-92.6) |
| “Opioid tolerant” means that a patient is already taking other opioid pain medicines around-the-clock and their body is used to these medicines (true) | 91.7 (86.8-95.2) | 88.1 (83.9-91.5) |
| For which of the following conditions should you use a TIRF medicine? | ||
| Headache or migraine pain (no) | 72.9 | 78.1 (73.0-82.5) |
| Breakthrough pain from cancer (yes) | 69.8 | 72.6 (67.3-77.5) |
| Dental pain (no) | 89.6 | 86.8 (82.5-90.3) |
| Pain after surgery (no) | 67.7 | 64.2 (58.6-69.5) |
| Long-lasting pain not from cancer (eg, arthritis joint pain) (no) | 24.5 | 39.0 (33.6-44.7) |
| It is OK for patients to take TIRF medicines for headache pain (no) | 70.8 (63.9-77.2) | 67.4 (61.9-72.6) |
Data derived from surveys of pharmacists, prescribers, and patients conducted by TIRF sponsors in fulfilment of US Food and Drug Administration (FDA) Risk Evaluation and Mitigation Strategy (REMS) Program.
Each survey question was reported as assessing a “key risk message” or not. The TIRF sponsors’ assessments we reviewed only reported CIs for key risk messages; some questions were considered key risk messages in the 60-mo assessment but not in the 12-mo assessment.
See Figure 1 footnote “c” and Technical Appendix in the Supplement for additional details on the pharmacist survey.
TIRF sponsors’ values purport to be the number of prescribers who responded “true” to the questions; however, we believe values were erroneously reverse coded because they are considerably inconsistent with reporting from other months, while their complement is not. We present the complement of the TIRF sponsors’ values here.
See Figure 1 footnote “b” and Technical Appendix in the Supplement for additional details on the prescriber survey.
Question and correct response rate derived from 48-mo assessment because the question modified at 60-mo from asking about clinical practice to “Per the approved labelling for TIRF medicines, for which of the following indication(s) are TIRF medicines approved.” The 60-mo results were similar to the 48-mo results, with the exception of breakthrough pain from cancer (99.3% [95% CI, 97.6-99.9%]) and chronic, noncancer pain (78.2% [95% CI, 73.1 – 82.8%]).
See Figure 1 footnote “d” and Technical Appendix in the Supplement for additional details on the patient survey.
The predetermined 300-respondent sample for each survey at each time point represented a small proportion of the representative subsample of all enrolled pharmacists, prescribers, and patients who were invited to complete surveys. Overall, across the 12-, 24-, 36-, 48-, and 60-month surveys, 1520 of 34 939 contacted pharmacists (4.4%) completed the survey before data collection was terminated at 300 responses for each time point. Before data collection ended, 1508 of 25 995 prescribers (5.8%) and 1343 of 12 872 patients (10.4%) responded. Although responders were generally reported to be geographically representative of all REMS participants, there were no data comparing responders to nonresponders at any time point, nor were there other assessments evaluating for the potential of nonresponse bias.
Minimizing TIRF Use Among Opioid-Nontolerant Patients
Knowledge
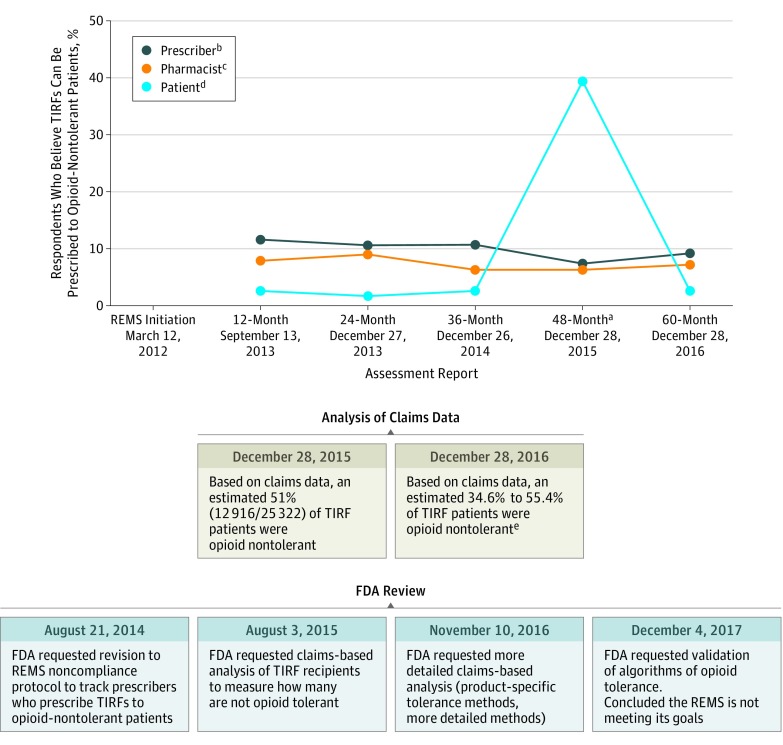
At 12 months, 24 of 302 pharmacists (7.9%), 35 of 302 prescribers (11.6%), and 5 of 192 patients (2.6%) incorrectly reported that TIRFs can be prescribed to opioid-nontolerant patients, with similar levels of misunderstanding maintained in the subsequent reports (Figure 1).
Figure 1. Pharmacists, Prescribers, and Patients Who Believe Transmucosal Immediate-Release Fentanyls (TIRFs) Can Be Prescribed to Opioid-Nontolerant Patients, Claims-Based Estimates of Rates of Opioid Tolerance, and Associated US Food and Drug Administration (FDA) Actions.
a Statement changed to the following (addition italicized): “TIRF medicines should only be taken by cancer patients who are opioid tolerant.” The question was changed back for the 60-month report.
b Recruitment of prescribers to complete online or phone-based survey continued until 300 respondents were recruited. The number of prescribers who responded before data cutoff was 302 of 5330 (5.7%) for the 12-month REMS assessment, 302 of 5108 (5.9%) for the 24-month, 300 of 4499 (6.7%) for 36-month, 310 of 8210 (3.8%) for 48-month, and 294 of 2848 (10.3%) for 60-month assessment. See Technical Appendix in the Supplement for additional details.
c Recruitment of pharmacists filling TIRFs to complete online or phone-based surveys continued until 300 respondents were recruited. The number of pharmacists who responded before data cutoff was 302 of 7236 (4.2%) for the 12-month REMS assessment, 300 of 7167 (4.2%) for the 24-month, 300 of 4022 (7.5%) for 36-month, 301 of 4906 (6.1%) for 48-month, and 318 of 11 598 (2.7%) for the 60-month assessment. See Technical Appendix in the Supplement for additional details.
d Recruitment of patients who filled a TIRF prescription within the past 90 days and their caregivers continued until 300 respondents were recruited. The number of invited patients who responded before data cutoff was 192 of 1112 (17.3%) for the 12-month REMS assessment, 302 of 1903 (15.8%) for the 24-month, 229 of 1343 (17.0%) for the 36-month, 310 of 5569 (5.6%) for the 48-month, and 310 of 2945 (10.5%) for the 60-month assessment. See Technical Appendix in the Supplement for additional details.
e Because of redactions in the source documents, the numerators and denominators used to calculate the opioid-nontolerance percentages reported in the 60-month report are not available. The opioid-nontolerance percentages reported at this time point were product-specific and differed on a product-by-product basis, which is why it is reported as a range.
Utilization
Analyses of health plan data indicated that thousands of patients receiving TIRFs were opioid-nontolerant. In their evaluation of the TRIG’s 36-month (3-year) assessment, the FDA requested an analysis of health plan claims “in order to assess the TIRF REMS goal of prescribing and dispensing TIRF products only to appropriate patients.”7 This analysis, provided as part of the 48-month (4-year) TRIG report, determined that 12 916 of 25 322 patients receiving TIRFs (51%) were opioid nontolerant, which was defined in the REMS protocol as patients who had not “had an opioid analgesic product prescription fill with at least 7 continuous days of sufficient daily dose immediately preceding the TIRF prescription date.”8 The FDA’s 48-month evaluation requested more detailed claims-based analyses for the 60-month report. In a supplement to the 60-month report, the TRIG reported that, on a product-by-product basis, between 34.6% and 55.4% of patients receiving TIRFs were opioid nontolerant.
FDA Response
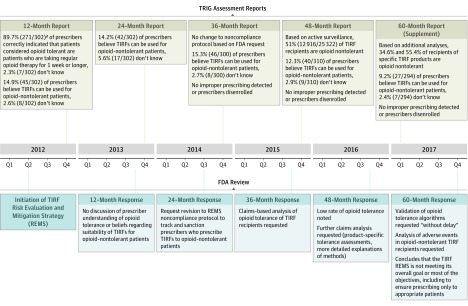
Figure 2 depicts the FDA’s iterative communications with the TRIG regarding TIRF use in opioid-nontolerant patients. Following review of the TRIG’s 12-month assessment, a REMS Modification Review showed that the FDA received complaints from both the TRIG and an unspecified number of TIRF-enrolled physicians that 2 requirements for patients to receive TIRFs (that the patient must be “currently using around-the-clock opioid medication” and be “opioid tolerant”) unduly restricted the clinical judgment of clinicians.9 In response, the FDA changed an attestation in the patient-provider agreement form from “My patient is opioid tolerant” to “I understand that patients considered opioid tolerant are those who are regularly taking at least: 60 mg oral morphine/day; 25 micrograms transdermal fentanyl/hour; 30 mg oral oxycodone/day; 8 mg oral hydromorphone/day; 25 mg oral oxymorphone/day; or an equianalgesic dose of another opioid for one week or longer.”9
Figure 2. Timeline of Communication Between Transmucosal Immediate-Release Fentanyl (TIRF) Sponsors and the US Food and Drug Administration (FDA) About TIRF Use Among Individuals Without Opioid Tolerance, 2012-2017.
Following the 24-month assessment, the FDA also requested changes to the TIRF REMS Non-Compliance Plan, including tracking instances of the “number of times a TIRF was prescribed to an opioid non-tolerant individual.”10 Based on these changes, the TRIG was expected to identify, investigate, and even deactivate prescribers who inappropriately prescribed TIRFs to opioid-nontolerant patients, according to a later iteration of the Non-Compliance Plan.11 The TRIG was responsible for monitoring noncompliance using “standard program reports, spontaneous reports identified via the program’s Call Center, vendor/sponsor reported events, outreach to relevant stakeholders to validate data/information and solicit further information, and investigation of the TIRF REMS Access database.”8 However, in the 24-month assessment and all subsequent reports, there were “no reports of TIRF medicines being prescribed to an opioid non-tolerant individual”12 from the noncompliance event information sources and no reports of prescribers being disenrolled from the REMS, despite claims-based data at 48 months suggesting that 12 916 of 25 322 patients receiving TIRFs (51%) were opioid-nontolerant.13
As part of its 60-month evaluation, the FDA stated that “the first objective [prescribing only to appropriate/opioid-tolerant patients] is not being achieved,” and “we have determined that the TIRF REMS is not meeting its overall goal or most of the objectives.”14 The FDA requested that the TRIG validate its algorithm for determining opioid tolerance and provide an additional analysis to evaluate adverse events in opioid-nontolerant patients.
Minimizing TIRF Use Among Patients Without Breakthrough Cancer Pain
Knowledge
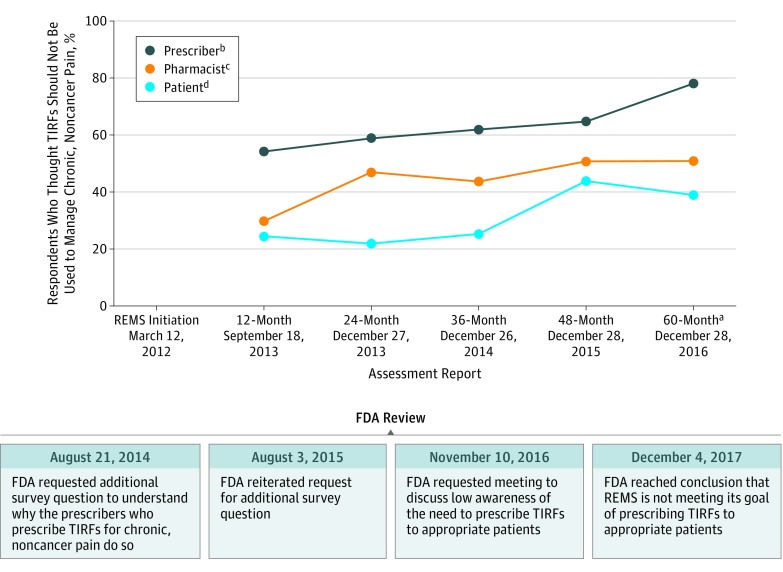
In data collected as part of the REMS, increasing numbers of pharmacists and patients over time correctly reported that TIRFs were not FDA-approved for patients without breakthrough cancer pain, and an increasing proportion of prescribers responded that they did not prescribe TIRFs off-label for that indication (Figure 3). Nonetheless, at 60 months, only 162 of 318 pharmacists (50.9%) (95% CI, 45.3%-56.6%) reported that TIRFs should not be prescribed to opioid-tolerant patients for chronic, noncancer pain. Similar patterns were observed with prescribers. At the 60-month assessment, 54 of 294 prescribers (18.4%) erroneously reported that TIRFs were FDA-approved for chronic, noncancer pain.
Figure 3. Pharmacists, Prescribers, and Patients Who Believe Transmucosal Immediate-Release Fentanyls (TIRFs) Should Not Be Used to Manage Chronic, Noncancer Pain and Associated US Food and Drug Administration (FDA) Actions.
a Patients and pharmacists were asked if you should use TIRFs to treat chronic, noncancer pain, prescribers were asked if they do prescribe TIRFs to treat chronic, noncancer pain. The question was modified at 60 months to ask prescribers if TIRFs are indicated for chronic, noncancer pain, rather than if they prescribe TIRFs to treat chronic, noncancer pain.
b Recruitment of prescribers to complete online or phone-based survey continued until 300 were respondents recruited. The number of prescribers who responded before data cutoff was 302 of 5330 (5.7%) for the 12-month REMS assessment, 302 of 5108 (5.9%) for the 24-month, 300 of 4499 (6.7%) for 36-month, 310 of 8210 (3.8%) for 48-month, and 294 of 2848 (10.3%) for 60-month assessment. See Technical Appendix in the Supplement for additional details.
c Recruitment of pharmacists filling TIRFs recruitment continued until 300 respondents were recruited. Number of pharmacists who responded before data cutoff was 302 of 7236 (4.2%) for the 12-Month REMS assessment, 300 of 7167 (4.2%) for the 24-month, 300 of 4022 (7.5%) for 36-month, 301 of 4906 (6.1%) for 48-month, and 318 of 11 598 (2.7%) for the 60-month assessment. See Technical Appendix in the Supplement for additional details.
d Recruitment of patients who filled TIRF prescription within the past 90 days and their caregivers continued until 300 respondents were recruited. The number of invited patients who responded before data cutoff was 192 of 1112 (17.3%) for the 12-Month REMS assessment, 302 of 1903 (15.8%) for the 24-month, 229 of 1343 (17.0%) for the 36-month, 310 of 5569 (5.6%) for the 48-month, and 310 of 2945 (10.5%) for the 60-month assessment. See Technical Appendix in the Supplement for additional details.
Overall, comprehension regarding appropriate use of TIRFs was lower in patients than in pharmacists or prescribers. In the 60-month assessment, 148 of 310 patients (47.7%) erroneously reported that TIRFs were FDA-approved for “long-lasting pain not from cancer, like arthritis joint pain.”
Utilization
In the 5 years of REMS documents available for analysis, there was no request by the FDA for the TRIG to conduct claims-based analyses quantifying the number of patients receiving TIRFs who did not have cancer; however, surveys did ask prescribers about the clinical indications for prescribing TIRFs. At 12 months, 261 of 302 (86.4%), 262 of 302 (86.7%), and 290 of 302 (96.0%) prescribers reported that they did not prescribe TIRFs for acute or postoperative pain, headaches, or dental pain, respectively. Comparable understanding of prescribing was reported in the 60-month assessment: 278 of 294 (94.6%), 276 of 294 (93.9%), and 283 of 294 (96.3%) prescribers reported that per the approved labeling, TIRFs were not approved for acute or postoperative pain, headaches, or dental pain, respectively. Despite these results, at 24 months, 119 of 302 prescribers (39.4%) reported that they prescribed TIRFs for opioid-tolerant patients with chronic, noncancer pain. Comparable levels of prescribers reported that they prescribed TIRFs for opioid-tolerant patients with chronic, noncancer pain in the 36-month (112 of 300 [37.3%]) and 48-month (106 of 310 [34.2%]) assessments.
FDA Response
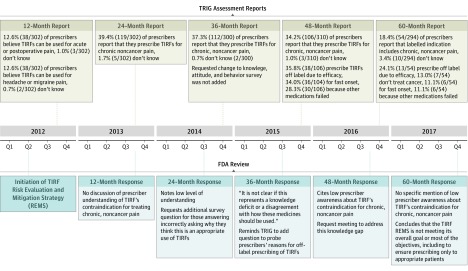
Following the 24-month prescriber knowledge, attitudes, and behavior assessment, in which 119 of 302 prescribers (39.4%) reported prescribing TIRFs for chronic, noncancer pain, the FDA requested that a follow-up question be added in the 36-month report to determine prescribers’ reasons for prescribing TIRFs off-label.15 In its review of the TRIG’s 36-month assessment, the FDA again requested that the question be added. The question was included in the 48-month assessment report. In this report, 106 of 310 prescribers (34.2%) reported prescribing TIRFs for chronic noncancer pain; of these 106 prescribers, 38 (35.8%) reported the reason for prescribing was TIRF efficacy, 36 (34.0%) reported fast onset, and 30 (28.3%) reported other medications failing. In response, the FDA requested a meeting with the TRIG to address the low prescriber awareness that TIRFs are not indicated for chronic, noncancer pain; the deliberations and outcomes of that meeting were not available for analysis.
Despite 54 of 294 prescribers (18.4%) erroneously reporting in the 60-month (5-year) assessment that the labeled indication for TIRFs includes chronic, noncancer pain, the FDA’s response to that report did not specifically mention prescriber awareness of TIRF’s labeled indications. But, the response did allude to a high prevalence of improper prescribing in its program critique.16
Before the submission of the 60-month assessment, there were no substantial changes made to the REMS program to address the proportions of pharmacists, prescribers, and patients indicating that TIRFs were appropriate for the treatment of chronic, noncancer pain (Figure 4). However, after the submission of that report, the FDA approved the following change in the patient-provider agreement form and prescriber enrollment form (modifications are italicized): “I understand that TIRF medicines are indicated only for the management of breakthrough pain in cancer patients, 18 years of age or older (Actiq and its generic equivalents are approved for 16 years of age and older), who are already receiving and who are tolerant to, around-the-clock opioid therapy for their underlying persistent pain.”17 The FDA also approved the addition of the following attestation to both the patient-provider agreement form and the prescriber enrollment form: “I understand that TIRF medicines must not be used to treat acute or postoperative pain, including headache/migraine and dental pain, or acute pain in the emergency department.”18
Figure 4. Timeline of Communication Between Transmucosal Immediate-Release Fentanyl (TIRF) Sponsors and the US Food and Drug Administration (FDA) About TIRF Use Among Individuals With Chronic, Noncancer Pain, 2012-2017.
Discussion
This review of documents obtained through a FOIA request that depict the assessment and management of the class-wide TIRF REMS program found that while REMS-mandated knowledge surveys indicated that large majorities of pharmacists, prescribers, and patients were aware that TIRFs are contraindicated in opioid-nontolerant patients, claims-based analyses indicated that 34.6% to 55.4% of patients receiving TIRFs were not opioid tolerant. Despite increasing evidence that the program was not achieving its stated goals, no substantive changes to the program were made by TIRF manufacturers or the FDA to address these shortcomings.
Beginning with the 12-month REMS assessment report, sequential assessments suggested unacceptably high rates of off-label prescribing, including prescribing of TIRFs contrary to absolute contraindication in opioid-nontolerant patients. In response, the FDA continually requested more data related to off-label prescribing, while stating that it could not determine whether the REMS program was successful. At the 60-month assessment, the FDA concluded that “the TIRF REMS is not meeting its overall goal or most of the objectives”12 and again requested further analyses of opioid tolerance among patients receiving TIRFs. Thus, after 5 years of data suggesting that the program did not effectively limit TIRF use to opioid-tolerant patients, few changes had been instituted, and changes that were instituted generally occurred after an 8- to 10-month lag in communication on the part of the FDA, preventing rapid, iterative revision of the program to improve safe TIRF use.
The REMS assessment protocol included a detailed noncompliance plan, empowering the TIRF sponsors to investigate and issue “corrective actions” against patients, prescribers, and pharmacies who violated the requirements of the program, including “notices, warnings, suspension and deactivation based on the requirements of the TIRF REMS Access Program.”9 In its Index of Non-Compliance Scenarios for Prescribers, the protocol lists “Prescribed TIRF medicine to an opioid non-tolerant individual” as an applicable scenario. However, as of the 60-month assessment, no instances of inappropriate prescribing, or corrective action against prescribers, were reported by TIRF manufacturers, while data suggested that as many as half of prescriptions had been for opioid-nontolerant patients.
REMS programs were the subject of a 2013 US Department of Health and Human Services Office of the Inspector General (OIG) report questioning their overall effectiveness.19 The OIG concluded its report with 7 recommendations for the FDA to improve its oversight of the REMS programs; the FDA concurred with 6. However, in this analysis there was little evidence that the FDA complied with these recommendations, particularly the recommendation stating that “if FDA cannot determine whether a REMS is meeting its goals, it should work with sponsors to obtain any additional information that it needs to make this determination,” and it “should not wait until it reviews the next sponsor assessment to determine whether a REMS is meeting its goals.”17 Likewise, there was little evidence that the FDA successfully met its commitment to “ensure that assessment reviews are timely,” consistent with the OIG’s suggestion that “FDA could prioritize assessment reviews for REMS with ETASUs as these REMS are required for drugs with the most serious risks.”17 These findings reinforce many of the concerns noted in the 2013 OIG report.
In August 2018, the FDA convened a Joint Meeting of the Drug Safety and Risk Management Advisory Committee and the Anesthetic and Analgesic Drug Products Advisory Committee to examine the TIRF REMS program. This meeting coincided with the submission of select preliminary findings from this FOIA in formal written testimony to the FDA that were also reported by the authors of the current study.20 Committee members were briefed on the background, execution, and outcomes of the program by the FDA and industry sources. The committees acknowledged that off-label prescribing appeared common and provided several recommendations to strengthen the program, including the conduct of additional claims-based analyses and more rigorous educational programs to mitigate “knowledge attrition” among trained TIRF prescribers.21
Limitations
This analysis has several limitations. First, some of the information requested, such as complete safety surveillance data at multiple time points, was redacted, preventing full visibility of the context for the regulatory decisions that were made. Similarly, there may be additional documents not provided that could give a clearer picture of this regulatory history, or relevant advice provided by the FDA in nonwritten contexts. Second, the data were limited. For example, the surveys of pharmacists, prescribers, and patients had small sample sizes, increasing the likelihood that the sample was not representative or that socially desirable response bias was operating. Third, qualitative data are subject to differential interpretation, despite efforts to triangulate and validate the findings through several rounds of extraction and analysis. Fourth, the documents analyzed did not measure, and the analysis did not consider, the potential burdens the program places on prescribers, pharmacists, and patients, and whether additional requirements might pose undue restrictions on individuals participating in the program.
Conclusions
In this review of FDA documents pertaining to the TIRF REMS, surveys of pharmacists, prescribers, and patients reflected generally high levels of knowledge regarding proper TIRF prescribing, yet some survey items as well as claims-based analyses indicated substantial rates of inappropriate TIRF use. Despite these findings, the FDA did not require substantive changes to the program.
eTable 1
Technical Appendix
TIRF REMS Provider Knowledge Assessment
12‐Month Patient KAB Survey
12‐Month Pharmacist KAB Survey
12‐Month Prescriber KAB Survey
60‐Month Patient KAB Survey
60‐Month Pharmacist KAB Survey
60‐Month Patient KAB Survey
References
- 1.Ahmad FB, Rossen LM, Spencer MR, Warner M, Sutton P Provisional drug overdose death counts. National Center for Health Statistics. 2018. https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm. Accessed September 15, 2018.
- 2.National Institute on Drug Abuse Overdose death rates. National Institute on Drug Abuse website. https://www.drugabuse.gov/related-topics/trends-statistics/overdose-death-rates. Revised August 2018. Accessed September 15, 2018.
- 3.Substance Abuse and Mental Health Services Administration Key Substance Use and Mental Health Indicators in the United States: Results from the 2016 National Survey on Drug Use and Health Rockville, MD: Center for Behavioral Health Statistics and Quality ; 2017. https://www.samhsa.gov/data/sites/default/files/NSDUH-FFR1-2016/NSDUH-FFR1-2016.htm#opioid. Accessed July 2, 2018.
- 4.US Food and Drug Administration. Approved risk evaluation and mitigation strategies (REMS). US Food and Drug Administration website. https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm?event=RemsDetails.page&REMS=60. Published 2018. Accessed April 1, 2018.
- 5.US Food and Drug Administration Questions and answers: FDA approves a class Risk Evaluation and Mitigation Strategy (REMS) for transmucosal immediate-release fentanyl (TIRF) medicines. US Food and Drug Administration Website. https://www.fda.gov/ForConsumers/ConsumerUpdates/ucm284717.htm. Updated April 30, 2017. Accessed June 8, 2018.
- 6.Transmucosal Immediate Release Fentanyl Risk Evaluation and Mitigation Strategy Industry Group 24-month REMS Assessment Report. Submitted to the FDA on December 27, 2013.(Obtained under the Freedom of Information Act from US Food and Drug Administration; requested as FDA FOIA 2012-7093 September 2012; received January 2018).
- 7.Transmucosal Immediate Release Fentanyl Risk Evaluation and Mitigation Strategy 36-month REMS Assessment Report. Submitted to the US Food and Drug Administration on December 26, 2014. (Obtained under the Freedom of Information Act from US Food and Drug Administration; requested as FDA FOIA 2012-7093 September 1 2012; received January 2018).
- 8.Transmucosal Immediate Release Fentanyl Risk Evaluation and Mitigation Strategy Response to 17MAY2016 FDA Questions on 48-Month Supplemental Report. Submitted to the FDA on January 30, 2017. (Obtained under the Freedom of Information Act from US Food and Drug Administration; requested as FDA FOIA 2012 7093 September 2012; received January 2018).
- 9.US Food and Drug Administration Interim Comments on Risk Evaluation and Mitigation Strategy (REMS) Set #1.February 1, 2013. (Obtained under the Freedom of Information Act from US Food and Drug Administration; requested as FDA FOIA 2012 7093 September 2012; received January 2018).
- 10.Transmucosal Immediate Release Fentanyl Risk Evaluation and Mitigation Strategy 36-month REMS Assessment Report. Submitted to the US Food and Drug Administration on December 26, 2014. (Obtained under the Freedom of Information Act from US Food and Drug Administration; requested as FDA FOIA 2012-7093 September 2012; received January 2018).
- 11.Transmucosal Immediate Release Fentanyl Risk Evaluation and Mitigation Strategy Transmucosal Immediate Release Fentanyl Risk Evaluation and Mitigation Strategy Access Program Non-Compliance Protocol. Version 7.0. October 26, 2015. (Obtained under the Freedom of Information Act from US Food and Drug Administration; requested as FDA FOIA 2012-7093 September 2012; received January 2018).
- 12.Transmucosal Immediate Release Fentanyl Risk Evaluation and Mitigation Strategy 48-month REMS Assessment Report. p. 64. Submitted to the FDA on December 28, 2015. (Obtained under the Freedom of Information Act from US Food and Drug Administration; requested as FDA FOIA 2012-7093 September 2012; received January 2018).
- 13.US Food and Drug Administration TIRF REMS Access Program 48-Month FDA Assessment Report. FDA Information Request 1 Response. p. 1. May 27, 2016. (Obtained under the Freedom of Information Act from US Food and Drug Administration; requested as FDA FOIA 2012-7093 September 2012; received January 2018).
- 14.US Food and Drug Administration Review of the sixth (60 month, October 29, 2015 to October 28, 2016) Risk Evaluation and Mitigation Strategy (REMS) Assessment Report for Transmucosal Immediate Release Fentanyl (TIRF) Agents. p. 8. December 4, 2017. (Obtained under the Freedom of Information Act from US Food and Drug Administration; requested as FDA FOIA 2012-7093 September 2012; received May 2018).
- 15.US Food and Drug Administration REMS Assessment Acknowledgment. p. 3. August 3, 2015. (Obtained under the Freedom of Information Act from US Food and Drug Administration; requested as FDA FOIA 2012-7093 September 2012; received January 2018).
- 16.US Food and Drug Administration Review of the sixth (60 month, October 29, 2015 to October 28, 2016) Risk Evaluation and Mitigation Strategy (REMS) Assessment Report for Transmucosal Immediate Release Fentanyl (TIRF) Agents. p. 101. December 4, 2017. (Obtained under the Freedom of Information Act from US Food and Drug Administration; requested as FDA FOIA 2012-7093 September 2012; received May 2018).
- 17.US Food and Drug Administration REMS Modification Review. p. 6. July 12, 2017. (Obtained under the Freedom of Information Act from US Food and Drug Administration; requested as FDA FOIA 2012-7093 September 2012; received January 2018).
- 18.US Food and Drug Administration REMS Modification Review. p. 7. July 12, 2017. (Obtained under the Freedom of Information Act from US Food and Drug Administration; requested as FDA FOIA 2012-7093 September 2012; received January 2018).
- 19.Office of Inspector General. FDA Lacks Comprehensive Data to Determine Whether Risk Evaluation and Mitigation Strategies Improve Drug Safety. Washington, DC: Dept of Health and Human Services; 2013. https://oig.hhs.gov/oei/reports/oei-04-11-00510.pdf. Accessed October 7, 2018.
- 20.Baumgaertner E. F.D.A. did not intervene to curb risky fentanyl prescriptions. New York Times https://www.nytimes.com/2018/08/02/health/fda-fentanyl-opioid-epidemic-overdose-cancer.html. August 2, 2018. Accessed October 7, 2018.
- 21.US Food and Drug Administration Center for Drug Evaluation and Research. Summary minutes of the joint meeting of the drug safety and risk management advisory committee and the anesthetic and analgesic drug products advisory committee. US Food and Drug Administration Center for Drug Evaluation and Research: Silver Spring, MD; 2018. https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/DrugSafetyandRiskManagementAdvisoryCommittee/UCM620615.pdf. Accessed October 7, 2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1
Technical Appendix
TIRF REMS Provider Knowledge Assessment
12‐Month Patient KAB Survey
12‐Month Pharmacist KAB Survey
12‐Month Prescriber KAB Survey
60‐Month Patient KAB Survey
60‐Month Pharmacist KAB Survey
60‐Month Patient KAB Survey