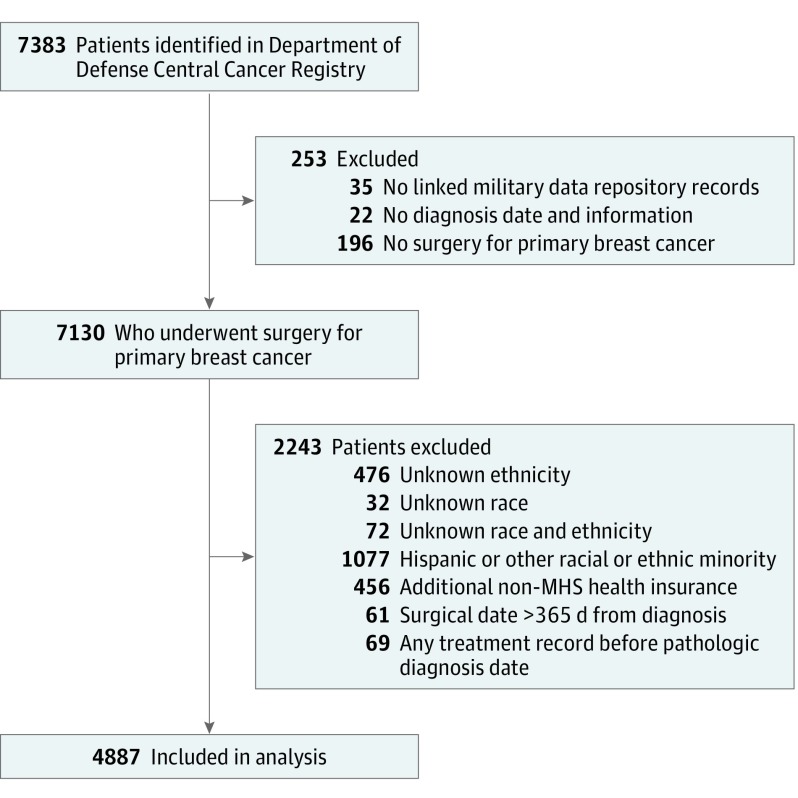
This cohort study uses data from the US Department of Defense Central Cancer Registry and Military Health System Data Repository to compare time to surgery with overall survival of non-Hispanic black and non-Hispanic white women diagnosed and treated for breast cancer.
Key Points
Questions
Do black and white women differ in time to breast cancer surgery, and could such differences explain racial disparities in overall survival?
Findings
This cohort study of 988 non-Hispanic black and 3899 non-Hispanic white women who received a diagnosis of and were treated for breast cancer in the US Military Health System found that black women had longer 75th and 90th percentile times to surgery than white women. The longer time to surgery, however, did not account for the observed racial disparity in overall survival.
Meaning
Future research on factors that influence surgical decisions, treatment delays, and short-term and long-term clinical outcomes is warranted to better understand racial disparities in breast cancer treatment and overall survival.
Abstract
Importance
Racial disparities in time to surgery (TTS) after a breast cancer diagnosis and whether these differences account for disparities in overall survival have been understudied in the US population.
Objectives
To compare TTS in non-Hispanic black (NHB) and non-Hispanic white (NHW) women with breast cancer and to examine whether racial differences in TTS may explain possible racial disparities in overall survival in a universal health care system.
Design, Setting, and Participants
Retrospective cohort identified from the Department of Defense Central Cancer Registry and Military Health System Data Repository linked databases containing records between January 1, 1998, and December 31, 2008, of 998 NHB women and 3899 NHW women who received a diagnosis of stages I to III breast cancer and underwent breast-conserving surgery (BCS) or mastectomy in the US Military Health System during the study period. Data analyses were conducted from July 5, 2017, to December 29, 2017.
Main Outcomes and Measures
The main outcome was time to breast cancer surgery. Non-Hispanic black and NHW women were compared at the 25th, 50th (median), 75th, and 90th percentiles of TTS by using multivariable quantile regression. Cox proportional hazards regression models were used to estimate hazard ratios (HRs) and 95% CIs for all-cause death in NHB compared with NHW women after controlling for potential confounders first without and then with TTS.
Results
Among the 4887 NHB and NHW women in the cohort, the mean (SD) age was 50.0 (9.4) years. The median TTS was 21 days (95% CI, 20.6-21.4 days) among NHW women and 22 days (95% CI, 20.6-23.4 days) among NHB women. Non-Hispanic black women had a significantly greater estimated TTS at the 75th (3.6 days; 95% CI, 1.6-5.5 days) and 90th (8.9 days; 95% CI, 5.1-12.6 days) percentiles than NHW women in multivariable models. The estimated differences were similar by surgery type. Non-Hispanic black women had a higher adjusted risk for death (HR, 1.45; 95% CI, 1.06-2.01) compared with NHW women among patients receiving breast-conserving surgery. The risks were similar between races among those receiving mastectomy (HR, 1.06; 95% CI, 0.76-1.48). The HRs remained similar after adding TTS to the Cox proportional hazards regression models.
Conclusions and Relevance
This study’s results indicate that time to breast cancer surgery was delayed for NHB compared with NHW women in the Military Health System. However, the racial differences in TTS did not explain the observed racial differences in overall survival among women who received breast-conserving surgery.
Introduction
Black women who receive a diagnosis of breast cancer have lower survival than their white counterparts in the general US population.1,2,3,4 Documented racial differences in tumor characteristics and receipt of recommended treatments3,5,6,7 may only partially explain survival disparities.3,7 Time to first treatment has gained attention as a possible explanatory factor for survival disparities in nonmetastatic disease.8,9,10,11,12 Treatment delays could lead to poorer treatment response, more rapid disease progression, or adverse health events13,14 and may contribute to reduced overall survival.8,12,15 Because surgery is the primary treatment for early-stage breast cancer,16,17 time to surgery (TTS) may be important for racial disparities research.
Recent investigations have evaluated whether there are racial differences in breast cancer TTS.10,18,19,20,21,22,23 In population-based studies using data from the Surveillance, Epidemiology, and End Results–Medicare (SEER-Medicare) database, Medicaid, and the National Cancer Database,10,18,19,21 non-Hispanic black (NHB) women had longer TTS or were less likely to receive surgery within 30 days of breast cancer diagnosis than non-Hispanic white (NHW) women. However, it is understudied whether these differences may be associated with racial disparities in survival. A California Cancer Registry study of 8860 young women reported that NHB women were more likely than NHW women to have surgery more than 6 weeks after diagnosis and that NHB women and those with treatment delays of more than 6 weeks also had poorer overall survival.20 Nevertheless, the study did not directly address whether TTS differences between NHB and NHW women could account for racial disparities in survival.
Less access to care and lower insurance coverage among black individuals compared with white individuals24 are main factors for racial disparities in breast cancer survival in the general US population.1,25,26,27,28 Access to care and insurance coverage may influence treatment timing.29,30 The California Cancer Registry study also reported that women with public or no health insurance had longer TTS and poorer overall survival than their counterparts with private insurance.20 Independent of access to care and insurance coverage, it is unclear whether NHBs and NHWs differ in TTS and, further, whether racial differences in TTS may account for disparities in survival. Research in universal health systems, in which different racial groups are provided equal access to health care, can help address this topic.
The US Military Health System (MHS) provides eligible beneficiaries with insurance coverage and access to medical care through a direct network of military treatment facilities or from approved civilian medical facilities.31 Therefore, beneficiaries have insurance and access to care regardless of racial background. Previous studies have shown that black women with breast cancer had worse overall survival than their white counterparts within the MHS.32,33 However, to our knowledge, TTS after a breast cancer diagnosis has not been evaluated in the MHS population in relation to race and clinical outcomes. The objectives of this study were to examine whether racial differences exist in TTS and to evaluate whether any differences in TTS may subsequently explain any observed disparities in overall survival between NHB and NHW women with a diagnosis of breast cancer in the MHS.
Methods
Data Sources
This study used linked data from the Department of Defense Central Cancer Registry (CCR) and the MHS Data Repository (MDR). The CCR contains information on demographics, cancer diagnosis, and treatment for patients diagnosed or treated at military treatment facilities beginning in 1998.34 The CCR compiles data and does follow-up on all patients for vital status using the North American Association of Central Cancer Registries reporting standards.35 The MDR includes administrative and medical claims data for inpatient, outpatient, laboratory, pharmacy, and other ancillary services provided at military treatment facilities or civilian facilities paid by the Department of Defense.36 The CCR and MDR data linkage was approved by the institutional review boards of the Walter Reed National Military Medical Center, the Defense Health Agency, and the National Institutes of Health. The requirement for informed consent from patients was waived by the Walter Reed National Military Medical Center Institutional Review Board because the study was based on data from a large existing database. Analyses were conducted from July 5, 2017, to December 29, 2017.
Study Population
Patients with a pathologic diagnosis of breast cancer (International Classification of Diseases for Oncology, Third Revision (ICD-O-3) topography codes C50.0-50.6 and C50.8 and C50.9) were identified in the CCR data (Figure). Eligible patients were NHB or NHW37 women aged 18 to 64 years who received a diagnosis of stages I to III disease between January 1, 1998, and December 31, 2007; had corresponding records in the MDR database; and had documented surgical treatment (mastectomy or breast-conserving surgery [BCS]) in either the CCR or MDR. Women with stage IV (metastatic) breast cancer were excluded because surgery is usually not the primary treatment.16 Women who were Hispanic/Latina were excluded to minimize the potential effects of ethnicity on racial comparisons in TTS and survival.4 Women older than 64 years at diagnosis were excluded to reduce the possibility of incomplete information owing to their Medicare eligibility. Patients with multiple primary cancer diagnoses were excluded to minimize potential effects of other cancers on results.
Figure. Selection of Patients in the Department of Defense Central Cancer Registry for Study Inclusion.
MHS indicates Military Health Service.
Study Variables
Cancer information in the CCR included diagnosis date, pathologic and clinical stage, tumor grade (well, moderately, poorly, or nondifferentiated), and estrogen receptor (ER) and progesterone receptor (PR) status. Tumor stage was defined using the American Joint Committee on Cancer sixth edition criteria as stage I, II, or III.38 Breast cancer treatments were identified from both databases. In the CCR data, site-specific Facility Oncology Registry Data Standards surgery codes were used to identify mastectomy (30, 40-49, 50-59, 60-69, and 70-76) and BCS (20-24). The date(s) of first treatment for chemotherapy, radiotherapy, and hormone therapy were also extracted from the CCR. In the MDR data, medical billing codes39 were used to identify the first surgical record with an algorithm to exclude those for diagnostic purposes and to identify the first date of other treatment(s). The cancer diagnosis and treatment dates were consolidated using a deliberate and systematic process. Chemotherapy and radiotherapy were classified as neoadjuvant or adjuvant based on timing with the first surgery. Hormone therapy was confined to those with hormone receptor–positive tumors (ER+, PR+, or both). Surveillance mammograms were identified in MDR records as any mammogram within the 3 years after diagnosis.
Comorbid conditions were identified in the MDR records using International Classification of Diseases, Ninth Revision, Clinical Modification codes for medical conditions included in the Charlson Comorbidity Index,40 with the exception of cancer because breast cancer was the condition of interest and other primary cancers were excluded. To reduce the possibility of false or unconfirmed diagnoses, comorbidities were included when there was at least 1 inpatient or 3 outpatient records with a relevant International Classification of Diseases, Ninth Revision, Clinical Modification diagnosis code. The number of conditions was categorized as 0, 1, or 2 or greater.
Other study variables included age at diagnosis, marital status (married, single, separated, divorced, or widowed), active-duty status, military service, or sponsor branch (Army, Navy, Marines, Air Force, or other), TRICARE service region (North, South, West, or overseas [Europe, Pacific, or Africa]), insurance benefit type in the 3 months surrounding diagnosis (prime, standard, or extra) and care source (military treatment facilities or civilian facilities) in the 3 months before diagnosis and up to the first surgery date. Women with other health insurance in addition to MHS-provided coverage (n = 456) were excluded owing to the possibility of missing records for cancer treatment.
Study Outcomes
The primary study outcomes were TTS and overall survival. The TTS was calculated as the difference in days between the diagnosis and first surgery dates. Women with a TTS longer than 365 days (n = 61) were excluded because surgery beyond this period is unlikely to be part of primary treatment based on clinical guidelines.16 Women with a surgery date prior to diagnosis (n = 28) were excluded because these cases may represent emergency procedures before a diagnosis could be made and did not have a valid time interval for analyses. Women with other treatment prior to diagnosis (n = 41) were excluded owing to the illogical ordering of diagnosis and treatment and its potential effects on the study exposures and outcomes. Vital status was determined from CCR records. Survival time was calculated as the time between diagnosis and the date of death for those who died, date of last contact recorded in CCR and MDR databases, or end date of the data on December 31, 2008, whichever occurred first.
Statistical Analysis
Distributions of patient demographic, tumor, and treatment characteristics by race (NHW and NHB) were examined using χ2 or Fisher exact tests. Time to surgery and associated 95% CIs were estimated at the 25th, 50th (median), 75th, and 90th percentiles for each race using model intercepts and parameter estimates from quantile regression with bootstrap method for SEs.41,42 Then, we compared TTS at the designated percentiles between NHB and NHW women using both univariable and multivariable models adjusted for demographic, tumor, and other treatment variables. Analyses were conducted for the first surgery and then separately for BCS or mastectomy, respectively, owing to inherent differences in treatment planning and excision techniques between surgery types and the potential implications for surgical delays. We repeated the quantile regression analyses by neoadjuvant treatment status because neoadjuvant treatment is indicated for more advanced or aggressive tumors16,17 and can markedly affect TTS.
We then compared overall survival between racial groups and whether TTS might be associated with racial differences in survival using Cox proportional hazards regression. Patients with less than 30 days’ follow-up after the surgery date (n = 14) were excluded from survival analysis owing to possibly incomplete information on course of treatment, such as adjuvant chemotherapy or radiotherapy, which may be associated with cancer outcomes. Individual hazard ratios (HRs) and 95% CIs for all-cause death were modeled for NHB compared with NHW women in univariable and multivariable models. To assess the association of TTS with racial differences in overall survival, we first fit the model without TTS. Then, we added TTS to the model to examine whether racial differences in survival were attenuated. Relative likelihood of the models was assessed using the Akaike information criterion.43 In this analysis, the overall sample TTS was divided into quartiles (Q1-Q4), with the shortest time interval defined by Q1. To reduce the possibility of residual confounding within TTS quartiles, we also modeled TTS as continuous time. Survival analyses were conducted for the entire sample and stratified by surgery type. Analyses were conducted using SAS, version 9.4 (SAS Institute Inc). Statistical tests were 2-sided, and associations were considered statistically significant at the α = .05 level.
Results
Patient Characteristics
Of the 4887 women included in the study, 3899 were NHW and 988 were NHB. The women in the study had a mean (SD) age at diagnosis of 50.0 (9.4) years and were followed up for a mean (SD) of 3.8 (2.1) years. Mean (SD) follow-up time was 3.86 (2.1) years for NHW and 3.5 (2.0) years for NHB women. Distributions of patient and tumor characteristics differed between NHB and NHW women on nearly every characteristic evaluated (Table 1). Notably, NHB women tended to be younger at diagnosis and were more likely to have stage II or stage III tumors, hormone receptor–negative tumors, comorbid conditions, or to have died during the study period compared with NHW women.
Table 1. Selected Demographic and Pathologic Characteristics by Race/Ethnicity for 4887 Women With a Diagnosis of Breast Cancer in the US Military Health System, 1998-2007 .
| Characteristic | Race/Ethnicity, No. (%) | P Value | |
|---|---|---|---|
| Non-Hispanic White | Non-Hispanic Black | ||
| Age at diagnosis, y | |||
| 18-39 | 508 (13.0) | 246 (24.9) | <.001 |
| 40-49 | 1148 (29.4) | 382 (38.7) | |
| 50-59 | 1343 (34.4) | 243 (24.6) | |
| 60-64 | 900 (23.1) | 117 (11.8) | |
| Marital status | |||
| Married | 3337 (85.6) | 777 (78.6) | <.001 |
| Single | 101 (2.6) | 59 (6.0) | |
| Divorced/widowed/separated | 367 (9.4) | 122 (12.4) | |
| Unknown | 94 (2.4) | 30 (3.0) | |
| Military service/sponsor branch | |||
| Army | 1184 (30.4) | 474 (48.0) | <.001 |
| Navy | 802 (20.6) | 142 (14.4) | |
| Marine Corps | 189 (4.9) | 42 (4.3) | |
| Air Force | 1329 (34.1) | 228 (23.1) | |
| Other | 367 (9.4) | 94 (9.5) | |
| Unknown | 28 (0.7) | 8 (0.8) | |
| Active duty at diagnosis | |||
| Yes | 237 (6.1) | 166 (16.8) | <.001 |
| No | 3635 (93.2) | 814 (82.4) | |
| Unknown | 27 (0.7) | 8 (0.8) | |
| TRICARE region | |||
| North | 1303 (33.4) | 400 (4.5) | <.001 |
| South | 1245 (31.9) | 352 (35.6) | |
| West | 1253 (32.1) | 204 (2.7) | |
| Overseas (Europe, Pacific, or Africa) | 98 (2.5) | 32 (3.2) | |
| Benefit type | |||
| Prime | 3245 (83.2) | 819 (82.9) | .91 |
| Nonprime (standard or extra) | 269 (6.9) | 72 (7.3) | |
| Unknown | 385 (9.9) | 97 (9.8) | |
| Medical care source | |||
| Direct (military) | 1838 (47.1) | 577 (58.4) | <.001 |
| Purchased (private) | 840 (21.5) | 135 (13.7) | |
| Both (military and private) | 1151 (29.5) | 256 (25.9) | |
| Unknown | 70 (1.8) | 20 (2.0) | |
| Breast cancer diagnosis year | |||
| 1998 | 471 (12.1) | 99 (1.0) | .01 |
| 1999 | 495 (12.7) | 113 (11.4) | |
| 2000 | 446 (11.4) | 94 (9.5) | |
| 2001 | 425 (10.9) | 93 (9.4) | |
| 2002 | 412 (10.6) | 105 (1.6) | |
| 2003 | 371 (9.5) | 97 (9.8) | |
| 2004 | 356 (9.1) | 91 (9.2) | |
| 2005 | 350 (9.0) | 110 (11.1) | |
| 2006 | 282 (7.2) | 85 (8.6) | |
| 2007 | 291 (7.5) | 101 (1.2) | |
| Tumor stage (AJCC) | |||
| I | 1921 (49.3) | 369 (37.4) | <.001 |
| II | 1563 (40.1) | 463 (46.9) | |
| III | 415 (10.6) | 156 (15.8) | |
| Tumor grade (AJCC) | |||
| G1 (well differentiated) | 794 (20.4) | 120 (12.2) | <.001 |
| G2 (moderately differentiated) | 1431 (36.7) | 322 (32.6) | |
| G3 (poorly differentiated) | 1196 (30.7) | 434 (43.9) | |
| G4 (nondifferentiated) | 49 (1.3) | 12 (1.2) | |
| GX (undetermined) | 429 (11.0) | 100 (1.1) | |
| Hormone receptor status | |||
| ER+/PR+ | 2206 (56.6) | 397 (4.2) | <.001 |
| ER+/PR− | 307 (7.9) | 107 (1.8) | |
| ER−/PR+ | 89 (2.3) | 46 (4.7) | |
| ER−/PR− | 741 (19.0) | 307 (31.1) | |
| Unknown | 556 (14.3) | 131 (13.3) | |
| Hormone therapya | |||
| Yes | 1505 (57.8) | 283 (51.5) | .01 |
| No | 1097 (42.4) | 267 (48.5) | |
| Surgery type | |||
| Breast conserving | 2507 (64.3) | 647 (65.5) | .49 |
| Mastectomy | 1392 (35.7) | 341 (34.5) | |
| With immediate reconstruction | 172 (12.4) | 41 (12.0) | .87 |
| Chemotherapy | |||
| Preoperative (neoadjuvant) | 215 (5.5) | 85 (8.6) | <.001 |
| Postoperative (adjuvant) | 2596 (66.6) | 718 (72.7) | |
| No | 1088 (27.9) | 185 (18.7) | |
| Radiotherapy | |||
| Preoperative (neoadjuvant) | 31 (0.8) | 18 (1.8) | .001 |
| Postoperative (adjuvant) | 2713 (69.6) | 714 (72.3) | |
| No | 1155 (29.6) | 256 (25.9) | |
| Surveillance mammogram | |||
| Yes | 1751 (44.9) | 414 (41.9) | .09 |
| No | 2148 (55.1) | 574 (58.1) | |
| Comorbid conditions | |||
| None | 3248 (83.3) | 786 (79.6) | .01 |
| 1 | 487 (12.5) | 157 (15.9) | |
| ≥2 | 164 (4.2) | 45 (4.6) | |
| Vital status | |||
| Died (all cause) | 334 (8.6) | 115 (11.6) | .002 |
| Alive | 3565 (91.4) | 873 (88.4) | |
Abbreviations: AJCC, American Joint Committee on Cancer; ER, estrogen receptor; PR, progesterone receptor.
Hormone therapy for women with hormone receptor–positive tumors (ER+ or PR+).
Time to Surgery
For NHW women, the overall 25th percentile TTS was 7 days (95% CI, 5.6-8.4 days), overall 50th percentile TTS was 21 days (95% CI, 20.6-21.4 days), overall 75th percentile TTS was 35 days (95% CI, 34.0-36.0 days), and overall 90th percentile TTS was 60 days (95% CI, 55.3-64.7 days). For NHB women, the overall 25th percentile TTS was 6 days (95% CI, 1.6-10.4 days), overall 50th percentile TTS was 22 days (95% CI, 20.6-23.4 days), overall 75th percentile TTS was 39.5 days (95% CI, 35.7-42.3 days), and overall 90th percentile TTS was 92 days (95% CI, 75.9-108.0 days) (Table 2). In multivariable models, NHB women had a longer TTS than NHW women by 3.6 days (95% CI, 1.6-5.5 days) at the 75th percentile and 8.9 days (95% CI, 5.1-12.6 days) at the 90th percentile (Table 2). The magnitudes of adjusted differences in TTS between NHB women and NHW women were similar in models stratified by surgery type (Table 2). We also considered patients with and without neoadjuvant treatment separately (Table 3). Among patients with neoadjuvant treatment, there were no significant racial differences in time to neoadjuvant treatment or in TTS, although there was a tendency toward longer TTS for NHB women at the 75th and 90th percentiles. Among patients without neoadjuvant treatment, NHB women had a significantly longer TTS at the 50th percentile of 1.9 days (95% CI, 0.3-3.5 days), at the 75th percentile of 3.1 days (95% CI, 0.8-5.4 days), and at the 90th percentile of 7.6 days (95% CI, 3.5-11.7 days) (Table 3).
Table 2. Quantile Regression Estimated Racial Difference in Time to Surgery Across Percentiles for Women With a Diagnosis of Breast Cancer in the US Military Health System, 1998-2007.
| Surgery Type and Time to Surgery, Percentile | Time to Surgery by Race/Ethnicity, (95% CI), d | Model-Estimated Difference (95% CI): Non-Hispanic Black − Non-Hispanic White | ||
|---|---|---|---|---|
| Non-Hispanic White | Non-Hispanic Black | Unadjusted | Adjusteda | |
| Breast conserving or mastectomy (n = 4887) | ||||
| 25th | 7 (5.6 to 8.4) | 6 (1.6 to 10.4) | −1.0 (−6.6 to 4.6) | −0.6 (−2.1 to 0.9) |
| 50th | 21 (20.6 to 21.4) | 22 (20.6 to 23.4) | 1.0 (−0.2 to 2.2) | 1.3 (−0.2 to 2.9) |
| 75th | 35 (34.0 to 36.0) | 39.5 (35.7 to 42.3) | 4.0 (0.7 to 7.2)b | 3.6 (1.6 to 5.5)b |
| 90th | 60 (55.3 to 64.7) | 92 (75.9 to 108.0) | 32 (12.3 to 51.7)b | 8.9 (5.1 to 12.6)b |
| Breast conserving (n = 3154) | ||||
| 25th | 0 (0 to 0) | 0 (0 to 0) | 0 (0 to 0) | 0 (−0.4 to 0.4) |
| 50th | 18 (16.9 to 19.1) | 19 (16.5 to 21.5) | 1.0 (−2.0 to 4.0) | 2.0 (0.0 to 4.0) |
| 75th | 31 (29.2 to 32.8) | 33 (30.1 to 35.9) | 2.0 (−1.5 to 5.5) | 3.5 (0.9 to 6.1)b |
| 90th | 48 (45.5 to 50.5) | 57 (49.1 to 64.9) | 9.0 (−0.7 to 18.7) | 7.9 (3.6 to 12.1b |
| Mastectomy (n = 1733) | ||||
| 25th | 15 (14.2 to 15.8) | 14 (12.5 to 15.5) | −1.0 (−2.8 to 0.8) | −0.3 (−3.5 to 2.8) |
| 50th | 26 (24.4 to 27.6) | 29 (25.7 to 32.3) | 3.0 (−0.7 to 6.7) | 2.0 (−0.8 to 4.9) |
| 75th | 43.5 (40.4 to 47.6) | 64 (52.2 to 75.8) | 20.0 (5.7 to 34.3)b | 4.1 (−0.1 to 8.5) |
| 90th | 102 (86.5 to 117.5) | 149 (125.9 to 172.1) | 47.0 (24.2 to 69.8)b | 9.2 (0.8 to 17.5)b |
Model adjusted for age, marital status, active duty status, military service/sponsor branch, care source, benefit type, TRICARE region, year of diagnosis, tumor stage, tumor grade, hormone receptor status, preoperative chemotherapy or radiotherapy, and comorbid conditions. (See the Study Variables subsection of the Methods section for a description of the variable levels.)
P < .05.
Table 3. Multivariable Quantile Regression–Estimated Racial Difference in Time to Treatment Across Percentiles by Neoadjuvant Treatment Status for Women With a Diagnosis of Breast Cancer in the US Military Health System, 1998-2007.
| Treatment Interval, Percentile | Time by Race/Ethnicity (95% CI), d | Model-Estimated Difference (95% CI)a | |
|---|---|---|---|
| Non-Hispanic White | Non-Hispanic Black | ||
| Patients With Neoadjuvant Treatment | |||
| Time to neoadjuvant treatment | |||
| 25th | 13 (11.2 to 14.8) | 18 (13.2 to 22.8) | 4.3 (0.6 to 8.0)b |
| 50th | 22 (19.5 to 24.5) | 24 (20.1 to 27.8) | 3.5 (−0.9 to 7.8) |
| 75th | 32 (28.0 to 35.9) | 36 (30.9 to 41.1) | 2.3 (−3.2 to 7.8) |
| 90th | 50 (43.3 to 56.7) | 45 (36.7 to 53.3) | −2.8 (−12.1 to 6.6) |
| Time to surgery | |||
| 25th | 100 (91.5 to 108.5) | 91 (71.5 to 110.5) | −13.1 (−29.8 to 3.6) |
| 50th | 133 (127.0 to 138.9) | 142 (128.1 to 155.9) | −12.2 (−28.8 to 4.3) |
| 75th | 166 (157.4 to 174.6) | 177 (164.9 to 189.1) | 9.3 (−10.2 to 28.9) |
| 90th | 201 (187.5 to 214.4) | 214 (176.9 to 251.1) | 24.2 (−2.7 to 51.2) |
| Patients Without Neoadjuvant Treatment | |||
| Time to surgery | |||
| 25th | 6.0 (4.3 to 7.7) | 0.0 (−3.3 to 3.3) | 0.4 (−0.7 to 1.5) |
| 50th | 20.0 (19.4 to 20.6) | 20.0 (18.4 to 21.6) | 1.9 (0.3 to 3.5)b |
| 75th | 33.0 (31.7 to 34.3) | 34.0 (31.5 to 36.5) | 3.1 (0.8 to 5.4)b |
| 90th | 47.0 (45.1 to 48.9) | 52.0 (47.7 to 56.3) | 7.6 (3.5 to 11.7)b |
Model adjusted for age, marital status, active duty status, military service/sponsor branch, care source, benefit type, TRICARE region, year of diagnosis, tumor stage, tumor grade, hormone receptor status, surgery type, and comorbid conditions. (See the Study Variables subsection of the Methods section for a description of the variable levels.)
P < .05.
Overall Survival
Non-Hispanic black women had a higher risk for all-cause death (HR, 1.53; 95% CI, 1.24-1.90) compared with NHW women in the univariable model. The higher mortality risk for NHB women was attenuated (HR, 1.21; 95% CI, 0.97-1.52) when adjusted for demographic, pathologic, and treatment variables (Table 4). The addition of TTS to the multivariable model did not substantially attenuate the HR estimates compared with the adjusted model. The results remained similar when TTS was modeled as continuous time (data not shown). When stratified by surgery type, NHB women had a higher adjusted risk of all-cause death compared with NHW women (HR, 1.45; 95% CI, 1.06-2.01) among those receiving BCS and a similar adjusted risk of all-cause death compared with NHW women (HR 1.06; 95% CI, 0.76-1.48) among those receiving mastectomy (Table 4). The addition of TTS to the models did not change the HR estimates associated with race by surgery type (Table 4).
Table 4. Cox Proportional Hazard for All-Cause Death for Non-Hispanic Black Compared With Non-Hispanic White Women With a Diagnosis of Breast Cancer in the US Military Health System, 1998-2007.
| Surgery Type, Race/Ethnicity | Deaths, No. (%) | Hazard Ratio (95% CI) | ||
|---|---|---|---|---|
| Unadjusted | Adjusteda | Adjustedb | ||
| Breast conserving or mastectomy (n = 4873) | ||||
| Non-Hispanic white | 334 (8.6) | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Non-Hispanic black | 115 (11.7) | 1.53 (1.24-1.90)c | 1.21 (0.97-1.52) | 1.19 (0.94-1.49) |
| Breast conserving (n = 3147) | ||||
| Non-Hispanic white | 158 (6.3) | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Non-Hispanic black | 61 (9.4) | 1.76 (1.31-2.36)c | 1.45 (1.06-2.01)c | 1.47 (1.06-2.03)c |
| Mastectomy (n = 1726) | ||||
| Non-Hispanic white | 176 (12.7) | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Non-Hispanic black | 54 (15.9) | 1.35 (0.99-1.83) | 1.06 (0.76-1.48) | 1.04 (0.75-1.45) |
Model adjusted for age at diagnosis, marital status, military service branch, active duty status, benefit type, care source, TRICARE region, year of diagnosis, tumor stage, tumor grade, hormone receptor status, surveillance mammography, chemotherapy treatment (none, preoperative, or postoperative), radiotherapy (none, preoperative, or postoperative), hormone treatment, and comorbid conditions. (See the Study Variables subsection of the Methods section for a description of the variable levels.)
Model adjusted for time to surgery (quartiles) in addition to the variables in footnote a.
P < .05.
Discussion
Racial differences in TTS have been previously reported, with black women experiencing a 3- to 15-day longer median TTS compared with white women.10,18,19,20,21,22,23 Insurance status and access to care have been identified as 2 system-driven factors that potentially contribute to treatment delays.10,15,20,22 The median TTS of 22 days among NHB women in our study was shorter than the median TTS of 27 to 37 days reported among NHB women in the literature.10,21,22,23,44 Nevertheless, in the MHS, which provides insurance and equal access to care regardless of racial background, we observed a longer TTS of nearly 4 and 9 days for NHB compared with NHW women at the 75th and 90th percentiles of treatment time, respectively. This finding suggests that factors other than access to care and insurance status may play a role in longer TTS for NHB compared with NHW women.
Patient and tumor factors, such as comorbid conditions or hormone receptor negative status, which tend to be more prevalent among NHB women than NHW women,45,46,47 could also influence surgical treatment decisions and delay treatment.5,48,49 Nevertheless, we controlled for comorbidities and ER and PR status in our data analysis. To further account for possible effects of underlying racial differences in tumor phenotype, we stratified the analysis by hormone receptor status and found similar results; NHB women tended to have longer TTS at the 75th and 90th percentiles despite ER and PR status (data not shown). We also considered effects of neoadjuvant treatment on racial differences in TTS and found a similar tendency for longer TTS among NHB women in patients with and without neoadjuvant treatment. Other factors, such as genetic or familial risk; social, behavioral, and cultural factors; and patient attitudes and perceptions not routinely captured in registry and medical claims data may be associated with cancer treatment49,50,51,52 and thus TTS. These factors may provide an area of future research to better understand racial disparities in treatment timing for breast cancer.50
Although NHB patients experienced longer TTS, the delays did not appear to account for a higher risk of all-cause mortality for NHB compared with NHW women. This may be related to the dilution of TTS-associated outcomes because the racial discrepancy in TTS was observed only at the 75th and 90th percentiles. To assess this possibility, we stratified the analysis by median TTS among patients with BCS, in whom racial differences in survival were found. We noted a slight attenuation in the HR comparing NHB with NHW women among patients with a TTS greater than the median, the group that had racial differences in TTS, after adding TTS to the model (eTable in the Supplement). We also explored whether differences in tumor phenotype or time to initiation of adjuvant chemotherapy after surgery may lead to different associations. Although sample sizes were relatively small in each stratum, the results for the association of TTS with overall survival remained similar by ER and PR status (data not shown). For patients with adjuvant treatment, we found longer time between surgery and adjuvant chemotherapy for NHB compared with NHW women. However, the addition of time to adjuvant chemotherapy in the multivariable Cox model containing key variables and TTS did not affect the HR estimates of all-cause death associated with race.
The difference in relative mortality risk associated with race by surgery type may be related to variation in characteristics of women eligible for BCS compared with mastectomy and underlying all-cause mortality risk. Patients receiving BCS may have earlier tumor stages16,17 and may be more likely to die of competing risks (ie, comorbid conditions) rather than breast cancer. In our data, NHB women were more likely to have comorbidities, which may be reflected in our outcome of all-cause death, although we adjusted for comorbidities in the analysis. Meanwhile, mastectomy is recommended for later-stage or poorly differentiated tumors,16,17 and mortality among patients receiving mastectomy may be more likely to be associated with breast cancer or its treatment. In our data, NHB patients tended to have more aggressive tumor features (ER−/PR−) but had similar time to initiation of neoadjuvant treatment and were equally likely to receive adjuvant chemotherapy or radiotherapy among patients receiving mastectomy (data not shown). Probably as a result of the combined effects of tumor features and breast cancer treatment, no racial differences in survival were observed among women receiving a mastectomy.
Limitations
To the best of our knowledge, this is the first study to evaluate racial differences in breast cancer TTS and its potential associations with disparities in overall survival by using comprehensive data from a universal health care system. Despite the strengths and implications for future research, our study had limitations. First, with no consensus guidelines on surgery timing,11 the clinical significance of racial differences in TTS observed in this study is unclear. Second, we were limited to all-cause death as the study outcome owing to incomplete data on breast cancer–specific death. Third, the relatively short follow-up period may have prevented observation of sufficient outcomes to evaluate the associations of TTS with racial differences in overall survival. Fourth, we cannot rule out the potential effects of unknown clinical details, such as ERBB2 (formerly HER2 or Her2/neu) status, on our results. Fifth, as with any administrative data, possible errors in coding and recording could result in inaccurate determination of diagnostic and treatment intervals. Nevertheless, such errors might not be differential by race and thus might not substantially affect our results.
Conclusions
In the universal access MHS, NHB women had longer TTS than NHW women. However, surgical delays did not appear to explain observed racial disparities in survival. Future research on factors that influence surgical decisions, treatment delays, and short-term and long-term clinical outcomes is warranted to better understand racial disparities in breast cancer treatment and overall survival.
eTable. Post-hoc Cox Proportional Hazards Analysis Comparing Overall Survival Between Non-Hispanic Black and White Women Receiving Breast Conserving Surgery as Treatment for Breast Cancer Grouped by Time-to-Surgery
References
- 1.Field TS, Buist DS, Doubeni C, et al. Disparities and survival among breast cancer patients. J Natl Cancer Inst Monogr. 2005;2005(35):-. doi: 10.1093/jncimonographs/lgi044 [DOI] [PubMed] [Google Scholar]
- 2.Deshpande AD, Jeffe DB, Gnerlich J, Iqbal AZ, Thummalakunta A, Margenthaler JA. Racial disparities in breast cancer survival: an analysis by age and stage. J Surg Res. 2009;153(1):105-113. doi: 10.1016/j.jss.2008.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iqbal J, Ginsburg O, Rochon PA, Sun P, Narod SA. Differences in breast cancer stage at diagnosis and cancer-specific survival by race and ethnicity in the United States. JAMA. 2015;313(2):165-173. doi: 10.1001/jama.2014.17322 [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention Breast cancer rates by race and ethnicity. https://www.cdc.gov/cancer/breast/statistics/race.htm. Published 2016. Accessed December 10, 2018.
- 5.Chen L, Li CI. Racial disparities in breast cancer diagnosis and treatment by hormone receptor and HER2 status. Cancer Epidemiol Biomarkers Prev. 2015;24(11):1666-1672. doi: 10.1158/1055-9965.EPI-15-0293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dietze EC, Sistrunk C, Miranda-Carboni G, O’Regan R, Seewaldt VL. Triple-negative breast cancer in African-American women: disparities versus biology. Nat Rev Cancer. 2015;15(4):248-254. doi: 10.1038/nrc3896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schinkel JK, Zahm SH, Jatoi I, et al. Racial/ethnic differences in breast cancer survival by inflammatory status and hormonal receptor status: an analysis of the Surveillance, Epidemiology, and End Results data. Cancer Causes Control. 2014;25(8):959-968. doi: 10.1007/s10552-014-0395-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neal RD, Tharmanathan P, France B, et al. Is increased time to diagnosis and treatment in symptomatic cancer associated with poorer outcomes? systematic review. Br J Cancer. 2015;112(suppl 1):S92-S107. doi: 10.1038/bjc.2015.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Del Turco MR, Ponti A, Bick U, et al. Quality indicators in breast cancer care. Eur J Cancer. 2010;46(13):2344-2356. doi: 10.1016/j.ejca.2010.06.119 [DOI] [PubMed] [Google Scholar]
- 10.Bleicher RJ, Ruth K, Sigurdson ER, et al. Preoperative delays in the US Medicare population with breast cancer. J Clin Oncol. 2012;30(36):4485-4492. doi: 10.1200/JCO.2012.41.7972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaufman CS, Shockney L, Rabinowitz B, et al. ; Quality Initiative Committee . National Quality Measures for Breast Centers (NQMBC): a robust quality tool: breast center quality measures. Ann Surg Oncol. 2010;17(2):377-385. doi: 10.1245/s10434-009-0729-5 [DOI] [PubMed] [Google Scholar]
- 12.Polverini AC, Nelson RA, Marcinkowski E, et al. Time to treatment: measuring quality breast cancer care. Ann Surg Oncol. 2016;23(10):3392-3402. doi: 10.1245/s10434-016-5486-7 [DOI] [PubMed] [Google Scholar]
- 13.Comber H, Cronin DP, Deady S, Lorcain PO, Riordan P. Delays in treatment in the cancer services: impact on cancer stage and survival. Ir Med J. 2005;98(8):238-239. [PubMed] [Google Scholar]
- 14.Brazda A, Estroff J, Euhus D, et al. Delays in time to treatment and survival impact in breast cancer. Ann Surg Oncol. 2010;17(suppl 3):291-296. doi: 10.1245/s10434-010-1250-6 [DOI] [PubMed] [Google Scholar]
- 15.Bleicher RJ, Ruth K, Sigurdson ER, et al. Time to surgery and breast cancer survival in the United States. JAMA Oncol. 2016;2(3):330-339. doi: 10.1001/jamaoncol.2015.4508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Comprehensive Cancer Network NCCN Clinical Practice Guidelines in Oncology: breast cancer. Version 1. Published 2017. https://www.nccn.org. Accessed April 6, 2017 [Google Scholar]
- 17.American Cancer Society Treatment of breast cancer by stage. https://www.cancer.org/cancer/breast-cancer/treatment/treatment-of-breast-cancer-by-stage.html?_ga=2.232950618.572366305.1544405452-241348340.1544405452. Revised February 2, 2018. Accessed September 13, 2017.
- 18.Fedewa SA, Edge SB, Stewart AK, Halpern MT, Marlow NM, Ward EM. Race and ethnicity are associated with delays in breast cancer treatment (2003-2006). J Health Care Poor Underserved. 2011;22(1):128-141. [DOI] [PubMed] [Google Scholar]
- 19.Gorin SS, Heck JE, Cheng B, Smith SJ. Delays in breast cancer diagnosis and treatment by racial/ethnic group. Arch Intern Med. 2006;166(20):2244-2252. doi: 10.1001/archinte.166.20.2244 [DOI] [PubMed] [Google Scholar]
- 20.Smith EC, Ziogas A, Anton-Culver H. Delay in surgical treatment and survival after breast cancer diagnosis in young women by race/ethnicity. JAMA Surg. 2013;148(6):516-523. doi: 10.1001/jamasurg.2013.1680 [DOI] [PubMed] [Google Scholar]
- 21.Selove R, Kilbourne B, Fadden MK, et al. Time from screening mammography to biopsy and from biopsy to breast cancer treatment among black and white, women Medicare beneficiaries not participating in a health maintenance organization. Womens Health Issues. 2016;26(6):642-647. doi: 10.1016/j.whi.2016.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.George P, Chandwani S, Gabel M, et al. Diagnosis and surgical delays in African American and white women with early-stage breast cancer. J Womens Health (Larchmt). 2015;24(3):209-217. doi: 10.1089/jwh.2014.4773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Halpern MT, Schrag D. Effects of state-level Medicaid policies and patient characteristics on time to breast cancer surgery among Medicaid beneficiaries. Breast Cancer Res Treat. 2016;158(3):573-581. doi: 10.1007/s10549-016-3879-8 [DOI] [PubMed] [Google Scholar]
- 24.Barnett JC, Berchick ER. Health Insurance Coverage in the United States, 2016. Washington, DC: US Government Printing Office; 2017:44. [Google Scholar]
- 25.Newman LA, Martin IK. Disparities in breast cancer. Curr Probl Cancer. 2007;31(3):134-156. doi: 10.1016/j.currproblcancer.2007.01.003 [DOI] [PubMed] [Google Scholar]
- 26.Newman LA, Griffith KA, Jatoi I, Simon MS, Crowe JP, Colditz GA. Meta-analysis of survival in African American and white American patients with breast cancer: ethnicity compared with socioeconomic status. J Clin Oncol. 2006;24(9):1342-1349. doi: 10.1200/JCO.2005.03.3472 [DOI] [PubMed] [Google Scholar]
- 27.Du XL, Fang S, Meyer TE. Impact of treatment and socioeconomic status on racial disparities in survival among older women with breast cancer. Am J Clin Oncol. 2008;31(2):125-132. doi: 10.1097/COC.0b013e3181587890 [DOI] [PubMed] [Google Scholar]
- 28.Akinyemiju TF, Soliman AS, Johnson NJ, et al. Individual and neighborhood socioeconomic status and healthcare resources in relation to black-white breast cancer survival disparities. J Cancer Epidemiol. 2013;2013:490472. doi: 10.1155/2013/490472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abdelsattar ZM, Hendren S, Wong SL. The impact of health insurance on cancer care in disadvantaged communities. Cancer. 2017;123(7):1219-1227. doi: 10.1002/cncr.30431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adamson AS, Zhou L, Baggett CD, Thomas NE, Meyer AM. Association of delays in surgery for melanoma with insurance type. JAMA Dermatol. 2017;153(11):1106-1113. doi: 10.1001/jamadermatol.2017.3338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jansen DJ. Military medical care: questions and answers. In: Congressional Research Service. Vol 7-5700. https://fas.org/sgp/crs/misc/RL33537.pdf. Published January 2, 2014. Accessed December 10, 2018.
- 32.Jatoi I, Becher H, Leake CR. Widening disparity in survival between white and African-American patients with breast carcinoma treated in the U. S. Department of Defense Healthcare system. Cancer. 2003;98(5):894-899. doi: 10.1002/cncr.11604 [DOI] [PubMed] [Google Scholar]
- 33.Ru Y, Liu J, Fantacone-Campbell JL, et al. Comparative survival analysis of invasive breast cancer patients treated by a U.S. Military Medical Center and matched patients from the U.S. general population. Mil Med. 2017;182(11):e1851-e1858. doi: 10.7205/MILMED-D-17-00097 [DOI] [PubMed] [Google Scholar]
- 34.Department of Defense Joint Pathology Center DoD Cancer Registry Program. https://www.jpc.capmed.mil/education/dodccrs/index.asp. Last modified November 4, 2014. Accessed October 26, 2017.
- 35.North American Association of Central Cancer Registries Central Registry Standards. https://www.naaccr.org/data-standards-data-dictionary/. Published 2015. Accessed October 26, 2017.
- 36.Defense Health Agency Military Health System Data Repository. https://www.health.mil/Military-Health-Topics/Technology/Clinical-Support/Military-Health-System-Data-Repository. Accessed October 26, 2017.
- 37.National Institutes of Health Racial and ethnic categories and definitions for NIH diversity programs and for other reporting purposes. Vol NOT-OD-15-089. https://grants.nih.gov/grants/guide/notice-files/NOT-OD-15-089.html. Published 2015. Accessed November 28, 2017.
- 38.American Joint Committee on Cancer Part VII: breast In: Greene FL, Page DL, Fleming ID, et al, eds. AJCC Cancer Staging Manual. 6th ed New York, NY: Springer; 2002:223-240. doi: 10.1007/978-1-4757-3656-4_25 [DOI] [Google Scholar]
- 39.Eaglehouse YL, Manjelievskaia J, Shao S, et al. Costs for breast cancer care in the military health system: an analysis by benefit type and care source. Mil Med. 2018;183(11-12):e500-e508. doi: 10.1093/milmed/usy052 [DOI] [PubMed] [Google Scholar]
- 40.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. doi: 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 41.Lê Cook B, Manning WG. Thinking beyond the mean: a practical guide for using quantile regression methods for health services research. Shanghai Arch Psychiatry. 2013;25(1):55-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Choi D, Hoffman KA, Kim M-O, McCarty D. A high-resolution analysis of process improvement: use of quantile regression for wait time. Health Serv Res. 2013;48(1):333-347. doi: 10.1111/j.1475-6773.2012.01436.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Findley DF. Model selection: Akaike’s information criterion In: Kotz S, Read CB, Balakrishnan N, Vidakovic B, Johnson NL, eds. Encyclopedia of Statistical Sciences. Hoboken, NJ: Wiley-Blackwell; 2006:1-6. doi: 10.1002/0471667196.ess0980.pub2. [DOI] [Google Scholar]
- 44.McGee SA, Durham DD, Tse CK, Millikan RC. Determinants of breast cancer treatment delay differ for African American and white women. Cancer Epidemiol Biomarkers Prev. 2013;22(7):1227-1238. doi: 10.1158/1055-9965.EPI-12-1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.National Center for Chronic Disease Prevention and Health Promotion Division of Diabetes Translation National Diabetes Statistics Report, 2017: Estimates of Diabetes and its Burden in the United States. Atlanta, GA: Centers for Disease Control and Prevention; 2017. [Google Scholar]
- 46.Saunders E. Hypertension in African-Americans. Circulation. 1991;83(4):1465-1467. doi: 10.1161/01.CIR.83.4.1465 [DOI] [PubMed] [Google Scholar]
- 47.Newman LA, Kaljee LM. Health disparities and triple-negative breast cancer in African American women: a review. JAMA Surg. 2017;152(5):485-493. doi: 10.1001/jamasurg.2017.0005 [DOI] [PubMed] [Google Scholar]
- 48.Zhou J, Enewold L, Zahm SH, et al. Breast conserving surgery versus mastectomy: the influence of comorbidities on choice of surgical operation in the Department of Defense health care system. Am J Surg. 2013;206(3):393-399. doi: 10.1016/j.amjsurg.2013.01.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gu J, Groot G, Boden C, Busch A, Holtslander L, Lim H. Review of factors influencing women’s choice of mastectomy versus breast conserving therapy in early stage breast cancer: a systematic review. Clin Breast Cancer. 2018;18(4):e539-e554. doi: 10.1016/j.clbc.2017.12.013 [DOI] [PubMed] [Google Scholar]
- 50.Wheeler SB, Reeder-Hayes KE, Carey LA. Disparities in breast cancer treatment and outcomes: biological, social, and health system determinants and opportunities for research. Oncologist. 2013;18(9):986-993. doi: 10.1634/theoncologist.2013-0243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mac Bride MB, Neal L, Dilaveri CA, et al. Factors associated with surgical decision making in women with early-stage breast cancer: a literature review. J Womens Health (Larchmt). 2013;22(3):236-242. doi: 10.1089/jwh.2012.3969 [DOI] [PubMed] [Google Scholar]
- 52.Forsythe LP, Alfano CM, Kent EE, et al. Social support, self-efficacy for decision-making, and follow-up care use in long-term cancer survivors. Psychooncology. 2014;23(7):788-796. doi: 10.1002/pon.3480 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Post-hoc Cox Proportional Hazards Analysis Comparing Overall Survival Between Non-Hispanic Black and White Women Receiving Breast Conserving Surgery as Treatment for Breast Cancer Grouped by Time-to-Surgery