Key Points
Question
Does a program addressing personal and environmental factors reduce disability among low-income older adults?
Findings
In this randomized clinical trial of 300 low-income older adults with disability, participation in a person-directed program resulted in a 30% reduction in disability scores compared with the results achieved in an attention control group.
Meaning
The findings suggest that disability may be modifiable through addressing both the person and the environment and such an intervention merits consideration for broader implementation.
Abstract
Importance
Disability among older adults is a strong predictor of health outcomes, health service use, and health care costs. Few interventions have reduced disability among older adults.
Objective
To determine whether a 10-session, home-based, multidisciplinary program reduces disability.
Design, Setting, and Participants
In this randomized clinical trial of 300 low-income community-dwelling adults with a disability in Baltimore, Maryland, between March 18, 2012, and April 29, 2016, 65 years or older, cognitively intact, and with self-reported difficulty with 1 or more activities of daily living (ADLs) or 2 or more instrumental ADLs (IADLs), participants were interviewed in their home at baseline, 5 months (end point), and 12 months (follow-up) by trained research assistants who were masked to the group allocation. Participants were randomized to either the intervention (CAPABLE) group (n = 152) or the attention control group (n = 148) through a computer-based assignment scheme, stratified by sex in randomized blocks. Intention-to-treat analysis was used to assess the intervention. Data were analyzed from September 2017 through August 2018.
Interventions
The CAPABLE group received up to 10 home visits over 5 months by occupational therapists, registered nurses, and home modifiers to address self-identified functional goals by enhancing individual capacity and the home environment. The control group received 10 social home visits by a research assistant.
Main Outcomes and Measures
Disability with ADLs or IADLs at 5 months. Each ADL and IADL task was self-scored from 0 to 2 according to whether in the previous month the person did not have difficulty and did not need help (0), did not need help but had difficulty (1), or needed help regardless of difficulty (2). The overall score ranged from 0 to 16 points.
Results
Of the 300 people randomized to either the CAPABLE group (n = 152) or the control group (n = 148), 133 of the CAPABLE participants (87.5%) were women with a mean (SD) age of 75.7 (7.6) years; 126 (82.9%) self-identified as black. Of the controls, 129 (87.2%) were women with a mean (SD) age of 75.4 (7.4) years; 133 (89.9%) self-identified as black. CAPABLE participation resulted in 30% reduction in ADL disability scores at 5 months (relative risk [RR], 0.70; 95% CI, 0.54-0.93; P = .01) vs control participation. CAPABLE participation resulted in a statistically nonsignificant 17% reduction in IADL disability scores (RR, 0.83; 95% CI, 0.65-1.06; P = .13) vs control participation. Participants in the CAPABLE group vs those in the control group were more likely to report that the program made their life easier (82.3% vs 43.1%; P < .001), helped them take care of themselves (79.8% vs 35.5%; P < .001), and helped them gain confidence in managing daily challenges (79.9% vs 37.7%; P < .001).
Conclusions and Relevance
Low-income community-dwelling older adults who received the CAPABLE intervention experienced substantial decrease in disability; disability may be modifiable through addressing both the person and the environment.
Trial Registration
ClinicalTrials.gov identifier: NCT01576133
This randomized clinical trial evaluates a patient-driven, home-based program to reduce disability for older adults with functional disabilities.
Introduction
Nearly 1 in 3 older Americans receives help with 1 or more basic daily activities, and as many as half of these individuals report difficulty.1 Disability is associated with poor quality of life, depression, hospitalization, nursing home placement, and further disability progression,2 with few exceptions.3 Despite all of this, geriatric clinic and transitional care models have not addressed or succeeded in reducing the difficulty or dependence when performing daily activities.
Disability is an especially pressing issue for low-income older adults because they have a higher prevalence of disability4,5 and often have housing conditions that exacerbate the effect of disability (such as broken flooring).6,7 Contemporary payment and delivery reform initiatives create opportunities to address social determinants, such as housing conditions, to improve health while saving costs.8 Drawing on evidence that person-directed strategies that target individual priorities are especially effective,3,9 we tested a person-directed, tailored intervention to improve daily function and to meet the needs of low-income older adults.10 This intervention, called Community Aging in Place—Advancing Better Living for Elders (CAPABLE), recognizes individual contextual factors, such as preferences, and home environmental factors related to health, family, and housing circumstances.
The CAPABLE program addresses personal and environmental factors that contribute to disability. It extends a successful program called Advancing Better Living for Elders (ABLE), which is designed to help older adults engage in everyday activities of their choice by reducing their difficulty with physical function, improving their quality of life, and lowering their mortality risk through a person-directed approach consisting of occupational therapy, physical therapy, and home environmental modifications.9,11 CAPABLE extends ABLE by including management of pain, medications, and depressive symptoms; communication with primary care practitioners; and home repair. Through pilot work, we identified these areas as crucial to amplifying the disability outcomes.12
CAPABLE has been studied in a randomized pilot study13 and in the Centers for Medicare & Medicaid Services (CMS) Innovation Center demonstration program.14 Both studies found CAPABLE to have a strong effect on reducing disability, but the pilot was underpowered with only 40 participants, whereas the CMS Innovation Center study was limited by a 1-arm study design with a matched comparison group. In this CAPABLE randomized clinical trial, we addressed both limitations. We hypothesized that participants randomized to the CAPABLE program would experience a reduction in disability 5 months after baseline compared with participants randomized to the attention control group.
Methods
This single-blind, 2-arm CAPABLE randomized clinical trial was conducted in Baltimore, Maryland, between March 18, 2012, and April 29, 2016. The trial protocol was approved by the Johns Hopkins University Institutional Review Board. Written informed consent was obtained from all study participants. The trial was monitored by a data and safety monitoring board. Details of the methods have been published elsewhere,12 and the full protocol is included in Supplement 1. All study participants were interviewed in their home at baseline, 5 months (main study end point), and 12-month follow-up by trained research assistants who were masked to the group allocation.
Study Population
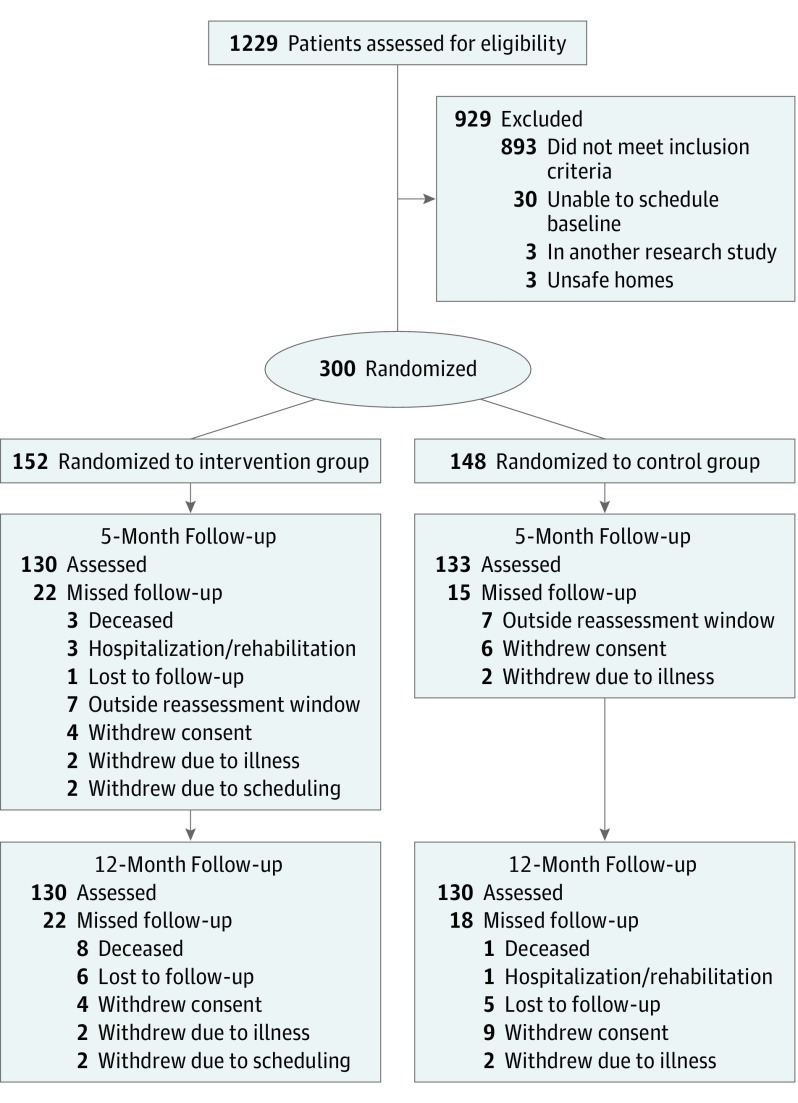
The sample was recruited using a variety of strategies, including direct mail marketing (35%), self-referral (30%), sign-ups through government programs (19%), word of mouth (15%), and enrollment through community programs (7%). The screening process for these community-dwelling participants has been described elsewhere12 and is depicted in Figure 1.
Figure 1. CONSORT Flow Diagram.
Eligible participants were 65 years of age or older; were cognitively intact, according to a Mini-Mental State examination15 score (from 0-30, with scores of 24-30 indicating no cognitive impairment) of at least 24 of 30 at the time of the in-person baseline visit; and reported difficulty with at least 1 activity of daily living (ADL)16 or at least 2 instrumental activities of daily living (IADLs).17 Eligible individuals also had to document income of less than 200% of the federal poverty level (in 2018, this amount is $22 980 annually for a 1-person household in the 48 contiguous US states).18
Exclusion criteria included self-report of active cancer treatment, more than 3 acute hospitalizations in the past year, inability to stand, apartment dwelling, plans to move within a year, or use of home-based physical or occupational therapy services at enrollment.12
Participants were randomized to either the intervention (CAPABLE) group or the attention control group through a computer-based assignment scheme, which was stratified by sex in randomized blocks. Investigators and study staff were masked to assignment scheme.
Intervention Group
The CAPABLE program has been described in detail elsewhere.12 Briefly, the program includes (1) a multidisciplinary assessment performed by an occupational therapist (OT), who evaluates the functional disability and home safety risks as well as asks participants about their functional goals, and by a registered nurse (RN), who inquires about participant goals regarding pain level, depression, medication understanding, primary care practitioner communication, and strength and balance; (2) development of an integrated plan that is based on individual assessments and participant goals and that includes tailored strategies that address those goals; (3) implementation of strategies that came from brainstorming with the participant; and (4) home repair, environmental modifications, and medical equipment that support the achievement of participant-identified functional goals.
The program targets individual functional goals (eg, walk downstairs, take a shower, and get dressed without pain) identified by each participant and barriers to achieving these goals. For instance, if a participant wants to prepare food rather than wait for a neighbor to help, then the OT and participant together identify feasible energy-conserving approaches and tools.3 To complement these strategies, the RN uses behavioral activation strategies to help the participant manage depressive symptoms19 and balance issues.20 In consultation with the team, the home modifier stabilizes stairs, levels flooring, and repairs floors to enable participants to practice newly learned mobility skills safely and efficiently.
For 5 months, CAPABLE participants receive up to 6 one-hour home sessions with the OT; up to 4 one-hour home sessions with the RN; and up to $1300 worth of home repairs, modifications, and assistive devices. The cost of delivering CAPABLE services in this trial was $2825 per person, which included visits, supplies, team coordination, mileage, and home improvement parts and labor.14
The OTs and RNs were trained in the CAPABLE approach through readings, didactic sessions, shadowing experienced OTs and RNs to observe the protocol in the field, and bimonthly supervision meetings. The OTs and RNs documented the duration and content of each home session within 24 hours of completion. All study visits were audiotaped to ensure fidelity; study staff listened to a random 10% of the recordings and evaluated the tapes on the basis of a priori criteria.
Attention Control Group
The attention control group was designed to match the amount of social engagement that the intervention group received (10 home visits of 60 minutes each). The group research assistant helped participants identify sedentary activities they would like to learn or enjoy. Common choices were reminiscing about life, learning to use the internet, playing board games, and listening to music. The duration of each session was monitored.
Measures and Outcomes
Race/ethnicity, age, and sex information was self-reported by each participant. The primary outcomes were disability as measured by difficulty or dependence in self-reported ADLs and IADLs at 5 months (after program completion).
Activities of daily living refer to self-reported difficulty or need for help when performing 8 essential ADLs: walking across a small room, bathing, upper-body dressing, lower-body dressing, eating, using the toilet, transferring in and out of bed, and grooming.16 This method of self-report, which has high test-retest reliability and sensitivity, predicts future morbidity.21 Functioning on each task was classified from 0 to 2, depending on whether in the previous month the person did not have difficulty and did not need help (0), did not need help but had difficulty (1), or needed help regardless of difficulty (2). A summary disability score for the 8 items ranged from 0 to 16 points; a 1-point change was considered clinically meaningful.22
Instrumental activities of daily living refer to self-reported difficulty or need for help when performing 8 tasks: using the phone, shopping, preparing food, light housekeeping, washing laundry, traveling independently, taking medications, and managing finances independently. The response category for each IADL task ranged from 0 to 2, depending on whether in the previous month the person did not have difficulty and did not need help (0), did not need help but had difficulty (1), or needed help regardless of difficulty (2). The score ranged from 0 to 16 points.
Perceived Program Benefits
We evaluated participant assessment of study benefits using a survey adapted from previous trials23 that addressed the following 10 questions: (1) How much benefit did you perceive from the CAPABLE program? (2-9) How much did the program help you … take care of yourself? keep living at home? make life easier? make home safer? gain confidence in managing daily challenges? be less upset, distressed, or overwhelmed? take care of others? help others in similar situations? (10) Did the study require too much work or effort? Each of these questions could be answered by 1 of 3 responses: not at all, some, or a great deal.
Statistical Analysis
On the basis of the pilot study,13 we assumed 15% attrition by 5 months and 90% power to detect a moderate effect size. We set the significance level at α = .05. Given these numbers, we planned to randomize 300 participants to the CAPABLE group or control group. The sample size calculation was based on 2-sided, 2-sample t tests at a P = .05 significance level and detected an effect size of 0.36 or greater with 80% power. The effect size was based on standardized mean difference in the ADL (or IADL) score at 5 months between the intervention and attention control groups.
We compared the baseline characteristics of study participants to assess the balance between the CAPABLE and control groups. Both crude and covariate-adjusted effect sizes were presented to evaluate the robustness of findings. We used intention-to-treat analysis to assess the intervention effects. We were unable to use analysis of covariance to test the differences in primary outcomes between the groups because so many participants scored a 0 on their disability assessment after the intervention. This result led to overdispersion, shown in a significant likelihood ratio test.24 Therefore, we used the negative binomial regression model, a generalized linear model that accounts for nonnegative integer-valued outcome variables.25
To implement the intention-to-treat analysis, we modeled the ADL and IADL scores as a longitudinal outcome consisting of up to 3 measurements taken at baseline, 5 months, and 12 months, using the random-effect overdispersion negative binomial regression model.26 In this model, the random effect refers to the distribution of the dispersion parameter on the assumption that the dispersion is constant within a person but varies from person to person, such that the inverse of 1 plus the dispersion follows a beta distribution. We included in the model a binary indicator for treatment allocation, dummy indicators of study visits (ie, baseline, 5 months, and 12 months), and interaction terms between the treatment indicator and visit time to allow varying effect size during the intervention phase compared with the maintenance phase. The effect-size estimates were presented, separately at 5 months and 12 months, as the ratio of means between the CAPABLE and the control groups after adjusting for race/ethnicity, sex, and prognostic factors such as pain distress, tiredness, and unintentional weight loss in the past year at baseline. These prognostic factors were determined by forward stepwise selection, with 0.1 significant level for variable entry into and with 0.15 significant level for removal from negative binomial models of posttreatment ADL and IADL scores, weighted by factors associated with the likelihood to drop out. Attrition at the primary end point (5 months) was low at 12.3%. With the maximum likelihood estimator, the random-effect model was robust to data missing at randomization, conditional on the covariates in the model. All analyses were conducted using STATA, version 15 (StataCorp LLC).
Results
We screened 1229 people for participation. Of these individuals, 300 were eligible, interested in participating, and then randomized to either the intervention group (n = 152) or the control group (n = 148) (Figure 1). Of the 152 participants in the intervention group, 133 (87.5%) were women and 19 (12.5%) were men, with a mean (SD) age of 75.7 (7.6) years, and 126 (82.9%) self-identified as black. Of the 148 participants in the control group, 129 (87.2%) were women and 19 (12.8%) were men, with a mean (SD) age of 75.4 (7.4) years, and 133 (89.9%) self-identified as black.
The study attrition rate was low, with 37 participants (12.3%) not completing the assessment at 5 months and 40 participants (13.3%) not completing the follow-up at 12 months. A total of 130 participants (85.5%) in the intervention group and 133 participants (89.9%) in the control group completed the study at 5 months, and 130 participants (85.5%) in the intervention group and 130 participants (87.8%) in the control group completed the study at 12 months. Those who did not get reassessed were older and had higher ADL disability scores. No demographic or functional differences were observed between the treatment and control groups at baseline (Table 1) except for pain distress in the previous week, tiredness, and unintentional weight loss, each of which was statistically significantly worse in the treatment group than in the control group.
Table 1. Baseline Characteristics by Treatment Group.
| Variable | Control Group (n = 148) | Intervention Group (n = 152) |
|---|---|---|
| Age, mean (SD), y | 75.4 (7.4) | 76.1 (7.8) |
| Female sex, No. (%) | 129 (87.2) | 133 (87.5) |
| Race/ethnicity, No. (%) | ||
| White | 14 (9.5) | 26 (17.1) |
| Black | 133 (89.9) | 126 (82.9) |
| Asian | 1 (0.7) | 0 |
| Educational level, No. (%) | ||
| <12 y | 54 (36.7) | 44 (29.0) |
| ≥12 y | 93 (63.3) | 108 (71.0) |
| Live alone, No. (%) | 70 (47.3) | 80 (52.6) |
| No. of medical conditions, mean (SD) | 3.3 (1.4) | 3.3 (1.4) |
| ADL score, mean (SD) | 4.0 (3.0) | 4.0 (3.1) |
| IADL score, mean (SD) | 5.6 (3.9) | 6.2 (4.2) |
| PHQ-9 score, mean (SD) | 6.6 (5.2) | 7.0 (5.0) |
| Falls efficacy | 36.5 (21.2) | 37.32 (21.2) |
| Pain over the past wk, No. (%)a | ||
| Intensity ≥5 | 100 (68.0) | 117 (77.0) |
| Distress ≥5 | 94 (64.0) | 113 (74.3) |
| Energy in past mo, No. (%) | ||
| Unusually low energy | 78 (56.5) | 76 (56.3) |
| Usually tired | 87 (63.0) | 83 (61.5) |
| Usually weak | 56 (42.8) | 72 (53.3) |
| Unintentional weight loss in the past year | 83 (60.1) | 89 (65.9) |
Abbreviations: ADL, activity of daily living; IADL, instrumental activity of daily living; PHQ-9, 9-item Patient Health Questionnaire (score range: 0-27, with higher scores indicating more depressive symptoms).
The pain variable is a component of the Brief Pain Inventory. The scale runs from 0 to 10. Higher scores indicate more intensity, or more distress.
Treatment Dose
Of the 152 participants in the intervention arm, 141 (92.8%) completed 8 to 10 sessions and only 6 (3.9%) had fewer than 3 sessions, considered the minimal treatment threshold. Participants in the intervention group received a mean (SD) of 9.1 (1.86) home visits. Of the 148 control group participants, 73 (49.3%) completed 8 to 10 visits, and 56 people (37.8) had fewer than 3 sessions.
Primary Outcome: Disability at 5 Months
In unadjusted analyses, participants in the intervention group reported a 26% reduction (relative risk [RR], 0.74; 95% CI, 0.57-0.97; P = .03) in ADL disability scores compared with participants in the control group. The 10% reduction in IADL disability scores among those in the intervention group, compared with the controls, was not statistically significant (RR, 0.90; 95% CI, 0.72-1.12; P = .35). In adjusted analyses, the CAPABLE treatment effect on ADL disability scores remained statistically significant (30% reduction; RR, 0.70; 95% CI, 0.54-0.93; P = .01) and the effect on IADL scores was still not statistically significant (17% reduction; RR, 0.83; 95% CI, 0.65-1.06; P = .13) (Table 2). These adjustments were made in sex; race/ethnicity; corresponding pre-intervention ADL (or IADL) score; and baseline treatment group differences in pain distress, tiredness, and unintentional weight loss; as well as after applying weights (to adjust for the likelihood of a reassessment dropout).
Table 2. Comparison of Control and Intervention Group Participants at 5 Months and 12 Months.
| Variable | Mean (SE) | Crude Effect Size | Adjusted Effect Sizeb | |||
|---|---|---|---|---|---|---|
| Control Group | Intervention Group | RR (95% CI)a | P Value | RR (95% CI)a | P Value | |
| Primary outcomes (baseline)c | ||||||
| ADL score | 3.99 (0.24) | 4.00 (0.25) | NA | NA | NA | NA |
| IADL score | 5.61 (0.32) | 6.17 (0.34) | NA | NA | NA | NA |
| Study end point (5 mo)d | ||||||
| ADL score | 2.83 (0.28) | 2.22 (0.26) | 0.74 (0.57-0.97) | .03 | 0.70 (0.54-0.93) | .01 |
| IADL score | 4.39 (0.34) | 3.86 (0.35) | 0.90 (0.72-1.12) | .35 | 0.83 (0.65-1.06) | .13 |
| Secondary outcomes (12 mo) | ||||||
| ADL score | 2.67 (0.27) | 2.65 (0.30) | 0.93 (0.72-1.21) | .60 | 0.90 (0.68-1.18) | .44 |
| IADL score | 4.28 (0.37) | 4.50 (0.41) | 1.06 (0.85-1.32) | .61 | 1.03 (0.81-1.131) | .80 |
Abbreviations: ADL, activity of daily living; IADL, instrumental activity of daily living; RR, relative risk.
The effect-size estimates are presented as the ratio of means between the intervention group (CAPABLE) and the attention control group after accounting for study attrition.
Adjusting for sex, race/ethnicity, baseline outcome value, pain distress, tiredness, and unintentional weight loss.
Functioning on each task is classified from 0 to 2, depending on whether in the previous month the person did not have difficulty and did not need help (0), did not need help but had difficulty (1), or needed help regardless of difficulty (2). Answers were summed to create the score.
The means and SEs for 5-month and 12-month data were weighted to adjust for missing data.
Secondary Outcome: Disability at 12 Months
Unadjusted analyses showed that participants in the intervention group reported a statistically nonsignificant 7% improvement in ADL disability scores (RR, 0.93; 95% CI, 0.72-1.21; P = .60) from baseline to 12-month measurement (7 months after the conclusion of CAPABLE visits), compared with the control participants. The RR of improvement in IADL disability scores at 12 months was also not statistically significant (RR, 1.06; 95% CI, 0.85-1.32; P = .61). In adjusted analyses, the effect on ADL (RR, 0.90; 95% CI, 0.68- 1.18; P = .44) and IADL (RR, 1.03; 95% CI, 0.81-1.31; P = .80) scores remained statistically nonsignificant (Table 2). The adjustments were the same as those for the assessment at 5 months, with adjustments made in sex; race/ethnicity; corresponding pre-intervention (ADL or IADL) score; baseline intervention group differences in pain distress, tiredness, and unintentional weight loss; and after applying weights (to adjust for the likelihood of a 12-month reassessment dropout).
Perception of Benefit
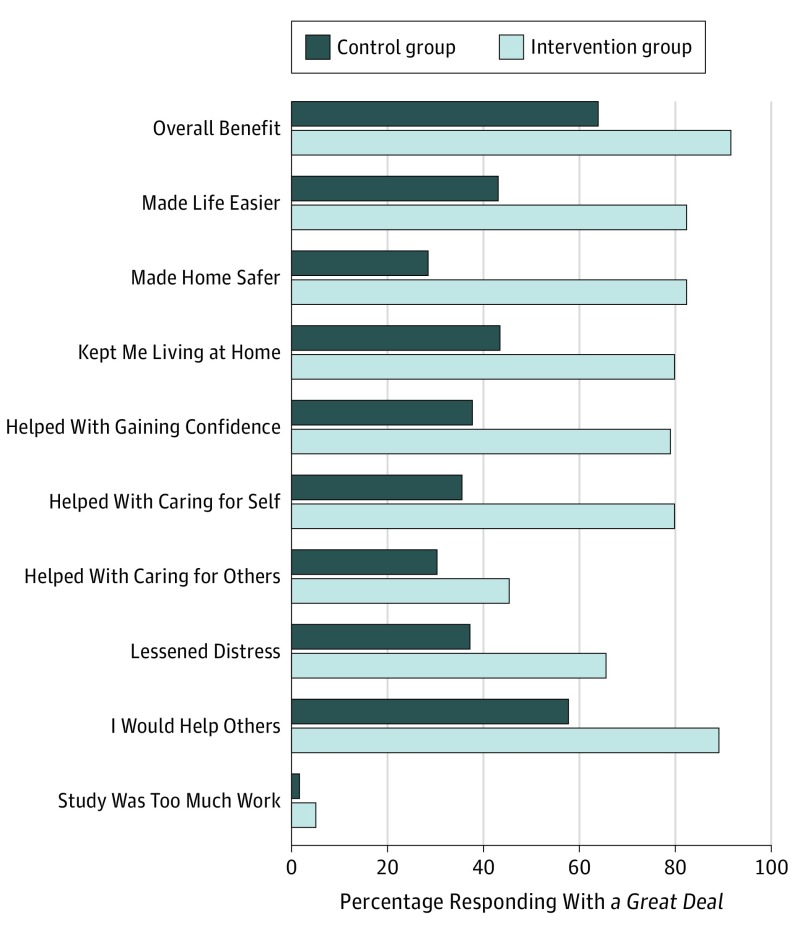
The intervention group participants (n=119), compared with controls (n=123), responded with a great deal to the following survey items: overall benefit (91.6% [109] vs 63.4% [78]; P < .001); the CAPABLE program made their life easier (82.3% [98] vs 43.1% [53]; P < .001), made their home safer (82.3% [98] vs 28.5% [35]; P < .001), kept them living at home (79.8% [95] vs 43.4% [53]; P < .001), helped them gain confidence in managing daily challenges (78.9% [94] vs 37.7% [46]; P < .001), helped them take care of themselves (79.8% [95] vs 35.5% [43]; P < .001), lessened their upset, distress, or overwhelmed feeling (65.5% [78] vs 37.2% [45]; P < .001), would help others in a similar situation (89.1% [106] vs 57.72% [71]; P < .001), and whether participants said the program helped them take care of others (45.4% [54] vs 30.3% [37]; P = .02). No statistical differences were found between the control and intervention groups in whether participants said the program required too much work or effort (5.0% [6] vs 1.6% [2]; P = .14) (Figure 2).
Figure 2. Survey Responses by Intervention and Control Group Participants .
At 5 months or program completion, participants were asked to complete a 10-item survey that assesses their satisfaction with aspects of the CAPABLE program, including (1) overall benefit, (2-9) how the program addressed specific functional goals, and (10) whether the program required too much work or effort. Participants could pick 1 of 3 responses for each question: not at all, some, or a great deal.
Discussion
In this randomized clinical trial, participants randomized to the CAPABLE (intervention) group reported a substantial reduction in disability scores after treatment (5-month outcome) compared with the attention control group. The 30% magnitude of the reduction is comparable to results of the 1-armed study of CAPABLE funded by the CMS Innovation Center, which used multiple matched Medicare beneficiaries for comparison. Participants in the control group who received individualized attention also improved, reporting smaller magnitude reductions in ADL and IADL disability scores.
Disabilities are common among adults aged 65 years or older and are associated with poor quality of life,27,28 further functional decline,2 hospitalization,29 increased mortality, and triple the medical costs.30 We tested the CAPABLE program, a person-directed intervention that helps older adults identify and achieve their own functional goals through a combination of strategies, including targeting the individual and the home environment. Our findings extend the body of literature on person-directed care,31 goal-directed care,32,33 and the power of both person and environment interventions34 to decrease disability scores by more than 4 times the SE of the primary outcomes. Other studies have used 0.5-SD reduction as a clinically meaningful cutoff in main outcomes.23,35
Reducing disability scores among low-income older adults has clinical, fiscal, and policy relevance. In a nonrandomized evaluation of the CMS Innovation Center demonstration of CAPABLE, CMS evaluators estimated cost savings to Medicare of $22 000 over 2 years for the average CAPABLE participant (at a total cost per participant of $2825), compared with a propensity score–matched comparison group.36 The CAPABLE program has subsequently been adopted by health care organizations in 22 cities and rural areas in 11 states, through varied innovations in payment policy such as Medicaid waivers (which provide community-based resources for people deemed eligible for a nursing home); accountable care organizations; and hospital readmission prevention programs. This well-powered, randomized trial provides further support that the CAPABLE intervention reduces disability scores in a high-risk subset of the older adult population. As such, the program merits consideration of inclusion in payment innovations, such as those from CMS that allow Medicare Advantage to pay for nonmedical costs with the medical budget37 or through a Special Needs Plan geared toward people with disabilities who are dually eligible for Medicaid and Medicare.
Despite its predictive value and strong relevance to value-based care and population health efforts, functional status is not commonly prioritized in primary and specialty care. Functional status is often a hidden feature in electronic medical records, if the feature exists at all,38 the reason for which may be the widely held belief that functional decline is not modifiable. Our work suggests that function can be improved.
The effect of the CAPABLE intervention diminished between 5 and 12 months, which may suggest that a booster visit or call could be useful. In addition, a screening for possible benefits like the Supplemental Nutrition Assistance Program or involvement by a social worker, community health worker, or physical therapist could augment the effect. Some of the new CAPABLE sites are experimenting with these extensions.
Limitations
This trial has a few limitations. Participants who responded to recruitment may be different in unmeasured ways from individuals who did not respond. Older adults who are referred to as high-cost utilizers are often harder to engage and may not have the same uptake or same results. In addition, this study was limited to low-income older adults in Baltimore, Maryland, and the sample was predominantly black women, which may limit generalizability. However, few studies of geriatric models have been conducted among low-income older adults and with a predominantly black sample.
Conclusions
Meeting the near-universal goal of supporting older adults to age in place will require models that address more than medical conditions. Findings from this trial suggest that disability may be modifiable through addressing both the person and the environment.
Trial Protocol
Data Sharing Statement
References
- 1.Freedman VA, Spillman BC. The residential continuum from home to nursing home: size, characteristics and unmet needs of older adults. J Gerontol B Psychol Sci Soc Sci. 2014;69(suppl 1):S42-S50. doi: 10.1093/geronb/gbu120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arnau A, Espaulella J, Serrarols M, Canudas J, Formiga F, Ferrer M. Risk factors for functional decline in a population aged 75 years and older without total dependence: A one-year follow-up. Arch Gerontol Geriatr. 2016;65:239-247. doi: 10.1016/j.archger.2016.04.002 [DOI] [PubMed] [Google Scholar]
- 3.Gitlin LN, Hauck WW, Winter L, Dennis MP, Schulz R. Effect of an in-home occupational and physical therapy intervention on reducing mortality in functionally vulnerable older people: preliminary findings. J Am Geriatr Soc. 2006;54(6):950-955. doi: 10.1111/j.1532-5415.2006.00733.x [DOI] [PubMed] [Google Scholar]
- 4.Fuller-Thomson E, Nuru-Jeter A, Minkler M, Guralnik JM. Black-White disparities in disability among older Americans: further untangling the role of race and socioeconomic status. J Aging Health. 2009;21(5):677-698. doi: 10.1177/0898264309338296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Minkler M, Fuller-Thomson E, Guralnik JM. Gradient of disability across the socioeconomic spectrum in the United States. N Engl J Med. 2006;355(7):695-703. doi: 10.1056/NEJMsa044316 [DOI] [PubMed] [Google Scholar]
- 6.Cho J, Cook C, Bruin MJ. Functional ability, neighborhood resources and housing satisfaction among older adults in the U.S. J Hous Elder. 2012;26(4):395-412. doi: 10.1080/02763893.2012.724376 [DOI] [Google Scholar]
- 7.Cho HY, MacLachlan M, Clarke M, Mannan H. Accessible Home Environments for People with Functional Limitations: A Systematic Review. Int J Environ Res Public Health. 2016;13(8):826. doi: 10.3390/ijerph13080826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alley DE, Asomugha CN, Conway PH, Sanghavi DM. Accountable Health Communities–Addressing Social Needs through Medicare and Medicaid. N Engl J Med. 2016;374(1):8-11. doi: 10.1056/NEJMp1512532 [DOI] [PubMed] [Google Scholar]
- 9.Gitlin LN, Hauck WW, Dennis MP, Winter L, Hodgson N, Schinfeld S. Long-term effect on mortality of a home intervention that reduces functional difficulties in older adults: results from a randomized trial. J Am Geriatr Soc. 2009;57(3):476-481. doi: 10.1111/j.1532-5415.2008.02147.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Starfield B. Is patient-centered care the same as person-focused care? Perm J. 2011;15(2):63-69. doi: 10.7812/TPP/10-148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gitlin LN, Winter L, Dennis MP, Corcoran M, Schinfeld S, Hauck WW. A randomized trial of a multicomponent home intervention to reduce functional difficulties in older adults. J Am Geriatr Soc. 2006;54(5):809-816. doi: 10.1111/j.1532-5415.2006.00703.x [DOI] [PubMed] [Google Scholar]
- 12.Szanton SL, Wolff JW, Leff B, et al. . CAPABLE trial: a randomized controlled trial of nurse, occupational therapist and handyman to reduce disability among older adults: rationale and design. Contemp Clin Trials. 2014;38(1):102-112. doi: 10.1016/j.cct.2014.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Szanton SL, Thorpe RJ, Boyd C, et al. . Community aging in place, advancing better living for elders: a bio-behavioral-environmental intervention to improve function and health-related quality of life in disabled older adults. J Am Geriatr Soc. 2011;59(12):2314-2320. doi: 10.1111/j.1532-5415.2011.03698.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Szanton SL, Leff B, Wolff JL, Roberts L, Gitlin LN. Home-Based Care Program Reduces Disability And Promotes Aging In Place. Health Aff (Millwood). 2016;35(9):1558-1563. doi: 10.1377/hlthaff.2016.0140 [DOI] [PubMed] [Google Scholar]
- 15.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198. [DOI] [PubMed] [Google Scholar]
- 16.Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. the index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185:914-919. doi: 10.1001/jama.1963.03060120024016 [DOI] [PubMed] [Google Scholar]
- 17.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179-186. doi: 10.1093/geront/9.3_Part_1.179 [DOI] [PubMed] [Google Scholar]
- 18.HealthCare.Gov Federal poverty level. Healthcare.gov. https://www.healthcare.gov/glossary/federal-poverty-level-FPL/. Accessed March 15, 2012.
- 19.Gitlin LN, Harris LF, McCoy M, Chernett NL, Jutkowitz E, Pizzi LT; Beat the Blues Team . A community-integrated home based depression intervention for older African Americans: [corrected] description of the Beat the Blues randomized trial and intervention costs. BMC Geriatr. 2012;12:4. doi: 10.1186/1471-2318-12-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frick KD, Kung JY, Parrish JM, Narrett MJ. Evaluating the cost-effectiveness of fall prevention programs that reduce fall-related hip fractures in older adults. J Am Geriatr Soc. 2010;58(1):136-141. doi: 10.1111/j.1532-5415.2009.02575.x [DOI] [PubMed] [Google Scholar]
- 21.Wallace M, Shelkey M. Monitoring functional status in hospitalized older adults. Am J Nurs. 2008;108(4):64-71. doi: 10.1097/01.NAJ.0000315266.66521.e7 [DOI] [PubMed] [Google Scholar]
- 22.Gill TM, Baker DI, Gottschalk M, Peduzzi PN, Allore H, Byers A. A program to prevent functional decline in physically frail, elderly persons who live at home. N Engl J Med. 2002;347(14):1068-1074. doi: 10.1056/NEJMoa020423 [DOI] [PubMed] [Google Scholar]
- 23.Gitlin LN, Winter L, Dennis MP, Hodgson N, Hauck WW. A biobehavioral home-based intervention and the well-being of patients with dementia and their caregivers: the COPE randomized trial. JAMA. 2010;304(9):983-991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gutierrez RG, Carter S, Drukker DM. On boundary-value likelihood-ratio tests. Stata Tech Bull. 2001;10(60):15-20. [Google Scholar]
- 25.McCullagh P, Nelder J. Generalized Linear Models. 2nd ed London, UK: Chapman and Hall; 1994. [Google Scholar]
- 26.Hausman JA, Hall BH, Griliches Z. Econometric models for count data with an application to the patents-R&D relationship. Econometrica. 1984;52(4):909-938. doi: 10.2307/1911191 [DOI] [Google Scholar]
- 27.Bentley JP, Brown CJ, McGwin G Jr, Sawyer P, Allman RM, Roth DL. Functional status, life-space mobility, and quality of life: a longitudinal mediation analysis. Qual Life Res. 2013;22(7):1621-1632. doi: 10.1007/s11136-012-0315-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barile JP, Thompson WW, Zack MM, Krahn GL, Horner-Johnson W, Haffer SC. Activities of daily living, chronic medical conditions, and health-related quality of life in older adults. J Ambul Care Manage. 2012;35(4):292-303. doi: 10.1097/JAC.0b013e31826746f5 [DOI] [PubMed] [Google Scholar]
- 29.Perrin NA, Stiefel M, Mosen DM, Bauck A, Shuster E, Dirks EM. Self-reported health and functional status information improves prediction of inpatient admissions and costs. Am J Manag Care. 2011;17(12):e472-e478. [PubMed] [Google Scholar]
- 30.Alecxih L, Shen S, Chan I Individuals living in the community with chronic conditions and functional limitations: a closer look. Office of The Assistant Secretary for Planning and Evaluation. http://aspe.hhs.gov/daltcp/reports/2010/closerlook.pdf. Published January 1, 2010. Accessed September 10, 2017.
- 31.Lines LM, Lepore M, Wiener JM. Patient-centered, Person-centered, and Person-directed Care: They are Not the Same. Med Care. 2015;53(7):561-563. doi: 10.1097/MLR.0000000000000387 [DOI] [PubMed] [Google Scholar]
- 32.Bodenheimer T, Lorig K, Holman H, Grumbach K. Patient self-management of chronic disease in primary care. JAMA. 2002;288(19):2469-2475. doi: 10.1001/jama.288.19.2469 [DOI] [PubMed] [Google Scholar]
- 33.Reuben DB, Tinetti ME. Goal-oriented patient care–an alternative health outcomes paradigm. N Engl J Med. 2012;366(9):777-779. doi: 10.1056/NEJMp1113631 [DOI] [PubMed] [Google Scholar]
- 34.Wahl HW, Fänge A, Oswald F, Gitlin LN, Iwarsson S. The home environment and disability-related outcomes in aging individuals: what is the empirical evidence? Gerontologist. 2009;49(3):355-367. doi: 10.1093/geront/gnp056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Belle SH, Burgio L, Burns R, et al. ; Resources for Enhancing Alzheimer’s Caregiver Health (REACH) II Investigators . Enhancing the quality of life of dementia caregivers from different ethnic or racial groups: a randomized, controlled trial. Ann Intern Med. 2006;145(10):727-738. doi: 10.7326/0003-4819-145-10-200611210-00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ruiz S, Snyder LP, Rotondo C, Cross-Barnet C, Colligan EM, Giuriceo K. Innovative home visit models associated with reductions in costs, hospitalizations, and emergency department use. Health Aff (Millwood). 2017;36(3):425-432. doi: 10.1377/hlthaff.2016.1305 [DOI] [PubMed] [Google Scholar]
- 37.Gleckman H. Today’s massive budget deal makes big Medicare changes. Forbes https://www.forbes.com/sites/howardgleckman/2018/02/09/todays-massive-budget-deal-makes-big-medicare-changes/#7e9880d237bf. Published February 9, 2018. Accessed March 4, 2018.
- 38.Bayliss EA, McQuillan DB, Ellis JL, et al. . Using Electronic Health Record Data to Measure Care Quality for Individuals with Multiple Chronic Medical Conditions. J Am Geriatr Soc. 2016;64(9):1839-1844. doi: 10.1111/jgs.14248 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Data Sharing Statement