Key Points
Question
Are mobile phone–delivered smoking cessation interventions efficacious for economically disadvantaged individuals?
Findings
In this 3-group randomized clinical trial of 624 current cigarette smokers, biochemically verified abstinence was found in 13 (12.0%) in the nicotine replacement therapy group, 19 (12.0%) with the addition of text messaging, and 28 (25.5%) with the addition of text messaging plus call. Participants in the group receiving all 3 interventions were approximately 1.5 to 2.0 times more likely to be abstinent than those receiving nicotine replacement therapy alone.
Meaning
An intervention consisting of text messaging and nicotine replacement therapy may not be adequate to increase cessation rates in economically disadvantaged smokers, but the addition of proactive counseling may be an appropriate option.
This randomized clinical trial assesses the efficacy of mobile phone–delivered cessation interventions targeted to current cigarette smokers at neighborhood sites serving racial/ethnic minority and socioeconomically disadvantaged individuals.
Abstract
Importance
Limited evidence supports mobile phone–delivered cessation interventions for socioeconomically disadvantaged individuals.
Objective
To assess the efficacy of mobile phone–delivered cessation interventions targeted to smokers at neighborhood sites serving racial/ethnic minority and socioeconomically disadvantaged individuals.
Design, Setting, and Participants
This group-randomized clinical trial with neighborhood site serving as the sampling unit compared smoking cessation interventions that included (1) nicotine replacement therapy (NRT), (2) NRT plus text messaging, and (3) NRT plus text messaging plus proactive counseling via mobile phone. Recruitment took place at churches, public housing complexes, and community centers located throughout the Houston, Texas, area. A total of 624 current cigarette smokers 18 years or older were enrolled at neighborhood sites from August 13, 2011, through December 12, 2014. Final follow-up was completed on June 12, 2015, and data were analyzed from August 17, 2017, through May 10, 2018, based on intention to treat.
Interventions
Nicotine replacement therapy consisted of transdermal nicotine patches; NRT plus text messages, transdermal nicotine patches and individually tailored mobile phone text messages; and NRT plus text plus call, transdermal patches, individually tailored mobile phone text messages, and proactive counseling via mobile phone.
Main Outcomes and Measures
The primary outcome was smoking abstinence at 6 months, defined as (1) biochemically verified smoking abstinence (calculated among a subgroup of 377 participants) as determined by saliva cotinine level; and (2) self-reported 30-day abstinence (calculated among all 624 participants).
Results
The study sample included 624 current cigarette smokers (50.6% female; mean [SD] age, 45.8 [12.8] years). Among the 377 participants eligible for biochemical verification, 127 self-reported 30-day abstinence and were asked to provide saliva samples. Of these, 98 samples were returned (participants who did not return samples were coded as smoking). Biochemically verified abstinence rates were 12.0% for NRT, 12.0% for NRT plus text, and 25.5% for NRT plus text plus call. Participants in the NRT plus text plus call group were 2.11 (95% CI, 1.00-4.48) times more likely to be biochemically verified as abstinent compared with the NRT group. No differences in biochemically verified abstinence between the NRT plus text group and the NRT group were observed. Similar associations were observed with the self-report cessation outcomes.
Conclusions and Relevance
Findings indicate that assignment to an intervention consisting of text messaging alone may not increase cessation rates for socioeconomically disadvantaged smokers. However, text messaging plus proactive counseling may be an efficacious option.
Trial Registration
ClinicalTrials.gov identifier: NCT00948129
Introduction
Cigarette smoking remains the leading cause of preventable death in the United States and is responsible for 480 000 deaths and $156 billion in lost productivity annually.1 Although reductions in smoking prevalence have been observed in recent decades, these reductions have not occurred equally in all population subgroups. Current trends indicate that smoking is increasingly concentrated in socioeconomically disadvantaged groups and is responsible for numerous health disparities.2 Substantial efforts have been made to develop and implement cessation programs, including cessation quitlines. These quitlines offer the promise of great reach, while providing a cost-effective and evidence-based treatment option.3 Despite widespread adoption of quitlines, only a small minority of current smokers, typically 1% to 3%, use this treatment.4,5 Thus, additional efforts to identify acceptable, cost-effective, and efficacious smoking cessation services are warranted.
One treatment approach increasingly supported by government agencies is text messaging.6 Findings from the Pew Research Center indicate that in 2017, 95% of adults owned a mobile phone. Moreover, the proportion of mobile phone ownership exceeded 85% in every age, racial/ethnic, educational attainment, income, and geographic subgroup.7 These trends indicate that mobile phone interventions may be an effective tool for reaching underserved populations (eg, minority, low income, low educational attainment, and rural). In addition, the efficacy of text messaging interventions for smoking cessation is supported by results of several randomized clinical trials.8,9 Findings indicate that text messaging interventions (vs a control condition) are associated with a 2-fold increase in cessation rates.9,10,11 Also of note, a recent economic analysis12 indicated that text messaging interventions for smoking cessation are cost-effective. Indeed, mobile phone–delivered text messaging has been identified as one of the most affordable interventions for global tobacco control13 and has been endorsed by the World Health Organization.14,15
Despite the promise, few efforts to assess the efficacy of text messaging interventions for smoking cessation among socioeconomically disadvantaged individuals appear in the scientific literature.8,10 The purpose of the present study was to address this gap. Specifically, an additive design was used to assess the efficacy of mobile phone–delivered cessation interventions targeted to smokers at neighborhood sites serving racial/ethnic minority and socioeconomically disadvantaged individuals.
Methods
Study Design
The study protocol is found in Supplement 1. A group-randomized clinical trial with neighborhood site serving as the sampling unit was used to compare 3 smoking cessation interventions. From August 13, 2011, through December 12, 2014, participant recruitment took place at churches, public housing sites, and community centers located in the Houston, Texas area. Participants received a US $20 gift card for completing an in-person baseline assessment and a $10 card for completing a mobile phone–delivered 6-month follow-up. Participants were provided a mobile phone with active voice and text messaging service for the 6-month study and follow-up period. The institutional review board at The University of Texas MD Anderson Cancer Center, Houston, Texas, approved the study and provided oversight of all study-related activities. All participants provided written informed consent.
Participant Eligibility
Participant inclusion criteria consisted of (1) being 18 years or older; (2) smoking at least 100 cigarettes in their lifetime; (3) English or Spanish speaking; (4) smoking at least 5 cigarettes per day; and (5) willing to schedule a quit date within 1 week of enrollment. Exclusion criteria consisted of (1) a history of a condition that precluded nicotine patch use; (2) current use of nicotine replacement therapy (NRT) or other smoking cessation medications; (3) current enrollment in another smoking cessation program; and (4) pregnancy or breastfeeding.
Procedures
Study staff screened and recruited potential participants at neighborhood sites located throughout the Houston metropolitan area. After providing informed consent, participants completed a baseline audio computer-assisted self-interview. Variables assessed included sociodemographic characteristics and depressive symptoms (as measured by the Center for Epidemiological Studies–Depression Scale).16 Smoking-associated variables included age of initiation, number of quit attempts, use of other tobacco products, and nicotine dependence (as measured by the Fagerström Test of Nicotine Dependence).17 Alcohol use was assessed with the Alcohol Use Disorders Identification Test.18 Biochemical verification of smoking status at the 6-month follow-up was not initiated until the second year of accrual. Therefore, all participants enrolled after accrual year 1 (377 [60.4%] of the overall sample) who self-reported abstinence at the time of the 6-month assessment were asked to provide, by mail, a saliva sample for cotinine testing to confirm their stated smoking status. All assessments and intervention content were available in English and Spanish.
Interventions
Nicotine Replacement Therapy
The NRT group components were based on recommendations included in the US Public Health Service’s Treating Tobacco Use and Dependence clinical practice guideline.19 Specifically, participants randomized to the NRT group received brief advice to quit smoking (delivered by trained research staff), self-help written materials, a referral (ie, a card with a telephone number to the Texas Quitline), and a 10-week supply of NRT in the form of transdermal patches.
NRT Plus Text
Participants in the NRT plus text group received all NRT group components plus tailored text messaging. Message delivery began several days before a scheduled quit date and continued for a 12-week period. Frequency of messages was highest (ie, 5 per day) near the time of the quit date, but gradually reduced to 1 per day. Message content was informed by cognitive-behavioral and motivational enhancement principles and was designed to increase health knowledge, quit motivation, use of coping skills, support, and self-efficacy.20,21 Messages were tailored based on participants’ first name and current smoking status (proactively assessed weekly by mobile phone), and on disease history, future disease concerns, and preferred coping skills (each assessed at the baseline audio computer-assisted self-interview).
NRT Plus Text Plus Call
Participants in the NRT plus text plus call group received all NRT plus text group components, plus a proactive telephone-counseling regimen consisting of 11 counseling sessions during a 12-week period. Consistent with the US Public Health Service’s guidelines and numerous state quitlines, session topics included coping with withdrawal, maintaining a commitment to continued abstinence, and relapse prevention.19 Each call lasted approximately 10 to 12 minutes. All counselors completed training in tobacco cessation counseling and motivational interviewing.19,22 Details about the study interventions have been previously published.23
Outcomes
The primary outcome was smoking abstinence at 6 months, with follow-up completed by June 12, 2015. Abstinence was defined as (1) biochemically verified smoking abstinence, defined as a negative finding of a saliva cotinine (<20 ng/mL [to convert to nanomoles per liter, multiply by 0.176]) sample24 and (2) self-reported 30-day abstinence (ie, not a single puff of a cigarette in the past 30 days).25
Statistical Considerations
Randomization
Neighborhood sites were stratified based on type (ie, church, community center, or public housing complex) and racial/ethnic composition, then randomized to a treatment group using a random number list generated by a staff statistician (S.K.M.). Research staff who recruited, consented, and administered the assessments were blinded to the treatment group assignment.
Effect size was estimated based on previously published quit rates with text messaging interventions and on the intensity of the intervention (6% in the NRT group; 15% in the NRT plus text group; and 25% in the NRT plus text plus call group).8,26,27 To detect a significant difference in quit rates between the NRT plus text group vs the NRT group and the NRT plus text plus call group vs the NRT group with 80% power, we used a Holm-Bonferroni method for significance (α of 5.0% and 2.5%, respectively).28 After adjusting for the clustering effect of individuals being group randomized from the same study site (an intracluster correlation coefficient of 0.001) and accounting for a retention rate of 80% at the 6-month follow-up, 600 participants (200 per group) were needed.
Statistical Analysis
Data were analyzed from August 17, 2017, through May 10, 2018, based on intention to treat (ITT). Descriptive statistics were generated, as appropriate, to characterize the sample overall and by treatment group. We used χ2 tests or 1-way analysis of variance tests to identify differences in baseline characteristics between treatment groups. To estimate the effect of treatment group on the outcomes of interest while accounting for the group-randomized nature of the study, generalized linear mixed-model analyses were performed in which treatment group was estimated as a fixed effect while the study site type was modeled as a random effect nested within treatment condition using a variance components covariance structure. Log-binomial mixed models were used to estimate the relative risk (RR) of smoking abstinence (ie, how much more likely a participant is to be abstinent vs smoking) in the NRT plus text group vs the NRT group and in the NRT plus text plus call group vs the NRT group.
Unadjusted and adjusted models for biochemically verified and self-reported abstinence were estimated. Unadjusted models included only treatment group as a fixed effect and study site type as a random effect. We explored various adjusted models to identify which variables influenced the interpretation of the associations of interest (treatment group with the outcomes of 6-month self-report and biochemically verified abstinence). The covariates explored included those collected at baseline that differed among the randomized groups, as well as those known to affect the associations of interest. Final models were identified by following general guidelines for retaining covariates (>20% change in association of interest, significant association with outcome).29 Covariates are listed in Table 1.
Table 1. Sociodemographic, Behavioral, and Psychosocial Characteristics of the Sample at Study Enrollment.
| Characteristic | Treatment Groupa | |||
|---|---|---|---|---|
| All (n = 624) | NRT (n = 223) | NRT Plus Text (n = 213) | NRT Plus Text Plus Call (n = 188) | |
| Age, mean (SD), y | 45.8 (12.8) | 45.6 (12.4) | 45.7 (13.1) | 46.1 (13.1) |
| Sex, No. (%) | ||||
| Male | 308 (49.4) | 112 (50.2) | 107 (50.2) | 89 (47.3) |
| Female | 316 (50.6) | 111 (49.8) | 106 (49.8) | 99 (52.7) |
| Married/living with significant other, No. (%) | 167 (26.8) | 57 (25.6) | 65 (30.5) | 45 (23.9) |
| Race/ethnicity, No. (%) | ||||
| Latino or Hispanic | 55 (8.8) | 18 (8.1) | 19 (8.9) | 18 (9.6) |
| Non-Hispanic black | 486 (77.9) | 185 (83.0) | 162 (76.1) | 139 (73.9) |
| Non-Hispanic white | 64 (10.2) | 15 (6.7) | 24 (11.3) | 25 (13.3) |
| Other | 19 (3.0) | 5 (2.2) | 8 (3.8) | 6 (3.2) |
| Spanish language preference, No. (%) | 15 (2.4) | 4 (1.8) | 8 (3.8) | 3 (1.6) |
| Employment status, No. (%) | ||||
| Regular full-time | 137 (22.0) | 45 (20.2) | 48 (22.5) | 44 (23.4) |
| Regular part-time | 121 (19.4) | 49 (22.0) | 38 (17.8) | 34 (18.1) |
| Not working because of health problems | 101 (16.2) | 40 (17.9) | 34 (16.0) | 27 (14.4) |
| Not working (reasons unrelated to health) | 134 (21.5) | 41 (18.4) | 50 (23.5) | 43 (22.9) |
| Out of work | 131 (21.0) | 48 (21.5) | 43 (20.2) | 40 (21.3) |
| Monthly household income, $, No. (%) | ||||
| <10 000 | 345 (55.3) | 126 (56.5) | 111 (52.1) | 108 (57.4) |
| 10 000 to 19 999 | 112 (17.9) | 35 (15.7) | 38 (17.8) | 39 (20.7) |
| 20 000 to$29 999 | 64 (10.2) | 22 (9.9) | 25 (11.7) | 17 (9.0) |
| 30 000 to 39 999 | 44 (7.1) | 14 (6.3) | 21 (9.9) | 9 (4.8) |
| ≥40 000 | 58 (9.3) | 26 (11.7) | 17 (8.0) | 15 (8.0) |
| Did not answer | 1 (0.2) | 0 | 1 (0.5) | 0 |
| Educational level, No. (%) | ||||
| Less than high school | 178 (28.5) | 74 (33.2) | 65 (30.5) | 39 (20.7) |
| High school or GED | 259 (41.5) | 88 (39.5) | 91 (42.7) | 80 (42.6) |
| Some college or technical degree or more | 187 (30.0) | 61 (27.4) | 57 (26.8) | 69 (36.7) |
| Age smoking was initiated, mean (SD), y | 18.5 (7.3) | 18.4 (7.2) | 18.9 (7.9) | 18.3 (6.8) |
| Time smoked, mean (SD), y | 21.14 (12.81) | 21.06 (12.77) | 20.37 (12.21) | 22.09 (13.50) |
| No. of cigarettes smoked per day, No. (%) | ||||
| 1-10 | 188 (30.1) | 56 (25.1) | 77 (36.2) | 55 (29.2) |
| 11-20 | 285 (45.7) | 104 (46.6) | 96 (45.1) | 85 (45.2) |
| ≥21 | 151 (24.2) | 63 (28.3) | 40 (18.8) | 48 (25.5) |
| Nicotine dependence score, mean (SD)b | 5.59 (2.38) | 5.67 (2.31) | 5.45 (2.25) | 5.65 (2.61) |
| No. of quit attempts in past 12 mo, mean (SD) |
2.83 (5.43) | 3.04 (6.62) | 2.83 (5.20) | 2.59 (3.94) |
| Risky drinking, No. (%)c,d | 106 (17.0) | 43 (19.3) | 42 (19.7) | 21 (11.2) |
| Used illicit drugs in last 30 d, No. (%) | 112 (17.9) | 45 (20.2) | 43 (20.2) | 24 (12.8) |
| At risk of depression, No. (%)e | 292 (46.8) | 109 (48.9) | 102 (47.9) | 81 (43.1) |
Abbreviations: GED, General Education Development; NRT, nicotine replacement therapy.
Groups are described in the Interventions subsection of the Methods section. Percentages have been rounded and may not total 100. Unless otherwise indicated, no significant between-group differences were observed.
Calculated using the Fagerström Test of Nicotine Dependence (range, 0-10, with higher scores indicating greater nicotine dependence).
Defined as an Alcohol Use Disorders Identification Test score of at least 8 (range, 0-40, with higher scores indicating greater likelihood of hazardous and harmful drinking).
P < .04 for differences among groups.
Defined as a Center for Epidemiological Studies–Depression Scale score of at least 16 (range, 0-60, with higher scores indicating greater depressive symptoms).
Several methods were used to handle missing data, including (1) ITT, such that missing abstinence outcomes were considered nonabstinent; and (2) sequential multiple imputation.30,31,32 All analyses were conducted using SAS software (version 9.4; SAS Institute). Two-sided P values were calculated using χ2 test and 1-way analysis of variance for descriptive statistics and t test for the log-binomial mixed model fixed-effects parameter estimates, with P < .05 indicating significance.
Results
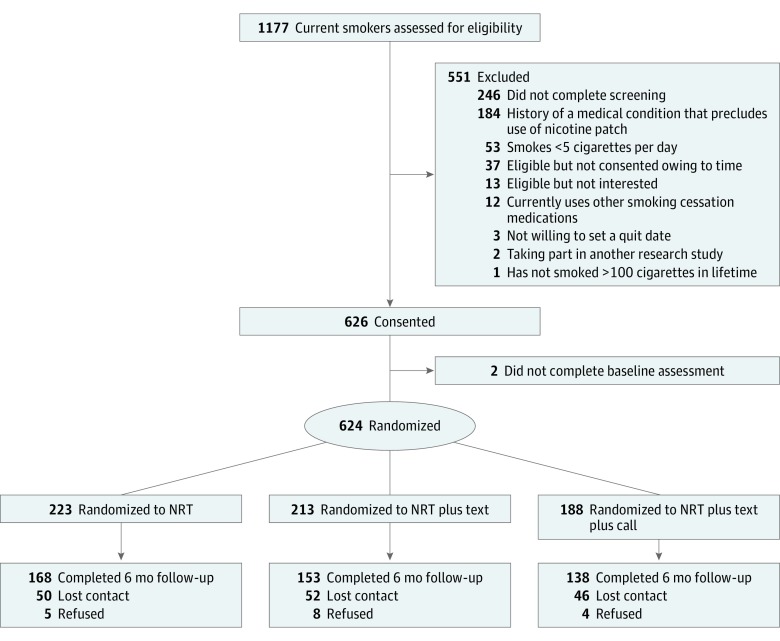
A total of 1177 individuals underwent screening for eligibility at 46 sites, including 9 churches, 14 public housing complexes, and 23 community centers. A total of 624 participants were randomized, including 223 in the NRT group, 213 in the NRT plus text group, and 188 in the NRT plus text plus call group. The overall 6-month follow-up rate was 73.6%, and no significant group differences (P > .57 for all) were observed (Figure).
Figure. Participant Flow Diagram.
Results of screening, study enrollment, and retention through the 6-month follow-up are shown. NRT indicates nicotine replacement therapy. Groups are described in the Interventions subsection of the Methods section.
Mean (SD) age at the time of study enrollment was 45.8 (12.8) years. Three hundred sixteen participants (50.6%) were female and 308 (49.4%) were male, and most (486 [77.9%]) were African American. Markers of socioeconomic status indicated 366 participants (58.6%) were not employed; 345 (55.3%) had an annual household income of less than $10 000; and 437 (70.0%) had an educational level no higher than high school or equivalency. Mean (SD) age at smoking initiation was 18.5 (7.3) years, and 151 participants (24.2%) reported smoking more than 20 cigarettes per day. Risky drinking was reported by 106 participants (17.0%), whereas 112 (17.9%) reported using illicit drugs in the past 30 days. With the exception of the proportion of risky drinking (43 [19.3%] in the NRT group vs 42 [19.7%] in the NRT plus text group vs 21 [11.2%] in the NRT plus text plus call group), no statistically significant group differences in demographic, behavioral, or psychosocial variables were observed among groups (Table 1). At the 6-month follow-up, no significant treatment group differences in NRT use (77 [34.5%] in the NRT group vs 72 [33.8%] in the NRT plus text group vs 67 [35.6%] in the NRT plus text plus call group) were observed.
Biochemically Verified Cessation
Among the 377 participants eligible for biochemical verification, 127 self-reported 30-day abstinence and were asked to provide saliva samples. Of these, 98 samples were returned (participants who did not return samples were coded as smoking). Results from the ITT analyses indicated biochemically verified abstinence in 13 participants (12.0%) in the NRT group, 19 (12.0%) in the NRT plus text group, and 28 (25.5%) in the NRT plus text plus call group. Results from the unadjusted ITT models indicated that participants in the NRT plus text group were no more likely to be abstinent than participants in the NRT group (RR, 0.99; 95% CI, 0.43-2.27). However, participants in the NRT plus text plus call group were twice as likely to be abstinent (vs the NRT group) (RR, 2.11; 95% CI, 1.00-4.48). Results from the adjusted ITT models and the sequential multiple imputation method revealed similar results (Table 2).
Table 2. Intention-to-Treat Analyses for Biochemically Verified Abstinence and Self-reported 30-Day Abstinencea.
| Treatment Groupb | Biochemically Verified Abstinence (n = 377)c | Self-reported 30-d Abstinence (n = 624)d | ||
|---|---|---|---|---|
| Proportion Abstinent, No. (%) | Unadjusted RR (95% CI) | Proportion Abstinent, No. (%) | Unadjusted RR (95% CI) | |
| NRT | 13 (12.0) | 1 [Reference] | 64 (28.7) | 1 [Reference] |
| NRT plus text | 19 (12.0) | 0.99 (0.43-2.27) | 70 (32.9) | 1.15 (0.81-1.63) |
| NRT plus text plus call | 28 (25.5) | 2.11 (1.00-4.48) | 80 (42.5) | 1.48 (1.06-2.06) |
Abbreviations: NRT, nicotine replacement therapy; RR, relative risk.
Models use only treatment group as a fixed effect and study site type as a random effect. Missing abstinence data are coded as smoking.
Groups are described in the Interventions subsection of the Methods section.
Defined as a negative finding of a saliva cotinine (<20 ng/mL [to convert to nanomoles per liter, multiply by 0.176]) sample.
Defined as not a single puff of a cigarette in the past 30 days.
Self-reported Smoking Abstinence
At the 6-month follow-up, 30-day abstinence was self-reported by 64 participants (28.7%) in the NRT group, 70 (32.9%) in the NRT plus text group, and 80 (42.5%) in the NRT plus text plus call group. Results of the ITT unadjusted models indicated that participants in the NRT plus text group were no more likely to be abstinent compared with participants in the NRT group (RR, 1.15; 95% CI, 0.81-1.63). However, participants in the NRT plus text plus call group were significantly more likely to be abstinent compared with the NRT group (RR, 1.48; 95% CI, 1.06-2.06). Findings from the fully adjusted ITT models and results using the sequential multiple imputation method yielded consistent treatment group effects (Table 2).
Discussion
In this study, we observed higher cessation rates among participants assigned to tailored interactive text messages and proactive counseling via mobile phone compared with a lower-intensity control condition. Specifically, participants in a text messaging and phone counseling group were approximately 1.5 to 2.0 times more likely to be abstinent 6 months after study enrollment compared with participants in a control intervention, which consisted of nicotine patches and a passive referral to quitline services. Our findings also indicated that a text messaging intervention delivered on mobile phones provided to underserved smokers (without proactive counseling) did not significantly improve cessation rates compared with the control condition.
Current evidence supports the efficacy of mobile phone–delivered text messaging for smoking cessation treatment.9 As a result, text messaging interventions for smoking cessation are supported at the state and federal level (eg, the National Cancer Institute’s SmokefreeTXT), and additional efforts have been made to adapt text messaging interventions to vulnerable and/or high-risk populations, such as pregnant women, teens, and veterans.33 Similarly, an abundance of evidence supports the efficacy and cost-effectiveness of proactive counseling.34,35 In fact, quitline services are available throughout the United States.36,37 Findings from the present trial support the feasibility of text messaging as a component of an intensive smoking cessation program. Implementation of the text messaging component was feasible, consisting of programming a web-based message delivery system and minimal maintenance.23 This ease of implementation is a well-recognized benefit of text-based interventions.38 Although the design of the present trial only allows for a comparison of text messaging alone vs text messaging plus proactive counseling, other trials have compared proactive counseling alone with proactive counseling plus text messaging. Specifically, Boal and colleagues26 concluded that combining text messaging with a proactive counseling regimen did not result in higher cessation rates compared with proactive counseling alone. Findings from a trial conducted with smokers in Denmark39 also indicated that participants who received proactive counseling were more likely to successfully quit smoking compared with participants who received self-help materials. However, cessation rates in a group that received text messaging did not differ from the self-help group.39
To our knowledge, the present study is among the first to proactively recruit socioeconomically disadvantaged smokers directly from community sites for mobile phone–delivered interventions. As a result, levels of education and income in our sample were low, whereas rates of unemployment and depressive symptoms were high. These same characteristics, which are overrepresented among smokers, are well-recognized factors associated with poor cessation outcomes.40,41 Our experience suggests that it is feasible to team with community sites to increase the reach of cessation services. Our results indicated high 6-month self-reported cessation rates in all 3 treatment groups. Thus, the findings suggest that offering even low-intensity cessation treatment, such as nicotine patches and a referral to quitline services, may be an effective approach to treating underserved smokers if delivered in neighborhood sites. Although point estimates of the treatment effects were similar across all cessation definitions used, a comparison of self-reported (vs biochemically verified) abstinence suggested high rates of discordance. Although biochemically verified abstinence was lower than self-reported abstinence, the results showed similar patterns, with models indicating that participants who received text messaging plus proactive counseling (vs controls) were about twice as likely to be abstinent. Others42,43,44 have reported similar discordance in self-report vs biochemically abstinence, particularly among medical populations. The difference in these rates likely has major implications on the potential public health effect of treatment programs.45 Although discordance did not differ among our groups, efforts to explore factors influencing misreporting (eg, recruitment site, stigma, and social desirability bias) are needed in this era of decreasing smoking prevalence.
Limitations
Results from this trial should be interpreted in consideration of several limitations. First, our design did not include a group receiving proactive mobile phone counseling only. Therefore, we cannot completely disentangle the effects of the texting and counseling components. A factorial trial would be necessary to evaluate the unique contributions of each of the 2 treatment elements. In addition, the original plans for this trial were to use 12-month abstinence as the primary outcome. However, owing to problems achieving adequate retention through month 12, we decided to focus on 6-month outcomes and added saliva cotinine assessment at that time point. This change was made in year 2 of participant accrual; thus, we were able to assess biochemical abstinence on only 60% of our sample. No treatment group differences in retention were observed at 6 months. However, the overall rate was lower than anticipated (73.6% vs 80%). This may have resulted in slightly lower ITT quit rates than expected. Another limitation involves our use of research staff to deliver the counseling component. This decision was made to allow for monitoring of fidelity; however, counseling delivered by quitline staff (vs research staff) may have yielded different cessation outcomes. Several design aspects, including the provision of mobile phones, baseline assessment length, and the recruitment region, may limit generalizability. For example, participants who owned mobile phones may have been more (or less) likely to have benefitted from the study interventions. The analyses only considered the effects of group assignment. Future efforts to explore the effects of treatment engagement are needed. Finally, other than at the time of biochemical verification, we did not consider e-cigarette use. Future efforts to characterize the effects that current and past e-cigarette use may have on mobile phone–delivered cessation interventions for socioeconomically disadvantaged populations are warranted.
Conclusions
Although text messaging interventions for smoking cessation are proliferating in the United States and in many other high-income countries,9,46 findings from this trial indicate that text messaging alone may not be adequate to increase cessation rates in economically disadvantaged smokers. However, our results suggest that an intervention consisting of text messaging plus proactive counseling may be an appropriate option. Future efforts are needed to evaluate the unique contribution of text messaging and the role of treatment mediators and moderators. Such efforts may identify subgroups of smokers for whom text messaging is most appropriate. Similarly, future efforts are needed to determine whether adapting text messaging interventions to the needs of socioeconomically disadvantaged smokers may improve efficacy.
Trial Protocol.
Data Sharing Supplement.
References
- 1.USDHHS The Health Consequences of Smoking: 50 Years of Progress: A Report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. [Google Scholar]
- 2.Jamal A, King BA, Neff LJ, Whitmill J, Babb SD, Graffunder CM. Current cigarette smoking among adults: United States, 2005-2015. MMWR Morb Mortal Wkly Rep. 2016;65(44):1205-1211. doi: 10.15585/mmwr.mm6544a2 [DOI] [PubMed] [Google Scholar]
- 3.Fu SS, van Ryn M, Nelson D, et al. Proactive tobacco treatment offering free nicotine replacement therapy and telephone counselling for socioeconomically disadvantaged smokers: a randomised clinical trial. Thorax. 2016;71(5):446-453. doi: 10.1136/thoraxjnl-2015-207904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marshall LL, Zhang L, Malarcher AM, Mann NH, King BA, Alexander RL. Race/ethnic variations in quitline use among US adult tobacco users in 45 states, 2011-2013. Nicotine Tob Res. 2017;19(12):1473-1481. [DOI] [PubMed] [Google Scholar]
- 5.Keller PA, Feltracco A, Bailey LA, et al. Changes in tobacco quitlines in the United States, 2005-2006. Prev Chronic Dis. 2010;7(2):A36. [PMC free article] [PubMed] [Google Scholar]
- 6.Hoeppner BB, Hoeppner SS, Abroms LC. How do text-messaging smoking cessation interventions confer benefit? a multiple mediation analysis of Text2Quit. Addiction. 2017;112(4):673-682. doi: 10.1111/add.13685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pew Research Center Pew Internet Project Survey: Mobile Technology Fact Sheet. Washington, DC: Pew Research Center; 2017. [Google Scholar]
- 8.Ybarra ML, Jiang Y, Free C, Abroms LC, Whittaker R. Participant-level meta-analysis of mobile phone–based interventions for smoking cessation across different countries. Prev Med. 2016;89:90-97. doi: 10.1016/j.ypmed.2016.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whittaker R, McRobbie H, Bullen C, Rodgers A, Gu Y. Mobile phone–based interventions for smoking cessation. Cochrane Database Syst Rev. 2016;4:CD006611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Free C, Knight R, Robertson S, et al. Smoking cessation support delivered via mobile phone text messaging (txt2stop): a single-blind, randomised trial. Lancet. 2011;378(9785):49-55. doi: 10.1016/S0140-6736(11)60701-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abroms LC, Boal AL, Simmens SJ, Mendel JA, Windsor RA. A randomized trial of Text2Quit: a text messaging program for smoking cessation. Am J Prev Med. 2014;47(3):242-250. doi: 10.1016/j.amepre.2014.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guerriero C, Cairns J, Roberts I, Rodgers A, Whittaker R, Free C. The cost-effectiveness of smoking cessation support delivered by mobile phone text messaging: Txt2stop. Eur J Health Econ. 2013;14(5):789-797. doi: 10.1007/s10198-012-0424-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.West R, Raw M, McNeill A, et al. Health-care interventions to promote and assist tobacco cessation: a review of efficacy, effectiveness and affordability for use in national guideline development. Addiction. 2015;110(9):1388-1403. doi: 10.1111/add.12998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raw M, Mackay J, Reddy S. Time to take tobacco dependence treatment seriously. Lancet. 2016;387(10017):412-413. doi: 10.1016/S0140-6736(15)00950-2 [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization Mobile health (mHealth) for tobacco control. 2011; http://www.who.int/tobacco/mhealth/en/. Accessed February 1, 2017.
- 16.Radloff L. The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385-401. doi: 10.1177/014662167700100306 [DOI] [Google Scholar]
- 17.Heatherton TF, Kozlowski LT, Frecker RC, Fagerström K-O. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86(9):1119-1127. doi: 10.1111/j.1360-0443.1991.tb01879.x [DOI] [PubMed] [Google Scholar]
- 18.Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption. Addiction. 1993;88(6):791-804. doi: 10.1111/j.1360-0443.1993.tb02093.x [DOI] [PubMed] [Google Scholar]
- 19.Fiore MC, Jaen CR, Baker TB, et al. Treating Tobacco Use and Dependence: 2008 Update. Rockville, MD: US Department of Health and Human Services, Public Health Service; 2008. [Google Scholar]
- 20.Miller WR, Rollnick S. Motivational Interviewing: Preparing People to Change Addictive Behavior. New York, NY: Guilford Press; 1991. [Google Scholar]
- 21.Bandura A. Social Foundations of Thought and Action. Englewood Cliffs, NJ: Prentice Hall; 1986. [Google Scholar]
- 22.Stead LF, Hartmann-Boyce J, Perera R, Lancaster T. Telephone counselling for smoking cessation. Cochrane Database Syst Rev. 2013;(8):CD002850. [DOI] [PubMed] [Google Scholar]
- 23.Vidrine DJ, Fletcher FE, Danysh HE, et al. A randomized controlled trial to assess the efficacy of an interactive mobile messaging intervention for underserved smokers: Project ACTION. BMC Public Health. 2012;12:696. doi: 10.1186/1471-2458-12-696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jarvis MJ, Fidler J, Mindell J, Feyerabend C, West R. Assessing smoking status in children, adolescents and adults: cotinine cut-points revisited. Addiction. 2008;103(9):1553-1561. doi: 10.1111/j.1360-0443.2008.02297.x [DOI] [PubMed] [Google Scholar]
- 25.Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: issues and recommendations. Nicotine Tob Res. 2003;5(1):13-25. doi: 10.1080/1462220031000070552 [DOI] [PubMed] [Google Scholar]
- 26.Boal AL, Abroms LC, Simmens S, Graham AL, Carpenter KM. Combined quitline counseling and text messaging for smoking cessation: a quasi-experimental evaluation. Nicotine Tob Res. 2016;18(5):1046-1053. doi: 10.1093/ntr/ntv249 [DOI] [PubMed] [Google Scholar]
- 27.Cobos-Campos R, Apiñaniz Fernández de Larrinoa A, Sáez de Lafuente Moriñigo A, Parraza Diez N, Aizpuru Barandiaran F. Effectiveness of text messaging as an adjuvant to health advice in smoking cessation programs in primary care: a randomized clinical trial. Nicotine Tob Res. 2017;19(8):901-907. [DOI] [PubMed] [Google Scholar]
- 28.Holm SA. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6(2):65-70. [Google Scholar]
- 29.Hosmer DW, Lemeshow S, Sturdivant RX. Applied Logistic Regression. 3rd ed Hoboken, NJ: John Wiley and Sons, Inc; 2013. doi: 10.1002/9781118548387 [DOI] [Google Scholar]
- 30.Raghunathan TE, Lepkowski JM, Van Hoewyk J, Solenberger P. A multivariate technique for multiply imputing missing values using a sequence of regression models. Surv Methodol. 2001;24(1):85-95. [Google Scholar]
- 31.Zhu J, Raghunathan TE. Convergence properties of a sequential regression multiple imputation algorithm. J Am Stat Assoc. 2015;110(511):1112-1124. doi: 10.1080/01621459.2014.948117 [DOI] [Google Scholar]
- 32.Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York, NY: John Wiley & Sons Inc; 1987. doi: 10.1002/9780470316696 [DOI] [Google Scholar]
- 33.Abroms LC, Chiang S, Macherelli L, Leavitt L, Montgomery M. Assessing the National Cancer Institute’s SmokefreeMOM text-messaging program for pregnant smokers: pilot randomized trial. J Med Internet Res. 2017;19(10):e333. doi: 10.2196/jmir.8411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Graham AL, Chang Y, Fang Y, et al. Cost-effectiveness of internet and telephone treatment for smoking cessation: an economic evaluation of the iQUITT Study. Tob Control. 2013;22(6):e11. doi: 10.1136/tobaccocontrol-2012-050465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McAlister AL, Rabius V, Geiger A, Glynn TJ, Huang P, Todd R. Telephone assistance for smoking cessation: one year cost effectiveness estimations. Tob Control. 2004;13(1):85-86. doi: 10.1136/tc.2003.004515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hollis JF, McAfee TA, Fellows JL, Zbikowski SM, Stark M, Riedlinger K. The effectiveness and cost effectiveness of telephone counselling and the nicotine patch in a state tobacco quitline. Tob Control. 2007;16(suppl 1):i53-i59. doi: 10.1136/tc.2006.019794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cummins SE, Bailey L, Campbell S, Koon-Kirby C, Zhu SH. Tobacco cessation quitlines in North America: a descriptive study. Tob Control. 2007;16(suppl 1):i9-i15. doi: 10.1136/tc.2007.020370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Keoleian V, Polcin D, Galloway GP. Text messaging for addiction: a review. J Psychoactive Drugs. 2015;47(2):158-176. doi: 10.1080/02791072.2015.1009200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Skov-Ettrup LS, Dalum P, Bech M, Tolstrup JS. The effectiveness of telephone counselling and internet- and text-message-based support for smoking cessation: results from a randomized controlled trial. Addiction. 2016;111(7):1257-1266. doi: 10.1111/add.13302 [DOI] [PubMed] [Google Scholar]
- 40.Weinberger AH, Bandiera FC, Leventhal AM, et al. Socioeconomic disparities in smoking among US adults with depression, 2005-2014. Am J Prev Med. 2018;54(6):765-775. doi: 10.1016/j.amepre.2018.02.008 [DOI] [PubMed] [Google Scholar]
- 41.Babb S, Malarcher A, Schauer G, Asman K, Jamal A. Quitting smoking among adults: United States, 2000-2015. MMWR Morb Mortal Wkly Rep. 2017;65(52):1457-1464. doi: 10.15585/mmwr.mm6552a1 [DOI] [PubMed] [Google Scholar]
- 42.Morales NA, Romano MA, Michael Cummings K, et al. Accuracy of self-reported tobacco use in newly diagnosed cancer patients. Cancer Causes Control. 2013;24(6):1223-1230. doi: 10.1007/s10552-013-0202-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scheuermann TS, Richter KP, Rigotti NA, et al. ; Consortium of Hospitals Advancing Research on Tobacco (CHART) . Accuracy of self-reported smoking abstinence in clinical trials of hospital-initiated smoking interventions. Addiction. 2017;112(12):2227-2236. doi: 10.1111/add.13913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spencer K, Cowans NJ. Accuracy of self-reported smoking status in first trimester aneuploidy screening. Prenat Diagn. 2013;33(3):245-250. doi: 10.1002/pd.4053 [DOI] [PubMed] [Google Scholar]
- 45.Connor Gorber S, Schofield-Hurwitz S, Hardt J, Levasseur G, Tremblay M. The accuracy of self-reported smoking: a systematic review of the relationship between self-reported and cotinine-assessed smoking status. Nicotine Tob Res. 2009;11(1):12-24. doi: 10.1093/ntr/ntn010 [DOI] [PubMed] [Google Scholar]
- 46.Augustson E, Cole-Lewis H, Sanders A, et al. Analysing user-reported data for enhancement of SmokefreeTXT: a national text message smoking cessation intervention. Tob Control. 2017;26(6):683-689. doi: 10.1136/tobaccocontrol-2016-052945 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol.
Data Sharing Supplement.