Key Points
Question
What are the health care utilization and cost outcomes of a comprehensive dementia care program for Medicare beneficiaries?
Findings
In a case control study that used a quasiexperimental design to assess the health care utilization of 3249 patients with dementia, those patients in a dementia care program were less likely to be admitted to a long-term care facility than patients in a propensity score–matched control group. After accounting for implementation costs, the program was cost neutral.
Meaning
Comprehensive dementia care programs may help more patients stay in community-based settings and was cost neutral.
This case-control study compares health care utilization and costs in patients participating in a dementia care program with patients in a propensity score–matched cohort.
Abstract
Importance
An estimated 4 to 5 million Americans have Alzheimer disease or another dementia.
Objective
To determine the health care utilization and cost outcomes of a comprehensive dementia care program for Medicare fee-for-service beneficiaries.
Design, Setting, and Participants
In this case-control study, we used a quasiexperimental design to compare health care utilization and costs for 1083 Medicare fee-for-service beneficiaries enrolled in the University of California Los Angeles Health System Alzheimer and Dementia Care program between July 1, 2012, and December 31, 2015, with those of 2166 similar patients with dementia not participating in the program. Patients in the comparison cohort were selected using the zip code of residence as a sampling frame and matched with propensity scores, which included demographic characteristics, comorbidities, and prior-year health care utilization. We used Medicare claims data to compare utilization and cost outcomes for the 2 groups.
Interventions
Patients in the dementia care program were comanaged by nurse practitioners and physicians, and the program consisted of structured needs assessments of patients and their caregivers, creation and implementation of individualized dementia care plans with input from primary care physicians, monitoring and revising care plans, referral to community organizations for dementia-related services and support, and access to a clinician for assistance and advice 24 hours per day, 7 days per week.
Main Outcomes and Measures
Admissions to long-term care facilities; average difference-in-differences per quarter over the 3-year intervention period for all-cause hospitalization, emergency department visits, 30-day hospital readmissions, and total Medicare Parts A and B costs of care. Program costs were included in the cost estimates.
Results
Program participants (n = 382 men, n = 701 women; mean [SD] age, 82.10 [7.90] years; age range 54-101 years) were less likely to be admitted to a long-term care facility (hazard ratio, 0.60; 95% CI, 0.59-0.61) than those not participating in the dementia care program (n = 759 men, n = 1407 women; mean [SD] age, 82.42 [8.50] years; age range, 34-103 years). There were no differences between groups in terms of hospitalizations, emergency department visits, or 30-day readmissions. The total cost of care to Medicare, excluding program costs, was $601 less per patient per quarter (95% CI, −$1198 to −$5). After accounting for the estimated program costs of $317 per patient per quarter, the program was cost neutral for Medicare, with an estimated net cost of −$284 (95% CI, −$881 to $312) per program participant per quarter.
Conclusions and Relevance
Comprehensive dementia care may reduce the number of admissions to long-term care facilities, and depending on program costs, may be cost neutral or cost saving. Wider implementation of such programs may help people with dementia stay in their communities.
Introduction
In the United States, Alzheimer disease and related dementias affect an estimated 4 to 5 million persons.1 Dementia is a chronic disease that requires comprehensive medical and social services to provide high-quality care and prevent complications and hospitalizations.2 This care is expensive, with annual estimated costs of $157 billion to $215 billion in 2010. The total societal costs of dementia are expected to increase nearly 80% by 2040.3
Treating patients with dementia requires coordinating social services and medical care, instructing caregivers, and counseling families. The quality of care for dementia is poor compared with that for other diseases that affect older persons.4,5,6 Community resources (eg, the Alzheimer Association) can help improve the quality of care, especially by providing patient education and support for caregivers.7 However, community-based resources are underutilized7 and are poorly integrated within the health care system.
Several dementia care programs have been developed to more comprehensively meet the needs of patients and their families. These programs have relied on care coordinators and software to increase the quality of dementia care, improve patient health-related quality of life, and reduce caregiver stress.8,9,10,11 Most of these programs have not evaluated effects on health care utilization; however, the program at Indiana University’s Healthy Aging Brain Center reports potential cost savings owing to the lower numbers of emergency department visits, inpatient hospitalizations, and 30-day readmissions in the program participation group than in the standard care group.12
The Alzheimer and Dementia Care program at the University of California, Los Angeles (UCLA),13 a comprehensive dementia care program with comanagement by nurse practitioners, was launched in November 2011. In July 2012, the program received additional support from the Centers for Medicare & Medicaid Services (CMS). The program provides high-quality dementia care, with pass rates exceeding 90% on widely accepted dementia quality indicators.14 To determine the health care utilization and cost outcomes of the program, CMS contracted with NORC at the University of Chicago to conduct an independent external evaluation.
Methods
The UCLA institutional review board did not consider this study to be human subject research, and patient written informed consent was not required. The UCLA and NORC institutional review boards approved secondary analyses of health care utilization data.
Description of the Clinical Program
The program is based at an academic health care system and partners with community-based organizations to provide comprehensive, coordinated, patient-centered dementia care. Program goals are to maximize patient function, independence, and dignity; minimize caregiver strain; and reduce unnecessary costs through improved care. The program uses a comanagement model between nurse practitioners, who are dementia care managers, and physicians.13 The Box lists the 5 key program components. Each dementia care manager is responsible for up to 250 patients, with 2 assistants supporting 5 dementia care managers.
Box. Components and Key Elements of the UCLA Alzheimer and Dementia Care Program.
Patient Referrala
-
Program entry requirements:
University of California, Los Angeles physician who partners with program
Community-dwelling status at entry
Established diagnosis of dementia
Needs Assessments of Patients and Their Caregivers
90-minute in-person session during which additional information is obtained through a semistructured interview and examination
Creation and Implementation of Individualized Dementia Care Plans
-
Care management by a dementia care manager supervised by a physician dementia specialist, which may include:
In-person sessions
Telephone follow-up to monitor implementation of dementia care plans
Facilitation of appointments with consultants when the treatment plan needs to be reassessed
Teaching dementia management skills to caregivers through individual counseling
Referral to community-based organizations for services such as support groups, adult day care, care/case management, counseling, and transportation assistance as well as caregiver training through evidence-based programs
Vouchers to pay community-based organizations for time-limited services for patients and caregivers who have financial barriers
Monitoring and Revising Care Plans
Calls at a minimum frequency of every 3 mo
In-person visits at a minimum of yearly
More frequent contacts per initial care plans and revisions if needed
Access to Assistance and Advice 24 Hours a Day, 365 Days a Year
Daytime calls go to a dedicated phone number with triage to the dementia care manager
Nights and weekend calls managed by University of California, Los Angeles geriatricians
Data Sources
Primary data sources were Medicare Part A and B claims files (ie, inpatient, outpatient, home-health, skilled-nursing facility, and hospice claims) and the Medicare Master Beneficiary Summary File (demographics, mortality, Medicare enrollment status, and chronic conditions). Program records and participant enrollment dates were linked to Medicare claims on the Chronic Conditions Data Warehouse Virtual Research Data Center. Secondary data sources used to calculate the total program costs included financial reports detailing operating costs within awardee progress reports to CMS.
Participants
The intervention cohort included 1083 (n = 382 men, n = 701 women; mean [SD] age, 82.10 [7.90] years; age range, 54-101 years) Medicare fee-for-service beneficiaries enrolled from July 1, 2012, through December 31, 2015. Patients in the comparison cohort (n = 759 men, n = 1407 women; mean [SD] age, 82.42 [8.50] years; age range, 34-103 years) were selected from a list of Medicare fee-for-service beneficiaries living in the same zip codes as the treatment population who had a claim for at least 1 of the following International Classification of Diseases, Ninth Revision (ICD-9) dementia codes: 331.0, 331.11, 331.19, 331.2, 331.7, 290.0, 290.10, 290.11, 290.12, 290.13, 290.20, 290.21, 290.3, 290.40, 290.41, 290.42, 290.43, 294.0, 294.10, 294.11, 294.20, 294.21, 294.8, and 797. For each patient in the comparison cohort, the pseudoenrollment date was established as the date of the patient’s first visit related to dementia care in 2013 (ie, claims for hospitalization, hospital outpatient visit, or ambulatory facility visit that included an ICD-9 dementia code). Participants had to be enrolled in fee-for-service Medicare for at least 1 quarter after the date of enrollment (intervention group) or pseudoenrollment (comparison cohort). To match the inclusion criteria for the intervention group, we excluded patients residing in a nursing home from the comparison group.15
We selected 2 patients from the comparison group for each program participant using propensity scores with nearest-neighbor matching without replacement. The propensity score used logistic regression to model the odds of program enrollment, based on the patient’s covariates. The propensity score model included Alzheimer-type dementia, age, sex, race/ethnicity, prior cancer diagnosis, chronic obstructive pulmonary disease, heart disease, arthritis, hyperlipidemia, chronic kidney disease, hip fracture, depression, prior-year health care utilization (emergency department visits, hospitalizations), dual Medicare and Medicaid eligibility, prior-year Hierarchical Condition Category (HCC) score,16 prior costs (costs during the prior quarter, costs for the prior year, and a ratio of costs in the prior quarter compared with total costs over the last year), and duration of dementia (years since the first claim with a dementia ICD-9 diagnosis code looking back to 1999). Overlap in distribution of estimated propensity scores across intervention and comparison groups and covariate balance were assessed before and after applying the scores.17
Outcome Measures
Entry to a long-term care facility was defined at the patient level as at least 3 consecutive months of Medicare Part B claims, with a skilled-nursing facility listed as the place of service.18 All-cause hospitalizations, ambulatory care–sensitive hospitalizations,19 30-day readmissions, and emergency department visits were calculated for each patient per quarter.
The total cost of care (Medicare Part A and B) per patient per quarter was expressed in 2013 dollars.20 Program costs for 1250 patients included salary and benefits for 5 full-time equivalent (FTE) dementia care managers, 0.33 FTE medical director, 0.05 FTE support group leader, 0.25 FTE program manager, 2 FTE dementia care manager assistants, and 2 FTE clinical office staff; payments for services to community-based organizations, and other nonpersonnel costs, including maintenance of care management software (eAppendix 1 in the Supplement). To obtain program quarterly costs per patient, total annual program costs were adjusted to 2013 dollars, divided by 1250 enrollees, and then divided by 4. To determine the net relative costs for Medicare, program quarterly costs per patient were subtracted from the quarterly Medicare total cost of care difference-in-differences estimate.
Time Periods
Preintervention and postintervention time periods were defined based on the enrollment or pseudoenrollment date for each patient. Preintervention time included the 8 quarters (2 years) prior to the enrollment or pseudoenrollment date. The postintervention period was limited to 12 quarters (3 years); the sample size became too small to conduct analyses beyond 12 quarters.
Analysis
For analysis of long-term care placement, we used the stratified extension to the Fine-Gray proportional hazard models adjusted for age, sex, race (black), ethnicity (Hispanic), HCC score, and Alzheimer-type dementia to estimate the relative subhazard of long-term care placement for patients in the intervention and comparison groups.21,22,23 This competing risk regression model accounted for 198 patients in the intervention group (18.2%) and 614 patients in the comparison group (28.3%) who died without entering a long-term care facility.
For the other utilization measures and cost of care, we used difference-in-difference analyses to estimate the average treatment effect by comparing the average outcomes of patients in the program and comparison groups across preintervention and postintervention periods.24
We used multilevel mixed-effects generalized linear models with the appropriate functional form (ie, binomial for utilization outcomes and linear for costs) to estimate the program’s influence. Random intercepts were allowed for matched sets of program and comparison patients. Fixed effects in the model included a treatment group indicator; a vector of dummy variables for time, including up to 8 quarters before and 12 quarters after enrollment; difference-in-difference estimator in each quarter after implementation (ie, treatment and time vector interaction term); and a vector of participant characteristics including age, sex, race (black), ethnicity (Hispanic), HCC score, Alzheimer-type dementia, and days of fee-for-service coverage in a quarter.
Similar to the total estimates obtained in survey sampling, we obtained overall estimates for the entire implementation period by weighting each quarterly estimate by the number of participants enrolled in that quarter. We then estimated the program’s average quarterly influence using weighted quarter-specific estimates. All analyses were performed using Stata software, version 14 (StataCorp).25
Results
Of the 1426 program participants who were enrolled in the program on or before December 31, 2015, 1083 were enrolled in fee-for-service Medicare. Table 1 summarizes demographic and other baseline information for patients in the intervention and comparison groups. About two-thirds of patients in the intervention group were women (n = 701), 28.3% were non-white (n = 308), and 15.3% were dual enrolled in Medicare and Medicaid (n = 166). Additional demographic and clinical characteristics of the program cohort and their caregivers are provided in eAppendix 2 in the Supplement.
Table 1. Characteristics of Patients in the UCLA Alzheimer and Dementia Care Program and Comparison Cohort.
| Variable | Group, No. (%) | |
|---|---|---|
| Treatment | Comparison | |
| No. of persons | 1083 | 2166 |
| No. of quarters, mean (range) | 7.1 (1-12) | 9.3 (1-12) |
| Alzheimer diagnosis | 744 (68.7) | 1465 (67.6) |
| Duration of disease, y | 2.9 | 3.0 |
| Sex | ||
| Female | 701 (64.7) | 1407 (65.0) |
| Age group, y | ||
| 54-64 | 21 (1.9) | 40 (1.8) |
| 65-69 | 56 (5.2) | 125 (5.8) |
| 70-74 | 95 (8.8) | 205 (9.5) |
| 75-79 | 212 (19.6) | 347 (16.0) |
| 80-84 | 243 (22.4) | 476 (22.0) |
| ≥85 | 456 (42.1) | 973 (44.9) |
| Race/ethnicity | ||
| White | 775 (71.6) | 1600 (73.9) |
| Black | 102 (9.4) | 218 (10.1) |
| Hispanic | 98 (9.0) | 109 (5.0) |
| Asian | 86 (7.9) | 178 (8.2) |
| Other | 22 (2.0) | 60 (2.8) |
| Dual Medicare and Medicaid enrolled | 166 (15.3) | 304 (14.0) |
| Medicare coverage reason | ||
| Age ≥65 y | 1019 (94.1) | 2035 (94.0) |
| Disability | 62 (5.7) | 129 (6.0) |
| Hierarchical Condition Category, mean (SD) | ||
| HCC score | 1.8 (1.2) | 1.8 (1.4) |
| Count of HCCs | 3.1 (2.4) | 3.0 (2.7) |
| Utilization and cost in year prior to program enrollment, mean (SD) | ||
| Total Medicare cost, $ | 17 260 (27 526) | 17 193 (30 108) |
| Hospitalizations per 1000 patients | 494 (1000) | 497 (992) |
| ED visits per 1000 patients | 1082 (196) | 929 (214) |
Abbreviations: ED, emergency department; HCC, Hierarchical Condition Category; UCLA, University of California, Los Angeles.
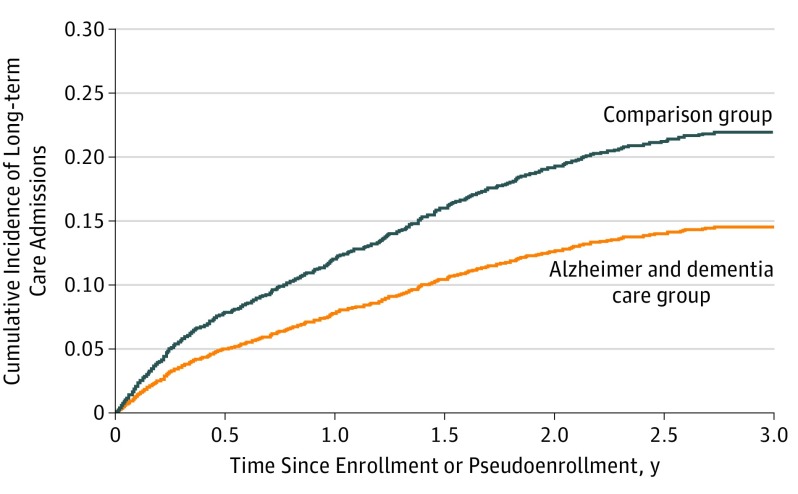
The Figure shows the cumulative incidence of admission to a long-term care nursing home for patients in the program and comparison group. Time to nursing home admission was delayed for program participants. Program participants were less likely than patients in the comparison group to be admitted to a long-term care facility (hazard ratio, 0.60; 95% CI, 0.59-0.61).
Figure. Cumulative Incidence of Long-term Care Nursing Home Admission.
Cumulative incidence curves show that patients enrolled in the University of California, Los Angeles Alzheimer and Dementia Care program had a lower incidence of admission to long-term care nursing homes compared with patients in the comparison group.
In regression models, hospital and emergency department utilization did not differ between intervention and comparison groups (Table 2). Total costs of care for Medicare were lower for program participants than for patients in the comparison group ($601 less per patient per quarter, 95% CI, −$1198 to −$5; P < .048). Total program costs per patient per quarter were estimated to be $317. After accounting for program costs, the program was cost neutral for Medicare, with an estimated net cost of −$284 (95% CI, −$881 to $312) per program participant per quarter.
Table 2. Difference-in-Differences Estimates for Cost and Utilizationa.
| Outcome Measure | DID Estimate per Quarter (95% CI) |
|---|---|
| Hospitalizations per 1000 patients | −1 (−13 to 11) |
| ED visits per 1000 patients | −1 (−15 to 12) |
| 30-d readmissions per 1000 patients hospitalized | 8 (−35 to 51) |
| ACS hospitalization per 1000 patients | −2 (−7 to 3) |
| Total Medicare cost of care per patient, $ | −601 (−1198 to −5) |
Abbreviations: ACS, ambulatory care sensitive; DID, difference-in-differences; ED, emergency department.
All models were adjusted for age, sex, race (black), ethnicity (Hispanic), Hierarchical Condition Category score, Alzheimer disease diagnosis and number of days of fee-for-service coverage in quarter. All models included 12 quarters of postintervention follow-up.
Discussion
In this study of a comprehensive dementia care comanagement program, the number of long-term care nursing home placements was reduced for Medicare fee-for-service beneficiaries, and the total costs of care for Medicare were lower for program participants than for patients in the comparison group. However, after accounting for program costs, the program was cost neutral.
Comanagement of dementia care allows nurse practitioners to focus on comprehensive care of dementia while allowing the primary care physician to retain responsibility for the clinical treatment of dementia and other conditions. Similar collaborative models of care have been valuable in improving the quality of care, outcomes, and sometimes the cost of care for conditions such as depression26 and heart failure.27,28
This study built on the earlier findings from the Indiana University Healthy Aging Brain Center, which initially relied on nurse practitioners to comanage treatment of patients with dementia and was conducted in a safety-net population.9,12 We found that it is feasible to provide collaborative care for dementia that is cost neutral in a predominantly fee-for-service health care environment. If implemented widely, program return on investment would depend on local costs of implementation, particularly local labor costs.29 At the time of the study, fee-for-service billing, however, did not generate sufficient revenues to fully pay for program costs. Through new payment mechanisms, the adoption and dissemination of dementia care management services could be facilitated.
Limitations
The limitations of the study should be noted. First, the study was a controlled before-and-after comparison conducted at a single institution and limited to Medicare fee-for-service beneficiaries. Because claims data were lacking, beneficiaries in Medicare Advantage plans were not included in the analysis. The quasiexperimental design is not as strong as a randomized clinical trial from an analytic perspective, but it has pragmatic design features (eg, implementation in real clinical settings, virtually no exclusionary criteria) that are difficult to implement in clinical trials. Although we used a zip code and propensity score–matched comparison group that was similar to the intervention group with regard to sociodemographic and clinical characteristics within claims data, these data do not provide detailed clinical information on disease severity, functional status, or the characteristics of caregivers or referring physicians. All of these factors may affect health care utilization and costs. Although the mean follow-up was about 21 months (7 3-month quarters), only one-third of participants (n = 357) were enrolled in the intervention group for more than 2 years, and analysis was truncated at 3 years of follow-up. Finally, the reduction of long-term nursing home admissions may have been partially or fully offset by increased use of assisted-living facilities; we were unable to assess whether this was the case. For many people, however, the use of assisted living and other community long-term services and support would be preferable to long-term care in a nursing home.
Conclusions
New models of care that are effective without substantial cost increases are needed for patients with Alzheimer disease and other dementias. Comprehensive dementia care addresses several goals of the National Plan to Address Alzheimer Disease,30 can reduce the number of admissions to long-term care facilities, and depending on program costs, may be cost neutral or cost saving. Wider implementation of such programs may help more people with dementia remain in their communities.
eAppendix 1. Detailed Annual UCLA Alzheimer’s and Dementia Care Program Costs
eAppendix 2. Additional Demographic and Clinical Characteristics of UCLA Alzheimer’s and Dementia Care (ADC) Program Participants
Footnotes
All referrals (n = 1083) to the Alzheimer and Dementia Care program were made by UCLA physicians, with most patients (91%, n = 986) being referred by a primary care physician. Patients were not required to have a caregiver to enroll in the program, though nearly all identified a family or friend caregiver. Caregivers were most often spouses (30%, n = 320) or adult children (41%, n = 440).
References
- 1.Langa KM, Larson EB, Crimmins EM, et al. . A comparison of the prevalence of dementia in the United States in 2000 and 2012. JAMA Intern Med. 2017;177(1):51-58. doi: 10.1001/jamainternmed.2016.6807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Phelan EA, Borson S, Grothaus L, Balch S, Larson EB. Association of incident dementia with hospitalizations. JAMA. 2012;307(2):165-172. doi: 10.1001/jama.2011.1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hurd MD, Martorell P, Delavande A, Mullen KJ, Langa KM. Monetary costs of dementia in the United States. N Engl J Med. 2013;368(14):1326-1334. doi: 10.1056/NEJMsa1204629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chodosh J, Mittman BS, Connor KI, et al. . Caring for patients with dementia: how good is the quality of care? results from three health systems. J Am Geriatr Soc. 2007;55(8):1260-1268. doi: 10.1111/j.1532-5415.2007.01249.x [DOI] [PubMed] [Google Scholar]
- 5.Belmin J, Min L, Roth C, Reuben D, Wenger N. Assessment and management of patients with cognitive impairment and dementia in primary care. J Nutr Health Aging. 2012;16(5):462-467. doi: 10.1007/s12603-012-0026-z [DOI] [PubMed] [Google Scholar]
- 6.Wenger NS, Solomon DH, Roth CP, et al. . The quality of medical care provided to vulnerable community-dwelling older patients. Ann Intern Med. 2003;139(9):740-747. doi: 10.7326/0003-4819-139-9-200311040-00008 [DOI] [PubMed] [Google Scholar]
- 7.Reuben DB, Roth CP, Frank JC, et al. . Assessing care of vulnerable elders—Alzheimer’s disease: a pilot study of a practice redesign intervention to improve the quality of dementia care. J Am Geriatr Soc. 2010;58(2):324-329. doi: 10.1111/j.1532-5415.2009.02678.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vickrey BG, Mittman BS, Connor KI, et al. . The effect of a disease management intervention on quality and outcomes of dementia care: a randomized, controlled trial. Ann Intern Med. 2006;145(10):713-726. doi: 10.7326/0003-4819-145-10-200611210-00004 [DOI] [PubMed] [Google Scholar]
- 9.Callahan CM, Boustani MA, Weiner M, et al. . Implementing dementia care models in primary care settings: the aging brain care medical home. Aging Ment Health. 2011;15(1):5-12. doi: 10.1080/13607861003801052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Callahan CM, Boustani MA, Unverzagt FW, et al. . Effectiveness of collaborative care for older adults with Alzheimer disease in primary care: a randomized controlled trial. JAMA. 2006;295(18):2148-2157. doi: 10.1001/jama.295.18.2148 [DOI] [PubMed] [Google Scholar]
- 11.Bass DM, Judge KS, Snow AL, et al. . A controlled trial of partners in dementia care: veteran outcomes after six and twelve months. Alzheimers Res Ther. 2014;6(1):9. doi: 10.1186/alzrt242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boustani MA, Sachs GA, Alder CA, et al. . Implementing innovative models of dementia care: the healthy aging brain center. Aging Ment Health. 2011;15(1):13-22. doi: 10.1080/13607863.2010.496445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reuben DB, Evertson LC, Wenger NS, et al. . The University of California at Los Angeles alzheimer’s and dementia care program for comprehensive, coordinated, patient-centered care: preliminary data. J Am Geriatr Soc. 2013;61(12):2214-2218. doi: 10.1111/jgs.12562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jennings LA, Tan Z, Wenger NS, et al. . Quality of care provided by a comprehensive dementia care comanagement program. J Am Geriatr Soc. 2016;64(8):1724-1730. doi: 10.1111/jgs.14251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Intrator O, Hiris J, Berg K, Miller SC, Mor V. The residential history file: studying nursing home residents’ long-term care histories(*). Health Serv Res. 2011;46(1 Pt 1):120-137. doi: 10.1111/j.1475-6773.2010.01194.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pope GC, Kautter J, Ellis RP, et al. . Risk adjustment of Medicare capitation payments using the CMS-HCC model. Health Care Financ Rev. 2004;25(4):119-141. [PMC free article] [PubMed] [Google Scholar]
- 17.Thoemmes FJ, Kim ES. A systematic review of propensity score methods in the social sciences. Multivariate Behav Res. 2011;46(1):90-118. doi: 10.1080/00273171.2011.540475 [DOI] [PubMed] [Google Scholar]
- 18.Yun H, Kilgore ML, Curtis JR, et al. . Identifying types of nursing facility stays using Medicare claims data: an algorithm and validation. Health Serv Outcomes Res Methodol. 2010;10:100-110. doi: 10.1007/s10742-010-0060-4 [DOI] [Google Scholar]
- 19.Agency for Healthcare Research and Quality: Prevention quality indicators #92, technical specifications, prevention quality chronic composite. http://www.qualityindicators.ahrq.gov/Downloads/Modules/PQI/V45/TechSpecs/PQI%2092%20Prevention%20Quality%20Chronic%20Composite.pdf. Published May 2013. Accessed May 3, 2017.
- 20.Health Resources and Services Administration Consumer price index for medical care. https://www.hrsa.gov/get-health-care/affordable/hill-burton/cpi.html. Accessed October 20, 2017.
- 21.Berry SD, Ngo L, Samelson EJ, Kiel DP. Competing risk of death: an important consideration in studies of older adults. J Am Geriatr Soc. 2010;58(4):783-787. doi: 10.1111/j.1532-5415.2010.02767.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496-509. doi: 10.1080/01621459.1999.10474144 [DOI] [Google Scholar]
- 23.Zhou B, Latouche A, Rocha V, Fine J. Competing risks regression for stratified data. Biometrics. 2011;67(2):661-670. doi: 10.1111/j.1541-0420.2010.01493.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ho D, Imai K, King G, Stuart E. Matching as nonparametric preprocessing for reducing model dependence in parametric causal inference. Polit Anal. 2007;15:199-236. doi: 10.1093/pan/mpl013 [DOI] [Google Scholar]
- 25.Stata [computer program]. Version 14. College Station, TX: StataCorp LP.
- 26.Unutzer J, Katon WJ, Fan MY, et al. . Long-term cost effects of collaborative care for late-life depression. Am J Manag Care. 2008;14(2):95-100. [PMC free article] [PubMed] [Google Scholar]
- 27.Takeda A, Taylor SJ, Taylor RS, Khan F, Krum H, Underwood M. Clinical service organisation for heart failure. Cochrane Database Syst Rev. 2012;(9):CD002752. [DOI] [PubMed] [Google Scholar]
- 28.Stewart S, Wiley JF, Ball J, et al. . Impact of nurse-led, multidisciplinary home-based intervention on event-free survival across the spectrum of chronic heart disease: composite analysis of health outcomes in 1226 patients from 3 randomized trials. Circulation. 2016;133(19):1867-1877. doi: 10.1161/CIRCULATIONAHA.116.020730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.US Department of Labor, Bureau of Labor Statistics Occupational employment statistics, May 2017. https://www.bls.gov/oes/current/oes291171.htm. Accessed May 18, 2018.
- 30.US Department of Health and Human Services National plan to address Alzheimer’s disease: 2015. update. https://aspe.hhs.gov/pdf-document/national-plan-address-alzheimer%E2%80%99s-disease-2015-update. Accessed May 3, 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix 1. Detailed Annual UCLA Alzheimer’s and Dementia Care Program Costs
eAppendix 2. Additional Demographic and Clinical Characteristics of UCLA Alzheimer’s and Dementia Care (ADC) Program Participants