This randomized clinical trial examines the incidence of incisional hernia in patients undergoing prophylactic intraperitoneal mesh implantation vs patients undergoing standard abdominal closure.
Key Points
Question
Is prophylactic intraperitoneal mesh implantation effective in preventing incisional hernia after elective open abdominal surgery?
Finding
An open-label randomized clinical trial was performed in 169 patients undergoing elective open abdominal surgery. The trial found that prophylactic intraperitoneal mesh implantation reduces the incidence of incisional hernia but with increased early postoperative pain and prolonged wound healing of surgical site infection.
Meaning
Incisional hernia is a frequent complication after open abdominal surgery; intraperitoneal mesh implantation is a simple and effective measure to prevent this complication.
Abstract
Importance
Incisional hernia is a frequent complication after open abdominal surgery. Prophylactic mesh implantation in the onlay or sublay position requires dissection of the abdominal wall, potentially leading to wound-associated complications.
Objective
To compare the incidence of incisional hernia among patients after prophylactic intraperitoneal mesh implantation with that among patients after standard abdominal closure.
Design, Setting, and Participants
An open-label randomized clinical trial was performed in 169 patients undergoing elective open abdominal surgery from January 1, 2011, to February 29, 2014. Follow-up examinations were performed 1 year and 3 years after surgery. The study was conducted at Bern University Hospital, Bern, Switzerland, a referral center that offers the whole spectrum of abdominal surgical interventions. Patients with 2 or more of the following risk factors were included: overweight or obesity, diagnosis of neoplastic disease, male sex, or history of previous laparotomy. Patients were randomly assigned to prophylactic intraperitoneal mesh implantation or standard abdominal closure. Data were analyzed in August 2017.
Interventions
Intraperitoneal implantation of a polypropylene-polyvinylidene fluoride mesh with circumferential fixation.
Main Outcomes and Measures
The primary end point was the incidence of incisional hernia 3 years after surgery. Secondary end points included mesh-related complications.
Results
After the exclusion of 19 patients, 150 patients (81 in the control group and 69 in the mesh group; mean [SD] age, 64.2 [11.1] years; 102 [68.0%] male) were studied. The cumulative incidence of incisional hernia was significantly lower in the mesh group compared with the control group (5 of 69 [7.2%] vs 15 of 81 [18.5%], log-rank test P = .03). Abdominal pain was observed in significantly more patients in the mesh group compared with the control group at 6 weeks (34 of 52 [65%] vs 26 of 59 [44%], P = .04) but not at 12 and 36 months postoperatively. No difference in surgical site infections was observed, but time to complete wound healing of surgical site infection was significantly longer in patients with mesh implantation (median [interquartile range], 8 [6-24] weeks compared with 5 [1-9] weeks; P = .03). Trunk extension was significantly decreased after mesh implantation compared with the control group (mean [SD], 1.73 [0.97] cm vs 2.40 [1.23] cm, P = .009).
Conclusions and Relevance
In patients at elevated risk for incisional hernia, prophylactic intraperitoneal mesh implantation reduces the incidence of hernia formation but with increased early postoperative pain and prolonged wound healing of surgical site infection.
Trial Registration
ClinicalTrials.gov Identifier: NCT01203553
Introduction
Under current guidelines, a running, slowly absorbable suture is recommended for the closure of the abdominal wall after laparotomy.1 However, this technique is associated with a considerable incidence of incisional hernia. Despite a wide variability of reported incidence, relevant studies2,3,4,5 indicate a frequency between 10% and 30% on long-term follow-up. Novel techniques, including the use of small bites, may reduce the incidence of incisional hernia after midline laparotomies.6,7 However, despite technical modifications, the rate of incisional hernia remains clinically significant, with an incidence of up to 13%6,7; therefore, it is important to reduce incisional hernia in patients undergoing open abdominal surgery.
The benefit of prophylactic mesh implantation in reducing the incidence of incisional hernia after laparotomy has been reported in previous studies.8,9,10 Onlay or sublay mesh position was investigated in most studies,10,11,12,13 which requires additional dissection of the abdominal wall, is time consuming and can therefore be a hindrance in routine prophylactic mesh implantation. Furthermore, an elevated rate of seroma formation was reported after onlay and sublay mesh implantation, potentially because of the increased wound surface area.8,14 Intraperitoneal placement of modern dual-layered meshes could potentially overcome these limitations by reducing the time required for mesh implantation and minimizing dissection of the abdominal wall.15,16 Therefore, the efficacy of prophylactic intraperitoneal mesh implantation for the prevention of incisional hernia after laparotomy was tested in a randomized clinical trial.
To maximize the benefit-risk ratio, prophylactic mesh implantation would ideally be performed in patients at elevated risk for hernia development. Previous studies17,18 explored the outcome of prophylactic mesh implantation after specific procedures with elevated risk for incisional hernia, such as bariatric or abdominal vascular surgery. The current study explores a population undergoing general abdominal surgery, including hepatobiliary, pancreatic, and upper and lower gastrointestinal tract surgery. A population at risk was defined by patient-related risk factors that can be recognized preoperatively. Such stratification allows identification of patients at elevated risk for incisional hernia development, who potentially benefit the most from mesh implantation. Numerous preoperative recognizable risk factors for incisional hernia have been described in the literature, including overweight or obesity, low hemoglobin level, uremia, male sex, old age, smoking, previous laparotomy, and diagnosis of neoplastic disease.2,3,4,19,20,21,22,23,24,25 To ensure practicability in daily clinical routine, only strong risk factors were included for stratification that are dichotomous, occur frequently, and are easy to identify. Thus, the following risk factors were used for patient stratification: overweight or obesity, diagnosis of neoplastic disease, male sex, and history of laparotomy. Patients with at least 2 of these risk factors were included. With this study design, we tested the hypothesis that primary mesh implantation in an intraperitoneal position reduces the risk of incisional hernia in a population at risk.
Methods
Trial Design
An open-label randomized clinical trial with 1:1 randomization in 2 parallel groups was performed at the Department of Visceral Surgery and Medicine in the University Hospital of Bern, Bern, Switzerland. The trial protocol can be found in Supplement 1. The study was conducted under the working title ProphMesh. The study, including the increase of sample size, was approved by the Ethical Committee of the Canton of Bern.
Participants
Patients seen at the visceral surgical outpatient department who were scheduled for open abdominal surgery from January 1, 2011, to February 29, 2014, were assessed for eligibility. Inclusion criteria were age older than 18 years, elective operation, and the presence of at least 2 of the following risk factors for incisional hernia development: body mass index above 25 (calculated as weight in kilograms divided by height in meters squared), diagnosis of neoplastic disease, male sex, and history of laparotomy. Exclusion criteria were previous intraperitoneal mesh placement, previous incisional hernia, emergency procedures, and patients with inflammatory bowel disease. All patients had to sign informed consent forms before inclusion in the study. Data were deidentified with a key according to Swiss regulations. Data were analyzed in August 2017.
Interventions
The scheduled operation was performed as planned. In the control group, the abdominal wall was closed according to the institutional standard operating procedure, using a slowly absorbable running suture (USP 1 PDS II loop, Ethicon, Johnson & Johnson). The distance of the stitches to the fascial border was 1 cm, and the distance between stitches was also 1 cm. In the intervention group, a mesh was implanted before closure of the abdominal wall in a standardized fashion. A double-layered polypropylene-polyvinylidene fluoride mesh (Dynamesh-IPOM, FEG Textiltechnik) was tailored to overlap lateral and cranial-caudal borders by at least 5 cm. The mesh was placed intraperitoneally and fixed to the abdominal wall using single stitches with polypropylene sutures (USP 2-0 Prolene; Ethicon) in all 4 corners. After the initial fixation, the borders of the mesh were fixed to the abdominal wall circumferentially using running polypropylene suture to prevent any intestinal structures to herniate onto the mesh. Afterward, the abdominal wall was closed as described in the control group. In both groups, the subcutaneous tissue was not adapted separately; the skin was closed using interrupted stitches with polypropylene sutures (USP 3-0 Prolene; Ethicon) or skin staples, according to the surgeon’s choice.
Outcomes
Data collection was performed at the outpatient department preoperatively and at 6 weeks, 1 year, and 3 years postoperatively. Patients were interviewed according to the respective questionnaires, and a clinical examination was performed by observers not otherwise involved in the planning or conduct of the study.
The primary outcome of the study was the incidence of an incisional hernia as defined by the European Hernia Society: an abdominal wall gap with or without bulge in the area of the postoperative scar palpable by clinical examination.26 The patients were seen for follow-up visits at the outpatient department and examined by a qualified physician with at least 2 years of surgical training. Imaging studies were performed if there was uncertainty about the diagnosis. Mean follow-up time was 16.9 months in the control group and 18.7 months in the intervention group.
Secondary outcomes were the occurrence of postoperative complications; hematoma, intestinal paralysis, and in-hospital mortality were reported until 30 days postoperatively. Fistula formation, small-bowel obstruction, and surgical site infection (SSI) were reported for the whole follow-up period of 3 years.27 Pain levels were assessed during the hospital stay and at 6 weeks, 1 year, and 3 years postoperatively. During follow-up consultations, patients were asked whether they still felt pain in the abdominal region and to rate the intensity of pain on a visual analog scale (VAS) from 0 to 10, with 0 indicating no pain and 10 indicating worst imaginable pain. Furthermore, they were asked to report whether they perceived pain at least once a day. Range of motion for trunk flexion and extension was assessed before and after surgery by measuring finger-to-floor distance and umbilicus-xiphoid distance in upright position and in maximal trunk retroflection.
Sample Size
On the basis of existing studies,2,4,28 we expected 25% of control patients from our at-risk population to develop an incisional hernia during the 3 years. In patients with an implanted mesh, an incidence of approximately 5% was expected, as observed in patients undergoing similar operations in our department before the initiation of the study.29
We calculated that a total of 150 patients needed to be included in the study to demonstrate the difference, presuming a 2-sided level of significance at 5% and a power of 80%. This number also allowed for a loss to follow-up of up to 20% in the patient sample. Because of the unexpectedly high number of patients (n = 19) excluded from the study during surgery, the aimed sample size was increased from 150 to 170 to ensure that 150 patients were treated according to randomization. An amendment was written and approved by the local ethics committee on February 12, 2013.
Randomization
Randomization was performed in permuted blocks of 30 patients, using the online tool randomization.com by an external study nurse. Sealed and numbered envelopes that contained the allocated group were prepared and opened during surgery before abdominal wall closure.
Statistical Analysis
Continuous variables were described using means (SDs). For categorical values, numbers (percentages) were calculated. Differences between groups were compared using the 2-tailed, unpaired t test or Mann-Whitney test for continuous variables and the Fisher exact test for categorical variables. Statistical significance was defined as 2-sided P < .05. In this intent-to-treat analysis, patients randomized to the mesh group were analyzed in the mesh group even if the mesh was explanted during follow-up. Patients in the control group were analyzed in the control group even if they underwent additional surgery with eventual prophylactic mesh implantation. Analysis was performed using SPSS statistical software, version 21 (IBM Corp) and Prism software, version 6 (GraphPad). A Kaplan-Meier curve of the occurrence of incisional hernias stratified by treatment group was plotted, and a log-rank test was used to compare the hernia incidence between the groups.
Results
Demographics
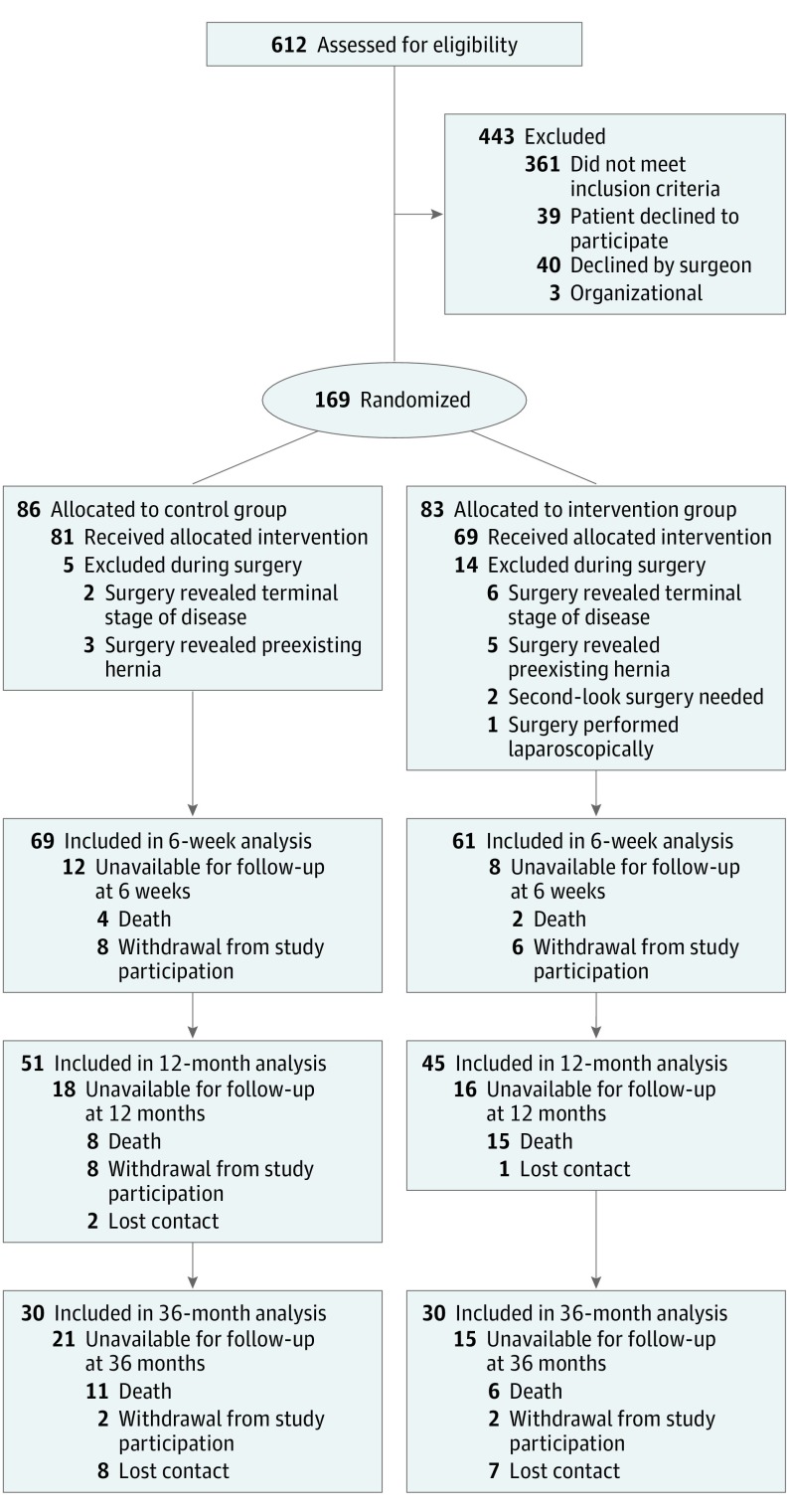
A total of 169 patients were randomized into a control group (n = 86) or a mesh group (n = 83). Nineteen patients were excluded from the study during surgery because new information that conflicted with the inclusion or exclusion criteria became evident (existing incisional hernia, finding of an implanted mesh), second-look surgery became necessary, or surgery revealed a terminal stage of disease (peritoneal carcinomatosis) with the surgeon disagreeing with mesh implantation. Therefore, 81 patients in the control group and 69 in the mesh group were studied (mean [SD] age, 64.2 [11.1] years; 102 [68.0%] male). Details are shown in Figure 1. Baseline data were similar between groups (Table 1).
Figure 1. CONSORT Diagram.
Table 1. Baseline Patient Characteristicsa.
| Characteristic | Control Group (n = 81) | Mesh Group (n = 69) |
|---|---|---|
| Age, median (IQR), y | 65 (56.5-70) | 67 (58-72) |
| BMI, mean (SD) | 26.7 (4.8) | 27.6 (4.6) |
| ASA classification | ||
| 1-2 | 19 (23.5) | 18 (26.1) |
| 3 | 55 (67.9) | 47 (68.1) |
| 4 | 7 (8.6) | 4 (5.8) |
| Immunosuppressants or corticosteroid therapy | 3 (3.7) | 4 (5.8) |
| Incisional hernia risk factors | ||
| BMI>25 | 50 (61.7) | 51 (73.9) |
| Diagnosis of neoplastic disease | 67 (82.7) | 54 (78.3) |
| Male | 56 (69.1) | 46 (66.7) |
| Previous laparotomy | 60 (74.1) | 51 (73.9) |
| Type of surgery | ||
| Upper GI tract | 12 (14.8) | 14 (20.3) |
| Lower GI tract | 19 (23.5) | 17 (24.6) |
| Hepatobiliary | 18 (22.2) | 20 (29.0) |
| Pancreatic | 30 (37.0) | 15 (21.7) |
| Other | 2 (2.5) | 3 (4.3) |
| Type of incision | ||
| Midline laparotomy | 30 (37.0) | 31 (44.9) |
| Transverse laparotomy | 51 (63.0) | 38 (55.1) |
| Operation duration, mean (SD), min | 293 (109) | 275 (102) |
| Mesh implantation duration, mean (SD), min | NA | 25 (8) |
Abbreviations: ASA, American Society of Anesthesiologists; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); GI, gastrointestinal; IQR, interquartile range; NA, not applicable.
Data are presented as number (percentage) of patients unless otherwise specified.
Primary Outcome
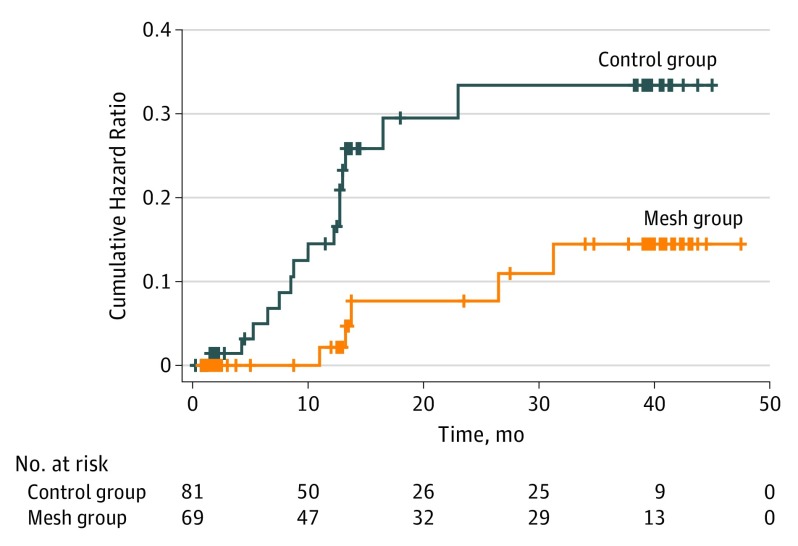
The incidence of incisional hernia 3 years after surgery was significantly higher in the control group vs the mesh group (15 of 81 [18.5%] vs 5 of 69 [7.2%]). Comparison of the cumulative hazard curves revealed a statistically significant difference between the groups (log-rank test P = .03) (Figure 2). In both groups, most hernias occurred during the first year after surgery. Follow-up at 1 year resulted in incisional hernia in 13 of 51 individuals (25.5%) in the control group vs 3 of 45 individuals (6.7%) in the mesh group. At the 3-year follow-up visit, 7 of the 30 control group patients (23.3%) presented with a hernia compared with 3 of 30 mesh group patients (10.0%).
Figure 2. Cumulative Hazard Curves for Incisional Hernia in the Mesh and Control Groups.
The curves are significantly different (P = .03, log-rank test).
Reasons for hernia occurrence in the mesh group were mesh removal in one patient and an additional laparotomy with abdominal wall closure using resorbable suture material in another patient. For the other patients with hernia occurence in the intervention group, the reason for failure of the implanted mesh remains unclear.
Secondary Outcomes
There was no statistically significant difference between the control and intervention groups concerning occurrence of hematoma (1.2% vs 1.4%; P > .99), intestinal paralysis (4.9% vs 4.4%; P > .99), length of hospital stay (13.5 vs 13 days; P = .46), or in-hospital mortality (3.7% vs 1.4%; P = .62) (Table 2). The incidence of small-bowel obstruction was similar between the groups during the entire observation time (Table 2). No enterocutaneous fistula was observed in either group during follow-up. The incidence of SSI was not statistically different between the control (18 of 69 [26.1%]) and mesh (11 of 61 [18.0%]) groups (P = .30). However, in patients with SSI, completion of wound healing took longer in the mesh group compared with the control group (median [interquartile range], 8 [6-24] weeks compared with 5 [1-9] weeks; P = .03). Surgical wound revisions were more frequent in the mesh group (Table 2).
Table 2. Surgical Complications, Length of Hospital Stay, and Mortality .
| Variable | Control Groupa | Mesh Groupa | Effect Size, % (95% CI) | P Value |
|---|---|---|---|---|
| Hematoma | 1/81 (1.2) | 1/69 (1.4) | 0.2 (−7 to 7) | >.99b |
| Intestinal postoperative paralysis | 4/81 (4.9) | 3/69 (4.4) | 0.5 (−9 to 9) | >.99b |
| Subsequent operation | 8/81 (9.9) | 5/69 (7.3) | 2.6 (−8 to 13) | .77b |
| Pain on VAS, mean (SD)c | ||||
| POD1 | 1.2 (1.2) | 1.6 (1.5) | 0.4 (−0.04 to 0.88) | .07d |
| POD3 | 0.6 (0.8) | 0.9 (1.4) | 0.3 (−0.11 to 0.69 | .15d |
| POD5 | 1.24 (1.1) | 1.26 (1.6) | 0.02 (−0.57 to 0.61) | .94d |
| Length of hospital stay, median (IQR), d | 13.5 (8.75-23.5) | 13 (9-17) | 0.5 (−3 to 1) | .46d |
| In-hospital mortality | 3/81 (3.7) | 1/69 (1.4) | 2.3 (−6 to 10) | .62b |
| Patients with SSIs | ||||
| Superficial incision | 7/69 (10.1) | 5/61 (8.2) | 8.1 (−8 to 23) | .30b |
| Deep incisional | 3/69 (4.4) | 2/61 (3.3) | ||
| Organ or space | 8/69 (11.6) | 4/61 (6.5) | ||
| Total | 18/69 (26.1) | 11/61 (18.0) | ||
| Time to final wound healing among patients with SSI, median (IQR), wk | 5 (1-9) | 8 (6-24) | 3 (0 to 14) | .03e |
| Subsequent surgery for chronic woundf | 1/69 (1.5) | 4/61 (6.6) | 5.1 (−5 to 14) | .19b |
| Small-bowel obstruction | ||||
| 6 wk to 12 mo | 1/50 (2.0) | 1/45 (2.2) | 0.2 (−11 to 10) | >.99b |
| 12 mo to 36 mo | 2/29 (6.9) | 1/27 (3.7) | 3.2 (−15 to 21) | >.99b |
Abbreviations: IQR, interquartile range; POD, postoperative day; SSI, surgical site infection; VAS, visual analog scale.
Data are presented as number per total number (percentage) of patients unless otherwise specified.
Fisher exact test.
Mean scores for all patients in each group from 0 to 10, with 0 indicating no pain and 10 indicating worst imaginable pain.
Two-tailed, unpaired t test.
Mann-Whitney test.
Equivalent to number of mesh explantations in mesh group.
Pain was similar between the groups during hospital stay (Table 3). At 6 weeks, significantly more patients in the mesh group reported postoperative pain compared with patients in the control group (34 of 52 [65.4%] vs 26 of 59 [44.1%]; effect size, 21.3%; 95% CI, 4%-41%; P = .04). Pain intensity was higher in the mesh group compared with the control group at 6 weeks (mean VAS score, 1.61 vs 0.83; VAS score difference, 0.78; 95% CI, 0.10-1.46; P = .02). At 1 and 3 years after surgery, no difference in pain perception was observed between the groups (Table 3).
Table 3. Abdominal Pain Perceptiona.
| Pain | Control Group | Mesh Group | Effect Size, % (95% CI) | P Value |
|---|---|---|---|---|
| Presence of Abdominal Pain | ||||
| 6 wk | 26/59 (44.1) | 34/52 (65.4) | 21.3 (4 to 41) | .04b |
| 12 mo | 16/45 (35.6) | 14/40 (35.0) | 0.6 (−20 to 22) | >.99b |
| 36 mo | 7/30 (23.3) | 6/29 (20.7) | 2.6 (−20 to 25) | >.99b |
| Daily Abdominal Pain (Among Patients With Pain) | ||||
| 6 wk | 10/26 (38.5) | 17/34 (50.0) | 11.5 (−13 to 39) | .44b |
| 12 mo | 4/16 (25) | 7/14 (50) | 25 (−6 to 63) | .26b |
| 36 mo | 3/7 (42.8) | 2/6 (33.3) | 9.5 (−43 to 55) | >.99b |
| Intensity of Pain Measured With VAS Scorec (Among All Patients) | ||||
| 6 wk | 0.83 (1.61) | 1.61 (2.27) | 0.78 (0.10 to 1.46) | .02d |
| 12 mo | 0.86 (1.88) | 1.2 (2.23) | 0.34 (−0.50 to 1.17) | .42d |
| 36 mo | 0.67 (1.56) | 0.73 (2.02) | 0.07 (−0.87 to 1.00) | .89d |
Abbreviation: VAS, visual analog scale.
Data are presented as number per total number (percentage) of patients for presence of abdominal pain and daily abdominal pain and mean (SD) for intensity of pain.
Fisher exact test.
VAS scores from 0 to 10, with 0 indicating no pain and 10 indicating worst imaginable pain.
Two-tailed, unpaired t test.
Trunk extension, assessed by elongation of umbilicus-xiphoid distance, was significantly reduced in the mesh group compared with the control group at 12 months (2.40 vs 1.73 cm; effect size, 0.675; 95% CI, 0.18-1.1; P = .009) but not at 36 months after surgery. Trunk flexion, as assessed by finger-to-floor distance, was not significantly different between the groups at all follow-up time points (eFigure in Supplement 2).
Discussion
Our findings reveal that prophylactic intraperitoneal mesh implantation significantly reduces the incidence of incisional hernia 3 years after laparotomy compared with standard abdominal closure in a high-risk population. This study confirms previous reports8,9,10,29 that found similar effects of prophylactic mesh implantation. The current study has 2 main differences to most previous reports.8,9 First, the study population represents patients with elevated risk profile for hernia development that are patient and not procedure related. Second, a double-layered mesh was implanted in an intraperitoneal position, whereas previous studies10,11,12 explored mesh implantation in onlay or sublay positions.
The incidence of incisional hernia in the control group was above average for an elective surgical population but in the same range as reported in current studies9,10,11,12 on prophylactic mesh implantation in at-risk patient collectives. This finding indicates that the simple scoring system used allows identification of a population at elevated risk for hernia development based on personal risk factors that can be recognized preoperatively.
The incidence of incisional hernia was significantly lower in the mesh group, showing that intraperitoneal mesh implantation is effective for prevention of hernia development. Among the 5 hernias that developed in the mesh group, 2 occurred because of technical aspects during secondary surgery, including mesh removal and mesh adaptation using resorbable suture material.
In our study, the calculated number needed to treat is 8.9; accordingly, 9 patients have to undergo prophylactic mesh implantation to prevent 1 incisional hernia. This number is in line with the current literature that shows a number needed to treat between 5 and 10 for prophylactic mesh implantation in patients undergoing surgical procedures with an increased risk of hernia formation.8 Because not every incisional hernia needs surgical repair, the number needed to treat to prevent 1 hernia repair is likely to be higher. We believe that risk assessment for incisional hernia should be performed to keep the number needed to treat low and to reduce unnecessary mesh implantations.
The recently published results of the Small Bites Versus Large Bites for Closure of Abdominal Midline Incisions (STITCH) trial revealed a decrease of incisional hernias after 1 year of follow-up with a small-bite technique.6 Because of the focus on a high-risk population in the current study, a direct comparison of hernia rates is not accurate. Prophylactic mesh implantation vs the small-bite technique should be compared directly in a future study.
Implantation of a mesh in an intraperitoneal position was not associated with an increase in the incidence of SSI. However, patients who developed SSI experienced delayed wound healing (Table 2). Such delayed wound healing is most likely the consequence of secondary infection of the prosthesis. The median healing time in the mesh group was 2 months; 5 of 12 patients had a chronic wound, defined as a healing time of more than 3 months.30 The fact that more than half of patients with mesh infection healed without mesh removal may be because the mesh was based on polypropylene and not on polyester that is more prone to chronic infection.31,32,33 Small-bowel obstruction, which potentially may occur after mesh implantation, was not significantly different between the 2 groups at long-term follow-up. No fistula was detected in either group, which is in line with the literature.34,35,36
Chronic postoperative pain is a potential challenge in hernia surgery and after prophylactic mesh placement. Prevalence and intensity of pain 6 weeks postoperatively were significantly higher in the mesh group compared with the control group. This elevated pain might be caused by tension through mesh fixation sutures in the abdominal wall or by inflammatory reaction to the implanted mesh. However, there was no difference in pain at long-term follow-up.
A preventive measure should be simple and fast in its application, which is where we see the advantage of the intraperitoneal onlay mesh technique. The mesh can be implanted without creating an additional wound surface area because no further dissection of the abdominal wall is needed. In the present study, clinically relevant long-term mesh-associated complications, such as erosion and formation of adhesions, were not observed.
Limitations
Limitations of the study include the high rate of unavailability for follow-up, the exclusion of patients during surgery, and lack of quality-of-life assessment. Because this study investigated a high-risk population with a significant fraction of patients with malignant disease, mortality was observed in some patients before reaching the end point. In the power calculation, a loss to follow-up of 20% was initially calculated. Therefore, the actual loss of patients was underestimated, mainly because of mortality and withdrawal from study participation. However, there was no difference in loss to follow-up between the 2 groups. Mesh implantation needed to be documented in the operation report to inform medical personnel responsible for aftercare and to ensure patient safety. Therefore, masking of the patient and the assessor during follow-up visits was not possible. To minimize bias, the assessor was a person not involved in the study and patient care. The elevated exclusion of patients randomized to receive mesh implantation may represent a reluctance of surgeons to implant a mesh prophylactically, especially in patients excluded because of a terminal stage of disease. This approach represents another limitation of this study. However, post hoc analysis revealed no major different baseline characteristics in patients intraoperatively excluded. Such reluctance to implant a mesh highlights the need for a better definition of patients who benefit from mesh implantation.
Conclusions
Prophylactic intraperitoneal mesh implantation in patients at risk for incisional hernia is feasible and effective to prevent hernia formation. Adverse effects of mesh implantation include delayed wound healing of SSI, early postoperative pain, and reduced trunk extension.
Trial Protocol
eFigure. Range of Motion for Trunk Extension/Flexion
References
- 1.Muysoms FE, Antoniou SA, Bury K, et al. ; European Hernia Society . European Hernia Society guidelines on the closure of abdominal wall incisions. Hernia. 2015;19(1):1-24. doi: 10.1007/s10029-014-1342-5 [DOI] [PubMed] [Google Scholar]
- 2.Höer J, Lawong G, Klinge U, Schumpelick V. [Factors influencing the development of incisional hernia. A retrospective study of 2,983 laparotomy patients over a period of 10 years] [in German]. Chirurg. 2002;73(5):474-480. [DOI] [PubMed] [Google Scholar]
- 3.Le Huu Nho R, Mege D, Ouaïssi M, Sielezneff I, Sastre B. Incidence and prevention of ventral incisional hernia. J Visc Surg. 2012;149(5)(suppl):e3-e14. doi: 10.1016/j.jviscsurg.2012.05.004 [DOI] [PubMed] [Google Scholar]
- 4.Sørensen LT, Hemmingsen UB, Kirkeby LT, Kallehave F, Jørgensen LN. Smoking is a risk factor for incisional hernia. Arch Surg. 2005;140(2):119-123. doi: 10.1001/archsurg.140.2.119 [DOI] [PubMed] [Google Scholar]
- 5.O’Dwyer PJ, Courtney CA. Factors involved in abdominal wall closure and subsequent incisional hernia. Surgeon. 2003;1(1):17-22. doi: 10.1016/S1479-666X(03)80004-5 [DOI] [PubMed] [Google Scholar]
- 6.Deerenberg EB, Harlaar JJ, Steyerberg EW, et al. Small bites versus large bites for closure of abdominal midline incisions (STITCH): a double-blind, multicentre, randomised controlled trial. Lancet. 2015;386(10000):1254-1260. doi: 10.1016/S0140-6736(15)60459-7 [DOI] [PubMed] [Google Scholar]
- 7.Millbourn D, Cengiz Y, Israelsson LA. Effect of stitch length on wound complications after closure of midline incisions: a randomized controlled trial. Arch Surg. 2009;144(11):1056-1059. doi: 10.1001/archsurg.2009.189 [DOI] [PubMed] [Google Scholar]
- 8.Bhangu A, Fitzgerald JE, Singh P, Battersby N, Marriott P, Pinkney T. Systematic review and meta-analysis of prophylactic mesh placement for prevention of incisional hernia following midline laparotomy. Hernia. 2013;17(4):445-455. doi: 10.1007/s10029-013-1119-2 [DOI] [PubMed] [Google Scholar]
- 9.Timmermans L, de Goede B, Eker HH, van Kempen BJ, Jeekel J, Lange JF. Meta-analysis of primary mesh augmentation as prophylactic measure to prevent incisional hernia. Dig Surg. 2013;30(4-6):401-409. doi: 10.1159/000355956 [DOI] [PubMed] [Google Scholar]
- 10.Jairam AP, Timmermans L, Eker HH, et al. ; PRIMA Trialist Group . Prevention of incisional hernia with prophylactic onlay and sublay mesh reinforcement versus primary suture only in midline laparotomies (PRIMA): 2-year follow-up of a multicentre, double-blind, randomised controlled trial. Lancet. 2017;390(10094):567-576. doi: 10.1016/S0140-6736(17)31332-6 [DOI] [PubMed] [Google Scholar]
- 11.Muysoms FE, Detry O, Vierendeels T, et al. Prevention of incisional hernias by prophylactic mesh-augmented reinforcement of midline laparotomies for abdominal aortic aneurysm treatment: a randomized controlled trial. Ann Surg. 2016;263(4):638-645. doi: 10.1097/SLA.0000000000001369 [DOI] [PubMed] [Google Scholar]
- 12.García-Ureña MA, López-Monclús J, Hernando LA, et al. Randomized controlled trial of the use of a large-pore polypropylene mesh to prevent incisional hernia in colorectal surgery. Ann Surg. 2015;261(5):876-881. doi: 10.1097/SLA.0000000000001116 [DOI] [PubMed] [Google Scholar]
- 13.Strzelczyk JM, Szymański D, Nowicki ME, Wilczyński W, Gaszynski T, Czupryniak L. Randomized clinical trial of postoperative hernia prophylaxis in open bariatric surgery. Br J Surg. 2006;93(11):1347-1350. doi: 10.1002/bjs.5512 [DOI] [PubMed] [Google Scholar]
- 14.Kurmann A, Visth E, Candinas D, Beldi G. Long-term follow-up of open and laparoscopic repair of large incisional hernias. World J Surg. 2011;35(2):297-301. doi: 10.1007/s00268-010-0874-9 [DOI] [PubMed] [Google Scholar]
- 15.Klink CD, Junge K, Binnebösel M, et al. Comparison of long-term biocompability of PVDF and PP meshes. J Invest Surg. 2011;24(6):292-299. doi: 10.3109/08941939.2011.589883 [DOI] [PubMed] [Google Scholar]
- 16.Berger D, Bientzle M. Polyvinylidene fluoride: a suitable mesh material for laparoscopic incisional and parastomal hernia repair! a prospective, observational study with 344 patients. Hernia. 2009;13(2):167-172. doi: 10.1007/s10029-008-0435-4 [DOI] [PubMed] [Google Scholar]
- 17.Bevis PM, Windhaber RA, Lear PA, Poskitt KR, Earnshaw JJ, Mitchell DC. Randomized clinical trial of mesh versus sutured wound closure after open abdominal aortic aneurysm surgery. Br J Surg. 2010;97(10):1497-1502. doi: 10.1002/bjs.7137 [DOI] [PubMed] [Google Scholar]
- 18.Abo-Ryia MH, El-Khadrawy OH, Abd-Allah HS. Prophylactic preperitoneal mesh placement in open bariatric surgery: a guard against incisional hernia development. Obes Surg. 2013;23(10):1571-1574. doi: 10.1007/s11695-013-0915-1 [DOI] [PubMed] [Google Scholar]
- 19.Weissler JM, Lanni MA, Hsu JY, et al. Development of a clinically actionable incisional hernia risk model after colectomy using the Healthcare Cost and Utilization Project. J Am Coll Surg. 2017;225(2):274-284.e1. doi: 10.1016/j.jamcollsurg.2017.04.007 [DOI] [PubMed] [Google Scholar]
- 20.Goodenough CJ, Ko TC, Kao LS, et al. Development and validation of a risk stratification score for ventral incisional hernia after abdominal surgery: hernia expectation rates in intra-abdominal surgery (the HERNIA Project). J Am Coll Surg. 2015;220(4):405-413. doi: 10.1016/j.jamcollsurg.2014.12.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Armañanzas L, Ruiz-Tovar J, Arroyo A, et al. Prophylactic mesh vs suture in the closure of the umbilical trocar site after laparoscopic cholecystectomy in high-risk patients for incisional hernia: a randomized clinical trial. J Am Coll Surg. 2014;218(5):960-968. doi: 10.1016/j.jamcollsurg.2014.01.049 [DOI] [PubMed] [Google Scholar]
- 22.Yamada T, Okabayashi K, Hasegawa H, et al. Age, preoperative subcutaneous fat area, and open laparotomy are risk factors for incisional hernia following colorectal cancer surgery. Ann Surg Oncol. 2016;23(suppl 2):S236-S241. doi: 10.1245/s10434-015-4462-y [DOI] [PubMed] [Google Scholar]
- 23.Seo GH, Choe EK, Park KJ, Chai YJ. Incidence of clinically relevant incisional hernia after colon cancer surgery and its risk factors: a nationwide claims study. World J Surg. 2018;42(4):1192-1199. doi: 10.1007/s00268-017-4256-4 [DOI] [PubMed] [Google Scholar]
- 24.Itatsu K, Yokoyama Y, Sugawara G, et al. Incidence of and risk factors for incisional hernia after abdominal surgery. Br J Surg. 2014;101(11):1439-1447. doi: 10.1002/bjs.9600 [DOI] [PubMed] [Google Scholar]
- 25.Bosanquet DC, Ansell J, Abdelrahman T, et al. Systematic review and meta-regression of factors affecting midline incisional hernia rates: analysis of 14,618 patients. PLoS One. 2015;10(9):e0138745. doi: 10.1371/journal.pone.0138745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muysoms FE, Miserez M, Berrevoet F, et al. Classification of primary and incisional abdominal wall hernias. Hernia. 2009;13(4):407-414. doi: 10.1007/s10029-009-0518-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horan TC, Gaynes RP, Martone WJ, Jarvis WR, Emori TG. CDC definitions of nosocomial surgical site infections, 1992: a modification of CDC definitions of surgical wound infections. Am J Infect Control. 1992;20(5):271-274. doi: 10.1016/S0196-6553(05)80201-9 [DOI] [PubMed] [Google Scholar]
- 28.Veljkovic R, Protic M, Gluhovic A, Potic Z, Milosevic Z, Stojadinovic A. Prospective clinical trial of factors predicting the early development of incisional hernia after midline laparotomy. J Am Coll Surg. 2010;210(2):210-219. doi: 10.1016/j.jamcollsurg.2009.10.013 [DOI] [PubMed] [Google Scholar]
- 29.Kurmann A, Barnetta C, Candinas D, Beldi G. Implantation of prophylactic nonabsorbable intraperitoneal mesh in patients with peritonitis is safe and feasible. World J Surg. 2013;37(7):1656-1660. doi: 10.1007/s00268-013-2019-4 [DOI] [PubMed] [Google Scholar]
- 30.Werdin F, Tennenhaus M, Schaller HE, Rennekampff HO. Evidence-based management strategies for treatment of chronic wounds. Eplasty. 2009;9:e19. [PMC free article] [PubMed] [Google Scholar]
- 31.Gonzalez R, Ramshaw BJ. Comparison of tissue integration between polyester and polypropylene prostheses in the preperitoneal space. Am Surg. 2003;69(6):471-476. [PubMed] [Google Scholar]
- 32.Majumder A, Petro CC, Liu L, Fayezizadeh M, Novitsky YW. Development of a novel murine model for treatment of infected mesh scenarios. Surg Endosc. 2017;31(2):922-927. doi: 10.1007/s00464-016-5056-x [DOI] [PubMed] [Google Scholar]
- 33.Petersen S, Henke G, Freitag M, Faulhaber A, Ludwig K. Deep prosthesis infection in incisional hernia repair: predictive factors and clinical outcome. Eur J Surg. 2001;167(6):453-457. doi: 10.1080/110241501750243815 [DOI] [PubMed] [Google Scholar]
- 34.Brandi CD, Roche S, Bertone S, Fratantoni ME. No enterocutaneous fistula development in a cohort of 695 patients after incisional hernia repair using intraperitoneal uncoated polyproylene mesh. Hernia. 2017;21(1):101-106. doi: 10.1007/s10029-016-1530-6 [DOI] [PubMed] [Google Scholar]
- 35.Lasses Martínez B, Peña Soria MJ, Cabeza Gómez JJ, et al. Surgical treatment of large incisional hernias with intraperitoneal composite mesh: a cohort study. Hernia. 2017;21(2):253-260. doi: 10.1007/s10029-016-1557-8 [DOI] [PubMed] [Google Scholar]
- 36.Scholtes M, Kurmann A, Seiler CA, Candinas D, Beldi G. Intraperitoneal mesh implantation for fascial dehiscence and open abdomen. World J Surg. 2012;36(7):1557-1561. doi: 10.1007/s00268-012-1534-z [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eFigure. Range of Motion for Trunk Extension/Flexion