This multicenter cohort study investigates the use of parenteral anticoagulation therapy and its association with in-hospital all-cause death and major bleeding among Chinese patients with non–ST-segment elevation acute coronary syndrome undergoing percutaneous coronary intervention.
Key Points
Question
Is parenteral anticoagulation therapy beneficial for patients with non–ST-segment elevation acute coronary syndrome undergoing percutaneous coronary intervention?
Findings
In this multicenter cohort study that included 6804 consecutive patients from 5 centers in China, parenteral anticoagulation therapy was not associated with lower all-cause death or myocardial infarction but was significantly associated with a higher risk of major bleeding.
Meaning
The findings suggest that the role of parenteral anticoagulation therapy should be reevaluated in patients undergoing percutaneous coronary intervention for non–ST-segment elevation acute coronary syndrome.
Abstract
Importance
The association of parenteral anticoagulation therapy with improved outcomes in patients with non–ST-segment elevation acute coronary syndrome was previously established. This benefit has not been evaluated in the era of dual antiplatelet therapy and percutaneous coronary intervention.
Objective
To evaluate the association between parenteral anticoagulation therapy and clinical outcomes in patients with non–ST-segment elevation acute coronary syndrome undergoing percutaneous coronary intervention.
Design, Setting, and Participants
This cohort study included 8197 adults who underwent percutaneous coronary intervention for non–ST-segment elevation acute coronary syndrome from January 1, 2010, to December 31, 2014, at 5 medical centers in China. Patients receiving parenteral anticoagulation therapy only after percutaneous coronary intervention were excluded.
Exposures
Parenteral anticoagulation therapy.
Main Outcomes and Measures
The primary outcome was in-hospital all-cause death and in-hospital major bleeding as defined by the Bleeding Academic Research Consortium definition (grades 3-5).
Results
Of 6804 patients who met the final criteria, 5104 (75.0%) were male, with a mean (SD) age of 64.2 (10.4) years. The incidence of in-hospital death was not significantly different between the patients who received and did not receive parenteral anticoagulation therapy (0.3% vs 0.1%; P = .13) (adjusted odds ratio, 1.27; 95% CI, 0.38-4.27; P = .70). A similar result was found for myocardial infarction (0.3% vs 0.3%; P = .82) (adjusted odds ratio, 0.77; 95% CI, 0.29-2.07; P = .61). In-hospital major bleeding was more frequent in the parenteral anticoagulation group (2.5% vs 1.0%; P < .001) (adjusted odds ratio, 1.94; 95% CI, 1.24-3.03; P = .004). At a median (interquartile range) follow-up of 2.96 years (1.93-4.46 years), all-cause death was not significantly different between the 2 groups (adjusted hazards ratio, 0.87; 95% CI, 0.71-1.07; P = .19), but the incidence of major bleeding was higher in the parenteral anticoagulation group (adjusted hazards ratio, 1.43; 95% CI, 1.01-2.02; P = .04). The propensity score analysis confirmed these primary analyses.
Conclusions and Relevance
In the patients undergoing percutaneous coronary intervention for non–ST-segment elevation acute coronary syndrome, parenteral anticoagulation therapy was not associated with a lower risk of all-cause death or myocardial infarction but was significantly associated with a higher risk of major bleeding. These findings raise important safety questions about the current practice of routine parenteral anticoagulation therapy while we await randomized trials of this practice.
Introduction
Previous studies have shown that parenteral anticoagulation therapy (PACT) was associated with a lower risk of adverse cardiovascular events in patients with non–ST-segment elevation (NSTE) acute coronary syndrome (ACS).1,2,3 Most of the above-cited studies were conducted before the establishment of dual antiplatelet therapy and percutaneous coronary intervention (PCI) as standards of care. The advantage of PACT was driven only by the reduction in ischemic end points, such as recurrent ischemia and emergency revascularization.1,2,3 However, these end points might not be appropriate in an era when timely revascularization is recommended and widely performed to reduce the risk of ischemia. To our knowledge, controlled studies to evaluate the role of PACT among patients undergoing PCI for NSTE-ACS are lacking. This study aimed to evaluate the association between PACT and clinical outcomes in this context.
Methods
Study Design and Patients
This retrospective cohort study included 8197 consecutively enrolled patients from January 1, 2010, to December 31, 2014, at 5 hospitals in China. The study protocol was approved by the central ethics committee of the Guangdong General Hospital, Guangzhou, China, with a waiver of informed consent. Data relevant to the study were analyzed on the basis of population. Information pertaining to the specific identity of patients was strictly concealed during the study. Central ethical approval was applicable at the other collaborating hospitals as well. The definitions of unstable angina and NSTE myocardial infarction and the method to search and identify appropriate candidates are shown in eAppendix 1 in the Supplement. Patients were excluded if they were pregnant, in cardiogenic shock, required an intra-aortic balloon pump, or had other indications for anticoagulation. Patients who received PACT only after PCI were also excluded.
Data Extraction and Processing
Data were extracted from the hospital records by trained study coordinators. Data collected included patient demographics, laboratory test results, PCI procedural details, clinical events, and medical treatment. Data pertaining to antithrombotic therapy, such as PACT and dual antiplatelet therapy dosages, dates of prescriptions, and durations of therapy, were also collected.
All patients were followed up by trained nurses via telephone interviews or clinic visits from November 7, 2015, through December 30, 2016. Relevant information was also collected from the residence registration system and the clinical records for the patients who were readmitted to the hospital. For events that occurred more than once, only the index event was used for statistical analysis. All adverse clinical events were evaluated by an independent clinical events committee that was masked to the treatment details. Key variables (such as medical treatment and clinical events) were double recorded, and inconsistent data were verified by a third researcher. The remainder of the collected data was monitored by random auditing of the medical records.
Treatment and Procedure
Patients who received PACT before PCI were classified into the PACT group. Patients only receiving PACT during PCI were classified into the non-PACT group. The type and duration of non-PACT (low-molecular-weight heparin or pentasaccharide fondaparinux) was prescribed at the discretion of the clinicians. Patients received either fondaparinux, 2.5 mg, subcutaneously once daily or low-molecular-weight heparin, 1 mg/kg, subcutaneously twice daily (dosage was reduced to 1 mg/kg once daily among patients with creatinine clearance <30 mL/min [to convert creatinine clearance to mL/s/m2, multiply by 0.0167]).4 The dosage of low-molecular-weight heparin was also adjusted according to crossover of different anticoagulants, time of admission and discharge, and timing of PCI at the discretion of the physicians.
Unfractionated heparin was chosen as the standard PACT for PCI and was administered in a bolus dose of 70 to 100 U/kg according to current guidelines.5 An exception was made for 3 patients who received bivalirudin at a dosage that was determined as previously described.6 Antithrombotic therapy and other medications were administered at the discretion of the physicians.
Outcomes
The primary outcome was in-hospital all-cause death and in-hospital major bleeding as defined by the Bleeding Academic Research Consortium definition (grades 3-5).7 The prespecified secondary outcomes included the following: any bleeding as defined by the Bleeding Academic Research Consortium (grades 1-5); myocardial infarction (MI); death or MI; death, MI, or major bleeding in hospital; or death or major bleeding during follow-up. The definitions of all outcomes are detailed8 in eAppendix 1 in the Supplement.
Statistical Analyses
The sample size calculation for in-hospital all-cause death was based on a statistical rule: the events per variable should be 10 or more. According to previous studies,6,9 a total of 6667 patients should be included for an estimated incidence of 1.5% for all-cause death, with the expected clinically important factors included in the multivariable analysis to be no more than 10. The sample size determination for in-hospital major bleeding was conducted based on the logistic regression of major bleeding. A sample size of 6133 patients achieves 80% power at a .05 significance level to detect a change in probability from a 1.5% in the non-PACT group to an estimated 3.0% in the PACT group. The details are given in eAppendix 1 in the Supplement.
For in-hospital outcomes, a multivariable analysis was performed using logistic regression. Potential confounders that were significant in the univariate analysis or clinically important were included in the multivariable models. All significant interactions were also examined. A 2-sided P < .05 was considered statistically significant. We also introduced the Global Registry of Acute Coronary Events (GRACE)10 (scores range from 15 to 330, with higher scores indicating a higher risk of death) and Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes With Early Implementation of the American College of Cardiology/American Heart Association Guidelines (CRUSADE)11 score (scores range from 1 to 96, with higher scores indicating a higher risk of bleeding) and compared the outcomes among different risk groups. Subgroup analyses were conducted for in-hospital outcomes. For the long-term clinical outcomes, univariate analyses were performed using the log-rank test, and multivariable analyses were performed using the Cox proportional hazards regression model. An additive hazards model was used to detect time-varying associations. Factors of clinical importance were included in the Cox proportional hazards regression and additive hazards models.
Propensity score analyses were conducted to test the robustness of the results. Details of the propensity score model are shown in eAppendix 1 in the Supplement. The heterogeneity analysis between the centers was conducted using meta-analysis methods. A multivariable analysis stratified by centers and including the random associations among centers for in-hospital and follow-up outcomes was also conducted. The statistical analysis protocol is presented in eAppendix 2 in the Supplement.
Results
Baseline Characteristics
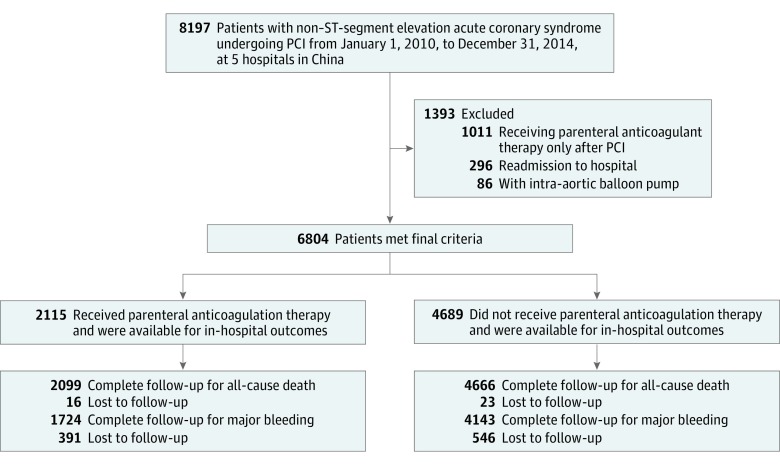
From January 1, 2010, to December 31, 2014, a total of 8197 consecutive patients with NSTE-ACS underwent PCI at 5 hospitals in China. Of 6804 patients who met the final criteria, 5104 (75.0%) were male, with a mean (SD) age of 64.2 (10.4) years. Of these patients, 3901 with NSTE myocardial infarction (57.3%) and 2903 with unstable angina (42.7%) met the inclusion criteria (Figure 1). Dual antiplatelet therapy was given to 6590 (96.9%) patients, among whom 6504 (98.7%) received therapy before diagnostic catheter placement. The mean (SD) GRACE score was 126.60 (28.70), and the mean (SD) CRUSADE score was 42.35 (11.97).
Figure 1. Flowchart.
Patients lost to follow-up were treated as censored at the time of discharge in analysis. PCI indicates percutaneous coronary intervention.
Baseline characteristics are presented in Table 1. PACT was administered to 2115 (31.1%) patients. Low-molecular-weight heparin was the most commonly used parenteral anticoagulant (79.1%), followed by fondaparinux (16.3%) and a combination of the 2 anticoagulants (4.6%). The median duration of PACT was 6 days (interquartile range, 4-9 days). Patients in the PACT group had higher mean (SD) GRACE scores (132.14 [30.23] vs 123.97 [27.56]; P < .001) but similar CRUSADE scores (42.39 [11.87] vs 42.34 [12.02]; P = .87) compared with those in the non-PACT group.
Table 1. Characteristics of Patients at Index Hospitalization.
| Characteristic | All Patients (N = 6804)a | P Value | Propensity-Matched Patients | P Value | Standard Difference (%) | ||
|---|---|---|---|---|---|---|---|
| Non-PACT (n = 4689) | PACT (n = 2115) | Non-PACT (n = 997) | PACT (n = 997) | ||||
| Age | |||||||
| Mean (SD), y | 64.01 (10.34) | 64.48 (10.62) | .08 | 64.41 (10.63) | 64.14 (10.36) | .57 | 2.53 |
| ≥65 y, No. (%) | 2283 (48.7) | 1098 (51.9) | .01 | 509 (51.1) | 499 (50.1) | .65 | NA |
| Female, No. (%) | 1189 (25.4) | 511 (24.2) | .29 | 225 (22.6) | 243 (24.4) | .34 | −4.26 |
| Weight, mean (SD), kg | 65.89 (11.53) | 66.37 (11.78) | .12 | 66.37 (12.51) | 65.95 (12.01) | .45 | 3.41 |
| Heart rate, mean (SD), bpm | 73.53 (10.84) | 74.05 (12.40) | .10 | 73.56 (11.05) | 74.01 (11.41) | .38 | −3.93 |
| Blood pressure, mean (SD), mm Hg | |||||||
| Systolic | 134.01 (19.01) | 135.09 (20.33) | .04 | 134.82 (19.46) | 134.58 (19.80) | .78 | 1.23 |
| Diastolic | 77.21 (11.37) | 78.02 (11.94) | .01 | 77.65 (11.60) | 77.42 (11.48) | .66 | 1.99 |
| Disease type, No. (%) | |||||||
| NSTEMI | 2647 (56.5) | 1254 (59.3) | .03 | 577 (57.9) | 577 (57.9) | >.99 | 0 |
| Unstable angina | 2042 (43.5) | 861 (40.7) | NA | 420 (42.1) | 420 (42.1) | NA | NA |
| Heart failure, No. (%) | 439 (9.4) | 403 (19.1) | <.001 | 131 (13.1) | 140 (14.0) | .56 | −2.63 |
| LVEF, mean (SD), % | 62.34 (10.41) | 60.36 (9.62) | <.001 | 61.58 (10.46) | 60.75 (10.66) | .12 | NA |
| eGFR | |||||||
| Mean (SD), mL/min/1.73 m2 | 81.67 (24.70) | 83.40 (25.91) | .01 | 80.87 (24.07) | 81.49 (25.55) | .58 | −2.49 |
| ≤60 mL/min/1.73 m2, No. (%) | 811 (17.3) | 366 (17.3) | .99 | 164 (16.4) | 175 (17.6) | .51 | NA |
| Serum creatinine level, mean (SD), μmol/dL | 1.05 (0.68) | 1.03 (0.60) | .17 | 1.06 (0.70) | 1.08 (0.78) | .71 | NA |
| Hematocrit, mean (SD) | 0.40 (0.11) | 0.40 (0.19) | .25 | 0.41 (0.22) | 0.40 (0.15) | .24 | −2.95 |
| Anemia, No. (%) | 1474 (31.4) | 678 (32.1) | .61 | 314 (31.5) | 325 (32.6) | .60 | NA |
| Medical history and risk factors, No. (%) | |||||||
| Current smoker | 1207 (25.7) | 690 (32.6) | <.001 | 308 (30.9) | 303 (30.4) | .81 | 1.09 |
| Cardiac arrest | 7 (0.1) | 5 (0.2) | .43 | 2 (0.2) | 2 (0.2) | >.99 | 0 |
| Myocardial infarction | 661 (14.1) | 394 (18.6) | <.001 | 170 (17.1) | 175 (17.6) | .77 | −1.33 |
| Percutaneous coronary intervention | 902 (19.2) | 338 (16.0) | .001 | 172 (17.3) | 162 (16.2) | .55 | 2.69 |
| Coronary artery bypass surgery | 61 (1.3) | 16 (0.8) | .049 | 10 (1.0) | 11 (1.1) | .83 | −0.98 |
| Stroke | 301 (6.4) | 167 (7.9) | .03 | 70 (7.0) | 64 (6.4) | .59 | 2.40 |
| Atrial fibrillation | 121 (2.6) | 108 (5.1) | <.001 | 40 (4.0) | 31 (3.1) | .28 | 4.87 |
| Hypertension | 3092 (65.9) | 1457 (68.9) | .02 | 687 (68.9) | 665 (66.7) | .29 | 4.72 |
| Diabetes | 1434 (30.6) | 678 (32.1) | .22 | 323 (32.4) | 311 (31.2) | .56 | 2.58 |
| In-hospital medication, No. (%) | |||||||
| Clopidogrel or ticagrelor loading dose | 2948 (62.9) | 1494 (70.6) | <.001 | 686 (68.8) | 668 (67.0) | .39 | 3.87 |
| Dual antiplatelet therapy | 4514 (96.3) | 2076 (98.2) | <.001 | 970 (97.3) | 968 (97.1) | .79 | 1.21 |
| Clopidogrel | 4514 (100) | 2068 (99.6) | NA | 970 (97.3) | 967 (97.0) | NA | NA |
| Ticagrelorb | 0 | 8 (0.4) | NA | 0 | 1 (0.1) | NA | NA |
| Type of parenteral anticoagulant, No. (%) | |||||||
| Low-molecular-weight heparin | NA | 1673 (79.1) | NA | NA | 744 (74.6) | NA | NA |
| Fondaparinux | NA | 345 (16.3) | NA | NA | 237 (23.8) | NA | NA |
| Low-molecular-weight heparin and fondaparinux | NA | 97 (4.6) | NA | NA | 16 (1.6) | NA | NA |
| Glycoprotein IIb/IIIa inhibitor | 428 (9.1) | 273 (12.9) | <.001 | 83 (8.3) | 80 (8.0) | .81 | 1.10 |
| Warfarin sodium | 22 (0.5) | 17 (0.8) | .09 | 11 (1.1) | 6 (0.6) | .22 | 5.46 |
| Statin | 4588 (97.8) | 2086 (98.6) | .03 | 980 (98.3) | 980 (98.3) | >.99 | 0 |
| ACE inhibitor or ARB | 3594 (76.6) | 1719 (81.3) | <.001 | 802 (80.4) | 802 (80.4) | >.99 | 0 |
| Calcium-channel blocker | 1071 (22.8) | 573 (27.1) | <.001 | 248 (24.9) | 243 (24.4) | .80 | 1.16 |
| β-Blocker | 3886 (82.9) | 1787 (84.5) | .10 | 836 (83.9) | 835 (83.8) | .95 | 0.27 |
| Procedure characteristic, No. (%) | |||||||
| Radial access | 4172 (89.0) | 1710 (80.9) | <.001 | 880 (88.3) | 880 (88.3) | >.99 | 0 |
| Coronary anatomy | |||||||
| Any left main disease | 570 (12.2) | 246 (11.6) | <.001 | 132 (13.2) | 123 (12.3) | .83 | 2.70 |
| Multivessel disease | 2898 (61.8) | 1445 (68.3) | 634 (63.6) | 640 (64.2) | −1.25 | ||
| Other | 1221 (26.0) | 424 (20.0) | 231 (23.2) | 234 (23.5) | −0.71 | ||
| Treated vessel | |||||||
| Any left main disease | 378 (8.1) | 177 (8.4) | .91 | 102 (10.2) | 92 (9.2) | .42 | 3.38 |
| Multivessel disease | 1547 (33.0) | 694 (32.8) | 322 (32.3) | 348 (34.9) | −5.52 | ||
| Other | 2764 (58.9) | 1244 (58.8) | 573 (57.5) | 557 (55.9) | 3.24 | ||
| Stent type, No. (%) | |||||||
| Drug eluting stent | 4681 (99.8) | 2091 (98.9) | <.001 | 994 (99.7) | 996 (99.9) | .32 | −4.48 |
| First generation | 2447 (52.2) | 1128 (53.3) | 503 (50.5) | 546 (54.8) | NA | ||
| Second generation | 2234 (47.6) | 963 (45.5) | 491 (49.2) | 449 (45.0) | NA | ||
| Bare metal stent, No. (%) | 3 (0.1) | 1 (0.0) | 0 | 0 | 0 | ||
| PTCA or aspiration only, No. (%) | 5 (0.1) | 23 (1.1) | 3 (0.3) | 1 (0.1) | 4.48 | ||
| Stents, No. (IQR) | 2 (1-3) | 2 (1-3) | .33 | 2 (1-3) | 2 (1-3) | .51 | −3.24 |
| Total length of stents, median (IQR), mm | 41 (24-66) | 44 (25-69) | .03 | 42 (24-71) | 43 (28-71) | .73 | −2.23 |
| Thrombus aspiration, No. (%) | 47 (1.0) | 24 (1.1) | .62 | 13 (1.3) | 10 (1.0) | .53 | 2.82 |
| Time to procedure | |||||||
| Median (IQR), d | 1 (1-3) | 3 (2-5) | <.001 | 2 (1-4) | 2 (1-4) | .35 | NA |
| <24 h, No. (%) | 2511 (53.6) | 440 (20.8) | <.001 | 330 (33.1) | 322 (32.3) | .85 | 1.71 |
| 24-72 h, No. (%) | 1417 (30.2) | 720 (34.0) | 353 (35.4) | 365 (36.6) | −2.51 | ||
| >72 h, No. (%) | 761 (16.2) | 955 (45.2) | 314 (31.5) | 310 (31.1) | 0.87 | ||
Abbreviations: ACE, angiostensin-converting enzyme; ARB, angiotensin receptor blocker; eGFR, estimated glomerular filtration rate; IQR, interquartile range; LVEF, left ventricular ejection fraction; NA, not applicable; NSTEMI, non–ST-segment elevation myocardial infarction; PACT, parenteral anticoagulation therapy; PTCA, percutaneous transluminal coronary angioplasty.
Data were missing for the following categories: current smoker (n = 22), LVEF (n = 1210), hematocrit (n = 138), weight (n = 177), and eGFR (n = 79).
Ticagrelor was available in only 1 of the 5 centers until late 2014.
In-Hospital Outcomes
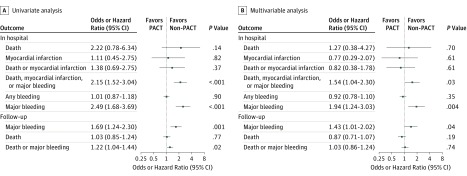
There was no significant difference in in-hospital all-cause death between the PACT and the non-PACT groups (0.3% vs 0.1%; P = .13) (adjusted odds ratio [OR], 1.27; 95% CI, 0.38-4.27; P = .70). The incidence of major bleeding in the PACT group was higher than that in the non-PACT group (2.5% vs 1.0%; P < .001) (adjusted OR, 1.94; 95% CI, 1.24-3.03; P = .004) (Table 2 and Figure 2). After adjustment for the GRACE or CRUSADE score, similar results were shown (eTables 1 and 2 in the Supplement).
Table 2. In-Hospital and Long-term Clinical Outcomes.
| Outcome | No. (%) | P Value | |
|---|---|---|---|
| Non-PACT (N = 4689) | PACT (N = 2115) | ||
| In-hospital outcome | |||
| Deatha | 7 (0.1) | 7 (0.3) | .13 |
| Myocardial infarction | 14 (0.3) | 7 (0.3) | .82 |
| Death or myocardial infarction | 21 (0.4) | 13 (0.6) | .37 |
| Major bleeding | 48 (1.0) | 53 (2.5) | <.001 |
| Any bleeding | 607 (12.9) | 276 (13.0) | .91 |
| Death, myocardial infarction, or major bleeding | 67 (1.4) | 64 (3.0) | <.001 |
| Long-term outcome | |||
| 30 d | |||
| Death | 13 (0.3) | 10 (0.5) | .20 |
| Major bleeding | 48 (1.0) | 53 (2.5) | <.001 |
| Death or major bleeding | 59 (1.3) | 61 (2.9) | <.001 |
| 1 y | |||
| Death | 91 (1.9) | 39 (1.8) | .79 |
| Major bleeding | 61 (1.3) | 58 (2.7) | <.001 |
| Death or major bleeding | 146 (3.1) | 93 (4.4) | .01 |
| 3 y | |||
| Death | 227 (4.8) | 111 (5.2) | .47 |
| Major bleeding | 91 (1.9) | 68 (3.2) | .001 |
| Death or major bleeding | 310 (6.6) | 169 (8.0) | .04 |
Abbreviation: PACT, parenteral anticoagulant therapy.
All-cause death.
Figure 2. Univariate and Multivariable Logistic or Cox Proportional Hazards Regression Analyses for Clinical Outcomes.
PACT indicates parenteral anticoagulant therapy.
The incidence of MI (0.3% vs 0.3%; P = .82) (adjusted OR, 0.77; 95% CI, 0.29-2.07; P = .61), any bleeding (13.0% vs 12.9%; P = .91) (adjusted OR, 0.92; 95% CI, 0.78-1.10; P = .35), and the composite end point of death or MI (0.6% vs 0.4%; P = .37) (adjusted OR, 0.82; 95% CI, 0.38-1.78; P = .61) were not significantly different between the 2 groups. However, the incidence of the composite end point of death, MI, or major bleeding was higher in the PACT group than in the non-PACT group (3.0% vs 1.4%; P < .001) (adjusted OR, 1.54; 95% CI, 1.04-2.30; P = .03) (Table 2 and Figure 2).
Long-term Outcomes
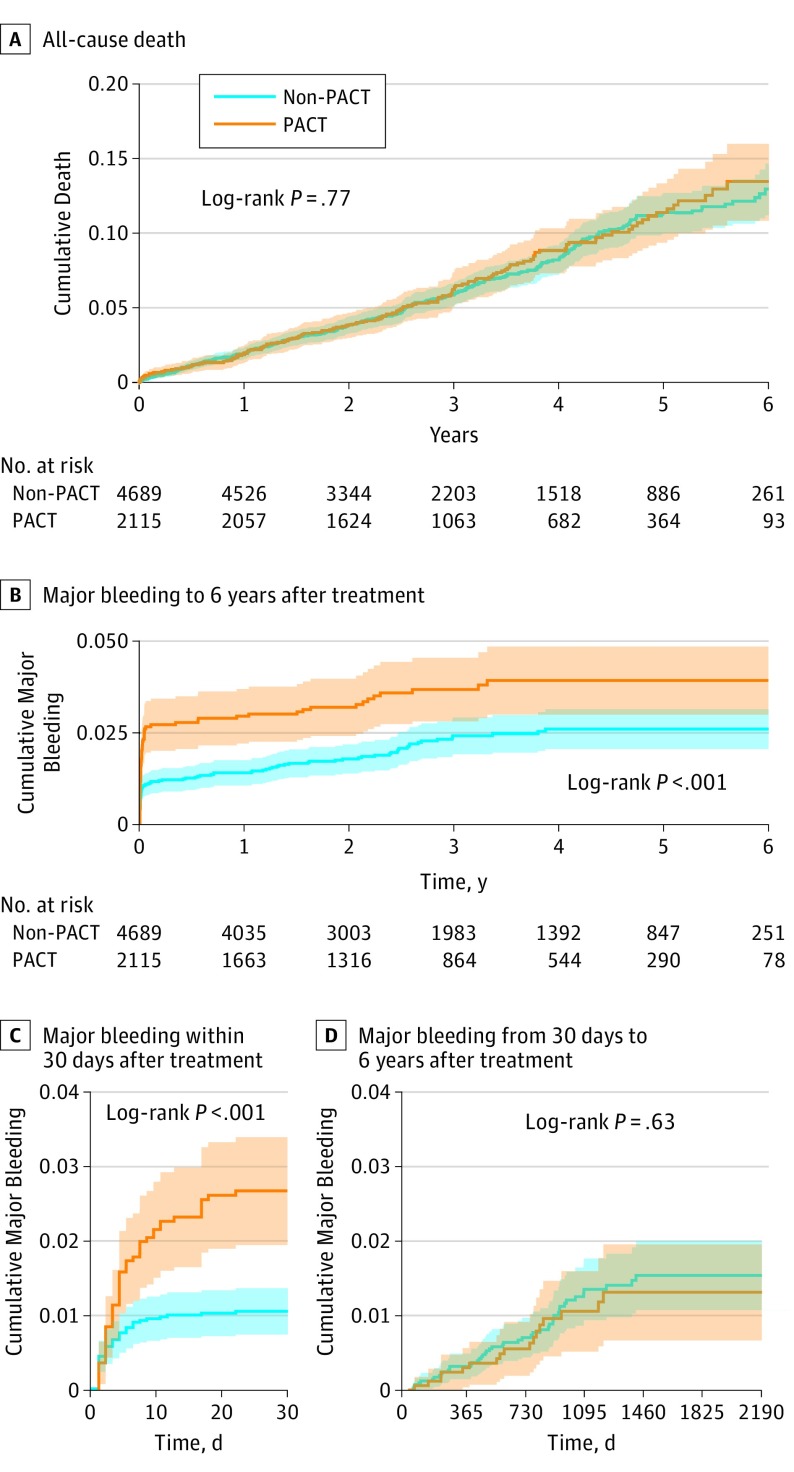
Complete follow-up (mean [SD] period, 3.25 [1.56] years; median [interquartile range], 2.96 years [1.93-4.46 years]) for all-cause death was achieved for 6765 patients (99.4%) and for major bleeding was achieved for 5867 patients (86.2%). All-cause death was not significantly different between the PACT and the non-PACT groups (adjusted hazard ratio [HR], 0.87; 95% CI, 0.71-1.07; P = .19) (Table 2 and Figure 2 and Figure 3A). However, patients in the PACT group tended to have higher risk of major bleeding (adjusted HR, 1.43; 95% CI, 1.01-2.02; P = .04). (Table 2 and Figure 2 and Figure 3B). The bleeding episodes in the PACT group mostly occurred in the first 30 days, and no further difference in new bleeding events between the groups was found in long-term follow-up (Figure 3C and D and eFigure 1 in the Supplement). The additive hazards model confirmed these results (eFigures 2 and 3 in the Supplement). The incidence of the composite end point of death or major bleeding did not differ between the 2 groups (adjusted HR, 1.03; 95% CI, 0.86-1.24; P = .74) (Figure 2 and eFigure 4 in the Supplement).
Figure 3. Kaplan-Meier Estimated Event Rates of All-Cause Death and Major Bleeding.
PACT indicates parenteral anticoagulation therapy.
Subgroup Analyses
The association of PACT with all-cause mortality, MI, and major bleeding was not significantly different among patients with different GRACE or CRUSADE scores (low, medium, or high risk) (eFigure 5 in the Supplement). Other subgroup analyses did not identify any significant difference in all-cause death or in the composite outcomes of death or MI (eFigures 6 and 7 in the Supplement). The higher incidence of major bleeding associated with PACT was consistent across all the subgroups with the exception of patients with NSTEMI. Major bleeding appeared to be more pronounced in the subgroup without anemia or heart failure (eFigure 8 in the Supplement).
Propensity Score Analyses
We matched 997 patients receiving PACT to those receiving non-PACT in a 1:1 ratio (Table 1 and eTable 3 and eFigure 9 in the Supplement). The propensity-matched results showed an absence of a significant difference in all-cause death between the PACT and non-PACT groups (in-hospital OR, 1.33; 95% CI, 0.30-5.98; long-term HR, 0.97; 95% CI, 0.67-1.39). PACT was associated with a higher risk of major bleeding during the hospital stay (OR, 2.33; 95% CI, 1.07-5.09), and a similar result was found at follow-up, although it was not statistically significant (HR, 1.47; 95% CI, 0.82-2.64) (eTable 4 in the Supplement). A similar result was also found in the covariate adjustment using the propensity score and after stratification of the quintiles of the propensity score (eTables 3 and 4 and eFigure 10 in the Supplement).
Heterogeneity Analyses Between Centers
The heterogeneity analyses showed low heterogeneity for in-hospital and long-term outcomes among the centers (I2≤30%, where I2 indicates the percentage of variation across centers that is the result of heterogeneity) (eFigures 11 and 12 in the Supplement). Furthermore, consistent results were found in the multivariable analyses stratified by centers and including the random association of the centers (eTable 5 in the Supplement).
Discussion
This study, to our knowledge, is the first to evaluate the association between PACT and clinical outcomes in patients undergoing PCI for NSTE-ACS. Our results showed that PACT was not associated with a lower risk of in-hospital death or MI or long-term death but was associated with a higher risk of major bleeding.
Unfractionated heparin has been previously proven to lower the risk of adverse cardiovascular events in patients with NSTE-ACS.1 Newer PACT, such as low-molecular-weight heparin and fondaparinux, is considered to be superior to unfractionated heparin.2,4,12,13 Although recommended for all patients with NSTE-ACS, PACT was reportedly used in 72.7% of patients with NSTE-ACS in China.14 It is difficult to determine the reason behind the variety of management. A possible explanation could be that physicians in PCI centers are reluctant to administer PACT before the procedure because of concern for bleeding attributable to aggressive antithrombotic treatment and the crossover between different anticoagulants. Although anticoagulation therapy has been proven to be beneficial among patients with NSTE-ACS, all studies comparing PACT with placebo or control were conducted more than 20 years ago,2,3 when neither dual antiplatelet therapy nor PCI was commonly used. Contrary to the previous findings, our study found no significant difference in effectiveness outcomes between the PACT and non-PACT groups. The difference in the findings may be explained by the difference in the end points over time and the changes in clinical practice. In previous studies, the benefit of PACT was mainly attributed to a reduction in recurrent angina and emergency revascularization.1,2,3 The PCI is now being widely accepted as an important measure to effectively prevent ischemic events, particularly in high-risk groups.15,16,17,18 Therefore, the protective effect of PACT might become less significant.
Conversely, our results reinforce the findings of previous studies,1,2,3,19 wherein no significant difference of mortality between the 2 groups was identified. Moreover, the results of previous studies were inconsistent even with respect to the composite end points, with some of them finding no protective effect of PACT in patients with NSTE-ACS.20,21,22,23 It could be argued that the efficacy of PACT might have been underestimated because fondaparinux, which has been found to be superior to enoxaparin because of fewer bleeding events,4 was used in less than 20% of patients in our study. Fondaparinux, enoxaparin, and bivalirudin have shown superiority compared with the older anticoagulants. However, studies comparing these agents with placebo are still lacking.14,15,16,24 Our results are hypothesis generating, and further studies are needed to evaluate the role of PACT in patients with NSTE-ACS for whom invasive management is planned.
An assessment of the role of PACT in patients with NSTE-ACS also requires consideration of antiplatelet therapy. Antiplatelet agents are the core of medical management in patients with ACS owing to their ability to prevent acute coronary events. Dual antiplatelet therapy consisting of aspirin and clopidogrel has been proven to improve patient prognoses and is recommended for patients with NSTE-ACS.25,26,27 Another study28 showed that ticagrelor was associated with further reduction in adverse events compared with clopidogrel, especially in those undergoing PCI. The P2Y12 inhibitors were not used in any of the previous studies that have shown the advantage of PACT over placebo.1,2,3 In our study, more than 96% of patients were prescribed P2Y12 inhibitors (96.4% of clopidogrel and 0.4% of ticagrelor) in addition to aspirin, with more than 62% receiving a loading dose of P2Y12 inhibitors before PCI. When platelet function is fundamentally inhibited by more aggressive antiplatelet therapy, the association of PACT might be attenuated.
Although the association between PACT and increased bleeding has been well established,29 it is possible that, in our study, more major bleeding in the anticoagulation group occurred because of an imbalance of the baseline characteristics. The association between PACT and major bleeding remained significant in both the logistic regression analyses and the propensity analyses. Moreover, the difference between the groups was based on mainly more major bleeding episodes within 30 days in the PACT group, and after 30 days, a similar incidence of new bleeding was observed. This finding suggests that the difference in management rather than an imbalance of baseline characteristics could be the main reason for higher bleeding in the PACT group. Conversely, excessive anticoagulation resulting from a crossover between different anticoagulants could not be ruled out as a contributing factor owing to the routine use of unfractionated heparin (99.9%) in our study for procedures in catheter laboratories regardless of the choice of preprocedure anticoagulation. A Korean study9 reported no extra bleeding events among patients who were routinely given unfractionated heparin in the catheter laboratory when low-molecular-weight heparin was administered before PCI. The incidence of major bleeding in the anticoagulation group was comparable to that in a previous study without crossover anticoagulation therapy.30 Therefore, anticoagulation itself rather than crossover anticoagulation therapy may have been the main cause of more major bleeding in the PACT group.
Limitations
Although many measures were taken to control inherent bias in this retrospective study, residual confounders secondary to unmeasured variables were possible. Bleeding events could not be determined in 13.8% of the study population during follow-up. However, our finding that PACT was not associated with long-term bleeding after hospital discharge is similar to previous studies, implying that the missing follow-up data were unlikely to change the results.4,31 Most of the patients were classified as low-moderate risk, which might underestimate the efficacy of PACT among patients with NSTE-ACS. However, the neutral effect of PACT in this study is still relevant to clinical practice because PACT is recommended in all patients with NSTE-ACS by current guidelines regardless of risk stratification. The low event rate means that our study had insufficient power to exclude a substantial associated increase in the risk of death or MI, which justifies further studies to determine the efficacy of PACT. Finally, because we only included patients undergoing PCI, the findings could not be generalized to all patients with NSTE-ACS.
Conclusions
In patients undergoing PCI for NSTE-ACS, PACT was not associated with lower risk of all-cause death or MI but was significantly associated with higher risk of major bleeding. Further studies are warranted to determine the role of PACT in this context.
eAppendix 1. Sample Size Calculations
eAppendix 2. Statistical Analysis Protocol
eTable 1. GRACE and Other Risk Factors Adjusted Result
eTable 2. CRUSADE and Other Risk Factors Adjusted Result
eFigure 1. Kaplan-Meier Estimated Event Rates of Major Bleeding in the First 30-Day Period
eFigure 2. Cumulative Regression Curves of Intercept and PACT Treatment in Univariate Analysis of the Additive Hazards Model
eFigure 3. Cumulative Regression Curves of PACT Treatment in the Multivariable Analysis of the Additive Hazards Model
eFigure 4, Kaplan-Meier Estimated Event Rates of All-Cause-Death or Major Bleeding
eFigure 5. Subgroup Analysis Based on the Different Risk of GRACE and CRUSADE score
eFigure 6. Subgroup Analysis of In-Hospital All-Cause Death
eFigure 7. Subgroup Analysis of In-Hospital All-Cause Death or Myocardial Infarction
eFigure 8. Subgroup Analysis of In-Hospital Major Bleeding
eFigure 9 Distributions of Propensity Scores Between the Two Groups Before and After Matching
eTable 3. Standardized Difference Between the Two Groups After Matching and Stratifying by Propensity Score (%)
eFigure 10. Quantile-Quartile Plots That Compare the Distribution of the Propensity Score for the PACT Group of Patients to Those of the NPACT Group Patients Within Each Quintile of the Propensity Score
eTable 4. Results of Propensity Score Analysis
eFigure 11. Heterogeneity Analysis by Stratifying Centers for In-Hospital Outcomes
eFigure 12. Heterogeneity Analysis by Stratifying Centers for Long-Term Outcomes
eTable 5. Multivariable Analysis by Centers and Including Random Effects of Centers
References
- 1.Cohen M, Adams PC, Parry G, et al. ; Antithrombotic Therapy in Acute Coronary Syndromes Research Group . Combination antithrombotic therapy in unstable rest angina and non–Q-wave infarction in nonprior aspirin users: primary end points analysis from the ATACS trial. Circulation. 1994;89(1):81-88. doi: 10.1161/01.CIR.89.1.81 [DOI] [PubMed] [Google Scholar]
- 2.Gurfinkel EP, Manos EJ, Mejaíl RI, et al. Low molecular weight heparin versus regular heparin or aspirin in the treatment of unstable angina and silent ischemia. J Am Coll Cardiol. 1995;26(2):313-318. doi: 10.1016/0735-1097(95)80001-W [DOI] [PubMed] [Google Scholar]
- 3.Fragmin during Instability in Coronary Artery Disease (FRISC) study group. Low–molecular-weight heparin during instability in coronary artery disease. Lancet. 1996;347(9001):561-568. doi: 10.1016/S0140-6736(96)91270-2 [DOI] [PubMed] [Google Scholar]
- 4.Yusuf S, Mehta SR, Chrolavicius S, et al. Comparison of fondaparinux and enoxaparin in acute coronary syndromes. N Engl J Med. 2006;354(14):1464-1476. doi: 10.1056/NEJMoa055443 [DOI] [PubMed] [Google Scholar]
- 5.Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation. 2011;124(23):e574-e651. [DOI] [PubMed] [Google Scholar]
- 6.Han Y, Guo J, Zheng Y, et al. ; BRIGHT Investigators . Bivalirudin vs heparin with or without tirofiban during primary percutaneous coronary intervention in acute myocardial infarction: the BRIGHT randomized clinical trial. JAMA. 2015;313(13):1336-1346. doi: 10.1001/jama.2015.2323 [DOI] [PubMed] [Google Scholar]
- 7.Mehran R, Rao SV, Bhatt DL, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123(23):2736-2747. doi: 10.1161/CIRCULATIONAHA.110.009449 [DOI] [PubMed] [Google Scholar]
- 8.Cannon CP, Brindis RG, Chaitman BR, et al. 2013 ACCF/AHA key data elements and definitions for measuring the clinical management and outcomes of patients with acute coronary syndromes and coronary artery disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on clinical data standards (writing committee to develop acute coronary syndromes and coronary artery disease clinical data standards). J Am Coll Cardiol. 2013;61(9):992-1025. doi: 10.1016/j.jacc.2012.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li YJ, Rha SW, Chen KY, et al. ; Korea Acute Myocardial Infarction Registry Investigators . Low molecular weight heparin versus unfractionated heparin in patients with acute non-ST-segment elevation myocardial infarction undergoing percutaneous coronary intervention with drug-eluting stents. J Cardiol. 2012;59(1):22-29. doi: 10.1016/j.jjcc.2011.09.005 [DOI] [PubMed] [Google Scholar]
- 10.Granger CB, Goldberg RJ, Dabbous O, et al. ; Global Registry of Acute Coronary Events Investigators . Predictors of hospital mortality in the Global Registry of Acute Coronary Events. Arch Intern Med. 2003;163(19):2345-2353. doi: 10.1001/archinte.163.19.2345 [DOI] [PubMed] [Google Scholar]
- 11.Subherwal S, Bach RG, Chen AY, et al. Baseline risk of major bleeding in non-ST-segment-elevation myocardial infarction: the CRUSADE (Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes With Early Implementation of the ACC/AHA Guidelines) bleeding score. Circulation. 2009;119(14):1873-1882. doi: 10.1161/CIRCULATIONAHA.108.828541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen M, Demers C, Gurfinkel EP, et al. ; Efficacy and Safety of Subcutaneous Enoxaparin in Non–Q-Wave Coronary Events Study Group . A comparison of low-molecular-weight heparin with unfractionated heparin for unstable coronary artery disease. N Engl J Med. 1997;337(7):447-452. doi: 10.1056/NEJM199708143370702 [DOI] [PubMed] [Google Scholar]
- 13.Ferguson JJ, Califf RM, Antman EM, et al. ; SYNERGY Trial Investigators . Enoxaparin vs unfractionated heparin in high-risk patients with non–ST-segment elevation acute coronary syndromes managed with an intended early invasive strategy: primary results of the SYNERGY randomized trial. JAMA. 2004;292(1):45-54. [DOI] [PubMed] [Google Scholar]
- 14.Yang Q, Wang Y, Liu J, et al. ; CCC-ACS Investigators . Invasive management strategies and antithrombotic treatments in patients with non–ST-segment-elevation acute coronary syndrome in China: findings from the Improving CCC Project (Care for Cardiovascular Disease in China). Circ Cardiovasc Interv. 2017;10(6):e004750. doi: 10.1161/CIRCINTERVENTIONS.116.004750 [DOI] [PubMed] [Google Scholar]
- 15.Cannon CP, Weintraub WS, Demopoulos LA, et al. ; TACTICS (Treat Angina with Aggrastat and Determine Cost of Therapy With an Invasive or Conservative Strategy)—Thrombolysis in Myocardial Infarction 18 Investigators . Comparison of early invasive and conservative strategies in patients with unstable coronary syndromes treated with the glycoprotein IIb/IIIa inhibitor tirofiban. N Engl J Med. 2001;344(25):1879-1887. doi: 10.1056/NEJM200106213442501 [DOI] [PubMed] [Google Scholar]
- 16.Mehta SR, Granger CB, Boden WE, et al. ; TIMACS Investigators . Early versus delayed invasive intervention in acute coronary syndromes. N Engl J Med. 2009;360(21):2165-2175. doi: 10.1056/NEJMoa0807986 [DOI] [PubMed] [Google Scholar]
- 17.Stefanini GG, Holmes DR Jr. Drug-eluting coronary-artery stents. N Engl J Med. 2013;368(3):254-265. doi: 10.1056/NEJMra1210816 [DOI] [PubMed] [Google Scholar]
- 18.Wallentin L, Lindhagen L, Ärnström E, et al. ; FRISC-II study group . Early invasive versus non-invasive treatment in patients with non–ST-elevation acute coronary syndrome (FRISC-II): 15 year follow-up of a prospective, randomised, multicentre study. Lancet. 2016;388(10054):1903-1911. doi: 10.1016/S0140-6736(16)31276-4 [DOI] [PubMed] [Google Scholar]
- 19.Andrade-Castellanos CA, Colunga-Lozano LE, Delgado-Figueroa N, Magee K. Heparin versus placebo for non-ST elevation acute coronary syndromes. Cochrane Database Syst Rev. 2014;(6):CD003462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Théroux P, Ouimet H, McCans J, et al. Aspirin, heparin, or both to treat acute unstable angina. N Engl J Med. 1988;319(17):1105-1111. doi: 10.1056/NEJM198810273191701 [DOI] [PubMed] [Google Scholar]
- 21.Cohen M, Adams PC, Hawkins L, Bach M, Fuster V. Usefulness of antithrombotic therapy in resting angina pectoris or non–Q-wave myocardial infarction in preventing death and myocardial infarction (a pilot study from the Antithrombotic Therapy in Acute Coronary Syndromes Study Group). Am J Cardiol. 1990;66(19):1287-1292. doi: 10.1016/0002-9149(90)91155-Y [DOI] [PubMed] [Google Scholar]
- 22.The RISC Group Risk of myocardial infarction and death during treatment with low dose aspirin and intravenous heparin in men with unstable coronary artery disease. Lancet. 1990;336(8719):827-830. doi: 10.1016/0140-6736(90)92336-G [DOI] [PubMed] [Google Scholar]
- 23.Holdright D, Patel D, Cunningham D, et al. Comparison of the effect of heparin and aspirin versus aspirin alone on transient myocardial ischemia and in-hospital prognosis in patients with unstable angina. J Am Coll Cardiol. 1994;24(1):39-45. doi: 10.1016/0735-1097(94)90539-8 [DOI] [PubMed] [Google Scholar]
- 24.Stone GW, McLaurin BT, Cox DA, et al. ; ACUITY Investigators . Bivalirudin for patients with acute coronary syndromes. N Engl J Med. 2006;355(21):2203-2216. doi: 10.1056/NEJMoa062437 [DOI] [PubMed] [Google Scholar]
- 25.Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC guideline for the management of patients with non–ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64(24):e139-e228. doi: 10.1016/j.jacc.2014.09.017 [DOI] [PubMed] [Google Scholar]
- 26.Roffi M, Patrono C, Collet JP, et al. ; ESC Scientific Document Group . 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting Without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J. 2016;37(3):267-315. doi: 10.1093/eurheartj/ehv320 [DOI] [PubMed] [Google Scholar]
- 27.Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK; Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial Investigators . Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001;345(7):494-502. doi: 10.1056/NEJMoa010746 [DOI] [PubMed] [Google Scholar]
- 28.Wallentin L, Becker RC, Budaj A, et al. ; PLATO Investigators . Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361(11):1045-1057. doi: 10.1056/NEJMoa0904327 [DOI] [PubMed] [Google Scholar]
- 29.Fragmin Fast Revascularisation During Instability in Coronary Artery Disease (FRISC II) Investigators Long-term low-molecular-mass heparin in unstable coronary-artery disease: FRISC II prospective randomised multicentre study. Lancet. 1999;354(9180):701-707. doi: 10.1016/S0140-6736(99)07350-X [DOI] [PubMed] [Google Scholar]
- 30.Mehta SR, Granger CB, Eikelboom JW, et al. Efficacy and safety of fondaparinux versus enoxaparin in patients with acute coronary syndromes undergoing percutaneous coronary intervention: results from the OASIS-5 trial. J Am Coll Cardiol. 2007;50(18):1742-1751. doi: 10.1016/j.jacc.2007.07.042 [DOI] [PubMed] [Google Scholar]
- 31.Stone GW, Ware JH, Bertrand ME, et al. ; ACUITY Investigators . Antithrombotic strategies in patients with acute coronary syndromes undergoing early invasive management: one-year results from the ACUITY trial. JAMA. 2007;298(21):2497-2506. doi: 10.1001/jama.298.21.2497 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix 1. Sample Size Calculations
eAppendix 2. Statistical Analysis Protocol
eTable 1. GRACE and Other Risk Factors Adjusted Result
eTable 2. CRUSADE and Other Risk Factors Adjusted Result
eFigure 1. Kaplan-Meier Estimated Event Rates of Major Bleeding in the First 30-Day Period
eFigure 2. Cumulative Regression Curves of Intercept and PACT Treatment in Univariate Analysis of the Additive Hazards Model
eFigure 3. Cumulative Regression Curves of PACT Treatment in the Multivariable Analysis of the Additive Hazards Model
eFigure 4, Kaplan-Meier Estimated Event Rates of All-Cause-Death or Major Bleeding
eFigure 5. Subgroup Analysis Based on the Different Risk of GRACE and CRUSADE score
eFigure 6. Subgroup Analysis of In-Hospital All-Cause Death
eFigure 7. Subgroup Analysis of In-Hospital All-Cause Death or Myocardial Infarction
eFigure 8. Subgroup Analysis of In-Hospital Major Bleeding
eFigure 9 Distributions of Propensity Scores Between the Two Groups Before and After Matching
eTable 3. Standardized Difference Between the Two Groups After Matching and Stratifying by Propensity Score (%)
eFigure 10. Quantile-Quartile Plots That Compare the Distribution of the Propensity Score for the PACT Group of Patients to Those of the NPACT Group Patients Within Each Quintile of the Propensity Score
eTable 4. Results of Propensity Score Analysis
eFigure 11. Heterogeneity Analysis by Stratifying Centers for In-Hospital Outcomes
eFigure 12. Heterogeneity Analysis by Stratifying Centers for Long-Term Outcomes
eTable 5. Multivariable Analysis by Centers and Including Random Effects of Centers