Key Points
Questions
Is lymph node positivity associated with disease recurrence in patients with small-bowel neuroendocrine tumors, and how many lymph nodes are needed to accurately stage patients undergoing curative resection?
Findings
This case series involving 199 patients who underwent curative-intent resection of a primary small-bowel neuroendocrine tumor found that lymph node positivity alone was not associated with disease recurrence; rather, a threshold of 4 positive lymph nodes was needed to see earlier disease recurrence. Retrieval of 8 or more total lymph nodes accurately discriminates recurrence-free survival in patients with 4 or more, 1 to 3, or 0 positive lymph nodes.
Meaning
Having 4 or more positive lymph nodes is associated with earlier disease recurrence, and a minimum of 8 lymph nodes are needed to stage patients with small-bowel neuroendocrine tumors.
Abstract
Importance
Little information is available regarding the minimum number of lymph nodes needed to accurately stage patients when performing a mesenteric lymphadenectomy for small-bowel neuroendocrine tumors.
Objectives
To determine the prognostic role of lymph node positivity and the ideal number of lymph nodes for accurately staging patients with small-bowel neuroendocrine tumors.
Design, Setting, and Participants
This case series from the US Neuroendocrine Tumor Study Group, a collaboration among
8 US-based, academic tertiary care referral centers, obtained demographic, perioperative, and pathologic data from the group’s database, Social Security Death Index, and publicly available obituaries. All patients in these institutions with small-bowel neuroendocrine tumors who underwent curative-intent surgical resection of a primary tumor between January 1, 2000, and December 31, 2015, were included (n = 199). Patients with duodenal or ampullary tumors, other nonneuroendocrine concurrent malignant neoplasms, mortality of fewer than 30 days after the surgical procedure, and distant metastatic disease were excluded. Data analysis was conducted from September 1, 2017, to December 1, 2017.
Main Outcomes and Measures
Primary study outcome was recurrence-free survival. Hypothesis was generated after data collection and data entry into the US Neuroendocrine Tumor Study Group database.
Results
Of the 199 patients included, 112 (56.3%) were male and 87 (43.7%) female with a mean (SD) age of 60.3 (12.5) years and a mean (SD) body mass index of 29.5 (6.0). One hundred fifty-four patients (77.4%) had lymph node–positive disease. No difference in 3-year recurrence-free survival was found between patients with lymph node–positive and lymph node–negative disease. Patients with 4 positive lymph nodes had a worse 3-year recurrence-free survival compared with those with 1 to 3 or 0 positive lymph nodes (81.6% vs 91.4% vs 92.1%; P = .01). When examining patients with fewer than 8 resected lymph nodes, no difference in 3-year recurrence-free survival was observed among patients with 4 or more, 1 to 3, or 0 positive lymph nodes (100% vs 93.8% vs 91.7%; P = .87). Retrieval of 8 or more lymph nodes, however, accurately discriminated patients with 4 or more, 1 to 3, or 0 positive lymph nodes (3-year recurrence-free survival: 79.9% vs 89.6% vs 92.9%; P = .05).
Conclusions and Relevance
The findings from this study suggest that, for patients undergoing curative-intent resection of small-bowel neuroendocrine tumors, accurate lymph node staging requires a minimum of 8 lymph nodes for examination, and 4 or more positive lymph nodes are associated with decreased 3-year recurrence-free survival compared with 1 to 3 or 0 positive lymph nodes; a thorough regional lymphadenectomy may be critical for accurate staging and management of this disease.
This case series evaluates demographic, perioperative, and pathologic data from the US Neuroendocrine Tumor Study Group database to identify the role of positive lymph nodes in small-bowel neuroendocrine tumors.
Introduction
Small-bowel neuroendocrine tumors (SB-NETs) are a rare subset of tumors of the gastrointestinal tract. Neuroendocrine tumors of the jejunum and ileum have more than 3 times higher incidence and an 11-month lower median overall survival than tumors in the duodenum.1 Although some SB-NETs secrete bioactive products, most are clinically silent until they present late in their course with mass effects. These tumors consist of a complex heterogeneity of disease, and they have not been associated with a substantial improvement in overall survival in the past 30 years.2 A surgical procedure has remained the primary method of cure in patients with SB-NETs, even with advancements in the therapeutic armamentarium.3,4
As with other solid-organ malignant neoplasms, the presence of lymph node metastasis plays a substantial prognostic role in SB-NETs. Changes in the cytokine milieu, the expansion of immunosuppressive cell lineages, and an increase in lymphangiogenesis all contribute to the seeding, survival, and subsequent expansion of tumor cells within the lymph node.5,6 Surgical resection of tumor-draining lymph nodes has come under scrutiny, especially in more superficial tumors, where an extended lymph node dissection is often associated with substantial morbidity for patients.7 In SB-NETs, however, regional mesenteric lymphadenectomy has been associated with improved survival.8,9 Nonetheless, the extent of lymphadenectomy has come under question. Patients with too few resected lymph nodes can be understaged, whereas the resection of a greater number of lymph nodes may enable the detection of clinically silent lymph node metastases.10,11,12 To circumvent the limitation of the number of lymph nodes procured, several groups have proposed using the lymph node ratio, or the number of tumor-positive lymph nodes divided by the total number of lymph nodes resected, as a way to place patients in prognostic stage groups.13,14,15,16,17 Increasing the lymph node ratio, as opposed to the total number of positive lymph nodes, may aid in a more accurate prognosis for patients with a solid-organ malignant neoplasm.
Little information is available regarding the minimum number of lymph nodes needed to accurately stage patients when performing a mesenteric lymphadenectomy for SB-NETs. Furthermore, few studies address the role of lymph node ratio in these patients. In this study, we aimed to (1) use a large, multi-institutional database to define the prognostic role of lymph node positivity and the number of positive lymph nodes in SB-NETs; (2) determine the ideal number of lymph nodes for accurately staging patients with these tumors; and (3) determine the prognostic value of the lymph node ratio in these patients.
Methods
The US Neuroendocrine Tumor Study Group (US-NETSG) was established in 2016 as a collaboration among 8 institutions: Emory University (Atlanta, Georgia), The Ohio State University (Columbus), Stanford University (Stanford, California), Virginia Mason University (Seattle, Washington), Vanderbilt University (Nashville, Tennessee), University of Michigan (Ann Arbor), University of Wisconsin (Madison), and Washington University in St Louis. The purpose of the US-NETSG was to build a platform to collect clinical, pathologic, and follow-up data retrospectively from academic and quaternary referral centers that treat high volumes of NETs. This study obtained approval from the institutional review board at each US-NETSG institution, which also waived the need for informed consent from patients. All patients with SB-NETs who underwent curative-intent surgical resection of a primary tumor between January 1, 2000, and December 31, 2015, were included. Patients with duodenal or ampullary tumors, other nonneuroendocrine concurrent malignant neoplasms, mortality of fewer than 30 days after the surgical procedure, and distant metastatic disease were excluded. Data analysis was conducted from September 1, 2017, to December 1, 2017.
Baseline demographic, perioperative, and pathologic data were collected retrospectively. Surgical pathologic specimens were reviewed by expert gastrointestinal pathologists at each institution. Staging was based on the guidelines of the American Joint Committee on Cancer (AJCC) Cancer Staging Manual, 7th edition.18 Survival and recurrence data were collected from the electronic medical record system of each US-NETSG institution, the Social Security Death Index, and publicly available obituaries.
The primary outcome studied was the association among variables of lymph node involvement (ie, lymph node positivity, number of positive lymph nodes, extent of lymphadenectomy, and lymph node ratio) with recurrence-free survival (RFS). Disease recurrence, defined as the radiographic evidence of recurrent disease, was detected through cross-sectional imaging (using either computed tomographic scan or magnetic resonance imaging); however, no standardized surveillance protocol between institutions was used during the study period.
Statistical Analysis
All statistical analysis was conducted using SPSS, version 22.0 (IBM Inc). The χ2 test was used to compare categorical variables, and an unpaired, 2-tailed t test was used for continuous variables. Univariable Cox proportional hazards regression analysis was used to determine the association of the variables of lymph node involvement with RFS. Kaplan-Meier survival plots for RFS were constructed to compare patients with differing variables of lymph node involvement. Statistical significance was defined as 2-sided P < .05.
Results
Of the 2182 total patients within the US-NETSG database, 199 underwent curative-intent resection of a primary SB-NET. Demographic characteristics are listed in Table 1. This cohort of patients consisted of 112 men (56.3%) and 87 women (43.7%), with a mean (SD) age of 60.3 (12.5) years and a mean (SD) body mass index of 29.5 (6.0) (calculated as weight in kilograms divided by height in meters squared). Pathologic characteristics are listed in Table 2. Notably, 20 patients (10.1%) had multifocal small-bowel tumors, 17 (8.7%) had R1 resections, and 13 (7.4%) had moderately differentiated tumors. One hundred fifty-four patients (77.4%) had lymph node–positive disease. The median (interquartile range [IQR]) number of lymph nodes retrieved was 13 (7-19), and the median (IQR) number of positive lymph nodes was 3 (1-5). The median (IQR) follow-up time was 39 (25-72) months, and the median RFS was not reached. Of the 32 patients (16.1%) who experienced disease recurrence, 9 (28.1%) were local recurrences, 22 (68.8%) were distant recurrences, and 1 (3.1%) was both a local and distant recurrence.
Table 1. Demographic Characteristics of All Patients.
| Baseline Variable | No. (%) |
|---|---|
| Age, mean (SD), y | 60.3 (12.5) |
| Male | 112 (56.3) |
| BMI, mean (SD) | 29.5 (6.0) |
| Race/ethnicity | |
| White | 149 (76.4) |
| Black | 43 (22.1) |
| Asian | 2 (1.0) |
| Other | 1 (0.5) |
| Functional status | |
| Independent | 190 (99.0) |
| Partially dependent | 2 (1.0) |
| Presenting symptom | |
| Pain | 107 (54.6) |
| Nausea/vomiting | 57 (29.1) |
| GI tract obstruction | 43 (21.8) |
| GI tract bleed | 41 (20.9) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); GI, gastrointestinal.
Table 2. Pathologic Characteristics of All Patients.
| Pathologic Variable | No. (%) |
|---|---|
| Tumor size, mean (SD), cm | 2.2 (1.7) |
| No. of tumors, mean (SD) | 2.2 (2.7) |
| Multifocal | 20 (10.1) |
| Tumor differentiation | |
| Well | 160 (91.4) |
| Moderate | 13 (7.4) |
| Poor | 2 (1.1) |
| Tumor grade | |
| Low | 97 (70.3) |
| Intermediate | 38 (27.5) |
| High | 3 (2.2) |
| Lymphovascular invasion | 98 (69.5) |
| Perineural invasion | 62 (50.8) |
| No. of lymph nodes retrieved, median (IQR) | 13 (7-19) |
| Lymph node positive | 154 (77.4) |
| No. of lymph nodes positive, median (IQR) | 3 (1-5) |
| Lymph node ratio, mean (SD) | 0.34 (0.28) |
| T stage | |
| TX | 3 (1.5) |
| T0 | 1 (0.5) |
| T1 | 22 (11.1) |
| T2 | 49 (24.7) |
| T3 | 89 (44.9) |
| T4 | 34 (17.2) |
| N stage | |
| NX | 19 (9.5) |
| N0 | 26 (13.1) |
| N1 | 154 (77.4) |
| M stage | |
| MX | 2 (1.0) |
| M0 | 193 (99) |
| Final resection status | |
| R0 | 179 (91.3) |
| R1 | 17 (8.7) |
Abbreviation: IQR, interquartile range.
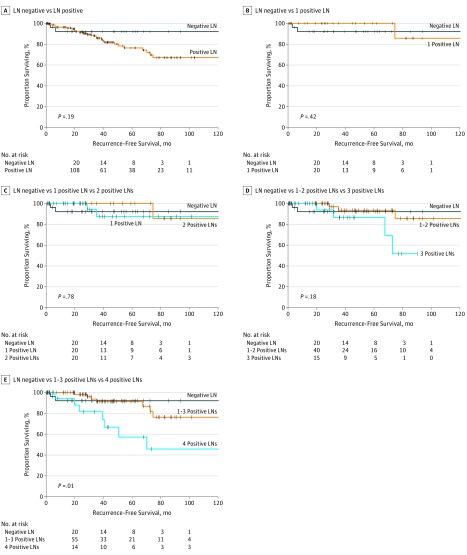
When examining lymph node positivity as a dichotomous variable, we observed no difference in RFS between patients who were lymph node positive and those who were lymph node negative (P = .19; Figure 1A). Further analysis of the number of positive lymph nodes revealed that 1, 1 to 2, 2, or 3 positive lymph nodes was or were not associated with a reduced RFS compared with lymph node–negative disease (Figure 1B-D). Once the threshold of 4 positive lymph nodes was reached, however, a substantial reduction in RFS occurred. Patients with 4 positive lymph nodes had a worse 3-year RFS compared with patients with 1 to 3 positive lymph nodes or with negative lymph nodes (81.6% vs 91.4% vs 92.1%; P = .01; Figure 1E). On univariable Cox regression analysis, the presence of 4 or more positive lymph nodes was significantly associated with decreased RFS (hazard ratio [HR], 3.32; 95% CI, 1.51-7.28; P = .003). When further analyzed in relation to other clinicopathologic factors in a multivariable model (specifically race/ethnicity, sex, age, tumor differentiation, and final resection status), only the presence of 4 or more positive lymph nodes was associated with decreased RFS (HR, 3.03; 95% CI, 1.30-7.06; P = .01).
Figure 1. Kaplan-Meier Survival Plots Comparing Increasing Number of Positive Lymph Nodes (LNs) With Decreased Recurrence-Free Survival (RFS).
A, No difference in RFS was found between patients with any number of positive LNs and those who were LN negative. B-D, The RFS for patients with an increasing number of positive LNs did not differ until a threshold of 4 positive LNs was reached. E, Patients with 4 positive LNs had decreased RFS compared with those with 1 to 3 or 0 positive LNs.
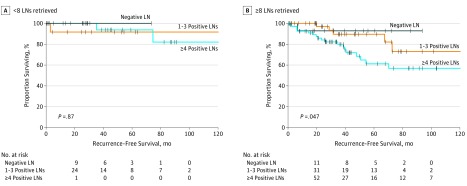
When considering the extent of lymphadenectomy, retrieval of 8 or more lymph nodes was associated with a higher positive lymph node count compared with retrieval of fewer than 8 nodes (4.2 vs 1.4; P < .001). Notably, when examining patients who had fewer than 8 lymph nodes resected, no difference was observed in 3-year RFS in patients with 4 or more positive lymph nodes compared with those with 1 to 3 positive lymph nodes or with negative lymph nodes (100% vs 93.8% vs 91.7%; P = .87; Figure 2A). However, in patients with 8 or more lymph nodes retrieved, there was accurate discrimination of RFS in patients with 4 or more positive lymph nodes, 1 to 3 positive lymph nodes, or negative lymph nodes (79.9% vs 89.6% vs 92.9%; P = .05; Figure 2B).
Figure 2. Kaplan-Meier Survival Plots Comparing Recurrence-Free Survival (RFS) and Lymph Node (LN) Positivity Stratified by Number of LNs Resected.
A, No difference in RFS was found among patients with 4 or more, 1 to 3, or 0 LNs if fewer than 8 LNs were retrieved. B, A total of 8 LNs resected accurately discriminated decreased RFS in patients with 4 or more, 1 to 3, or 0 positive LNs.
When evaluating lymph node ratio, we found that the median (IQR) lymph node ratio for the entire cohort was 0.25 (0.14-0.45). When performing Cox regression analysis to analyze the association between the lymph node ratio and RFS, we observed no association between the lymph node ratio and a decreased RFS (HR, 1.6; 95% CI, 0.422-5.83; P = .50). We further grouped patients by increasing the values of the lymph node ratio (≥0.25 vs <0.25, ≥0.33 vs <0.33, and ≥0.50 vs <0.50). No association was found between these lymph node ratio categories and decreased RFS (≥0.25 vs <0.25 HR, 1.8 [95% CI, 0.84-3.9; P = .13]; ≥0.33 vs <0.33 HR, 1.6 [95% CI, 0.77-3.5; P = .20]; ≥0.50 vs <0.50 HR, 0.96 [95% CI, 0.33-2.8; P = .93]).
Discussion
Small-bowel neuroendocrine tumors frequently metastasize to regional lymph nodes, but the association of lymph node involvement with disease recurrence has not been defined. This study found that most patients with SB-NETs who undergo resection have lymph node–positive disease (83.5%). Patients who undergo resection of these tumors have a median of 13 lymph nodes retrieved during regional mesenteric lymphadenectomy and a median of 3 lymph nodes with tumor involvement. Lymph node positivity alone is not associated with decreased RFS; rather, having 4 or more positive lymph nodes is significantly associated with earlier disease recurrence compared with having 1 to 3 positive lymph nodes or negative lymph nodes. To prevent understaging of these patients, an extended lymphadenectomy of at least 8 lymph nodes is required to adequately discriminate between those patients with 4 or more positive lymph nodes and patients with 1 to 3 positive lymph nodes or with negative lymph nodes.
Tumor nodal status is one of the most important prognostic markers for solid-organ malignant neoplasms. As tumors develop, they gain increased genetic heterogeneity, which is associated with tumor progression and subsequent spread.19 Tumors often travel in a predictable fashion, from local sites to sentinel lymph nodes, as they progress to distant sites.20 This pattern of spread has been used clinically in the treatment of melanoma as well as breast, head and neck, upper gastrointestinal tract, gynecologic, and genitourinary cancers.21 Whereas the goal of sentinel lymph node mapping is to minimize the morbidity of extended lymph node dissections in more superficial cancers, the goal of extended lymphadenectomy in gastrointestinal cancers is to harvest sentinel lymph nodes and remove all regional draining nodal basins and accurately stage patients.
Traditional staging systems for many solid-organ malignant neoplasms include lymph node positivity and the number of positive lymph nodes as prognostic variables. Several groups have found that separating patients by the number of tumor-positive lymph nodes after surgical resection, as opposed to just being positive or negative, can provide superior ability to estimate the likelihood of recurrence and survival.22,23 In SB-NETs, lymph node involvement is also characterized as a prognostic factor. In the AJCC Cancer Staging Manual, 7th edition, TNM classification places patients with regional lymph node metastasis (N1) as having stage IIIB disease.18 In the AJCC Cancer Staging Manual, 8th edition, patients are distinguished by N0 (no regional lymph node metastasis), N1 (lymph node involvement in fewer than 12 nodes), and N2 tumors (large mesenteric masses [>2 cm]) and/or by extensive nodal deposits in more than 12 lymph nodes (especially those that encase the superior mesenteric vessels).24 The AJCC Cancer Staging Manual, 8th edition, categorizes patients as having either stage III or IV disease according to the extent of nodal involvement. These staging guidelines, however, are designed to estimate overall survival rather than RFS. Given that neuroendocrine tumors are generally indolent tumors that are more likely to recur than cause death within a study period, this study focused on disease recurrence rather than overall survival. This study recapitulates the AJCC Cancer Staging Manual, 8th edition, in that the patients were also divided into 3 prognostic cohorts that were similar to the N0, N1, and N2 groups of the guidelines but differed in the number of positive lymph nodes. We found that patients with 4 or more positive lymph nodes had worse RFS compared with patients with 1 to 3 positive lymph nodes and those with negative lymph nodes. Furthermore, no difference in RFS was found among patients with 0, 1, 1 to 2, 2, or 3 positive lymph nodes. This finding may provide an accurate prognostic model for disease recurrence after resection and potentially guide surveillance strategies and inclusion criteria in future trials assessing the value of adjuvant therapy.
In several gastrointestinal cancers, the optimal regional lymph node dissection during curative-intent resection is debated, as extended lymphadenectomy may confer better staging, disease control, and survival. For gastric cancer, Smith et al25 reported that overall survival was dependent on the number of lymph nodes examined, with a survival advantage found with every 10 lymph nodes examined up to 40 lymph nodes. In colon cancer, more than 12 lymph nodes are needed to accurately stage tumors.26 In our study of SB-NETs, we found that a regional lymphadenectomy with retrieval of at least 8 nodes was necessary to accurately discriminate the differences in RFS between patients with 4 or more positive lymph nodes compared with patients with 1 to 3 positive lymph nodes or with negative lymph nodes.
To help offset limitations with lymph node retrieval, several groups have proposed the use of the lymph node ratio as opposed to the total number of disease-positive lymph nodes in the staging of patients.13,14,15,16,17 In this study, however, the lymph node ratio was not a useful tool in determining RFS in patients. This may partly be explained by the inclusion in this study of only high-volume academic centers with experienced fellowship-trained surgical oncologists who routinely perform an oncologic regional lymphadenectomy when resecting SB-NETs. The mean lymph node count was high (14 nodes retrieved), which may have limited the lymph node ratio value, despite the presence of multiple positive lymph nodes.
Limitations
This study has several limitations. The retrospective design presents a challenge in capturing complete RFS data. In addition, surgical conduct and pathologic examination of lymph nodes were not standardized across the 8 institutions, which may lead to variability in reporting. Nevertheless, to our knowledge, this study represents one of the largest in the literature focusing on SB-NETs and includes only academic centers that are leaders in the management of gastrointestinal malignant neoplasms. Furthermore, the multi-institutional collaborative design eliminates single-institution bias and includes data from all geographic regions in the United States.
Conclusions
In patients undergoing curative-intent surgical resection of SB-NETs, the presence of 4 or more positive lymph nodes is associated with a decreased RFS compared with 1 to 3 positive lymph nodes or negative lymph nodes. Accurate staging of these patients requires a minimum of 8 lymph nodes for examination. The lymph node ratio does not appear to be useful as a prognostic indicator in SB-NETs. Complete regional lymphadenectomy may need to be performed routinely.
References
- 1.Yao JC, Hassan M, Phan A, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26(18):3063-3072. doi: 10.1200/JCO.2007.15.4377 [DOI] [PubMed] [Google Scholar]
- 2.Modlin IM, Oberg K, Chung DC, et al. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol. 2008;9(1):61-72. doi: 10.1016/S1470-2045(07)70410-2 [DOI] [PubMed] [Google Scholar]
- 3.Eriksson B, Klöppel G, Krenning E, et al. ; Frascati Consensus Conference Participants . Consensus guidelines for the management of patients with digestive neuroendocrine tumors–well-differentiated jejunal-ileal tumor/carcinoma. Neuroendocrinology. 2008;87(1):8-19. doi: 10.1159/000111034 [DOI] [PubMed] [Google Scholar]
- 4.Ramage JK, Ahmed A, Ardill J, et al. ; UK and Ireland Neuroendocrine Tumour Society . Guidelines for the management of gastroenteropancreatic neuroendocrine (including carcinoid) tumours (NETs). Gut. 2012;61(1):6-32. doi: 10.1136/gutjnl-2011-300831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferris RL, Lotze MT, Leong SP, Hoon DS, Morton DL. Lymphatics, lymph nodes and the immune system: barriers and gateways for cancer spread. Clin Exp Metastasis. 2012;29(7):729-736. doi: 10.1007/s10585-012-9520-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pereira ER, Jones D, Jung K, Padera TP. The lymph node microenvironment and its role in the progression of metastatic cancer. Semin Cell Dev Biol. 2015;38:98-105. doi: 10.1016/j.semcdb.2015.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giuliano AE, Hunt KK, Ballman KV, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA. 2011;305(6):569-575. doi: 10.1001/jama.2011.90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Landry CS, Lin HY, Phan A, et al. Resection of at-risk mesenteric lymph nodes is associated with improved survival in patients with small bowel neuroendocrine tumors. World J Surg. 2013;37(7):1695-1700. doi: 10.1007/s00268-013-1918-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watzka FM, Fottner C, Miederer M, et al. Surgical treatment of NEN of small bowel: a retrospective analysis. World J Surg. 2016;40(3):749-758. doi: 10.1007/s00268-016-3432-2 [DOI] [PubMed] [Google Scholar]
- 10.Bunt AM, Hermans J, Smit VT, van de Velde CJ, Fleuren GJ, Bruijn JA. Surgical/pathologic-stage migration confounds comparisons of gastric cancer survival rates between Japan and Western countries. J Clin Oncol. 1995;13(1):19-25. doi: 10.1200/JCO.1995.13.1.19 [DOI] [PubMed] [Google Scholar]
- 11.de Manzoni G, Verlato G, Roviello F, et al. The new TNM classification of lymph node metastasis minimises stage migration problems in gastric cancer patients. Br J Cancer. 2002;87(2):171-174. doi: 10.1038/sj.bjc.6600432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feinstein AR, Sosin DM, Wells CK. The Will Rogers phenomenon. Stage migration and new diagnostic techniques as a source of misleading statistics for survival in cancer. N Engl J Med. 1985;312(25):1604-1608. doi: 10.1056/NEJM198506203122504 [DOI] [PubMed] [Google Scholar]
- 13.Berger AC, Sigurdson ER, LeVoyer T, et al. Colon cancer survival is associated with decreasing ratio of metastatic to examined lymph nodes. J Clin Oncol. 2005;23(34):8706-8712. doi: 10.1200/JCO.2005.02.8852 [DOI] [PubMed] [Google Scholar]
- 14.Marchet A, Mocellin S, Ambrosi A, et al. ; Italian Research Group for Gastric Cancer (IRGGC) . The ratio between metastatic and examined lymph nodes (N ratio) is an independent prognostic factor in gastric cancer regardless of the type of lymphadenectomy: results from an Italian multicentric study in 1853 patients. Ann Surg. 2007;245(4):543-552. doi: 10.1097/01.sla.0000250423.43436.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vinh-Hung V, Verschraegen C, Promish DI, et al. Ratios of involved nodes in early breast cancer. Breast Cancer Res. 2004;6(6):R680-R688. doi: 10.1186/bcr934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang J, Dang P, Raut CP, et al. Comparison of a lymph node ratio-based staging system with the 7th AJCC system for gastric cancer: analysis of 18,043 patients from the SEER database. Ann Surg. 2012;255(3):478-485. doi: 10.1097/SLA.0b013e31824857e2 [DOI] [PubMed] [Google Scholar]
- 17.Xu DZ, Geng QR, Long ZJ, et al. Positive lymph node ratio is an independent prognostic factor in gastric cancer after d2 resection regardless of the examined number of lymph nodes. Ann Surg Oncol. 2009;16(2):319-326. doi: 10.1245/s10434-008-0240-4 [DOI] [PubMed] [Google Scholar]
- 18.Neuroendocrine Tumors In: Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, eds. AJCC Cancer Staging Manual. 7th ed New York: Springer; 2010:181-185. [Google Scholar]
- 19.Hellman S. Darwin’s clinical relevance. Cancer. 1997;79(12):2275-2281. doi: [DOI] [PubMed] [Google Scholar]
- 20.Leong SP. Paradigm of metastasis for melanoma and breast cancer based on the sentinel lymph node experience. Ann Surg Oncol. 2004;11(3)(suppl):192S-197S. doi: 10.1245/ASO.2004.12.922 [DOI] [PubMed] [Google Scholar]
- 21.Leong SP, Zuber M, Ferris RL, et al. Impact of nodal status and tumor burden in sentinel lymph nodes on the clinical outcomes of cancer patients. J Surg Oncol. 2011;103(6):518-530. doi: 10.1002/jso.21815 [DOI] [PubMed] [Google Scholar]
- 22.Adam MA, Pura J, Goffredo P, et al. Presence and number of lymph node metastases are associated with compromised survival for patients younger than age 45 years with papillary thyroid cancer. J Clin Oncol. 2015;33(21):2370-2375. doi: 10.1200/JCO.2014.59.8391 [DOI] [PubMed] [Google Scholar]
- 23.Strobel O, Hinz U, Gluth A, et al. Pancreatic adenocarcinoma: number of positive nodes allows to distinguish several N categories. Ann Surg. 2015;261(5):961-969. doi: 10.1097/SLA.0000000000000814 [DOI] [PubMed] [Google Scholar]
- 24.Neuroendocrine Tumors of the Jejunum and Ileum In: Amin MB, Edge S, Greene FL, et al. , eds. AJCC Cancer Staging Manual. 8th ed New York: Springer; 2017:375-387. doi: 10.1007/978-3-319-40618-3_31 [DOI] [Google Scholar]
- 25.Smith DD, Schwarz RR, Schwarz RE. Impact of total lymph node count on staging and survival after gastrectomy for gastric cancer: data from a large US-population database. J Clin Oncol. 2005;23(28):7114-7124. doi: 10.1200/JCO.2005.14.621 [DOI] [PubMed] [Google Scholar]
- 26.Dillman RO, Aaron K, Heinemann FS, McClure SE. Identification of 12 or more lymph nodes in resected colon cancer specimens as an indicator of quality performance. Cancer. 2009;115(9):1840-1848. doi: 10.1002/cncr.24185 [DOI] [PubMed] [Google Scholar]