Key Points
Question
Is tramadol prescription associated with a higher risk of all-cause mortality than other pain relief medications among patients with osteoarthritis?
Findings
In this cohort study that included 88 902 patients with osteoarthritis, initial prescription of tramadol was associated with a significantly increased risk of mortality over 1 year compared with initial prescription of naproxen (hazard ratio [HR], 1.71), diclofenac (HR, 1.88), celecoxib (HR, 1.70), and etoricoxib (HR, 2.04), but not compared with codeine (HR, 0.94).
Meaning
Tramadol prescription may be associated with increased all-cause mortality compared with commonly prescribed nonsteroidal anti-inflammatory drugs, but further research is needed to determine if this relationship is causal.
Abstract
Importance
An American Academy of Orthopaedic Surgeons guideline recommends tramadol for patients with knee osteoarthritis, and an American College of Rheumatology guideline conditionally recommends tramadol as first-line therapy for patients with knee osteoarthritis, along with nonsteroidal anti-inflammatory drugs.
Objective
To examine the association of tramadol prescription with all-cause mortality among patients with osteoarthritis.
Design, Setting, and Participants
Sequential, propensity score–matched cohort study at a general practice in the United Kingdom. Individuals aged at least 50 years with a diagnosis of osteoarthritis in the Health Improvement Network database from January 2000 to December 2015, with follow-up to December 2016.
Exposures
Initial prescription of tramadol (n = 44 451), naproxen (n = 12 397), diclofenac (n = 6512), celecoxib (n = 5674), etoricoxib (n = 2946), or codeine (n = 16 922).
Main Outcomes and Measures
All-cause mortality within 1 year after initial tramadol prescription, compared with 5 other pain relief medications.
Results
After propensity score matching, 88 902 patients were included (mean [SD] age, 70.1 [9.5] years; 61.2% were women). During the 1-year follow-up, 278 deaths (23.5/1000 person-years) occurred in the tramadol cohort and 164 (13.8/1000 person-years) occurred in the naproxen cohort (rate difference, 9.7 deaths/1000 person-years [95% CI, 6.3-13.2]; hazard ratio [HR], 1.71 [95% CI, 1.41-2.07]), and mortality was higher for tramadol compared with diclofenac (36.2/1000 vs 19.2/1000 person-years; HR, 1.88 [95% CI, 1.51-2.35]). Tramadol was also associated with a higher all-cause mortality rate compared with celecoxib (31.2/1000 vs 18.4/1000 person-years; HR, 1.70 [95% CI, 1.33-2.17]) and etoricoxib (25.7/1000 vs 12.8/1000 person-years; HR, 2.04 [95% CI, 1.37-3.03]). No statistically significant difference in all-cause mortality was observed between tramadol and codeine (32.2/1000 vs 34.6/1000 person-years; HR, 0.94 [95% CI, 0.83-1.05]).
Conclusions and Relevance
Among patients aged 50 years and older with osteoarthritis, initial prescription of tramadol was associated with a significantly higher rate of mortality over 1 year of follow-up compared with commonly prescribed nonsteroidal anti-inflammatory drugs, but not compared with codeine. However, these findings may be susceptible to confounding by indication, and further research is needed to determine if this association is causal.
This cohort study uses EHR data from the practices of UK general practitioners to examine the association between an initial prescription for tramadol and all-cause mortality relative to other commonly prescribed NSAIDs (naproxen, diclofenac, celecoxib, etoricoxib) and codeine in patients with osteoarthritis (OA).
Introduction
Few safe and effective treatments are available for patients with osteoarthritis. The main goal of medical therapy for managing osteoarthritis is to control pain while avoiding therapeutic toxicity.1 Tramadol, a weak opioid agonist, has been considered a potential alternative to traditional opioid agonists in managing pain.2 Current American Academy of Orthopaedic Surgeons guidelines strongly recommended tramadol or nonsteroidal anti-inflammatory drugs (NSAIDs) for symptomatic knee osteoarthritis.3 The most recent American College of Rheumatology guidelines (from 2012) conditionally recommended tramadol as a first-line therapy for patients with knee osteoarthritis, along with NSAIDs.4 Tramadol prescription for management of knee osteoarthritis doubled from 5% to 10% from 2003 to 2009 in the United States.5
A meta-analysis showed no statistically significant association of tramadol vs NSAIDs for pain relief among patients with osteoarthritis,6 but tramadol was associated with more opioid-related adverse events (eg, nausea, dizziness, constipation, vomiting, somnolence, tiredness, headache).7 Few studies have examined the relationship between tramadol prescription and all-cause mortality, and current evidence regarding the association of tramadol with mortality rates compared with other analgesic medications is inconclusive.8,9,10,11,12,13 The present study examined the association of initial prescription of tramadol with all-cause mortality compared with alternative commonly prescribed analgesics in patients with osteoarthritis.
Methods
Data Source
The Health Improvement Network (THIN) is an electronic medical record database derived from the records of general practitioners (GPs) in the United Kingdom. THIN contains health information on approximately 11.1 million patients from 580 general practices in the United Kingdom. Health care information is recorded on site at each practice and includes information on sociodemographics, anthropometrics, lifestyle factors, details from GP visits (eg, disease diagnosis, medication prescription), diagnoses from specialists’ referrals and hospital admissions, as well as results of laboratory tests. The Read classification system is used to code specific diagnoses, and a drug dictionary based on data from the Multilex classification system is used to code drugs. The scientific review committee for the THIN database and the institutional review board at Xiangya Hospital approved this study, with waiver of informed consent.
Study Design and Cohort Definition
Eligible participants were patients aged 50 years or older with history of knee, hip, or hand osteoarthritis, based on Read codes, who visited the participating GP office between January 2000 and December 2015. All participants had at least 1 year of continuous enrollment with the general practice. Patients with a history of cancer or an opioid use disorder before study entry were excluded.
We conducted 5 sequential propensity score–matched cohort studies to compare all-cause mortality between participants who received an initial prescription of tramadol and participants who received initial prescription of 1 of the following medications: naproxen or diclofenac (commonly prescribed nonselective NSAIDs), celecoxib or etoricoxib (cyclooxygenase 2 [COX-2] inhibitors), or codeine (a commonly prescribed weak opioid). For example, to compare all-cause mortality between tramadol and naproxen, eligible participants were required to be prescribed neither tramadol nor naproxen 1 year before entering the study. The date of initial prescription of either tramadol or naproxen was considered the index date for the corresponding patient. We divided calendar time into 16 1-year blocks from January 2000 to December 2015. Follow-up ended on December 31, 2016. Within each time block, we calculated propensity scores for initial prescription of tramadol using logistic regression. The variables included in the model were sociodemographic factors (ie, age at index date, sex, Townsend Deprivation Index), body mass index (BMI), lifestyle factors (ie, drinking habits and smoking status), osteoarthritis duration, comorbidities and prescriptions prior to the index date, and health care utilization during the 2 years before the index date. Within each time block, tramadol prescriptions were matched 1:1 to naproxen prescriptions using the greedy matching method.14 We took the same approach to assemble 4 other propensity score–matched cohort studies: tramadol vs diclofenac, tramadol vs celecoxib, tramadol vs etoricoxib, and tramadol vs codeine.
Assessment of Outcome
The primary outcome was all-cause mortality 1 year (hereafter referred to as mortality) after initial prescription of tramadol or its comparators, defined by the death date recorded in THIN, linked to the National Health Service. The change in a patient’s vital status to “dead” is immediately updated in the patient’s electronic health record and requires no input by the practice staff in THIN.
Statistical Analysis
We described the annual prevalence and the treatment duration of prescriptions for tramadol, naproxen, diclofenac, celecoxib, etoricoxib, and codeine among patients with osteoarthritis in THIN between 2000 and 2015. We compared the baseline characteristics of the 5 tramadol cohorts with each of the 5 comparison cohorts. For each patient, we calculated person-years of follow-up as the amount of time from the index date to the first of the following events to occur: death, disenrollment from a GP practice participating in THIN (ie, transferring out of the GP practice; approximately 6% of the included individuals), or the end of a 1-year follow-up period. We calculated mortality for each cohort and plotted Kaplan-Meier mortality curves. We compared mortality in the tramadol cohort with each of the 5 comparison cohorts using Cox proportional hazard models adjusted for calendar year. Patients with missing values for BMI, drinking habits, smoking status, or Townsend Deprivation Index were excluded from analysis. We tested the proportional hazards assumption for each comparison cohort using the Kolmogorov supremum test.15 If the proportional hazard assumption was violated, we estimated the hazard ratio at 3 months, 6 months, 9 months, and 12 months. We also estimated absolute rate differences (RDs) in mortality between the tramadol cohorts and each of the 5 comparative cohorts.
We performed 6 sensitivity analyses to assess the robustness of our study findings. First, we used asymmetric trimming to exclude patients whose propensity score was below the 2.5th percentile of the propensity score of the tramadol cohort and above the 97.5th percentile of the propensity score of the comparator cohort.16 Second, to minimize residual confounding by indication when comparing mortality between each tramadol cohort with the comparison cohorts, we conducted a stratified analysis according to the prescription of other opioids before initiation of either tramadol or its comparator. Third, to account for nonadherence of medications under investigation during the study period, we conducted an “as-treated” analysis. Specifically, we censored the follow-up at the time when participants either changed (eg, switching from tramadol to naproxen or vice versa, when comparing tramadol with naproxen) or discontinued (ie, no prescription refill for the respective class of medication for more than 60 days) their initiated medication. Fourth, we performed an analysis among participants whose osteoarthritis was diagnosed during the study period (ie, incident osteoarthritis) to minimize potential misclassification of the duration of osteoarthritis. Fifth, because individuals with missing values were not included in our primary analyses, we performed imputation analyses to account for missing data. Specifically, missing values of the variables listed above were imputed by a sequential regression method based on a set of covariates as predictors. To minimize random error, we imputed 5 data sets, calculating effect estimates from each imputed data set and averaging estimates and their CIs obtained from each imputed data set using Rubin’s rules.17 Sixth, to minimize the potential reverse-causality bias (ie, protopathic bias) we introduced a 6-month or 1-year exposure lag period to account for a potential latency time window (eg, excluding cases of cancer that occurred within 6 months or 1 year).18
In addition, we compared cause-specific mortality in each tramadol cohort with each matched comparator cohort using a cause-specific Cox-proportional hazard model to account for competing risk of other causes of death. The cause-specific mortality was defined as either data set–documented cause of death or use of a death-attribution algorithm reliant on postmortem or premortem diagnostic codes when there was no documented cause of death.19
All P values were 2-sided and P < .05 was considered significant for all tests. All statistical analyses were conducted using SAS version 9.4.
Results
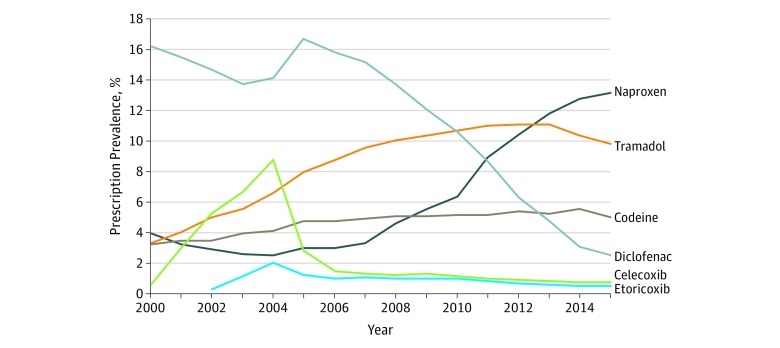
After propensity score matching, 88 902 patients were included in the analysis (mean [SD] age, 70.1 [9.5] years; 61.2% were women). Of the matched participants, 12 397 were included in the naproxen cohort, 6512 in the diclofenac cohort, 5674 in the celecoxib cohort, 2946 in the etoricoxib cohort, and 16 922 in the codeine cohort. As shown in Figure 1, the prevalence of participants with knee, hip, or hand osteoarthritis with prescriptions for tramadol increased from 1561 of 46 481 (3.4%) in 2000 to 12 633 of 113 856 (11.1%) in 2013, then decreased to 8407 of 86 014 (9.8%) in 2015. The prevalence of participants with naproxen prescriptions increased from 1830 of 46 481 (3.9%) in 2000 to 11 285 of 86 014 (13.1%) in 2015, whereas diclofenac prescription rates declined from 7512 of 46 481 participants (16.2%) in 2000 to 2161 of 86 014 (2.5%) in 2015. Participants with celecoxib prescriptions increased from 292 of 46 481 (0.6%) in 2000 to 6658 of 75 945 (8.8%) in 2004, then declined after 2005. Etoricoxib entered the UK market in 2002, and the annual prevalence of its prescription remained low during the study period (204 of 62 692 participants [0.3%] in 2002 and 479 of 86 014 [0.6%] in 2015). The prevalence of participants with codeine prescription increased over time from 1497 of 46 481 (3.2%) in 2000 to 4297 of 86 014 (5.0%) in 2015.
Figure 1. Prevalence of Tramadol, Naproxen, Diclofenac, Celecoxib, Etoricoxib, and Codeine Prescriptions Among Patients With Knee, Hip, or Hand Osteoarthritis in The Health Improvement Network Database From 2000 to 2015.
Of the matched participants, 12 397 were included in the naproxen cohort, 6512 in the diclofenac cohort, 5674 in the celecoxib cohort, 2946 in the etoricoxib cohort, and 16 922 in the codeine cohort.
The mean (range) treatment duration of a prescription for tramadol was 22 (5-67) days; naproxen, 24 (5-60) days; diclofenac, 24 (5-60) days; celecoxib, 31 (5-60) days; etoricoxib, 27 (5-60) days; and codeine, 25 (5-150) days among patients with osteoarthritis.
As shown in Table 1 and the Supplement, participants in the tramadol cohort, in general, were older; had a higher BMI; had a longer duration of osteoarthritis; and had a higher prevalence of comorbidities (eg, peptic ulcer, chronic kidney disease, diabetes, hypertension, and cardiovascular diseases), other prescriptions (eg, other NSAIDs, other opioids, aspirin, statin, antihypertensive medicine, and antidiabetic medicine), and health care utilization than participants in the NSAIDs cohorts before propensity score matching. After matching, the characteristics between the 2 matched cohorts were well balanced, with all standardized differences less than 0.10 (Table 2).
Table 1. Baseline Characteristics of Unmatched Patients With Osteoarthritis in a Study Comparing the Association of Tramadol and Other Analgesics With All-Cause Mortalitya .
| Nonselective NSAIDs | COX-2 Inhibitors | Weak Opioid | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tramadol | Naproxen | Standardized Differences | Tramadol | Diclofenac | Standardized Differences | Tramadol | Celecoxib | Standardized Differences | Tramadol | Etoricoxib | Standardized Differences | Tramadol | Codeine | Standardized Differences | |
| Participants, No. | 31 087 | 26 731 | 16 372 | 21 675 | 39 075 | 11 625 | 44 036 | 4006 | 34 353 | 23 899 | |||||
| Demographics | |||||||||||||||
| Age, mean (SD), y | 71.0 (9.7) | 67.6 (9.4) | 0.36 | 72.1 (9.7) | 67.5 (9.7) | 0.48 | 70.2 (9.7) | 70.8 (9.6) | 0.07 | 70.4 (9.7) | 69.0 (9.6) | 0.14 | 70.0 (9.7) | 71.6 (9.7) | 0.17 |
| Socioeconomic deprivation index score, mean (SD)b | 2.6 (1.4) | 2.4 (1.3) | 0.17 | 2.6 (1.4) | 2.4 (1.3) | 0.14 | 2.6 (1.4) | 2.5 (1.3) | 0.07 | 2.6 (1.4) | 2.5 (1.4) | 0.06 | 2.6 (1.4) | 2.5 (1.3) | 0.08 |
| Women, % | 63.1 | 55.5 | 0.15 | 63.7 | 55.4 | 0.17 | 61.0 | 66.9 | 0.12 | 62.4 | 61.4 | 0.02 | 60.9 | 61.4 | 0.01 |
| BMI, mean (SD) | 29.3 (5.9) | 28.9 (5.5) | 0.06 | 29.2 (5.9) | 28.3 (5.2) | 0.17 | 29.5 (5.9) | 28.2 (5.1) | 0.23 | 29.5 (5.9) | 28.6 (5.3) | 0.15 | 29.5 (5.9) | 28.6 (5.4) | 0.14 |
| Osteoarthritis duration, mean (SD), y | 6.8 (6.9) | 6.6 (6.5) | 0.03 | 6.8 (7.1) | 5.8 (6.5) | 0.14 | 6.9 (6.9) | 6.4 (6.5) | 0.08 | 7.0 (6.9) | 6.2 (6.3) | 0.12 | 6.8 (6.8) | 7.3 (7.0) | 0.08 |
| Lifestyle factors | |||||||||||||||
| Drinking alcohol, % | |||||||||||||||
| None | 22.8 | 17.0 | 0.13 | 23.8 | 17.4 | 0.17 | 21.4 | 21.3 | 0.03 | 21.8 | 20.9 | 0.03 | 21.2 | 20.4 | 0.01 |
| Past | 3.2 | 2.3 | 0.05 | 3.4 | 1.6 | 0.12 | 3.1 | 2.0 | 0.08 | 3.1 | 2.3 | 0.05 | 2.8 | 3.1 | 0.02 |
| Current | 74.0 | 80.7 | 0.16 | 72.8 | 81.0 | 0.10 | 75.4 | 76.7 | 0.05 | 75.1 | 76.8 | 0.01 | 76.0 | 76.5 | 0.03 |
| Smoking status, % | |||||||||||||||
| None | 53.5 | 56.8 | 0.08 | 53.6 | 56.7 | 0.03 | 52.8 | 59.5 | 0.08 | 53.3 | 57.1 | 0.06 | 53.3 | 56.4 | 0.06 |
| Past | 32.9 | 31.2 | 0.03 | 33.6 | 28.7 | 0.12 | 33.2 | 27.1 | 0.16 | 33.0 | 30.7 | 0.06 | 32.6 | 32.8 | 0.01 |
| Current | 13.6 | 12.0 | 0.04 | 12.7 | 14.6 | 0.04 | 13.9 | 13.4 | 0.03 | 13.7 | 12.2 | 0.05 | 14.0 | 10.8 | 0.10 |
| Comorbidity, % | |||||||||||||||
| CKD | |||||||||||||||
| No CKD | 86.7 | 90.0 | 0.10 | 85.4 | 95.6 | 0.36 | 86.9 | 97.5 | 0.40 | 87.0 | 93.3 | 0.21 | 87.9 | 87.3 | 0.02 |
| Stage 1 | 0.1 | 0.1 | 0.01 | 0.1 | 0.1 | 0.01 | 0.1 | 0.1 | 0.02 | 0.1 | 0.0 | 0.02 | 0.1 | 0.1 | 0.00 |
| Stage 2 | 1.1 | 1.4 | 0.02 | 1.2 | 0.5 | 0.08 | 1.2 | 0.2 | 0.12 | 1.2 | 0.6 | 0.06 | 1.1 | 1.0 | 0.01 |
| Stage 3 | 9.7 | 7.8 | 0.07 | 10.6 | 3.0 | 0.30 | 9.7 | 1.3 | 0.38 | 9.7 | 4.9 | 0.18 | 9.0 | 9.6 | 0.02 |
| Stage 4 | 0.8 | 0.2 | 0.08 | 1.0 | 0.1 | 0.12 | 0.7 | 0.1 | 0.10 | 0.7 | 0.2 | 0.06 | 0.6 | 0.6 | 0.00 |
| Stage 5 | 0.5 | 0.1 | 0.07 | 0.6 | 0.2 | 0.07 | 0.5 | 0.3 | 0.03 | 0.5 | 0.2 | 0.05 | 0.4 | 0.5 | 0.01 |
| Stage unknown | 1.0 | 0.4 | 0.07 | 1.1 | 0.4 | 0.08 | 0.9 | 0.6 | 0.04 | 0.9 | 0.6 | 0.04 | 0.9 | 0.9 | 0.00 |
| Hypertension | 54.3 | 44.6 | 0.20 | 56.7 | 39.8 | 0.34 | 53.6 | 44.6 | 0.18 | 53.8 | 46.8 | 0.14 | 52.2 | 53.3 | 0.02 |
| Other circulatory disease | 34.5 | 29.2 | 0.11 | 34.9 | 24.5 | 0.23 | 34.9 | 31.4 | 0.08 | 35.4 | 30.0 | 0.12 | 33.3 | 36.0 | 0.06 |
| Ischemic heart disease | 19.5 | 10.0 | 0.27 | 21.0 | 11.8 | 0.25 | 18.5 | 15.5 | 0.08 | 5.0 | 3.2 | 0.15 | 17.5 | 17.9 | 0.01 |
| Hyperlipidemia | 17.9 | 16.3 | 0.04 | 18.1 | 12.1 | 0.17 | 18.1 | 12.2 | 0.16 | 18.0 | 15.3 | 0.08 | 17.6 | 16.7 | 0.02 |
| Anxiety | 16.1 | 13.7 | 0.23 | 15.8 | 11.8 | 0.29 | 16.7 | 15.2 | 0.17 | 16.9 | 14.0 | 0.17 | 15.8 | 15.2 | 0.06 |
| Diabetes | 15.4 | 12.1 | 0.10 | 16.6 | 9.2 | 0.22 | 15.8 | 9.0 | 0.21 | 15.5 | 9.8 | Diabetes | 15.4 | 12.1 | 0.10 |
| Depression | 15.3 | 12.7 | 0.07 | 14.9 | 10.3 | 0.14 | 15.6 | 13.1 | 0.07 | 15.8 | 13.9 | 0.06 | 15.0 | 13.0 | 0.06 |
| Varicose veins | 14.1 | 12.6 | 0.05 | 13.1 | 12.2 | 0.03 | 13.9 | 14.6 | 0.02 | 14.2 | 14.2 | 0.00 | 13.5 | 14.9 | 0.04 |
| Angina | 13.2 | 6.5 | 0.23 | 13.9 | 8.3 | 0.18 | 12.6 | 11.6 | 0.03 | 2.5 | 1.4 | 0.11 | 11.6 | 12.2 | 0.020 |
| Peptic ulcer | 9.8 | 4.5 | 0.21 | 11.1 | 5.3 | 0.21 | 8.9 | 7.6 | 0.05 | 9.0 | 7.0 | 0.07 | 8.6 | 7.8 | 0.03 |
| Pneumonia or infection | 8.2 | 6.6 | 0.06 | 8.1 | 6.5 | 0.06 | 8.7 | 8.0 | 0.03 | 8.6 | 7.8 | 0.03 | 7.9 | 8.0 | 0.01 |
| Atrial fibrillation | 8.2 | 2.9 | 0.23 | 9.8 | 2.8 | 0.29 | 7.7 | 3.7 | 0.17 | 7.5 | 3.6 | 0.17 | 6.9 | 8.6 | 0.06 |
| Chronic obstructive pulmonary disease | 7.2 | 3.6 | 0.16 | 8.3 | 3.1 | 0.23 | 6.8 | 4.2 | 0.11 | 6.8 | 4.3 | 0.11 | 6.2 | 5.7 | 0.02 |
| Myocardial infarction | 6.9 | 3.5 | 0.16 | 7.5 | 4.1 | 0.15 | 6.5 | 4.7 | 0.08 | 4.3 | 2.6 | 0.10 | 6.2 | 6.6 | 0.02 |
| Venous thromboembolism | 5.5 | 2.9 | 0.13 | 5.8 | 2.9 | 0.15 | 5.3 | 4.3 | 0.05 | 5.4 | 3.8 | 0.07 | 5.0 | 5.0 | 0.00 |
| Congestive heart failure | 5.4 | 1.7 | 0.20 | 6.3 | 2.4 | 0.19 | 5.0 | 3.9 | 0.05 | 6.5 | 4.3 | 0.09 | 4.4 | 5.4 | 0.05 |
| Transient ischemic attack | 4.6 | 2.4 | 0.12 | 5.1 | 2.6 | 0.13 | 4.2 | 3.6 | 0.03 | 18.6 | 13.0 | 0.09 | 3.9 | 4.5 | 0.03 |
| Stroke | 4.5 | 2.3 | 0.12 | 5.2 | 2.5 | 0.15 | 4.2 | 3.1 | 0.06 | 12.6 | 9.3 | 0.09 | 3.9 | 4.7 | 0.04 |
| Medication, % | |||||||||||||||
| Other NSAIDsc | 85.4 | 84.7 | 0.02 | 72.0 | 67.6 | 0.10 | 88.4 | 88.8 | 0.01 | 89.7 | 93.4 | 0.14 | 89.5 | 85.4 | 0.13 |
| Antihypertensive | 72.2 | 57.8 | 0.31 | 73.6 | 52.1 | 0.46 | 71.4 | 63.8 | 0.16 | 71.8 | 64.2 | 0.17 | 69.8 | 69.6 | 0.00 |
| Statin | 48.1 | 41.5 | 0.13 | 49.2 | 28.3 | 0.44 | 47.9 | 28.3 | 0.41 | 48.0 | 37.1 | 0.22 | 46.1 | 44.9 | 0.03 |
| Aspirin | 42.0 | 28.6 | 0.28 | 42.0 | 26.2 | 0.34 | 40.7 | 31.1 | 0.20 | 41.0 | 30.4 | 0.22 | 39.0 | 40.0 | 0.02 |
| Other opioidsc | 40.7 | 27.4 | 0.28 | 38.7 | 19.1 | 0.44 | 42.6 | 30.0 | 0.26 | 43.4 | 30.8 | 0.26 | 23.7 | 15.5 | 0.21 |
| ACE inhibitors | 39.3 | 30.4 | 0.19 | 41.4 | 22.9 | 0.40 | 39.3 | 24.3 | 0.33 | 39.1 | 27.8 | 0.24 | 37.1 | 38.5 | 0.03 |
| Benzodiazepines | 39.2 | 31.4 | 0.16 | 36.6 | 24.0 | 0.28 | 40.2 | 34.1 | 0.13 | 41.0 | 36.4 | 0.09 | 38.9 | 33.2 | 0.12 |
| Calcium channel blockers | 38.2 | 28.3 | 0.21 | 40.4 | 22.8 | 0.39 | 38.0 | 27.8 | 0.22 | 38.1 | 29.3 | 0.19 | 35.9 | 36.7 | 0.02 |
| β-Receptor inhibitor | 38.0 | 29.8 | 0.18 | 37.5 | 26.7 | 0.23 | 37.9 | 32.6 | 0.11 | 38.4 | 32.7 | 0.12 | 36.4 | 38.4 | 0.04 |
| Loop diuretics | 26.7 | 12.8 | 0.36 | 28.1 | 13.0 | 0.38 | 25.4 | 21.6 | 0.09 | 25.9 | 19.6 | 0.15 | 24.1 | 23.4 | 0.02 |
| Glucocorticoids | 25.5 | 18.6 | 0.17 | 26.2 | 13.2 | 0.33 | 25.9 | 19.9 | 0.15 | 26.3 | 22.5 | 0.09 | 23.9 | 23.1 | 0.02 |
| SSRI | 23.3 | 20.4 | 0.07 | 21.6 | 13.7 | 0.21 | 23.9 | 17.6 | 0.16 | 24.3 | 21.2 | 0.07 | 23.2 | 19.5 | 0.09 |
| Nitrates | 18.2 | 9.9 | 0.24 | 18.9 | 9.8 | 0.26 | 17.4 | 13.9 | 0.10 | 17.8 | 12.0 | 0.17 | 16.1 | 16.5 | 0.01 |
| Angiotensin receptor blocker | 15.0 | 11.6 | 0.10 | 15.9 | 7.2 | 0.28 | 14.4 | 8.3 | 0.19 | 14.6 | 12.2 | 0.07 | 13.9 | 13.5 | 0.01 |
| Potassium-sparing diuretics | 12.4 | 5.3 | 0.25 | 12.7 | 7.0 | 0.19 | 11.4 | 12.6 | 0.04 | 11.8 | 10.5 | 0.04 | 11.0 | 10.4 | 0.02 |
| Antidiabetic | 11.1 | 8.4 | 0.09 | 11.9 | 6.7 | 0.18 | 11.4 | 6.6 | 0.17 | 11.1 | 7.1 | 0.14 | 10.6 | 10.5 | 0.01 |
| Anticoagulants | 10.9 | 4.0 | 0.27 | 12.4 | 3.3 | 0.34 | 10.5 | 4.2 | 0.24 | 10.3 | 4.5 | 0.22 | 9.3 | 10.3 | 0.04 |
| Thiazide-like diuretics | 6.3 | 4.6 | 0.07 | 6.9 | 4.0 | 0.13 | 6.4 | 5.6 | 0.04 | 6.5 | 6.7 | 0.01 | 6.3 | 6.3 | 0.00 |
| SNRI | 6.3 | 5.2 | 0.05 | 6.1 | 3.1 | 0.14 | 6.5 | 4.8 | 0.08 | 6.7 | 6.0 | 0.03 | 6.2 | 5.2 | 0.046 |
| Health care utilization, mean (SD)d | |||||||||||||||
| Hospitalizations | 0.8 (1.6) | 0.5 (1.1) | 0.21 | 0.9 (1.7) | 0.3 (0.9) | 0.42 | 0.8 (1.6) | 0.3 (0.9) | 0.44 | 0.8 (1.6) | 0.4 (1.0) | 0.31 | 0.8 (1.5) | 0.8 (1.6) | 0.01 |
| General practice visits | 14.2 (12.3) | 10.7 (9.0) | 0.32 | 14.3 (12.8) | 9.7 (8.7) | 0.43 | 14.2 (11.9) | 12.2 (10.1) | 0.18 | 14.5 (12.2) | 12.2 (9.4) | 0.21 | 13.6 (11.5) | 13.8 (11.5) | 0.02 |
| Specialist referrals | 1.3 (1.7) | 1.1 (1.5) | 0.11 | 1.3 (1.8) | 0.7 (1.1) | 0.44 | 1.3 (1.8) | 0.8 (1.3) | 0.35 | 1.3 (1.8) | 0.9 (1.4) | 0.27 | 1.3 (1.7) | 1.2 (1.6) | 0.05 |
Abbreviations: ACE, angiotensin-converting enzyme; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CKD, chronic kidney disease; COX-2, cyclooxygenase 2; NSAID, nonsteroidal anti-inflammatory drug; SNRI, serotonin–norepinephrine reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor.
The time block, site of osteoarthritis, and comorbidities occurring in <5% of patients are presented in the Supplement.
The socioeconomic deprivation index score (ie, Townsend Deprivation Index) ranged from 1 (least deprived) to 5 (most deprived).
Other NSAID or opioid prescriptions prior to the index date.
Frequency during the 2 years before index date.
Table 2. Baseline Characteristics of Propensity Score–Matched Patients With Osteoarthritis in a Study Comparing the Association of Tramadol and Other Analgesics With All-Cause Mortalitya .
| Nonselective NSAIDs | COX-2 Inhibitors | Weak Opioid | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tramadol | Naproxen | Standardized Differences | Tramadol | Diclofenac | Standardized Differences | Tramadol | Celecoxib | Standardized Differences | Tramadol | Etoricoxib | Standardized Differences | Tramadol | Codeine | Standardized Differences | |
| Participants, No. | 12 397 | 12 397 | 6512 | 6512 | 5674 | 5674 | 2946 | 2946 | 16 922 | 16 922 | |||||
| Demographics | |||||||||||||||
| Age, mean (SD), y | 69.4 (9.6) | 69.4 (9.5) | 0.00 | 70.3 (9.6) | 70.3 (9.5) | 0.00 | 69.9 (9.5) | 69.8 (9.5) | 0.01 | 68.9 (9.4) | 68.9 (9.4) | 0.00 | 70.9 (9.5) | 71.0 (9.5) | 0.00 |
| Socioeconomic deprivation index score, mean (SD)b | 2.5 (1.4) | 2.5 (1.4) | 0.01 | 2.5 (1.4) | 2.5 (1.4) | 0.01 | 2.6 (1.4) | 2.6 (1.4) | 0.01 | 2.5 (1.4) | 2.5 (1.4) | 0.03 | 2.5 (1.3) | 2.5 (1.4) | 0.01 |
| Women, % | 59.2 | 59.6 | 0.01 | 61.5 | 62.5 | 0.02 | 65.5 | 65.2 | 0.01 | 62.5 | 62.2 | 0.01 | 60.8 | 60.5 | 0.01 |
| BMI, mean (SD) | 29.1 (5.7) | 29.2 (5.6) | 0.01 | 28.7 (5.6) | 28.8 (5.6) | 0.01 | 28.6 (5.5) | 28.6 (5.3) | 0.00 | 28.7 (5.7) | 28.6 (5.3) | 0.00 | 28.8 (5.5) | 28.8 (5.5) | 0.01 |
| Osteoarthritis duration, mean (SD), y | 6.8 (6.8) | 6.8 (6.6) | 0.00 | 6.4 (6.8) | 6.4 (6.8) | 0.00 | 6.6 (6.4) | 6.6 (6.6) | 0.00 | 6.3 (6.3) | 6.3 (6.3) | 0.00 | 7.2 (7.0) | 7.1 (6.8) | 0.00 |
| Lifestyle factors | |||||||||||||||
| Drinking alcohol, % | |||||||||||||||
| None | 19.1 | 19.2 | 0.00 | 20.9 | 21.9 | 0.02 | 21.6 | 21.5 | 0.00 | 20.5 | 20.7 | 0.00 | 20.0 | 20.2 | 0.00 |
| Past | 2.8 | 2.8 | 0.00 | 2.4 | 2.5 | 0.00 | 2.6 | 2.4 | 0.02 | 2.9 | 2.4 | 0.03 | 2.9 | 3.0 | 0.01 |
| Current | 78.1 | 78.0 | 0.00 | 76.7 | 75.7 | 0.02 | 75.7 | 76.1 | 0.01 | 76.6 | 76.8 | 0.01 | 77.1 | 76.8 | 0.01 |
| Smoking status, % | |||||||||||||||
| None | 53.5 | 53.7 | 0.00 | 54.8 | 54.1 | 0.01 | 56.2 | 55.7 | 0.01 | 57.8 | 56.4 | 0.03 | 54.9 | 55.1 | 0.01 |
| Past | 33.9 | 33.7 | 0.00 | 32.4 | 32.9 | 0.01 | 29.6 | 30.0 | 0.01 | 30.9 | 31.9 | 0.02 | 34.0 | 33.9 | 0.00 |
| Current | 12.6 | 12.6 | 0.00 | 12.8 | 12.9 | 0.00 | 14.2 | 14.3 | 0.00 | 11.3 | 11.6 | 0.01 | 11.1 | 11.0 | 0.01 |
| Comorbidity, % | |||||||||||||||
| CKD | |||||||||||||||
| No CKD | 87.1 | 87.1 | 0.00 | 91.2 | 91.3 | 0.01 | 96.5 | 96.3 | 0.01 | 92.6 | 93.0 | 0.01 | 87.4 | 87.1 | 0.01 |
| Stage 1 | 0.1 | 0.1 | 0.01 | 0.0 | 0.1 | 0.01 | 0.1 | 0.0 | 0.02 | 0.1 | 0.1 | 0.01 | 0.1 | 0.1 | 0.00 |
| Stage 2 | 1.5 | 1.6 | 0.01 | 0.9 | 1.0 | 0.01 | 0.5 | 0.4 | 0.01 | 0.7 | 0.8 | 0.01 | 1.0 | 1.0 | 0.01 |
| Stage 3 | 10.1 | 10.1 | 0.00 | 6.4 | 6.2 | 0.01 | 1.8 | 2.0 | 0.01 | 5.5 | 5.1 | 0.02 | 9.7 | 9.9 | 0.01 |
| Stage 4 | 0.4 | 0.4 | 0.00 | 0.3 | 0.3 | 0.00 | 0.1 | 0.1 | 0.01 | 0.3 | 0.3 | 0.00 | 0.6 | 0.6 | 0.01 |
| Stage 5 | 0.3 | 0.2 | 0.01 | 0.3 | 0.3 | 0.00 | 0.4 | 0.4 | 0.01 | 0.2 | 0.2 | 0.02 | 0.4 | 0.4 | 0.00 |
| Stage unknown | 0.6 | 0.5 | 0.00 | 0.8 | 0.8 | 0.01 | 0.6 | 0.8 | 0.02 | 0.6 | 0.6 | 0.00 | 0.8 | 0.8 | 0.00 |
| Hypertension | 52.1 | 52.4 | 0.01 | 52.5 | 52.9 | 0.01 | 47.4 | 48.1 | 0.01 | 47.8 | 47.8 | 0.00 | 54.2 | 54.6 | 0.01 |
| Other circulatory disease | 32.7 | 32.4 | 0.01 | 32.3 | 32.5 | 0.00 | 34.9 | 34.1 | 0.02 | 33.1 | 31.7 | 0.03 | 36.4 | 36.7 | 0.01 |
| Ischemic heart disease | 14.2 | 14.6 | 0.01 | 18.0 | 18.0 | 0.00 | 17.9 | 18.1 | 0.01 | 13.1 | 13.7 | 0.02 | 18.0 | 18.2 | 0.00 |
| Hyperlipidemia | 18.4 | 18.2 | 0.00 | 17.2 | 17.2 | 0.00 | 14.1 | 14.9 | 0.02 | 16.5 | 16.3 | 0.01 | 18.3 | 18.3 | 0.00 |
| Anxiety | 15.8 | 15.6 | 0.02 | 15.2 | 14.6 | 0.01 | 17.2 | 17.6 | 0.01 | 15.3 | 15.1 | 0.01 | 15.7 | 15.7 | 0.01 |
| Diabetes | 14.6 | 14.5 | 0.00 | 13.2 | 13.8 | 0.02 | 11.7 | 11.4 | 0.01 | 11.2 | 10.8 | Diabetes | 14.6 | 14.5 | 0.00 |
| Depression | 14.4 | 14.5 | 0.00 | 13.7 | 13.9 | 0.01 | 15.3 | 15.2 | 0.00 | 14.5 | 14.8 | 0.01 | 13.5 | 13.7 | 0.01 |
| Varicose veins | 14.0 | 14.1 | 0.00 | 12.9 | 13.1 | 0.01 | 14.8 | 14.5 | 0.01 | 15.6 | 15.2 | 0.01 | 14.7 | 14.9 | 0.01 |
| Angina | 9.4 | 9.7 | 0.01 | 12.6 | 12.7 | 0.01 | 13.3 | 13.5 | 0.00 | 9.9 | 10.0 | 0.01 | 12.5 | 12.5 | 0.00 |
| Peptic ulcer | 6.5 | 6.1 | 0.01 | 8.9 | 8.6 | 0.01 | 8.9 | 8.8 | 0.00 | 6.1 | 6.6 | 0.02 | 7.8 | 7.9 | 0.00 |
| Pneumonia or infection | 7.3 | 7.2 | 0.00 | 7.6 | 7.6 | 0.00 | 9.5 | 9.2 | 0.01 | 8.8 | 8.4 | 0.02 | 7.9 | 8.1 | 0.01 |
| Atrial fibrillation | 4.8 | 4.5 | 0.02 | 5.6 | 5.3 | 0.01 | 4.8 | 4.7 | 0.01 | 3.6 | 3.9 | 0.01 | 7.8 | 8.0 | 0.01 |
| Chronic obstructive pulmonary disease | 5.0 | 4.8 | 0.01 | 6.0 | 5.5 | 0.02 | 5.5 | 5.1 | 0.02 | 5.0 | 4.2 | 0.04 | 5.8 | 5.9 | 0.00 |
| Myocardial infarction | 4.9 | 5.0 | 0.01 | 6.2 | 6.2 | 0.00 | 5.6 | 5.8 | 0.01 | 4.3 | 4.7 | 0.02 | 6.6 | 6.6 | 0.00 |
| Venous thromboembolism | 4.0 | 3.9 | 0.01 | 4.8 | 4.5 | 0.02 | 4.9 | 4.9 | 0.00 | 4.2 | 3.8 | 0.02 | 5.1 | 5.0 | 0.00 |
| Congestive heart failure | 2.8 | 2.6 | 0.02 | 4.5 | 4.3 | 0.01 | 4.6 | 4.5 | 0.00 | 3.3 | 3.2 | 0.01 | 4.8 | 4.8 | 0.00 |
| Transient ischemic attack | 3.2 | 3.3 | 0.01 | 4.2 | 4.1 | 0.01 | 4.0 | 4.0 | 0.00 | 2.1 | 2.7 | 0.04 | 4.2 | 4.2 | 0.00 |
| Stroke | 3.3 | 3.2 | 0.00 | 3.7 | 3.9 | 0.01 | 3.6 | 3.6 | 0.00 | 2.5 | 2.6 | 0.01 | 4.2 | 4.3 | 0.01 |
| Medication, % | |||||||||||||||
| Other NSAIDsc | 85.7 | 86.2 | 0.02 | 73.2 | 74.1 | 0.02 | 90.6 | 91.3 | 0.03 | 94.7 | 94.1 | 0.03 | 88.0 | 87.8 | 0.01 |
| Antihypertensive | 67.0 | 67.6 | 0.01 | 67.6 | 68.5 | 0.02 | 68.2 | 68.3 | 0.00 | 64.8 | 65.1 | 0.01 | 70.1 | 70.5 | 0.01 |
| Statin | 47.6 | 47.9 | 0.01 | 42.4 | 42.6 | 0.01 | 33.7 | 34.2 | 0.01 | 39.5 | 39.5 | 0.00 | 47.0 | 47.2 | 0.00 |
| Aspirin | 36.1 | 36.7 | 0.01 | 37.3 | 37.4 | 0.00 | 34.1 | 34.9 | 0.02 | 30.2 | 31.3 | 0.02 | 40.0 | 40.3 | 0.01 |
| Other opioidsc | 35.4 | 35.9 | 0.01 | 32.0 | 32.9 | 0.02 | 39.6 | 39.5 | 0.00 | 33.3 | 32.5 | 0.02 | 17.3 | 17.1 | 0.00 |
| ACE inhibitors | 36.7 | 36.9 | 0.01 | 34.4 | 35.3 | 0.02 | 28.6 | 28.9 | 0.01 | 28.8 | 29.2 | 0.01 | 39.0 | 39.1 | 0.00 |
| Benzodiazepines | 35.7 | 35.8 | 0.00 | 33.1 | 33.4 | 0.01 | 39.9 | 39.7 | 0.01 | 39.1 | 38.1 | 0.02 | 34.8 | 34.7 | 0.00 |
| Calcium channel blockers | 34.9 | 35.0 | 0.00 | 34.5 | 35.1 | 0.01 | 32.3 | 32.4 | 0.00 | 30.8 | 30.8 | 0.00 | 37.5 | 37.5 | 0.00 |
| β-Receptor inhibitor | 35.8 | 36.0 | 0.00 | 34.5 | 35.3 | 0.02 | 35.2 | 35.6 | 0.01 | 34.2 | 34.0 | 0.00 | 38.9 | 39.1 | 0.00 |
| Loop diuretics | 17.9 | 18.0 | 0.00 | 21.6 | 21.3 | 0.01 | 24.8 | 25.0 | 0.00 | 19.1 | 20.0 | 0.02 | 23.1 | 23.1 | 0.00 |
| Glucocorticoids | 22.7 | 22.9 | 0.00 | 21.3 | 21.0 | 0.01 | 23.9 | 22.7 | 0.03 | 22.6 | 23.2 | 0.01 | 24.1 | 24.0 | 0.00 |
| SSRI | 22.1 | 22.5 | 0.01 | 18.7 | 19.0 | 0.01 | 21.4 | 21.0 | 0.01 | 23.2 | 22.5 | 0.02 | 20.2 | 20.4 | 0.01 |
| Nitrates | 13.5 | 13.7 | 0.01 | 15.5 | 15.7 | 0.01 | 16.7 | 17.2 | 0.01 | 12.7 | 12.8 | 0.01 | 16.8 | 16.8 | 0.00 |
| Angiotensin receptor blocker | 14.2 | 14.3 | 0.00 | 12.7 | 12.6 | 0.00 | 8.8 | 8.9 | 0.00 | 11.8 | 12.1 | 0.01 | 14.1 | 14.2 | 0.00 |
| Potassium-sparing diuretics | 7.6 | 7.6 | 0.00 | 10.5 | 10.5 | 0.00 | 13.8 | 13.5 | 0.01 | 10.6 | 10.6 | 0.00 | 10.3 | 10.3 | 0.00 |
| Antidiabetic | 10.2 | 10.2 | 0.00 | 9.6 | 10.0 | 0.01 | 8.4 | 8.6 | 0.01 | 8.4 | 7.9 | 0.02 | 10.9 | 11.0 | 0.00 |
| Anticoagulants | 6.4 | 6.1 | 0.01 | 7.6 | 6.9 | 0.03 | 6.3 | 6.2 | 0.00 | 4.9 | 4.9 | 0.00 | 10.1 | 10.1 | 0.00 |
| Thiazide-like diuretics | 5.5 | 5.5 | 0.00 | 5.9 | 5.9 | 0.00 | 5.6 | 5.9 | 0.01 | 6.7 | 7.0 | 0.01 | 6.3 | 6.4 | 0.00 |
| SNRI | 5.8 | 6.0 | 0.01 | 4.3 | 4.6 | 0.01 | 5.5 | 5.4 | 0.00 | 5.7 | 5.9 | 0.01 | 5.2 | 5.3 | 0.01 |
| Health care utilization, mean (SD)d | |||||||||||||||
| Hospitalizations | 0.7 (1.3) | 0.7 (1.4) | 0.02 | 0.6 (1.1) | 0.6 (1.3) | 0.01 | 0.4 (1.0) | 0.4 (1.0) | 0.02 | 0.5 (1.0) | 0.4 (1.0) | 0.04 | 0.8 (1.5) | 0.8 (1.6) | 0.01 |
| General practice visits | 12.8 (10.0) | 12.7 (10.3) | 0.01 | 12.8 (9.7) | 12.9 (10.4) | 0.01 | 14.1 (10.7) | 13.9 (11.1) | 0.02 | 13.0 (9.7) | 12.8 (9.2) | 0.02 | 14.2 (11.5) | 14.1 (10.8) | 0.01 |
| Specialist referrals | 1.3 (1.6) | 1.3 (1.7) | 0.01 | 1.0 (1.4) | 1.0 (1.4) | 0.01 | 1.0 (1.3) | 1.0 (1.4) | 0.00 | 1.0 (1.4) | 1.0 (1.4) | 0.00 | 1.3 (1.6) | 1.3 (1.6) | 0.00 |
Abbreviations: ACE, angiotensin-converting enzyme; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CKD, chronic kidney disease; COX-2, cyclooxygenase 2; NSAID, nonsteroidal anti-inflammatory drug; SNRI, serotonin–norepinephrine reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor.
The time block, site of osteoarthritis, and comorbidities occurring in <5% of patients are presented in the Supplement.
The socioeconomic deprivation index score (ie, Townsend Deprivation Index) ranged from 1 (least deprived) to 5 (most deprived).
Other NSAID or opioid prescriptions prior to the index date.
Frequency during the 2 years before index date.
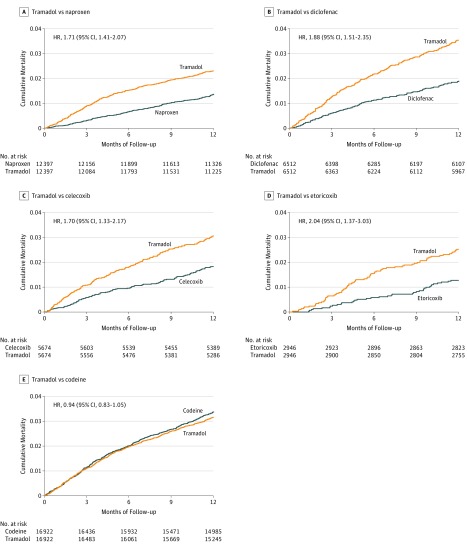
Mortality was higher in the tramadol cohort than in the naproxen cohort (Figure 2A). During the 1-year follow-up period, 278 deaths (23.5 per 1000 person-years) occurred in the tramadol cohort and 164 deaths (13.8 per 1000 person-years) occurred in the matched naproxen cohort (Table 3). Compared with the naproxen cohort, the RD of mortality for tramadol was 9.7 per 1000 person-years (95% CI, 6.3-13.2). Because the proportional hazard assumption was violated for the comparison of tramadol vs naproxen (P < .001), follow-up time was divided into less than or equal to 3, 6, 9, and 12 months, and the hazard ratios (HR) at 3 months was 2.93 (95% CI, 2.02-4.26), 6 months was 2.34 (95% CI, 1.80-3.05), 9 months was 1.93 (95% CI, 1.55-2.40), and 12 months was 1.71 (95% CI. 1.41-2.07). Tramadol was also associated with higher mortality than diclofenac in the matched cohorts (Figure 2B). Compared with the diclofenac cohort, the RD of mortality for tramadol prescription was 17.0 per 1000 person-years (95% CI, 11.2-22.8) and the HR was 1.88 (95% CI, 1.51-2.35) (Table 3).
Figure 2. Time to Death for Propensity Score–Matched Cohorts of Patients With Osteoarthritis and Initial Prescription of Tramadol Compared With Other Drugs .
Each patient can only be counted once in a category; however, the same patient could be selected multiple times in 5 categories. Each category represents a specific sequential propensity score–matched cohort study (ie, tramadol vs naproxen, tramadol vs diclofenac, tramadol vs celecoxib, tramadol vs etoricoxib, or tramadol vs codeine). The median (interquartile range) follow-up time for all drugs was 12.0 (0.0) months.
Table 3. All-Cause Mortality Within One Year Among Patients Initiating Tramadol Prescription Compared With Other Propensity Score–Matched Analgesics .
| Nonselective NSAIDs | COX−2 Inhibitors | Weak Opioid | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tramadol vs Naproxen | Tramadol vs Diclofenac | Tramadol vs Celecoxib | Tramadol vs Etoricoxib | |||||||||
| Tramadol (n = 12 397) | Naproxen (n = 12 397) | Tramadol (n = 6512) | Diclofenac (n = 6512) | Tramadol (n = 5674) | Celecoxib (n = 5674) | Tramadol (n = 2946) | Etoricoxib (n = 2946) | Tramadol (n = 16 922) | Codeine (n = 16 922) | |||
| Deaths, No. | 278 | 164 | 226 | 121 | 171 | 102 | 73 | 37 | 519 | 552 | ||
| Rate of death, per 1000 person-years | 23.5 | 13.8 | 36.2 | 19.2 | 31.2 | 18.4 | 25.7 | 12.8 | 32.2 | 34.6 | ||
| RD (95% CI), per 1000 person-years | 9.7 (6.3 to 13.2) | 17.0 (11.2 to 22.8) | 12.8 (6.9 to 18.7) | 12.8 (5.7 to 20.0) | −2.3 (−6.3 to 1.7) | |||||||
| HR (95% CI) | 1.71 (1.41 to 2.07) |
1 [Ref] | 1.88 (1.51 to 2.35) |
1 [Ref] | 1.70 (1.33 to 2.17) |
1 [Ref] | 2.04 (1.37 to 3.03) |
1 [Ref] | 0.94 (0.83 to 1.05) |
1 [Ref] | ||
| PS trimminga,b | 1.74 (1.42 to 2.13) |
1.87 (1.49 to 2.34) |
1.74 (1.35 to 2.24) |
2.00 (1.33 to 3.01) |
0.94 (0.83 to 1.06) |
|||||||
| History of other opioidsb | ||||||||||||
| Yes | 1.58 (1.17 to 2.14) | 1.83 (1.23 to 2.71) | 1.42 (0.98 to 2.06) | 2.39 (1.14 to 5.04) | 0.96 (0.83 to 1.09) | |||||||
| No | 1.80 (1.40 to 2.31) | 1.88 (1.44 to 2.46) | 1.93 (1.38 to 2.69) | 1.95 (1.21 to 3.14) | 0.88 (0.68 to 1.13) | |||||||
| As-treated approachb,c | 2.75 (1.86 to 4.06) | 2.04 (1.36 to 3.06) | 2.38 (1.54 to 3.69) | 2.55 (1.19 to 5.48) | 0.83 (0.68 to 1.02) | |||||||
| Incident OA patientsb,d | 1.50 (1.18 to 1.90) | 1.61 (1.19 to 2.16) | 1.91 (1.39 to 2.62) | 2.40 (1.44 to 4.01) | 0.97 (0.83 to 1.14) | |||||||
| Missing data imputationb,e | 1.63 (1.37 to 1.96) | 1.62 (1.33 to 1.96) | 1.92 (1.54 to 2.40) | 1.98 (1.28 to 3.06) | 0.92 (0.83 to 1.02) | |||||||
| Lagb,f | ||||||||||||
| Six months | 1.51 (1.22 to 1.88) | 1.87 (1.45 to 2.41) | 1.50 (1.15 to 1.96) | 1.96 (1.26 to 3.06) | 0.91 (0.80 to 1.05) | |||||||
| One year | 1.63 (1.3 to 2.05) | 1.95 (1.49 to 2.54) | 1.54 (1.16 to 2.05) | 1.92 (1.21 to 3.05) | 0.87 (0.76 to 1.01) | |||||||
Abbreviations: COX-2, cyclooxygenase 2; HR, hazard ratio; NSAIDs, nonsteroidal anti-inflammatory drugs; OA, osteoarthritis; PS, propensity score; RD, rate difference.
Asymmetric trimming was used to exclude participants whose propensity score was below the 2.5th percentile of the propensity score of the tramadol cohort and above the 97.5th percentile of the propensity-score of the comparator cohort.
The reference group is the second pair in each comparison.
This analysis censored the follow-up at the time when participants either changed (eg, switching from tramadol to naproxen or vice versa, when comparing tramadol with naproxen) or discontinued (ie, no prescription refill for the respective class of medication for over 60 d) their initiated medication.
This analysis was performed among participants whose osteoarthritis was diagnosed during the study period (ie, incident osteoarthritis) to minimize potential misclassification of duration of osteoarthritis.
Imputation analysis was performed to deal with missing data. Specifically, missing values of the variables (ie, body mass index, smoking, drinking status, or Townsend Deprivation Index) were imputed by a sequential regression method based on a set of covariates as predictors.
This analysis introduced a 6-month or 1-year exposure lag period to account for a potential latency time window (eg, excluding cancer cases that occurred within 6 months or 1 year.
Mortality in the tramadol cohort was higher than in the celecoxib cohort (Figure 2C). During the 1-year follow-up period, 171 deaths (31.2 per 1000 person-years) occurred in the tramadol cohort and 102 deaths (18.4 per 1000 person-years) occurred in the celecoxib cohort (Table 3). The RD of mortality for tramadol vs celecoxib was 12.8 per 1000 person-years (95% CI, 6.9-18.7) and the HR was 1.70 (95% CI, 1.33-2.17). Similar findings were observed when mortality in the tramadol cohort was compared with the etoricoxib cohort (Figure 2D and Table 3).
There was no statistically significant difference in mortality between the tramadol cohort and the matched codeine cohort (Figure 2E and Table 3). During the 1-year follow-up period, 519 deaths (32.2 per 1000 person-years) occurred in the tramadol cohort and 552 deaths (34.6 per 1000 person-years) occurred in the codeine cohort. The RD of mortality for tramadol was −2.3 per 1000 person-years (95% CI, −6.3 to 1.7) and the HR was 0.94 (95% CI, 0.83-1.05).
Results from sensitivity analyses also showed that participants in the tramadol cohort experienced significantly higher mortality than those in the naproxen, diclofenac, celecoxib, and etoricoxib cohorts, but not in the codeine cohorts (Table 3).
As shown in Table 4, mortality rates from cardiovascular, gastrointestinal, infection, cancer, and respiratory diseases were higher in the tramadol cohort than in the NSAIDs cohorts; however, because of the relatively small number of deaths from each specific cause, most associations were not statistically significant. No statistically significant difference in each cause-specific morality (except for infection) was observed between the tramadol cohort and the codeine cohort.
Table 4. Cause-Specific Mortality Within 1 Year Among Patients Initiating Tramadol Prescription Compared With Propensity Score–Matched Other Analgesics.
| Cause of Death, No. | Nonselective NSAIDs | COX-2 Inhibitors | Weak Opioid | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tramadol vs Naproxen | Tramadol vs Diclofenac | Tramadol vs Celecoxib | Tramadol vs Etoricoxib | |||||||||
| Tramadol (n = 12 397) | Naproxen (n = 12 397) | Tramadol (n = 6512) | Diclofenac (n = 6512) | Tramadol (n = 5674) | Celecoxib (n = 5674) | Tramadol (n = 2946) | Etoricoxib (n = 2946) | Tramadol (n = 16 922) | Codeine (n = 16 922) | |||
| Cardiovascular | 40 | 33 | 39 | 26 | 47 | 42 | 27 | 4 | 111 | 126 | ||
| HR (95% CI)a | 1.22 (0.77-1.94) | 1 [Ref] | 1.49 (0.90-2.44) | 1 [Ref] | 1.13 (0.74-1.70) | 1 [Ref] | 6.85 (2.43-19.30) | 1 [Ref] | 0.88 (0.68-1.13) | 1 [Ref] | ||
| Gastrointestinal | 20 | 10 | 20 | 10 | 11 | 6 | 3 | 4 | 38 | 49 | ||
| HR (95% CI)a | 1.99 (0.94-4.23) | 2.00 (0.94-4.28) | 1.82 (0.68-4.93) | 0.87 (0.18-4.20) | 0.77 (0.51-1.18) | |||||||
| Infection | 47 | 20 | 31 | 18 | 26 | 10 | 9 | 6 | 70 | 101 | ||
| HR (95% CI)a | 2.35 (1.38-3.98) | 1.73 (0.97-3.10) | 2.61 (1.27-5.38) | 1.64 (0.57-4.73) | 0.69 (0.51-0.93) | |||||||
| Cancer | 65 | 35 | 56 | 26 | 38 | 13 | 15 | 7 | 113 | 107 | ||
| HR (95% CI)a | 1.86 (1.24-2.81) | 2.10 (1.33-3.34) | 2.93 (1.57-5.47) | 2.16 (0.89-5.28) | 1.04 (0.8-1.36) | |||||||
| Respiratory | 23 | 19 | 23 | 8 | 25 | 11 | 13 | 3 | 63 | 75 | ||
| HR (95% CI)a | 1.22 (0.67-2.24) | 2.86 (1.28-6.41) | 2.27 (1.13-4.56) | 4.44 (1.30-15.17) | 0.84 (0.60-1.17) | |||||||
| Renal | 12 | 8 | 9 | 9 | 5 | 7 | 4 | 1 | 25 | 36 | ||
| HR (95% CI)a | 1.02 (0.40-2.58) | 0.69 (0.41-1.14) | ||||||||||
| Musculoskeletal | 12 | 12 | 6 | 5 | 10 | 1 | 4 | 1 | 21 | 21 | ||
| HR (95% CI)a | 1.02 (0.46-2.26) | |||||||||||
| Blood | 7 | 9 | 5 | 7 | 6 | 1 | 2 | 2 | 18 | 14 | ||
| Endocrine | 6 | 6 | 8 | 3 | 7 | 4 | 2 | 2 | 19 | 18 | ||
| Mental | 6 | 4 | 8 | 2 | 6 | 4 | 2 | 1 | 13 | 22 | ||
| Nervous system | 4 | 4 | 3 | 5 | 5 | 3 | 1 | 0 | 6 | 10 | ||
| Accidents | 6 | 3 | 5 | 2 | 2 | 0 | 0 | 0 | 8 | 5 | ||
| Sudden death | 4 | 4 | 5 | 0 | 5 | 3 | 4 | 2 | 14 | 7 | ||
| Unknown | 81 | 39 | 57 | 33 | 31 | 20 | 12 | 11 | 130 | 128 | ||
Abbreviations: COX-2, cyclooxygenase 2; HR, hazard ratio; NSAIDs, nonsteroidal anti-inflammatory drugs.
The HR (95% CI) was only estimated for the cause-specific death that contributed >5% of deaths to the total number of deaths within each matched-cohort except for unknown cause of death. The reference group is the second of the pair in each comparison.
Discussion
Using data collected from THIN, this study found that initial prescription of tramadol was associated with a significantly increased mortality rate over the next year compared with commonly prescribed NSAIDs among participants with osteoarthritis, but no statistically significant difference in mortality rate was observed between tramadol and codeine. Considering that participants with initial prescription of tramadol had a higher comorbidity burden than those with an initial prescription of NSAIDs before propensity score matching, these results were susceptible to confounding by indication. Thus, the present findings should be interpreted with caution, and future studies are needed.
Oral NSAIDs (nonselective NSAIDs and COX-2 inhibitors) are the predominant analgesic medications used to manage osteoarthritis worldwide; however, their safety, particularly with regard to cardiovascular and gastrointestinal risk, has raised concern. Similarly, opioids are commonly prescribed for managing osteoarthritis and their safety has been questioned because of a potential increase in mortality.20,21,22 Tramadol is a weak opioid agonist and has been considered a potential alternative to NSAIDs and traditional opioids because of its assumed relatively lower risk of serious cardiovascular and gastrointestinal adverse effects than NSAIDs,23 as well as a lower risk of addiction and respiratory depression compared with other opioids.2 Studies, including the present study, have shown that tramadol prescription among patients with osteoarthritis has been increasing since 2000.5
The few studies that have assessed the relationship between tramadol prescription and mortality among patients with different diseases have yielded conflicting results. One study of 1271 patients with perforated peptic ulcer found that tramadol prescription was associated with significantly higher in-hospital mortality than the absence of tramadol or NSAIDs prescription.9 Similar results were observed among 153 758 patients receiving dialysis.10 Furthermore, data on 11.3 million patients from the Clinical Practice Research Datalink showed that the tramadol-related death rate increased before tramadol was classified as a Schedule III controlled substance in 2014 in the United Kingdom, and decreased thereafter.13 However, a small study of 272 patients who underwent hip replacement due to fracture showed that tramadol prescription was not associated with increased mortality within 6 months after surgery compared with no prescription of tramadol.11 Another study failed to show a statistically significant mortality difference between prescription of tramadol alone or in combination with codeine compared with infrequent or no prescription of tramadol alone or combined with codeine among 8866 patients with Crohn disease and ulcerative colitis.12 In a large propensity score–matched cohort study that examined the safety of 5 commonly prescribed opioids among 31 375 Medicare beneficiaries in the United States, initial prescription of tramadol was not associated with a statistically significant higher mortality than hydrocodone prescription after a 180-day follow-up (rate ratio, 1.44 [95% CI, 0.96-2.17]).8
The biological mechanisms linking tramadol to mortality are unclear. Tramadol may activate μ opioid receptors and inhibit central serotonin and norepinephrine reuptake, and the latter may result in a unique adverse effect on the neurological system (ie, serotonin syndrome and seizures).2 Tramadol may also increase the risk of postoperative delirium, which tends to increase mortality.24 Fatal poisoning or respiratory depression may occur when tramadol users consume alcohol or use tramadol with other central nervous systems depressants.25,26,27,28 Furthermore, tramadol may increase the risk of hypoglycemia, hyponatremia, fracture, or fall, thus leading to an increased risk of death.29,30,31,32
The present findings may have clinical implications. First, if replicated and determined to likely be causal, these findings would indicate an unfavorable safety profile of tramadol. Second, various strategies have been proposed to minimize the adverse effects of analgesics use. For instance, coprescription of proton pump inhibitors with oral NSAIDs has been considered a cost-effective approach for patients with osteoarthritis with moderate or high gastrointestinal risk.4,33,34,35,36 For patients with high cardiovascular risk, naproxen may be preferred, owing to its relatively low cardiovascular risk.36,37 In general, based on results reported in the current study, nonopioid therapy could be preferred for management of chronic pain (eg, osteoarthritis).38
Limitations
This study has several limitations. First, 16.4% to 29.7% of causes of death could not be ascertained, and the current study did not have adequate statistical power to evaluate the relationship of initial prescription of tramadol to cause-specific mortality because of a small number of cause-specific deaths. Second, this study found a higher cancer-related mortality in the tramadol cohort than the NSAIDs cohorts. It is possible that some participants were experiencing pain from undetected early-stage cancer and therefore were given stronger pain medication to relieve the symptoms prior to cancer diagnosis (ie, protopathic bias). Although excluding cancer cases that occurred within 6 months or 1 year showed that all-cause mortality in the tramadol cohort was still significantly higher than in 4 NSAIDs cohorts, the increased rate of cancer mortality among patients prescribed tramadol suggests that confounding by indication, such as severity of other comorbidities, may be a potential explanation of the present findings. Third, participants with initial prescription of tramadol were older, had a higher BMI, had a longer duration of osteoarthritis, had a higher prevalence of comorbidities, received more prescriptions, and had more health care utilization than participants in the NSAIDs cohorts before propensity score matching. Thus, while techniques were used to try to control for the potential confounders, including propensity score matching, residual confounding still could affect the study findings. It is possible that comorbidities and illness severity associated with tramadol prescription may explain the higher mortality rate in this group. Fourth, this study was conducted among patients with osteoarthritis. Thus, these findings may not be generalizable to patients with other diseases whose disease pathophysiology may modify the effect of tramadol on mortality.
Conclusions
Among patients aged 50 years and older with osteoarthritis, initial prescription of tramadol was associated with a significantly higher risk of mortality over 1 year of follow-up compared with commonly prescribed NSAIDs, but not when compared with codeine. However, these findings may be susceptible to confounding by indication, and further research is needed to determine if this association is causal.
eTable 1. Baseline characteristics of unmatched and propensity-score matched osteoarthritis patients (≥ 50 years) initiating tramadol prescription or naproxen prescription.
eTable 2. Baseline characteristics of unmatched and propensity-score matched osteoarthritis patients (≥ 50 years) initiating tramadol prescription or diclofenac prescription.
eTable 3. Baseline characteristics of unmatched and propensity-score matched osteoarthritis patients (≥ 50 years) initiating tramadol prescription or celecoxib prescription.
eTable 4. Baseline characteristics of unmatched and propensity-score matched osteoarthritis patients (≥ 50 years) initiating tramadol prescription or etoricoxib prescription.
eTable 5. Baseline characteristics of unmatched and propensity-score matched osteoarthritis patients (≥ 50 years) initiating tramadol prescription or codeine prescription.
References
- 1.Glyn-Jones S, Palmer AJ, Agricola R, et al. Osteoarthritis. Lancet. 2015;386(9991):376-387. doi: 10.1016/S0140-6736(14)60802-3 [DOI] [PubMed] [Google Scholar]
- 2.Hassamal S, Miotto K, Dale W, Danovitch I. Tramadol: understanding the risk of serotonin syndrome and seizures. Am J Med. 2018;131(11):1382.e1-1382.e6. doi: 10.1016/j.amjmed.2018.04.025 [DOI] [PubMed] [Google Scholar]
- 3.Jevsevar DS. Treatment of osteoarthritis of the knee:evidence-based guideline, 2nd edition. J Am Acad Orthop Surg. 2013;21(9):571-576. doi: 10.5435/JAAOS-21-09-571 [DOI] [PubMed] [Google Scholar]
- 4.Hochberg MC, Altman RD, April KT, et al. ; American College of Rheumatology . American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken). 2012;64(4):465-474. doi: 10.1002/acr.21596 [DOI] [PubMed] [Google Scholar]
- 5.Wright EA, Katz JN, Abrams S, Solomon DH, Losina E. Trends in prescription of opioids from 2003-2009 in persons with knee osteoarthritis. Arthritis Care Res (Hoboken). 2014;66(10):1489-1495. doi: 10.1002/acr.22360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith SR, Deshpande BR, Collins JE, Katz JN, Losina E. Comparative pain reduction of oral non-steroidal anti-inflammatory drugs and opioids for knee osteoarthritis: systematic analytic review. Osteoarthritis Cartilage. 2016;24(6):962-972. doi: 10.1016/j.joca.2016.01.135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beaulieu AD, Peloso PM, Haraoui B, et al. Once-daily, controlled-release tramadol and sustained-release diclofenac relieve chronic pain due to osteoarthritis: a randomized controlled trial. Pain Res Manag. 2008;13(2):103-110. doi: 10.1155/2008/903784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Solomon DH, Rassen JA, Glynn RJ, et al. The comparative safety of opioids for nonmalignant pain in older adults. Arch Intern Med. 2010;170(22):1979-1986. doi: 10.1001/archinternmed.2010.450 [DOI] [PubMed] [Google Scholar]
- 9.Tørring ML, Riis A, Christensen S, et al. Perforated peptic ulcer and short-term mortality among tramadol users. Br J Clin Pharmacol. 2008;65(4):565-572. doi: 10.1111/j.1365-2125.2007.03038.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kimmel PL, Fwu CW, Abbott KC, Eggers AW, Kline PP, Eggers PW. Opioid prescription, morbidity, and mortality in United States dialysis patients. J Am Soc Nephrol. 2017;28(12):3658-3670. doi: 10.1681/ASN.2017010098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Härstedt M, Rogmark C, Sutton R, Melander O, Fedorowski A. Polypharmacy and adverse outcomes after hip fracture surgery. J Orthop Surg Res. 2016;11(1):151. doi: 10.1186/s13018-016-0486-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burr NE, Smith C, West R, Hull MA, Subramanian V. Increasing prescription of opiates and mortality in patients with inflammatory bowel diseases in England. Clin Gastroenterol Hepatol. 2018;16(4):534-541.e6. doi: 10.1016/j.cgh.2017.10.022 [DOI] [PubMed] [Google Scholar]
- 13.Chen TC, Chen LC, Knaggs RD. A 15-year overview of increasing tramadol utilisation and associated mortality and the impact of tramadol classification in the United Kingdom. Pharmacoepidemiol Drug Saf. 2018;27(5):487-494. doi: 10.1002/pds.4320 [DOI] [PubMed] [Google Scholar]
- 14.Seeger JD, Williams PL, Walker AM. An application of propensity score matching using claims data. Pharmacoepidemiol Drug Saf. 2005;14(7):465-476. doi: 10.1002/pds.1062 [DOI] [PubMed] [Google Scholar]
- 15.Lin DY, Wei LJ, Ying Z. Checking the Cox model with cumulative sums of Martingale based residuals. Biometrika. 1993;80:557-572. doi: 10.1093/biomet/80.3.557 [DOI] [Google Scholar]
- 16.Stürmer T, Rothman KJ, Avorn J, Glynn RJ. Treatment effects in the presence of unmeasured confounding: dealing with observations in the tails of the propensity score distribution--a simulation study. Am J Epidemiol. 2010;172(7):843-854. doi: 10.1093/aje/kwq198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York, NY: John Wiley & Sons; 1987. doi: 10.1002/9780470316696 [DOI] [Google Scholar]
- 18.Hicks BM, Filion KB, Yin H, Sakr L, Udell JA, Azoulay L. Angiotensin converting enzyme inhibitors and risk of lung cancer: population based cohort study. BMJ. 2018;363:k4209. doi: 10.1136/bmj.k4209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ogdie A, Maliha S, Shin D, et al. Cause-specific mortality in patients with psoriatic arthritis and rheumatoid arthritis. Rheumatology (Oxford). 2017;56(6):907-911. doi: 10.1093/rheumatology/kew502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Solomon DH, Rassen JA, Glynn RJ, Lee J, Levin R, Schneeweiss S. The comparative safety of analgesics in older adults with arthritis. Arch Intern Med. 2010;170(22):1968-1976. doi: 10.1001/archinternmed.2010.391 [DOI] [PubMed] [Google Scholar]
- 21.Dart RC, Surratt HL, Cicero TJ, et al. Trends in opioid analgesic abuse and mortality in the United States. N Engl J Med. 2015;372(3):241-248. doi: 10.1056/NEJMsa1406143 [DOI] [PubMed] [Google Scholar]
- 22.Ray WA, Chung CP, Murray KT, Hall K, Stein CM. Prescription of long-acting opioids and mortality in patients with chronic noncancer pain. JAMA. 2016;315(22):2415-2423. doi: 10.1001/jama.2016.7789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katz WA. Pharmacology and clinical experience with tramadol in osteoarthritis. Drugs. 1996;52(suppl 3):39-47. doi: 10.2165/00003495-199600523-00007 [DOI] [PubMed] [Google Scholar]
- 24.Brouquet A, Cudennec T, Benoist S, et al. Impaired mobility, ASA status and administration of tramadol are risk factors for postoperative delirium in patients aged 75 years or more after major abdominal surgery. Ann Surg. 2010;251(4):759-765. doi: 10.1097/SLA.0b013e3181c1cfc9 [DOI] [PubMed] [Google Scholar]
- 25.Randall C, Crane J. Tramadol deaths in Northern Ireland: a review of cases from 1996 to 2012. J Forensic Leg Med. 2014;23:32-36. doi: 10.1016/j.jflm.2014.01.006 [DOI] [PubMed] [Google Scholar]
- 26.Handley SA, Flanagan RJ. Drugs and other chemicals involved in fatal poisoning in England and Wales during 2000 – 2011. Clin Toxicol (Phila). 2014;52(1):1-12. doi: 10.3109/15563650.2013.872791 [DOI] [PubMed] [Google Scholar]
- 27.Tjäderborn M, Jönsson AK, Hägg S, Ahlner J. Fatal unintentional intoxications with tramadol during 1995-2005. Forensic Sci Int. 2007;173(2-3):107-111. doi: 10.1016/j.forsciint.2007.02.007 [DOI] [PubMed] [Google Scholar]
- 28.Häkkinen M, Launiainen T, Vuori E, Ojanperä I. Comparison of fatal poisonings by prescription opioids. Forensic Sci Int. 2012;222(1-3):327-331. doi: 10.1016/j.forsciint.2012.07.011 [DOI] [PubMed] [Google Scholar]
- 29.Fournier J-P, Azoulay L, Yin H, Montastruc J-L, Suissa S. Tramadol use and the risk of hospitalization for hypoglycemia in patients with noncancer pain. JAMA Intern Med. 2015;175(2):186-193. doi: 10.1001/jamainternmed.2014.6512 [DOI] [PubMed] [Google Scholar]
- 30.Fournier JP, Yin H, Nessim SJ, Montastruc JL, Azoulay L. Tramadol for noncancer pain and the risk of hyponatremia. Am J Med. 2015;128(4):418-425.e5. doi: 10.1016/j.amjmed.2014.10.046 [DOI] [PubMed] [Google Scholar]
- 31.Vestergaard P, Rejnmark L, Mosekilde L. Fracture risk associated with the use of morphine and opiates. J Intern Med. 2006;260(1):76-87. doi: 10.1111/j.1365-2796.2006.01667.x [DOI] [PubMed] [Google Scholar]
- 32.Costa-Dias MJ, Oliveira AS, Martins T, et al. Medication fall risk in old hospitalized patients: a retrospective study. Nurse Educ Today. 2014;34(2):171-176. doi: 10.1016/j.nedt.2013.05.016 [DOI] [PubMed] [Google Scholar]
- 33.McAlindon TE, Bannuru RR, Sullivan MC, et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis Cartilage. 2014;22(3):363-388. doi: 10.1016/j.joca.2014.01.003 [DOI] [PubMed] [Google Scholar]
- 34.National Institute for Health and Care Excellence Osteoarthritis: care and management. National Institute for Health and Care Excellence website. https://www.nice.org.uk/guidance/cg177. Published February 2014. Accessed June 21, 2017.
- 35.Latimer N, Lord J, Grant RL, O’Mahony R, Dickson J, Conaghan PG; National Institute for Health and Clinical Excellence Osteoarthritis Guideline Development Group . Cost effectiveness of COX 2 selective inhibitors and traditional NSAIDs alone or in combination with a proton pump inhibitor for people with osteoarthritis. BMJ. 2009;339:b2538. doi: 10.1136/bmj.b2538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Katz JN, Smith SR, Collins JE, et al. Cost-effectiveness of nonsteroidal anti-inflammatory drugs and opioids in the treatment of knee osteoarthritis in older patients with multiple comorbidities. Osteoarthritis Cartilage. 2016;24(3):409-418. doi: 10.1016/j.joca.2015.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scarpignato C, Lanas A, Blandizzi C, Lems WF, Hermann M, Hunt RH; International NSAID Consensus Group . Safe prescribing of non-steroidal anti-inflammatory drugs in patients with osteoarthritis--an expert consensus addressing benefits as well as gastrointestinal and cardiovascular risks. BMC Med. 2015;13:55. doi: 10.1186/s12916-015-0285-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain—United States, 2016. JAMA. 2016;315(15):1624-1645. doi: 10.1001/jama.2016.1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Baseline characteristics of unmatched and propensity-score matched osteoarthritis patients (≥ 50 years) initiating tramadol prescription or naproxen prescription.
eTable 2. Baseline characteristics of unmatched and propensity-score matched osteoarthritis patients (≥ 50 years) initiating tramadol prescription or diclofenac prescription.
eTable 3. Baseline characteristics of unmatched and propensity-score matched osteoarthritis patients (≥ 50 years) initiating tramadol prescription or celecoxib prescription.
eTable 4. Baseline characteristics of unmatched and propensity-score matched osteoarthritis patients (≥ 50 years) initiating tramadol prescription or etoricoxib prescription.
eTable 5. Baseline characteristics of unmatched and propensity-score matched osteoarthritis patients (≥ 50 years) initiating tramadol prescription or codeine prescription.