Key Points
Question
What is the association of aspirin use with cardiovascular events and bleeding events in individuals without cardiovascular disease?
Findings
In this meta-analysis of 13 trials with 164 225 participants without cardiovascular disease, aspirin use was associated with a lower risk of cardiovascular events, defined as cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke (hazard ratio [HR], 0.89; absolute risk reduction, 0.41%) and an increased risk of major bleeding (HR, 1.43; absolute risk increase, 0.47%).
Meaning
In individuals without cardiovascular disease, the use of aspirin was associated with a lower risk of cardiovascular events and an increased risk of major bleeding.
Abstract
Importance
The role for aspirin in cardiovascular primary prevention remains controversial, with potential benefits limited by an increased bleeding risk.
Objective
To assess the association of aspirin use for primary prevention with cardiovascular events and bleeding.
Data Sources
PubMed and Embase were searched on Cochrane Library Central Register of Controlled Trials from the earliest available date through November 1, 2018.
Study Selection
Randomized clinical trials enrolling at least 1000 participants with no known cardiovascular disease and a follow-up of at least 12 months were included. Included studies compared aspirin use with no aspirin (placebo or no treatment).
Data Extraction and Synthesis
Data were screened and extracted independently by both investigators. Bayesian and frequentist meta-analyses were performed.
Main Outcomes and Measures
The primary cardiovascular outcome was a composite of cardiovascular mortality, nonfatal myocardial infarction, and nonfatal stroke. The primary bleeding outcome was any major bleeding (defined by the individual studies).
Results
A total of 13 trials randomizing 164 225 participants with 1 050 511 participant-years of follow-up were included. The median age of trial participants was 62 years (range, 53-74), 77 501 (47%) were men, 30 361 (19%) had diabetes, and the median baseline risk of the primary cardiovascular outcome was 10.2% (range, 2.6%-30.9%). Aspirin use was associated with significant reductions in the composite cardiovascular outcome compared with no aspirin (60.2 per 10 000 participant-years with aspirin and 65.2 per 10 000 participant-years with no aspirin) (hazard ratio [HR], 0.89 [95% credible interval, 0.84-0.94]; absolute risk reduction, 0.41% [95% CI, 0.23%-0.59%]; number needed to treat, 241). Aspirin use was associated with an increased risk of major bleeding events compared with no aspirin (23.1 per 10 000 participant-years with aspirin and 16.4 per 10 000 participant-years with no aspirin) (HR, 1.43 [95% credible interval, 1.30-1.56]; absolute risk increase, 0.47% [95% CI, 0.34%-0.62%]; number needed to harm, 210).
Conclusions and Relevance
The use of aspirin in individuals without cardiovascular disease was associated with a lower risk of cardiovascular events and an increased risk of major bleeding. This information may inform discussions with patients about aspirin for primary prevention of cardiovascular events and bleeding.
This meta-analysis estimates the association between use of aspirin for primary prevention of cardiovascular disease and the rate of cardiovascular mortality, nonfatal myocardial infarction, nonfatal stroke, and bleeding.
Introduction
Despite reductions in death from cardiovascular disease over the past few decades, rates of death from stroke and myocardial infarction have plateaued in the United States.1 The health and economic burden of cardiovascular disease has triggered the Centers for Disease Control and Prevention and the Centers for Medicare & Medicaid Services to launch the Million Hearts 2022 initiative, aiming to prevent cardiovascular events through risk factor optimization.2,3 One target is to improve appropriate aspirin (acetylsalicylic acid) prescribing. The benefit of aspirin in the secondary prevention of stroke and myocardial infarction is well-established; however, its use in primary prevention remains controversial.4 Clinical trials of aspirin in patients without cardiovascular disease have inconsistently demonstrated improvements in cardiovascular outcomes,5,6 with potential benefits countered by increased risks of clinically significant bleeding.7 The uncertain role of aspirin in primary prevention of cardiovascular events is reflected in contrasting recommendations offered by guideline bodies.8,9 The overall effect of this uncertainty has been a decline in aspirin prescribing for primary prevention over the past 5 to 10 years.1,10
The purpose of this meta-analysis was to assess the association of aspirin use with cardiovascular events and bleeding events in populations without cardiovascular disease.
Methods
This article has been reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses.11 The protocol is available in Supplement 1. Ethical approval was not required for this study.
Data Sources
A systematic search of PubMed and Embase was conducted on Cochrane Central Register of Controlled Trials (CENTRAL) from the earliest publication date available through November 1, 2018 (eMethods 1 in Supplement 2). The reference lists of included studies and meta-analyses identified in the search were screened for additional studies.
After removal of duplicates, the titles and abstracts of the search results were screened for relevance by both authors. The full texts of the remaining results were individually assessed by both authors, independently, for inclusion based on predetermined criteria. The final list of included studies was decided on by discussion between authors, with full agreement required before inclusion. No disagreements required resolution by a third reviewer.
Study Selection
Trials were considered eligible if they (1) were a randomized clinical trial (RCT); (2) enrolled participants without known preexisting cardiovascular disease; (3) compared aspirin at any dose with no aspirin (defined as placebo or no treatment); (4) had a follow-up of at least 12 months; (5) enrolled over 1000 participants; (6) provided information on any of the prespecified primary and secondary cardiovascular outcomes, primary and secondary bleeding outcomes, or cancer outcomes; and (7) were published in the English language.
Data Extraction
Data were extracted using piloted forms, independently by both authors, and were transcribed into a dedicated database. The data extracted from each report included baseline participant characteristics, inclusion criteria, study drug and control treatment, follow-up duration, and end point data. Relevant subgroup data were extracted when available. The Antithrombotic Trialists’ (ATT) Collaboration provided additional outcome data on individual studies included in their individual patient data meta-analysis.4 Some event counts were updated by the ATT Collaboration from the original publications because of reclassification of International Classification of Diseases codes, with updated counts used in this analysis. Secondary publications of original studies were included if they reported on relevant outcomes.
Risk of bias assessment was done by the authors independently using the Cochrane Collaboration risk of bias tool across 5 domains (sequence generation, allocation concealment, blinding, detection bias, attrition bias, and reporting bias). No disagreements required resolution by a third reviewer. The Egger test was used to identify asymmetry of funnel plots for publication bias.12
Outcomes
The primary cardiovascular outcome was a composite of cardiovascular mortality, nonfatal myocardial infarction, and nonfatal stroke. Secondary cardiovascular outcomes included all-cause mortality, cardiovascular-related mortality, myocardial infarction, total stroke (ischemic, hemorrhagic, and unknown), and ischemic stroke. The primary bleeding outcome was major bleeding and was individually defined by studies. Secondary bleeding outcomes included intracranial bleeding (intracerebral, subarachnoid, and subdural) and major gastrointestinal bleeding.
Cardiovascular and bleeding outcome definitions used in studies were identified (eTable 1 in Supplement 2) and end point data were extracted with the aim to maintain consistency of definitions. For the primary cardiovascular outcome, stroke was defined as both ischemic and hemorrhagic for all studies except for A Study of Cardiovascular Events in Diabetes (ASCEND) trial, which included only ischemic subtypes.
Subgroup Analysis
Cardiovascular and bleeding outcomes were analyzed for study populations with low and high cardiovascular risk and in participants with diabetes. Cardiovascular risk subgroups were based at the trial level. The 10-year risk of the primary outcome was calculated by multiplying the annualized event rate for the primary outcome in the control group by 10 years (eMethods 2 in Supplement 2). A high cardiovascular risk was defined as an estimated 10-year risk of the primary outcome of at least 10%, and low cardiovascular risk was defined as an estimated 10-year risk of the primary outcome of less than 10%.9
Exploratory Cancer Outcomes
The effect of aspirin on cancer incidence and mortality is uncertain, with meta-analyses yielding contrasting results13,14 and the Aspirin in Reducing Events in the Elderly (ASPREE) study demonstrating an increased risk of cancer mortality with aspirin.15 In light of the uncertain evidence of aspirin on cancer outcomes generated from previous studies, incident cancer and cancer mortality were prespecified as exploratory outcomes.
Data Synthesis
A Bayesian meta-analysis was performed using the GeMTC package on R Software, version 3.4.1 (R Foundation for Statistical Computing). Analyses were performed using Markov chain Monte Carlo methods.16 Fixed-effects or random-effects models were selected for each outcome based on the deviance information criterion, using the model with the smallest value17 (eMethods 3 in Supplement 2). Between-study heterogeneity was assessed using the I2 statistic. When deviance information criterion values were similar, the I2 was used for model selection. Results are presented as hazard ratios (HRs) with 95% credible intervals (CrIs). For studies that reported event counts only, differences in follow-up duration between studies were incorporated using the trial patient-years follow-up to estimate HRs using Poisson likelihood and log link.17
Relative risk, with 95% CIs, was calculated using frequentist pairwise meta-analysis. Absolute risk reductions (ARRs) and absolute risk increases (ARIs) were calculated by multiplying the control risk by the calculated relative risk and 95% CIs. Numbers needed to treat and harm were derived by dividing 1 by the calculated ARRs and ARIs, respectively.
Sensitivity analyses were performed restricted to aspirin dose (excluding total daily doses >100 mg), double-blind placebo-controlled studies, and studies published after 2000. The year 2000 was chosen to represent the modern era of cardiovascular primary prevention. Post hoc sensitivity analyses, excluding the ASCEND trial and studies that enrolled participants with asymptomatic peripheral arterial disease from the primary cardiovascular outcome analysis, were also conducted. All data were represented graphically with network and forest plots using R software, version 3.4.1 and Microsoft Excel.
Results
Study Search and Study Characteristics
The systematic search of articles published before November 1, 2018, identified 1385 articles, of which 21 articles reporting on 13 studies were included5,6,18,19,20,21,22,23,24,25,26,27,28 (eFigure 1 in Supplement 2; Table). In total, 164 225 participants were enrolled, comprising 1 050 511 participant-years of follow-up. The median (interquartile range) duration of follow-up was 5.0 (4.7-6.7) years. The comparator treatment was placebo in 9 studies and no aspirin in 4 studies, while 6 studies used a factorial design (2 additionally tested vitamin E23,24; 1, n-3 fatty acid5; 1, antioxidant25; 1, warfarin22; and 1, hypertension treatment targets19). The Thrombosis Prevention Trial evaluated warfarin and aspirin alone and in combination22; data for participants who received warfarin were excluded from the analysis.
Table. Baseline Characteristics of Included Studiesa.
| Source | Aspirin Dose, mg | Comparator | Trial Design | Study Population | Country | Study Period | Total Randomized | Male Participants, No. (%) | Age at Entry, Mean (SD), y | Diabetes, No. (%) | Current Smokers | Hypertension | SBP, Mean (SD), mm H | Total Cholesterol, Mean (SD), mmol/L | BMI | 10-y Risk of Primary Outcome, % (95% CI)b | Overall Risk of Bias |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| British Doctors Study,19 1988 | 500 or 300 daily | No aspirin | Randomized, open-label, end point blind | Male physicians | United Kingdom | 1978-1984 | 5139 | 5139 (100) | 61 (7) | 101 (2) | 661 (13) | 508 (10) | 136 (17) | NR | 24.4 (2.5) | 13.9 (11.7-16.4) | High |
| Physicians’ Health Study,20 1989 | 325 alternate day | Placebo | Randomized, double-blind | Male physicians aged 40-84 y | United States | 1982-1988 | 22 071 | 22 071 (100) | 53 (10) | 533 (2) | 2438 (11) | 5297 (24) | 126 (12) | 5.5 (1.2) | 24.9 (3.0) | 6.7 (6.0-7.4) | Low |
| Hypertension Optimal Treatment,20 1998 | 75 daily | Placebo | Randomized, double-blind; factorial design with hypertension treatment targets | Individuals with hypertension aged 50-80 y | 26 Countries across Europe, North and South America, and Asia | 1992-1997 | 18 790 | 9959 (53) | 61 (7) | 1503 (8) | 2988 (16) | 18 790 (100) | 170 (14) | 6.0 (1.1) | 28.4 (4.7) | 10.7 (9.7-11.9) | Low |
| Thrombosis Prevention Trial,22 1998 | 75 daily | Placebo | Randomized, double-blind; factorial design with warfarin | Men aged 45-69 y in the top 20%-25% of CV risk score | United Kingdom | 1984-1997 | 5085c | 5085 (100) | 57 (7) | 102 (2) | 2100 (41) | 814 (16) | 139 (18) | 6.4 (1.0) | 27.4 (3.6) | 15.9 (14.0-18.0) | Low |
| Primary Prevention Project,23 2001 | 100 daily | No aspirin | Randomized, open-label, blind end point; factorial design with vitamin E | Individuals with ≥1 CV risk factor | Italy | 1994-1998 | 4495 | 1912 (42) | 64 (7.6) | 742 (17) | 667 (15) | 3065 (68) | 145.2 (16.0) | 6.1 (1.2) | 27.6 (4.7) | 8.1 (6.2-10.3) | High |
| Women’s Health Study,24 2005 | 100 alternate day | Placebo | Randomized, double-blind; factorial design with vitamin E | Female health professionals ≥45 y | United States | 1992-2004 | 39 876 | 0 (0) | 54 (7.1) | 1037 (3) | 5224 (13) | 10 328 (26) | NR | 5.2 (1.0) | 26.1 (5.2) | 2.6 (2.4-2.8) | Low |
| Prevention of Arterial Disease and Diabetes (POPADAD),25 2008 | 100 daily | Placebo | Randomized, double-blind; factorial design with antioxidant | Individuals with diabetes, ABPI ≤0.99, aged ≥40 y | United Kingdom | 1997-2006 | 1276 | 563 (44) | 60 (10) | 1276 (100) | NR | NR | 145 (21) | 5.5 (NR) | 29.2 (NR) | NA | Low |
| Japanese Primary Prevention of Atherosclerosis With Aspirin for Diabetes,28 2008 | 81 or 100 daily | No aspirin | Randomized, open-label, blind end point | Individuals with diabetes aged 30-85 y | Japan | 2002-2008 | 2539 | 1387 (55) | 65 (10) | 2539 (100) | 537 (21) | 1473 (58) | 135 (15) | 5.2 (0.9) | 24 (4) | 12.5 (9.8-15.9) | High |
| Aspirin for Asymptomatic Atherosclerosis,27 2010 | 100 daily | Placebo | Randomized, double-blind | Individuals aged 50-75 y with ABPI ≤0.95 | United Kingdom | 1998-2008 | 3350 | 954 (28) | 62 (6.7) | 88 (3) | 1085 (32) | NR | 147.5 (22) | 6.2 (1.1) | NR | 12.8 (11.0-14.8) | Low |
| Japanese Primary Prevention Project,26 2014 | 100 daily | No aspirin | Randomized, open-label, blind end point | Individuals aged 60-85y, with hypertension, dyslipidemia, or diabetes | Japan | 2005-2012 | 14 464 | 6123 (42) | 71 (6.2) | 4903 (34) | 1893 (13) | 12 278 (85) | 137.2 (15.7) | 5.3 (0.8) | 24.2 (3.5) | 5.7 (4.9-6.5) | High |
| A Study of Cardiovascular Events in Diabetes (ASCEND),5 2018 | 100 daily | Placebo | Randomized, double-blind; factorial design with n-3 fatty acid | Individuals with diabetes aged ≥40 y | United Kingdom | 2005-2017 | 15 480 | 9684 (63) | 63 (9.2) | 15 480 (100) | 1279 (8) | 9533 (62) | 136.2 (15.3) | 4.2 (0.9) | 30.7 (6.3) | 10.2 (9.4-11.1) | Low |
| Aspirin to Reduce Risk of Initial Vascular Events (ARRIVE),6 2018 | 100 daily | Placebo | Randomized, double-blind | Males with ≥2 and females with ≥3 CV risk factors. Aimed to recruit patients with 10-y CV risk of 10%-20% | Germany, Italy, Ireland, Poland, Spain, United Kingdom, and United States | 2007-2016 | 12 546 | 8838 (70) | 64 (7.1) | 0 (0) | 3594 (29) | 7866 (63) | 143.8 (90-199)d | NR | 28.4 (4.3) | 6.9 (6.1-7.9) | Low |
| Aspirin in Reducing Events in the Elderly (ASPREE),13,18 2018 | 100 daily | Placebo | Randomized, double-blind | Black or Hispanic individuals in the United States aged ≥65 y and other individuals aged ≥70 y | Australia and United States | 2010-2014 | 19 114 | 8331 (44) | 74 (NR)d | 2057 (11) | 735 (4) | 14 283 (74) | 139.2 (16.5) | 5.3 (1.0) | 28.1 (4.7) | 8.3 (7.4-9.1) | Low |
Abbreviations: ABPI, ankle-brachial pressure index; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CV, cardiovascular; NA, not applicable; NR, not reported in study publication; SBP, systolic blood pressure.
SI conversion factor: To convert cholesterol data to mg/dL, multiply by 0.0259.
Data are presented as mean (SD) unless otherwise specified.
10-Year risk of the primary cardiovascular outcome was calculated by multiplying the annualized event rate for the primary cardiovascular outcome in the control group by 10 years.
5085 Participants were randomized in a 2x2 factorial design warfarin, aspirin, warfarin and aspirin, or placebo. 2545 Were randomized to warfarin and excluded from analysis.
Data reported as median (range).
Three studies exclusively enrolled men19,20,22 and 1 exclusively enrolled women.24 Overall, 77 501 participants (47.2%) were men. Three studies exclusively enrolled participants with diabetes,5,25,28 with 30 361 participants (18.5%) having diabetes. The median study 10-year estimated cardiovascular event rate was 10.2% (range, 2.6%-30.9%).
Risk of Bias and Publication Bias
Of the 13 included studies, 9 were at low risk of bias and 4 were at high risk (eFigure 2 and eTable 2 in Supplement 2). There were 9 double-blind and 4 open-label studies, with the latter deemed high risk of bias. There was no evidence of publication bias for the primary outcome (Egger test: −0.47; P = .57) (eFigure 3 in Supplement 2).
Primary Cardiovascular Outcome
For the primary cardiovascular outcome, 13 studies reported a total of 6485 events (3143 with aspirin [60.2 per 10 000 participant-years] and 3342 with no aspirin [65.2 per 10 000 participant-years]). The use of aspirin was associated with reductions in the composite cardiovascular outcome (HR, 0.89 [95% CrI, 0.84-0.94]; ARR, 0.41% [95% CI, 0.23%-0.59%]; number needed to treat [NNT], 241) (Figure 1; eTable 3 in Supplement 2) compared with no aspirin, with low heterogeneity (I2 = 0%). Forest plots for frequentist meta-analysis used to derive absolute differences are presented in eFigure 4 in Supplement 2.
Figure 1. Cardiovascular and Bleeding Outcomes in All Participants.
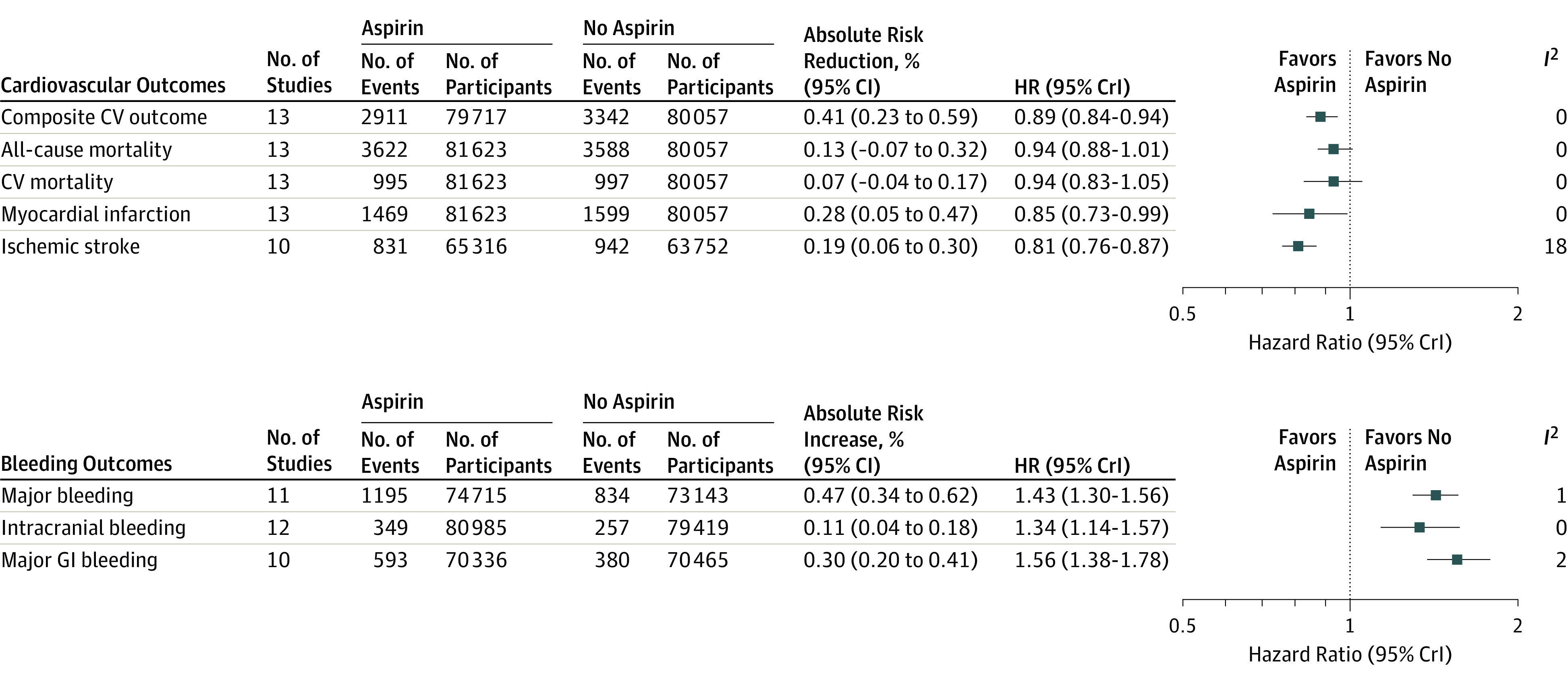
The composite cardiovascular (CV) outcome consisted of cardiovascular mortality, nonfatal myocardial infarction, and nonfatal stroke. Hazard ratios (HRs) and 95% credible interval variables (CrIs) were calculated using Bayesian meta-analysis of trial-level event counts. The absolute risk reductions and increases were calculated by multiplying the control event risk by the relative risk and 95% CIs derived by frequentist meta-analysis (eFigure 4 in Supplement 2). GI indicates gastrointestinal.
Secondary Cardiovascular Outcomes
All-cause and cardiovascular mortality were reported in all 13 studies. The use of aspirin was not associated with reductions in all-cause mortality (HR, 0.94 [95% CrI, 0.88-1.01]; ARR, 0.13% [95% CI, −0.07% to 0.32%]) or cardiovascular mortality (HR, 0.94 [95% CrI, 0.83-1.05]; ARR, 0.07% [95% CI, −0.04% to 0.17%]) compared with no aspirin, with low heterogeneity for both outcomes (I2 = 0%) (Figure 1; eTable 3 in Supplement 2).
The use of aspirin was associated with reduction in myocardial infarction (HR, 0.85 [95% CrI, 0.73-0.99]; ARR, 0.28% [95% CI, 0.05%-0.47%]; NNT, 361) and ischemic stroke (HR, 0.81 [95% CrI, 0.76-0.87]; ARR, 0.19% [95% CI, 0.06%-0.30%]; NNT, 540) compared with no aspirin (I2 = 0% and I2 = 18%, respectively) (Figure 1; eTable 3 in Supplement 2). There was no significant reduction in total stroke (HR, 0.93 [95% CrI, 0.86-1.02]) associated with aspirin use compared with no aspirin (I2 = 1%) (eTable 4 in Supplement 2).
Bleeding Outcomes
For the major bleeding outcome, 11 studies reported a total of 2029 events (1195 with aspirin [23.1 per 10 000 participant-years] and 834 with no aspirin [16.4 per 10 000 participant-years]). The use of aspirin was associated with an increased rate of major bleeding (HR, 1.43 [95% CrI, 1.30-1.56]; ARI, 0.47% [95% CI, 0.34%-0.62%]; number needed to harm [NNH], 210) compared with no aspirin (Figure 1; eTable 3 in Supplement 2). Intracranial hemorrhage (HR, 1.34 [95% CrI, 1.14-1.57]; ARI, 0.11% [95% CI, 0.04%-0.18%]; NNH, 927) and major gastrointestinal bleeding (HR, 1.56 [95% CrI, 1.38-1.78]; ARI, 0.30% [95% CI, 0.20%-0.41%]; NNH, 334) were also more common with aspirin use compared with no aspirin. Heterogeneity was low for all bleeding outcomes (I2 range, 0%-2%) (Figure 1; eTable 3 in Supplement 2).
Outcomes Across Population Cardiovascular Risk Range and in Participants With Diabetes
The estimated population 10-year risk of the primary composite cardiovascular outcome was less than 10% in 6 studies enrolling 112 566 participants (median, 6.8%; range, 2.6%-8.1%)6,18,20,23,24,26 and was at least 10% in 7 studies enrolling 48 934 participants (median, 12.8%; range, 10.2%-30.9%).5,19,21,22,25,27,28 The Women’s Health Study reported subgroup data for patients with a 10-year cardiovascular risk of 10% or more, which was included in the high-risk subgroup.24
In studies in which the risk of the cardiovascular outcome was low, aspirin use was associated with reductions in the primary composite cardiovascular outcome (HR, 0.91 [95% CrI, 0.84-0.98]; ARR, 0.63% [95% CI, 0.18%-1.03%]; NNT, 160) compared with no aspirin (Figure 2; eTable 3 in Supplement 2). Aspirin was not associated with reductions in any of the secondary outcomes. Aspirin use was associated with increased risk of major bleeding (HR, 1.45 [95% CrI, 1.28-1.63]; ARI, 0.40% [95% CI, 0.25%-0.57%]; NNH, 249), intracranial bleeding (HR, 1.41 [95% CrI, 1.16-1.71]; ARI, 0.13% [95% CI, 0.05%-0.22%]; NNH, 796), and major gastrointestinal bleeding (HR, 1.58 [95% CrI, 1.34-1.87]; ARI, 0.27% [95% CI, 0.15%-0.40%]; NNH, 376) (Figure 2; eTable 3 in Supplement 2). Heterogeneity was low for all outcomes in patients with low risk of the cardiovascular outcome (I2 range, 0%-11%).
Figure 2. Cardiovascular and Bleeding Outcomes for Studies With Patients at High and Low Risk for the Primary CV Outcome and With Diabetes.
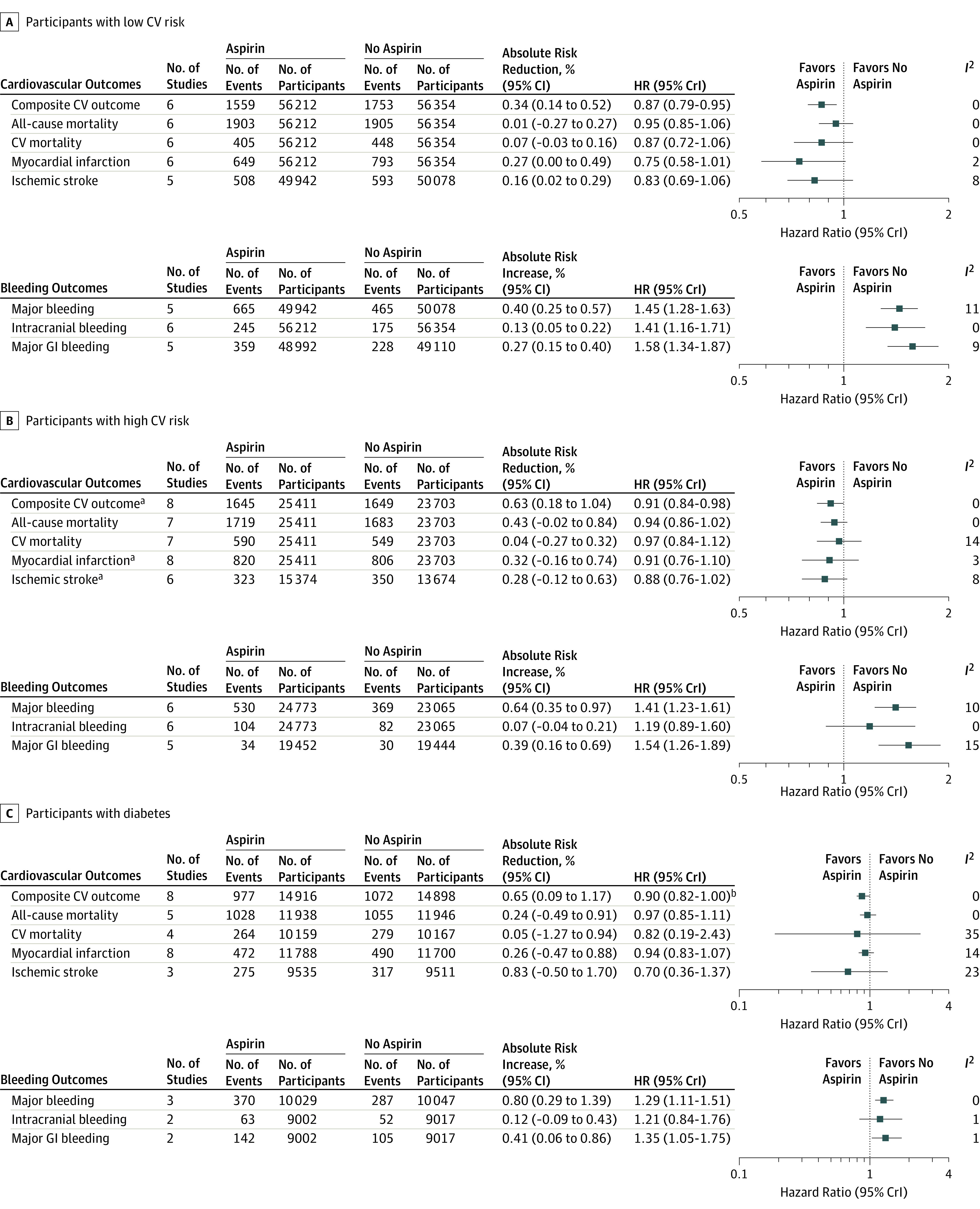
Cardiovascular and bleeding outcomes for studies in which the risk of the primary cardiovascular outcome were low and high, and in participants with diabetes. Trials were low or high risk if the 10-year cardiovascular (CV) risk for the primary CV outcome was less than 10% or greater than or equal to 10%, respectively. The composite CV outcome included CV mortality, nonfatal myocardial infarction, and nonfatal stroke. Hazard ratios (HRs) and 95% credible intervals (CrIs) were calculated using Bayesian meta-analysis of event counts. The absolute risk reductions and increases were calculated by multiplying the control event risk by the relative risk and 95% CIs derived by frequentist meta-analysis (eFigure 4 in Supplement 2). Heterogeneity was assessed using I2 statistics. GI indicates gastrointestinal. aThe number of participants randomized to each arm in the Women's Health Study24 was not reported, so event counts are omitted. bUpper CrI: 0.997.
In studies in which the risk of the cardiovascular outcome was high, aspirin use was associated with reductions in the primary composite cardiovascular outcome HR, 0.91 [95% CrI, 0.84-0.98]; ARR, 0.63% [95% CI, 0.18%-1.03%]; NNT, 160) compared with no aspirin, but not with reductions in any of the secondary outcomes (Figure 2; eTable 3 in Supplement 2). In participants with high cardiovascular risk, aspirin use was associated with an increase in major bleeding (HR, 1.41 [95% CrI, 1.23-1.61]; ARI, 0.64% [95% CI, 0.35%-0.97%]; NNH, 152) and major gastrointestinal bleeding (HR, 1.54 [95% CrI, 1.26-1.89]; ARI, 0.39% [95% CI, 0.16%-0.69%]; NNH, 255), but not in intracranial bleeding (HR, 1.19 [95% CrI, 0.89-1.60]; ARI, 0.07% [95% CI, −0.04% to 0.21%]) (Figure 2; eTable 3 in Supplement 2). Heterogeneity was low for all outcomes in participants with high risk of the cardiovascular outcome (I2 range, 0%-15%).
Data for participants with diabetes were reported in 10 studies, accounting for 30 448 participants. Among participants with diabetes, aspirin use was associated with reductions in the primary composite cardiovascular outcome (HR, 0.90 [95% CrI, 0.82-1.00]; ARR, 0.65% [95% CI, 0.09%-1.17%] NNT, 153) (Figure 2; eTable 3 in Supplement 2). Aspirin use was not associated with reductions in any of the secondary cardiovascular outcomes. Aspirin use was associated with an increase in major bleeding (HR, 1.29 [95% CrI, 1.11-1.51]; ARI, 0.80% [95% CI, 0.29%-1.39%]; NNH, 121) and major gastrointestinal bleeding (HR, 1.35 [95% CrI, 1.05-1.75]; ARI, 0.41% [95% CI, 0.06%-0.86%]; NNH, 243) in participants with diabetes, but not in intracranial bleeding (HR, 1.21 [95% CrI, 0.84-1.76]; ARI, 0.12% [95% CI, −0.09% to 0.43%]) (Figure 2; eTable 3 in Supplement 2). There was evidence of moderate heterogeneity for cardiovascular mortality in patients with diabetes (I2 = 35%). Heterogeneity was low for all other outcomes in patients with diabetes (I2 range, 0%-23%). Event rates and absolute risk differences for all outcomes are available in eTable 3 and eTable 5 in Supplement 2.
Sensitivity Analysis
Eleven studies (134 470 participants) used a total daily aspirin dose of 100 mg or less. Aspirin at a total daily dose of 100 mg or less was associated with reductions in the composite cardiovascular outcome (HR, 0.89 [95% CrI, 0.83-0.95]), myocardial infarction (HR, 0.87 [95% CrI, 0.76-1.00]), total stroke (HR, 0.90 [95% CrI, 0.82-0.98]), and ischemic stroke (HR, 0.79 [95% CrI, 0.74-0.85]). There was no significant difference in all-cause and cardiovascular mortality. There were associated increases in major bleeding (HR, 1.54 [95% CrI, 1.35-1.76]), intracranial bleeding (HR, 1.31 [95% CrI, 1.11-1.56]), and major gastrointestinal bleeding (HR, 1.55 [95% CrI, 1.36-1.77]).
For double-blind, placebo-controlled studies (9 studies; 135 043 participants), aspirin use was associated with reductions in the composite cardiovascular outcome (HR, 0.88 [95% CrI, 0.83-0.94]). No reduction was present in any of the secondary outcomes. Aspirin use was associated with increases in major bleeding (HR, 1.41 [95% CrI, 1.28-1.55]), intracranial bleeding (HR, 1.33 [95% CrI, 1.11-1.60]), and major gastrointestinal bleeding (HR, 1.52 [95% CrI, 1.31-1.78]).
In studies published since the year 2000 (9 studies; 113 140 participants), aspirin use was associated with reductions in the composite cardiovascular outcome (HR, 0.91 [95% CrI, 0.84-0.98]), total stroke (HR, 0.89 [95% CrI, 0.80-0.98), and ischemic stroke (HR, 0.80 [95% CrI, 0.74-0.86]) compared with no aspirin. There was no significant difference in all-cause and cardiovascular mortality or myocardial infarction. Aspirin use was associated with increased risk of major bleeding (HR, 1.39 [95% CrI, 1.26-1.53]), intracranial hemorrhage (HR, 1.34 [95% CrI, 1.13-1.60]), and major gastrointestinal bleeding (HR, 1.48 [95% CrI, 1.28-1.71]).
In a post hoc sensitivity analysis excluding studies that enrolled patients with asymptomatic peripheral arterial disease25,27 (11 studies; 156 874 participants), aspirin use was associated with reductions in the composite cardiovascular outcome (HR, 0.88 [95% CrI, 0.83-0.93]), myocardial infarction (HR, 0.80 [95% CrI, 0.68-0.95]), and ischemic stroke (HR, 0.81 [95% CrI, 0.76-0.87]) compared with no aspirin. Aspirin use was associated with increases in major bleeding (HR, 1.42 [95% CrI, 1.30-1.56]), intracranial bleeding (HR, 1.33 [95% CrI, 1.13-1.57]), and major gastrointestinal bleeding (HR, 1.57 [95% CrI, 1.38-1.79]) compared with no aspirin.
For the primary cardiovascular outcome, the ASCEND trial only included ischemic strokes. Post hoc sensitivity analysis excluding the ASCEND trial did not alter the primary outcome (HR, 0.88 [95% CrI, 0.83-0.94]). Data for all sensitivity analyses are available in eTable 6 in Supplement 2.
Exploratory Cancer Outcomes
Incident cancer and cancer mortality were reported in 10 and 12 studies, respectively. There was no significant difference in incident cancer (HR, 1.01 [95% CrI, 0.93-1.08]) or cancer mortality (HR, 1.03 [95% CrI, 0.96-1.11]) with aspirin use compared with no aspirin (Figure 3). There was evidence of low between-study heterogeneity for incident cancer (I2 = 14%) and cancer mortality (I2 = 17%). There was no significant difference in cancer outcomes associated with aspirin in studies in which the risk of the cardiovascular outcome was low (incident cancer: HR, 1.06 [95% CrI, 0.95-1.24]; cancer mortality: HR, 1.11 [95% CrI, 0.93-1.33]), in studies in which the risk of the cardiovascular outcome was high (incident cancer: HR, 0.96 [95% CrI, 0.90-1.03]; cancer mortality: HR, 0.96 [95% CrI, 0.86- 1.06]), and in participants with diabetes (incident cancer: HR, 0.95 [95% CrI, 0.74-1.14]; cancer mortality: HR, 1.05 [95% CrI, 0.80-1.43]). Exclusion of the ASPREE trial from the overall analysis did not affect the results but reduced between-study heterogeneity (incident cancer: HR, 1.00 [95% CrI, 0.90-1.09]; I2 = 13%) (cancer mortality: HR, 0.98 [95% CrI, 0.91-1.06]; I2 = 0%).
Figure 3. Exploratory Cancer Outcomes.
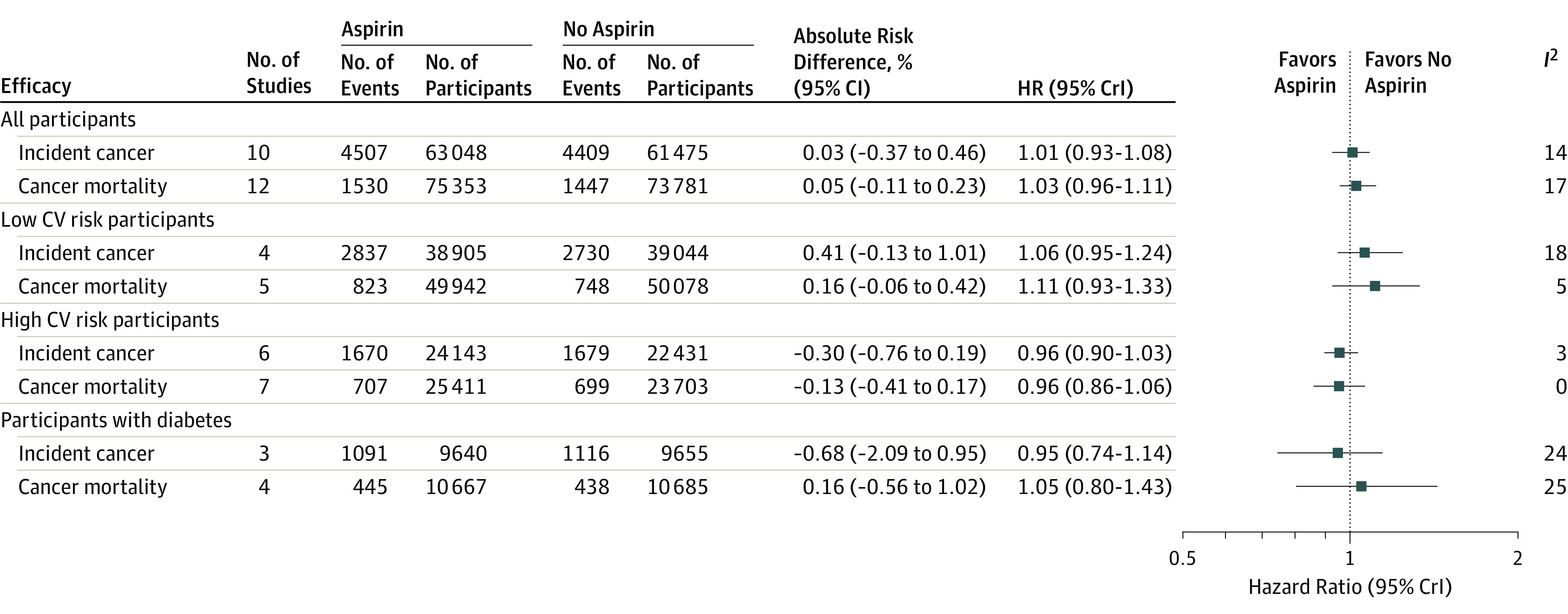
Cancer outcomes across all studies, in low and high cardiovascular risk populations, and in patients with diabetes. The absolute risk reductions and increases were calculated by multiplying the control event risk by the relative risk, and 95% CIs derived by frequentist meta-analysis (eFigure 4 in Supplement 2). Study heterogeneity was assessed using I2 statistics. HR indicates hazard ratio; CrI indicates credible interval; CV indicates cardiovascular. Data for the JPAD, JPPP, and WHS trials were extracted from subsequent trial publications on cancer outcomes.29,30,31 Data for the HOT, BDS, and PHS (cancer mortality) and the HOT, BDS, AAA, and POPADAD (incident cancer) trials were extracted from previous meta-analyses on cancer outcomes.14
Discussion
In this meta-analysis of 13 trials enrolling 164 225 participants without cardiovascular disease, aspirin use was associated with reductions in the composite cardiovascular outcome consisting of cardiovascular mortality, nonfatal myocardial infarction, and nonfatal stroke. However, aspirin use was associated with an increased risk of major bleeding, intracranial bleeding, and major gastrointestinal bleeding with comparable absolute risk estimates. Aspirin use was associated with reductions in the primary cardiovascular composite outcome and increases in major bleeding risks in both low and high cardiovascular risk populations and in participants with diabetes.
Compared with aspirin use in patients with established atherosclerotic cardiovascular disease, aspirin use for primary prevention has been controversial. This uncertainty has been reflected in contradictory guideline recommendations.8,9 The current study demonstrates that when considering the totality of evidence, cardiovascular benefits associated with aspirin were modest and equally balanced by major bleeding events.
This study builds on previous meta-analyses through the inclusion of 3 studies that enrolled participants for whom uncertainty regarding the efficacy of aspirin for primary prevention existed. The Aspirin to Reduce Risk of Initial Vascular Events (ARRIVE) trial enrolled participants with moderate to high estimated cardiovascular risk, the ASCEND trial enrolled participants with diabetes, and the ASPREE trial enrolled older participants (older than 65 or 70 years of age, depending on ethnicity). It has been suggested that patients with substantially increased cardiovascular risk may benefit from preventive aspirin use.9 The US Preventive Services Task Force recommends the initiation of low-dose aspirin in adults aged 50 to 69 years with a 10-year cardiovascular risk of 10% or more.9 However, the use of cardiovascular risk scores tends to overestimate an individual’s true risk,32 with poor agreement between different cardiovascular risk calculators.33 The difference in predicted and observed cardiovascular risk was demonstrated by the ARRIVE trial, in which moderate-risk participants (mean American College of Cardiology/American Heart Association 10-year atherosclerotic cardiovascular disease risk of 17.3%) had an observed event rate of less than 10%.6 This study showed that when analysis was restricted to populations with observation-derived 10-year cardiovascular event rate estimates of 10% or more, aspirin use was associated with an absolute risk decrease of 0.63% (95% CI, 0.18%-1.04%) for the cardiovascular composite outcome, but an absolute risk increase of 0.64% (95% CI, 0.35%-0.97%) for major bleeding.
The challenge of interpreting absolute risk across different outcomes lies in the interpretation of the severity of each outcome. The current study demonstrates that the absolute risk reduction for cardiovascular events and absolute risk increase for major bleeding associated with aspirin use were of similar magnitude. Aspirin use was not associated with a reduction in cardiovascular mortality, and deaths due to bleeding were rare. Consequently, the decision to use aspirin for primary prevention may need to be made on an individual basis, accounting for the patient’s risk of bleeding and their views on the balance of risk vs benefit.34
The role of additional measures to reduce the potential harms of long-term aspirin use is not clear from the present study. Coprescription of aspirin with a proton pump inhibitor (PPI) may limit the risk of significant gastrointestinal bleeding and, therefore, shift the risk-benefit ratio toward an overall benefit of aspirin use for primary prevention in patients without cardiovascular disease.27 However, the use of PPIs was inconsistently reported in the studies included in this analysis. Furthermore, this strategy has not been adequately tested in RCTs and is of uncertain cost effectiveness.28
The ASPREE study, which enrolled healthy older individuals (median age, 74), demonstrated an increased risk of death in patients randomized to receive aspirin, driven primarily by a 31% increased risk of cancer mortality.15 This finding is in contrast to an individual patient data meta-analysis of primary and secondary prevention aspirin trials that demonstrated a 15% reduction in cancer mortality associated with aspirin use.13 While the reduction in cancer mortality emerged after at least 5 years of follow-up, this result was not replicated in the ASCEND trial that followed up 15 480 participants with diabetes for a mean of 7.4 years. The findings of this study suggest that the association of aspirin with cancer outcomes is neutral, with no suggestion of harm or benefit from the available current evidence.
Limitations
This study has several limitations. First, the inherent limitations of meta-analyses exist, including the availability and quality of reported data.35 This was particularly apparent in the diabetes subgroup, in which cardiovascular and bleeding events were poorly reported in studies. Second, end point definitions between trials differed depending on contemporary consensus definitions, reflecting the long time frame and diagnostic advances encompassed by trials in this study. For the primary cardiovascular outcome, all studies except for ASCEND defined stroke events as including both ischemic and hemorrhagic etiologies. Post hoc sensitivity analysis excluding ASCEND did not alter the overall findings. Hemorrhagic stroke events could have been included as both a cardiovascular and a bleeding outcome.
Third, the total daily doses of aspirin varied in studies from 50 mg to 500 mg, with the majority using doses from 75 mg to 100 mg daily. Doses greater than 100 mg daily are not representative of current clinical practice. Importantly, sensitivity analysis restricted to daily aspirin doses of 100 mg or less demonstrated similar results to the overall analysis, including an increased risk of major bleeding outcomes. Fourth, 8 trials began randomizing study participants over 20 years ago, with 3 trials initiating recruitment in the 1970s and 1980s.19,20,22 Increasing adoption of additional primary prevention strategies, such as risk factor modification and development of public health initiatives, may limit the applicability of earlier studies to current practice. In studies published after 2000, aspirin was associated with reductions in the primary cardiovascular outcome and increases in all bleeding outcomes, but was no longer associated with reduced myocardial infarctions when only more contemporary studies were included.
Conclusions
In this meta-analysis, the use of aspirin in individuals without cardiovascular disease was associated with a lower risk of cardiovascular events and an increased risk of major bleeding. This information may inform discussions with patients about aspirin for primary prevention of cardiovascular events and bleeding.
Meta-analysis protocol
eMethods 1: Search strategy
eMethods 2: Detailed statistical methods
eMethods 3: Deviance information criterion and model selection
eTable 1: Outcome definitions
eTable 2: Risk of bias assessment
eTable 3: Absolute risk differences and numbers needed to treat
eTable 4: Total stroke outcomes
eTable 5: Event rates for efficacy and safety outcomes
eTable 6: Sensitivity analyses
eFigure 1: Study flow chart
eFigure 2: Risk of bias summary
eFigure 3: Funnel plot for primary cardiovascular outcome
eFigure 4: Frequentist analysis forest plots
References
- 1.Wall HK, Ritchey MD, Gillespie C, Omura JD, Jamal A, George MG. Vital signs: prevalence of key cardiovascular disease risk factors for million hearts 2022 - United States, 2011-2016. MMWR Morb Mortal Wkly Rep. 2018;67(35):983-991. doi: 10.15585/mmwr.mm6735a4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wright JS, Wall HK, Ritchey MD. Million Hearts 2022: small steps are needed for cardiovascular disease prevention. JAMA. 2018;320(18):1857-1858. doi: 10.1001/jama.2018.13326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ritchey MD, Wall HK, Owens PL, Wright JS. Vital signs: state-level variation in nonfatal and fatal cardiovascular events targeted for prevention by Million Hearts 2022. MMWR Morb Mortal Wkly Rep. 2018;67(35):974-982. doi: 10.15585/mmwr.mm6735a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antithrombotic Trialists’ (ATT) Collaboration, Baigent C, Blackwell L, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373(9678):1849-1860. doi: 10.1016/S0140-6736(09)60503-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.ASCEND Study Collaborative Group, Bowman L, Mafham M. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med. 2018;18(16):1529-1539. doi: 10.1056/NEJMoa1804988 [DOI] [PubMed] [Google Scholar]
- 6.Gaziano JM, Brotons C, Coppolecchia R, et al. Use of aspirin to reduce risk of initial vascular events in patients at moderate risk of cardiovascular disease (ARRIVE): a randomised, double-blind, placebo-controlled trial. Lancet. 2018;392(10152):1036-1046. doi: 10.1016/S0140-6736(18)31924-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whitlock EP, Burda BU, Williams SB, Guirguis-Blake JM, Evans CV. Bleeding risks with aspirin use for primary prevention in adults: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2016;164(12):826-835. doi: 10.7326/M15-2112 [DOI] [PubMed] [Google Scholar]
- 8.Piepoli MF, Hoes AW, Agewall S, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts). Eur Heart J. 2016;37(29):2315-2381. doi: 10.1093/eurheartj/ehw106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guirguis-Blake JM, Evans CV, Senger CA, O’Connor EA, Whitlock EP. Aspirin for the primary prevention of cardiovascular events: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2016;164(12):804-813. doi: 10.7326/M15-2113 [DOI] [PubMed] [Google Scholar]
- 10.Van’t Hof JR, Duval S, Walts A, Kopecky SL, Luepker RV, Hirsch AT. Contemporary primary prevention aspirin use by cardiovascular disease risk: impact of US Preventive Services Task Force recommendations, 2007-2015: a serial, cross-sectional study. J Am Heart Assoc. 2017;6(10):e006328. doi: 10.1161/JAHA.117.006328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629-634. doi: 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rothwell PM, Fowkes FGR, Belch JFF, Ogawa H, Warlow CP, Meade TW. Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet. 2011;377(9759):31-41. doi: 10.1016/S0140-6736(10)62110-1 [DOI] [PubMed] [Google Scholar]
- 14.Chubak J, Whitlock EP, Williams SB, et al. Aspirin for the prevention of cancer incidence and mortality: systematic evidence reviews for the U.S. Preventive Services Task Force. Ann Intern Med. 2016;164(12):814-825. doi: 10.7326/M15-2117 [DOI] [PubMed] [Google Scholar]
- 15.McNeil JJ, Nelson MR, Woods RL, et al. Effect of aspirin on all-cause mortality in the healthy elderly. N Engl J Med. 2018;379(16):1519-1528. doi: 10.1056/NEJMoa1803955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dias S, Sutton AJ, Ades AE, Welton NJ. Evidence synthesis for decision making 2: a generalized linear modeling framework for pairwise and network meta-analysis of randomized controlled trials. Med Decis Making. 2013;33(5):607-617. doi: 10.1177/0272989X12458724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng SL, Roddick AJ, Aghar-Jaffar R, et al. Association between use of sodium-glucose cotransporter 2 inhibitors, glucagon-like peptide 1 agonists, and dipeptidyl peptidase 4 inhibitors with all-cause mortality in patients with type 2 diabetes: a systematic review and meta-analysis. JAMA. 2018;319(15):1580-1591. doi: 10.1001/jama.2018.3024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McNeil JJ, Wolfe R, Woods RL, et al. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N Engl J Med. 2018;379(3):1509-1518. doi: 10.1056/NEJMoa1805819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peto R, Gray R, Collins R, et al. Randomised trial of prophylactic daily aspirin in British male doctors. Br Med J (Clin Res Ed). 1988;296(6618):313-316. doi: 10.1136/bmj.296.6618.313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steering Committee of the Physicians’ Health Study Research Group . Final report on the aspirin component of the ongoing Physicians’ Health Study. N Engl J Med. 1989;321(3):129-135. doi: 10.1056/NEJM198907203210301 [DOI] [PubMed] [Google Scholar]
- 21.Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. Lancet. 1998;351(9118):1755-1762. doi: 10.1016/S0140-6736(98)04311-6 [DOI] [PubMed] [Google Scholar]
- 22.Thrombosis prevention trial: randomised trial of low-intensity oral anticoagulation with warfarin and low-dose aspirin in the primary prevention of ischaemic heart disease in men at increased risk. The Medical Research Council’s General Practice Research Framework. Lancet. 1998;351(9098):233-241. doi: 10.1016/S0140-6736(97)11475-1 [DOI] [PubMed] [Google Scholar]
- 23.de Gaetano G, Collaborative Group of the Primary Prevention Project . Low-dose aspirin and vitamin E in people at cardiovascular risk: a randomised trial in general practice. Lancet. 2001;357(9250):89-95. doi: 10.1016/S0140-6736(00)03539-X [DOI] [PubMed] [Google Scholar]
- 24.Ridker PM, Cook NR, Lee I-M, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005;352(13):1293-1304. doi: 10.1056/NEJMoa050613 [DOI] [PubMed] [Google Scholar]
- 25.Belch J, MacCuish A, Campbell I, et al. The prevention of progression of arterial disease and diabetes (POPADAD) trial: factorial randomised placebo controlled trial of aspirin and antioxidants in patients with diabetes and asymptomatic peripheral arterial disease. BMJ. 2008;337:a1840. doi: 10.1136/bmj.a1840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ikeda Y, Shimada K, Teramoto T, et al. Low-dose aspirin for primary prevention of cardiovascular events in Japanese patients 60 years or older with atherosclerotic risk factors: a randomized clinical trial. JAMA. 2014;312(23):2510-2520. doi: 10.1001/jama.2014.15690 [DOI] [PubMed] [Google Scholar]
- 27.Fowkes FGR, Price JF, Stewart MCW, et al. Aspirin for prevention of cardiovascular events in a general population screened for a low ankle brachial index: a randomized controlled trial. JAMA. 2010;303(9):841-848. doi: 10.1001/jama.2010.221 [DOI] [PubMed] [Google Scholar]
- 28.Ogawa H, Nakayama M, Morimoto T, et al. Low-dose aspirin for primary prevention of atherosclerotic events in patients with type 2 diabetes: a randomized controlled trial. JAMA. 2008;300(18):2134-2141. doi: 10.1001/jama.2008.623 [DOI] [PubMed] [Google Scholar]
- 29.Okada S, Morimoto T, Ogawa H, et al. Effect of aspirin on cancer chemoprevention in Japanese patients with type 2 diabetes: 10-year observational follow-up of a randomized controlled trial. Diabetes Care. 2018;41(8):1757-1764. doi: 10.2337/dc18-0368 [DOI] [PubMed] [Google Scholar]
- 30.Yokoyama K, Ishizuka N, Uemura N, et al. Effects of daily aspirin on cancer incidence and mortality in the elderly Japanese. Res Pract Thromb Haemost. 2018;2(2):274-281. doi: 10.1002/rth2.12097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cook NR, Lee I-M, Gaziano JM, et al. Low-dose aspirin in the primary prevention of cancer: the Women’s Health Study: a randomized controlled trial. JAMA. 2005;294(1):47-55. doi: 10.1001/jama.294.1.47 [DOI] [PubMed] [Google Scholar]
- 32.Rana JS, Tabada GH, Solomon MD, et al. Accuracy of the atherosclerotic cardiovascular risk equation in a large contemporary, multiethnic population. J Am Coll Cardiol. 2016;67(18):2118-2130. doi: 10.1016/j.jacc.2016.02.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Allan GM, Nouri F, Korownyk C, Kolber MR, Vandermeer B, McCormack J. Agreement among cardiovascular disease risk calculators. Circulation. 2013;127(19):1948-1956. doi: 10.1161/CIRCULATIONAHA.112.000412 [DOI] [PubMed] [Google Scholar]
- 34.Li L, Geraghty OC, Mehta Z, Rothwell PM, Oxford Vascular Study . Age-specific risks, severity, time course, and outcome of bleeding on long-term antiplatelet treatment after vascular events: a population-based cohort study. Lancet. 2017;390(10093):490-499. doi: 10.1016/S0140-6736(17)30770-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zheng SL, Chan FT, Maclean E, Jayakumar S, Nabeebaccus AA. Reporting trends of randomised controlled trials in heart failure with preserved ejection fraction: a systematic review. Open Heart. 2016;3(2):e000449. doi: 10.1136/openhrt-2016-000449 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Meta-analysis protocol
eMethods 1: Search strategy
eMethods 2: Detailed statistical methods
eMethods 3: Deviance information criterion and model selection
eTable 1: Outcome definitions
eTable 2: Risk of bias assessment
eTable 3: Absolute risk differences and numbers needed to treat
eTable 4: Total stroke outcomes
eTable 5: Event rates for efficacy and safety outcomes
eTable 6: Sensitivity analyses
eFigure 1: Study flow chart
eFigure 2: Risk of bias summary
eFigure 3: Funnel plot for primary cardiovascular outcome
eFigure 4: Frequentist analysis forest plots


