Key Points
Question
What are the incidence, characteristics, and relevance of myocardial infarction (MI) in patients with peripheral artery disease (PAD)?
Findings
In this secondary analysis of the randomized clinical EUCLID trial, of 13 885 patients with symptomatic PAD, 683 (4.9%) experienced MI during a median follow-up of 30 months; type 1 MI (spontaneous) was the most common type of MI, and one-third of MIs were type 2 MI (secondary). Myocardial infarction was associated with an increased risk of cardiovascular death and acute limb ischemia events.
Meaning
More research is needed to identify therapies to reduce the risk of MI in patients with PAD and to improve management of type 2 MI.
This analysis of data from the EUCLID trial characterizes the incidence and types of myocardial infarction in patients with peripheral artery disease, identifies factors associated with myocardial infarction, and determines the association of myocardial infarction with cardiovascular mortality and acute limb ischemia.
Abstract
Importance
Patients with peripheral artery disease (PAD) are at high risk for myocardial infarction (MI).
Objective
To characterize the incidence and types of MI in a PAD population, identify factors associated with MI, and determine the association of MI with cardiovascular mortality and acute limb ischemia.
Design, Setting, and Participants
The Study Comparing Cardiovascular Effects of Ticagrelor and Clopidogrel in Patients With Peripheral Artery Disease (EUCLID) was a double-blind randomized clinical trial conducted at 811 sites in 28 countries that randomized 13 885 patients with symptomatic PAD to monotherapy with ticagrelor or clopidogrel. Participants had an ankle-brachial index (ABI) of 0.80 or less or previous lower extremity revascularization. Median follow-up was 30 months. For these analyses, patients were evaluated for MI occurrence during follow-up irrespective of treatment. Data were analyzed from June 2017 to September 2018.
Main Outcomes and Measures
An adjudication clinical events committee classified MI as type 1 (spontaneous), type 2 (secondary), type 3 (sudden cardiac death), type 4a (less than 48 hours after percutaneous coronary intervention), type 4b (definite stent thrombosis), or type 5 (less than 72 hours after coronary artery bypass graft). A multivariate regression model was developed by stepwise selection to identify factors associated with MI, and a time-dependent multivariate Cox regression analysis was performed to determine the association of MI with cardiovascular death and acute limb ischemia requiring hospitalization.
Results
Of the 13 885 patients included in this analysis, 9997 (72.0%) were male, and the median (interquartile range) age was 66 (60-73) years. Myocardial infarction occurred in 683 patients (4.9%; 2.4 events per 100 patient-years) during a median follow-up of 30 months. Patients experiencing MI were older (median [interquartile range] age, 69 [62-75] vs 66 [60-72] years), more likely to have diabetes (349 of 683 [51.1%] vs 4996 of 13 202 [37.8%]) or a previous lower extremity revascularization (466 of 683 [68.2%] vs 7409 of 13 202 [56.1%]), and had a lower ABI (if included by ABI) compared with censored patients. Of the 683 patients with MI during follow-up, the most common MI type was type 1 (405 [59.3%]), followed by type 2 (236 [34.6%]), type 4a (14 [2.0%]), type 3 (12 [1.8%]), type 4b (11 [1.6%]), and type 5 (5 [0.7%]). Postrandomization MI was independently associated with cardiovascular death (adjusted hazard ratio, 9.0; 95% CI, 7.3-11.2; P < .001) and acute limb ischemia requiring hospitalization (adjusted hazard ratio, 2.5; 95% CI, 1.3-5.0; P = .008).
Conclusions and Relevance
Approximately 5% of patients with symptomatic PAD had an MI during a median follow-up of 30 months. Type 1 MI (spontaneous) was the most common MI type; however, one-third of MIs were type 2 MI (secondary). More research is needed to identify therapies to reduce the risk of MI in patients with PAD and to improve management of type 2 MI.
Trial Registration
ClinicalTrials.gov Identifier: NCT01732822
Introduction
Peripheral artery disease (PAD) is a manifestation of systemic atherosclerosis. Compared with patients without PAD, those with PAD have a higher prevalence of comorbidities and atherosclerosis in other vascular beds, such as the coronary vessels.1 The risk of myocardial infarction (MI) is elevated in patients with PAD.2,3,4,5 Traditionally, MI is related to coronary artery disease (CAD) and the rupture of an atherosclerotic plaque resulting in an intraluminal thrombus. Partial or complete occlusion of the vessel decreases the blood flow and leads to myocyte necrosis. Conditions other than CAD, such as tachycardia, bradycardia, respiratory failure, hypotension, or anemia, can contribute to an imbalance of oxygen supply and/or demand with myocardial necrosis, especially in patients with comorbidities. Recent efforts have attempted to improve the diagnosis of MI through a universal definition of MI (UDMI).6,7 However, to our knowledge, the characterization of type, size, and relevance of MI in a population with PAD has not been extensively evaluated.
In the Study Comparing Cardiovascular Effects of Ticagrelor and Clopidogrel in Patients With Peripheral Artery Disease (EUCLID) trial,8 13 885 patients with symptomatic PAD were randomly assigned to receive either ticagrelor or clopidogrel. Compared with clopidogrel, ticagrelor did not reduce the risk of the primary efficacy end point—a composite of adjudicated cardiovascular death, MI, or ischemic stroke (hazard ratio, 1.02; 95% CI, 0.92-1.13; P = .65). The risk of MI was not significantly different between the groups (hazard ratio, 1.06; 95% CI, 0.91-1.23; P = .48).
This study sought to characterize the incidence and types of MI in patients with PAD from the EUCLID trial. Furthermore, this study aimed to identify factors associated with these events, and determine their association with cardiovascular mortality and acute limb ischemia (ALI).
Methods
Study Design
Data from patients enrolled in the EUCLID trial8 were included (ClinicalTrials.gov identifier: NCT01732822). Eligible participants had an ankle-brachial index of 0.80 or less or had undergone revascularization of the lower limbs.9 In the double-blind, event-driven EUCLID trial,8 13 885 patients were randomly assigned to receive monotherapy with ticagrelor or clopidogrel. All patients provided written informed consent, and the protocol was approved by ethics committees at participating sites. The EUCLID publications committee approved the post hoc analysis with a waiver of informed consent. For these analyses, we evaluated MI events after randomization and during follow-up irrespective of treatment.
End Point Definitions
Myocardial Infarction
Myocardial infarction was adjudicated by a central clinical events committee blinded to treatment assignment.9 The MI events were characterized by type, electrocardiographic changes, and peak troponin elevation. The MI definitions in the EUCLID trial were based on the third UDMI.6 Since then, the fourth UDMI has been published with similar definitions.7 Type 1 MI was defined as spontaneous MI; type 2, MI secondary to an ischemic imbalance; type 3, sudden cardiac death; type 4a, MI related to percutaneous coronary intervention (PCI); type 4b, MI related to stent thrombosis; and type 5, MI related to coronary artery bypass graft (CABG) surgery. For the detailed MI definitions used in the EUCLID trial, see the eMethods in the Supplement. Electrocardiographic changes were classified by ST-segment elevation MI (STEMI) or non-STEMI. Q-wave status was assessed by the site investigator only. The peak troponin elevation was measured as the ratio of the peak value to the upper limit of normal.
Cardiovascular Death
All deaths were adjudicated by the clinical events committee to be either cardiovascular or noncardiovascular in nature. Cardiovascular death included sudden cardiac death, death due to acute MI, death due to heart failure, death due to ischemic stroke, death due to other cardiovascular causes (eg, dysrhythmia unrelated to sudden cardiac death, pulmonary embolism, cardiovascular intervention, aortic aneurysm rupture, or PAD), and deaths for which there was no clearly documented noncardiovascular cause (ie, presumed cardiovascular death). Any death with unknown or uncertain cause within 30 days of an ischemic stroke, MI, or a procedure/surgery was considered a death due to ischemic stroke, MI, or procedure/surgery, respectively.
Acute Limb Ischemia
Acute limb ischemia was defined as requiring hospitalization with the clinical history of a symptomatic rapid or sudden decrease in limb perfusion. Additionally, either (1) a new pulse deficit with associated new rest pain, pallor, paresthesia, or paralysis or (2) confirmation of arterial obstruction by imaging, intraoperative findings, or pathological evaluation, including amputation findings was required.
Statistical Analysis
Categorical variables are summarized as frequencies and percentages, and continuous variables are summarized as medians and interquartile ranges or means and standard deviations, as appropriate. Hypothesis tests comparing patients with and without MI were performed using a univariate Cox regression model, with time to first MI censoring as the response variable and each covariate as the explanatory variables. We characterized the first incidence of type 1 MI or type 2 MI because these subtypes represented most events. A multivariate model was developed for the association of first MI (any subtype), first type 1 MI, or first type 2 MI with baseline variables that were selected by stepwise regression using an inclusion and removal criteria of α = .10. The Fine-Gray method was used to model MI, taking into account the competing risk of death with the chosen factors in the model. Results included hazard ratios and 95% CIs for all the variables included in the final model, χ2 statistics and associated P values for each variable, and C index values as a measure of discrimination. Continuous variables were tested for linearity, and linear piecewise splines were added as needed. The proportional hazards assumption was tested using Schoenfeld scaled residuals.
To assess the association of any MI during follow-up with cardiovascular death and subsequent ALI events, we used a multivariate Cox regression model with MI during follow-up as a time-dependent covariate. Once MI occurred, the indicator for MI was turned on for the remainder of follow-up. Unadjusted and adjusted hazard ratios for the association of MI with cardiovascular death and ALI events were derived. Adjustment variables were based on factors most associated with MI from the multivariate model and on factors identified to be important for cardiovascular death and ALI events (eTable 1 in the Supplement). All P values were 2-sided, and a P value less than .05 was considered statistically significant. All analyses were conducted by the Duke Clinical Research Institute using SAS version 9.4 (SAS Institute).
Results
Cohort and Baseline Characteristics
A total of 13 885 patients were analyzed; 9997 (72.0%) were male, and the median (interquartile range) age was 66 (60-73) years. Myocardial infarction occurred in 683 patients (4.9%; 2.4 MIs per 100 patient-years) during a median follow-up of 30 months. Baseline characteristics of patients with and without MI during the study are presented in Table 1. Patients with MI were typically older, had more advanced limb symptoms, and more frequently had a history of other cardiovascular conditions and revascularization. Cardiovascular risk factors were more prominent in patients with MI. Most patients were being treated with medications for cardiovascular disease or risk factors (ie, antiplatelet agents, β-blockers, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and statins). The baseline characteristics of patients by region are shown in eTable 2 in the Supplement. The MI incidence rates per 100 patient-years of follow-up by baseline Rutherford classification were 1.9 for asymptomatic patients (grade 0), 1.8 for patients with mild or moderate claudication (grade I or II), 2.4 for patients with severe claudication (grade III), 3.4 for patients with ischemic rest pain (grade IV), 2.9 for patients with minor tissue loss (grade V), and 2.5 for patients with major tissue loss (grade VI).
Table 1. Baseline Characteristics by Myocardial Infarction (MI).
| Characteristic | MI During Follow-up, No. (%) | Univariate Cox Regression, HR (95% CI) | |
|---|---|---|---|
| Yes (n = 683) | No (n = 13 202) | ||
| Age, median (IQR), y | 69 (62-75) | 66 (60-72) | 1.03 (1.02-1.04)a |
| Female | 175 (25.6) | 3713 (28.1) | 0.88 (0.74-1.04) |
| Region | |||
| Asia | 71 (10.4) | 1531 (11.6) | 1.17 (0.90-1.51) |
| Central/South America | 26 (3.8) | 1714 (13.0) | 0.41 (0.28-0.62) |
| Europe | 294 (43.0) | 7204 (54.6) | 1 [Reference] |
| North America | 292 (42.8) | 2753 (20.9) | 2.50 (2.12-2.94) |
| Weight, median (IQR), kg | 79 (67-90) | 76 (66-88) | NAb |
| Inclusion criteria for randomization | |||
| Previous revascularization | 466 (68.2) | 7409 (56.1) | 1.63 (1.38-1.91) |
| ABI value, mean (SD) | 0.77 (0.24) | 0.78 (0.23) | NA |
| ABI or TBI criteria | 217 (31.8) | 5793 (43.9) | 1 [Reference] |
| ABI value, mean (SD) | 0.60 (0.13) | 0.63 (0.15) | NA |
| TBI value, mean (SD) | 0.47 (0.18) | 0.52 (0.22) | NA |
| Limb symptoms by Rutherford classification | |||
| Asymptomatic | 121 (17.7) | 2480 (18.8) | 1 [Reference] |
| Mild or moderate claudication | 333 (48.8) | 7077 (53.6) | 0.98 (0.80-1.21) |
| Severe claudication | 184 (26.9) | 3044 (23.1) | 1.29 (1.02-1.62) |
| Pain while at rest | 29 (4.2) | 349 (2.6) | 1.82 (1.21-2.73) |
| Minor tissue loss | 13 (1.9) | 194 (1.5) | 1.54 (0.87-2.72) |
| Major tissue loss | 3 (0.4) | 55 (0.4) | 1.35 (0.43-4.24) |
| Major amputation above the ankle | 22 (3.2) | 317 (2.4) | 1.44 (0.94-2.21) |
| Minor amputation | 38 (5.6) | 567 (4.3) | 1.40 (1.01-1.95) |
| No. of vascular beds affected | |||
| 1 | 232 (34.0) | 7572 (57.4) | 1 [Reference] |
| 2 | 292 (42.8) | 4396 (33.3) | 2.14 (1.80-2.54) |
| 3 | 159 (23.3) | 1234 (9.3) | 4.03 (3.30-4.94) |
| Prior comorbidity | |||
| Stroke | 80 (11.7) | 1063 (8.1) | 1.53 (1.21-1.94) |
| TIA | 48 (7.0) | 459 (3.5) | 2.04 (1.52-2.73) |
| CAD | 349 (51.1) | 3683 (27.9) | 2.64 (2.27-3.07) |
| MI | 228 (33.4) | 2294 (17.4) | 2.37 (2.02-2.78) |
| PCI | 218 (31.9) | 1955 (14.8) | 2.60 (2.21-3.05) |
| CABG | 159 (23.3) | 1377 (10.4) | 2.52 (2.11-3.01) |
| Carotid stenosis/revascularization | 211 (30.9) | 2316 (17.5) | 2.04 (1.73-2.40) |
| Atrial fibrillation/flutter | 39 (5.7) | 457 (3.5) | 1.73 (1.25-2.39) |
| CHF | 136 (19.9) | 1792 (13.6) | 1.61 (1.34-1.95) |
| CCS angina class | |||
| 0 | 578 (84.6) | 11 441 (86.7) | 1 [Reference] |
| I | 62 (9.1) | 863 (6.5) | 1.44 (1.11-1.87) |
| II | 32 (4.7) | 709 (5.4) | 0.92 (0.64-1.31) |
| III | 10 (1.5) | 175 (1.3) | 1.16 (0.62-2.16) |
| IV | 1 (0.1) | 12 (0.1) | 1.55 (0.22-11.0) |
| Chronic kidney diseasec | 253 (38.3) | 3052 (23.9) | 2.03 (1.74-2.37) |
| Family history of CHD | 137 (27.0) | 1873 (19.4) | 1.50 (1.23-1.82) |
| Diabetes type 1 or 2 | 349 (51.1) | 4996 (37.8) | 1.73 (1.49-2.01) |
| Hypertension | 586 (85.8) | 10 271 (77.8) | 1.69 (1.36-2.09) |
| Hyperlipidemia | 568 (83.2) | 9912 (75.1) | 1.57 (1.28-1.91) |
| Tobacco use | |||
| Never | 121 (18.0) | 2863 (21.8) | 1 [Reference] |
| Former | 337 (50.0) | 6193 (47.2) | 1.24 (1.01-1.53) |
| Current | 216 (32.0) | 4073 (31.0) | 1.21 (0.97-1.52) |
| Medications before randomization | |||
| Aspirin | 519 (76.0) | 8752 (66.3) | 1.58 (1.33-1.89) |
| Clopidogrel | 288 (42.2) | 4185 (31.7) | 1.55 (1.33-1.80) |
| Statin | 544 (79.6) | 9637 (73.0) | 1.39 (1.15-1.67) |
| ACE inhibitor | 300 (43.9) | 5335 (40.4) | 1.14 (0.98-1.33) |
| Angiotensin receptor blocker | 200 (29.3) | 3288 (24.9) | 1.23 (1.04-1.45) |
| β-Blocker | 393 (57.5) | 5247 (39.7) | 2.00 (1.72-2.33) |
| Cilostazol | 95 (13.9) | 2000 (15.1) | 0.93 (0.75-1.16) |
Abbreviations: ABI, ankle-brachial index; ACE, angiotensin-converting enzyme; CABG, coronary artery bypass graft; CAD, coronary artery disease; CCS, Canadian Cardiovascular Society; CHD, coronary heart disease; CHF, congestive heart failure; HR, hazard ratio; IQR, interquartile range; PCI, percutaneous coronary intervention; TBI, toe-brachial index; TIA, transient ischemic attack.
The HR of age refers to the risk per 1-year increase.
This HR was not computed because of the nonlinear relationship between weight and the outcome.
Chronic kidney disease was defined as an estimated glomerular filtration rate of less than 60 mL/min/1.73m2.
Types of MI
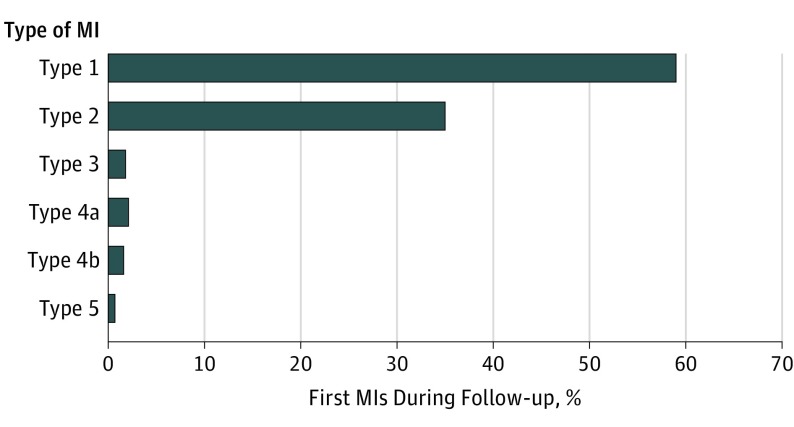
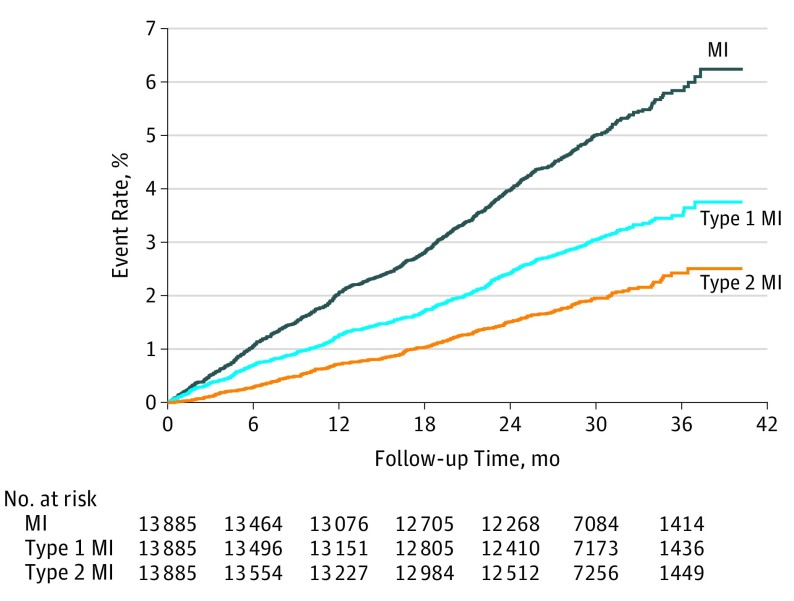
Types of first MI that occurred in 683 patients during the median follow-up of 30 months were adjudicated by the clinical events committee using the UDMI (Figure 1). Most of the MIs were type 1 MI (405 [59.3%]), but more than one-third were type 2 MI (236 [34.6%]). Few MIs were type 3 (12 [1.8%]), type 4a (14 [2.0%]), type 4b (11 [1.6%]), or type 5 (5 [0.7%]). Type 4a MI occurred in 14 of 651 patients (2.2%) undergoing a PCI procedure during follow-up. Type 5 MI occurred in 5 of 181 patients (2.8%) undergoing CABG. Figure 2 shows the time to first MI, first type 1 MI, and first type 2 MI. The incidence rates of MI by region are shown in eTable 3 in the Supplement.
Figure 1. Classification of the First Myocardial Infarction (MI) by the Universal Definition of MI.
Myocardial infarction occurred in 683 of 13 885 patients (4.9%; 2.4 MIs per 100 patient-years) with symptomatic peripheral artery disease during a median follow-up of 30 months. A total of 405 patients (59.3%) had type 1 MI; 236 (34.6%), type 2 MI; 12 (1.8%), type 3 MI; 14 (2.0%), type 4a MI; 11 (1.6%), type 4b MI; and 5 (0.7%), type 5 MI.
Figure 2. Kaplan-Meier Estimated Occurrence of First Myocardial Infarction (MI).
Myocardial infarction, type 1 MI, and type 2 MI occurred continuously over follow-up time.
Electrocardiographic changes and the ratio of the troponin I/T peak value to the upper limit of normal for the first MI, first type 1 MI, and first type 2 MI are shown in eTable 4 in the Supplement. A total of 522 of 683 first MIs (76.4%) were non-STEMI. Electrocardiography status was unknown in 98 patients (14.3%).
Q-wave status was assessed by the site investigator only and was available in 413 of 683 patients (60.5%) who had an MI, as adjudicated by the clinical events committee. Of these 413 patients, Q-waves were detected in 22 patients (5.3%). In 66 of 413 patients (16.0%), the site investigator determined that no electrocardiographic data were available for Q-wave assessment.
A peak troponin elevation greater than 10-fold the upper limit of normal was found in 312 of 683 first MIs (45.7%). Peak troponin values tended to be higher in type 1 MI vs type 2 MI (type 1: 221 of 405 [54.6%] with an elevation greater than 10-fold the upper limit of normal; type 2: 70 of 236 [29.7%] with an elevation greater than 10-fold the upper limit of normal).
Factors Associated With MI
The factors independently associated with first MI, first type 1 MI, and first type 2 MI are shown in Table 2. We identified region, age, diabetes, chronic kidney disease, current tobacco use, prior MI, prior revascularization procedures, severe claudication or prior minor amputation, use of β-blockers or angiotensin receptor blockers, and male sex as major factors associated with MI in this patient population. Most factors associated with first MI were also associated with first type 1 MI or with first type 2 MI. Prior PCI and prior MI were associated with first type 1 MI but not with first type 2 MI. Age, heart failure, sex, pain while at rest, β-blocker use, weight (<80 kg), and transient ischemic attack were associated with first type 2 MI but not with first type 1 MI. The C indices for the prediction model of MI, type 1 MI, and type 2 MI were 0.72, 0.71, and 0.77, respectively.
Table 2. Factors Associated With Myocardial Infarction (MI) in Patients With Peripheral Artery Disease.
| Variablea | MI (n = 683) | Type 1 MI (n = 415) | Type 2 MI (n = 267) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| χ2 | HR (95% CI) | P Value | χ2 | HR (95% CI) | P Value | χ2 | HR (95% CI) | P Value | |
| North America vs Europe | 33.0 | 1.72 (1.43-2.07) | <.001 | 10.2 | 1.47 (1.16-1.85) | .001 | 35.8 | 2.58 (1.89-3.52) | <.001 |
| Central America vs Europe | 22.6 | 0.36 (0.24-0.55) | <.001 | 13.4 | 0.39 (0.23-0.64) | <.001 | 9.8 | 0.27 (0.12-0.61) | .002 |
| Diabetes | 22.0 | 1.48 (1.26-1.74) | <.001 | 22.7 | 1.65 (1.34-2.02) | <.001 | 14.7 | 1.65 (1.28-2.14) | <.001 |
| Chronic kidney disease | 20.4 | 1.48 (1.25-1.76) | <.001 | 19.0 | 1.61 (1.30-1.99) | <.001 | 15.3 | 1.72 (1.31-2.26) | <.001 |
| Ageb | 16.8 | 1.12 (1.06-1.18) | <.001 | NA | NA | NA | 20.7 | 1.22 (1.12-1.32) | <.001 |
| Prior PCI | 16.0 | 1.49 (1.23-1.81) | <.001 | 15.9 | 1.64 (1.29-2.10) | <.001 | NA | NA | NA |
| Prior MI | 13.8 | 1.46 (1.20-1.78) | <.001 | 20.2 | 1.75 (1.37-2.23) | <.001 | NA | NA | NA |
| Inclusion criteria: prior revascularization vs ABI/TBI | 10.9 | 1.36 (1.13-1.64) | .001 | 6.2 | 1.34 (1.06-1.70) | .01 | 5.7 | 1.43 (1.07-1.91) | .02 |
| Carotid revascularization or stenosis | 10.2 | 1.33 (1.12-1.58) | .001 | 7.5 | 1.36 (1.09-1.70) | .006 | 6.4 | 1.42 (1.08-1.86) | .01 |
| Severe claudication vs asymptomatic | 7.3 | 1.41 (1.10-1.81) | .007 | 8.7 | 1.62 (1.18-2.23) | .003 | 3.7 | 1.49 (0.99-2.24) | .05 |
| Current tobacco use vs never | 6.0 | 1.37 (1.06-1.76) | .01 | 3.5 | 1.34 (0.99-1.81) | .06 | NA | NA | NA |
| Prior minor amputation | 5.8 | 1.54 (1.09-2.20) | .02 | NA | NA | NA | NA | NA | NA |
| Prior CABG | 5.3 | 1.28 (1.04-1.57) | .02 | 3.5 | 1.28 (0.99-1.65) | .06 | NA | NA | NA |
| β-Blockers within 30 d prior | 5.0 | 1.22 (1.02-1.46) | .03 | NA | NA | NA | 7.6 | 1.46 (1.12-1.92) | .006 |
| Prior angiotensin receptor blocker | 4.5 | 1.21 (1.02-1.43) | .03 | NA | NA | NA | NA | NA | NA |
| Female vs male | 4.2 | 0.82 (0.68-0.99) | .04 | NA | NA | NA | 9.2 | 0.60 (0.43-0.83) | .002 |
| Pain while at rest vs asymptomatic | 3.5 | 1.54 (0.98-2.42) | .06 | <1.0 | 1.14 (0.58-2.26) | .71 | 8.2 | 2.42 (1.32-4.43) | .004 |
| Prior TIA | 3.4 | 1.33 (0.98-1.81) | .07 | NA | NA | NA | 4.1 | 1.60 (1.02-2.51) | .04 |
| Minor tissue loss vs asymptomatic | 2.6 | 1.66 (0.89-3.08) | .11 | <1.0 | 1.38 (0.58-3.30) | .46 | 3.4 | 2.32 (0.95-5.63) | .06 |
| Major tissue loss vs asymptomatic | 1.1 | 1.90 (0.58-6.25) | .29 | <1.0 | 1.13 (0.15-8.27) | .91 | 3.3 | 3.80 (0.90-16.0) | .07 |
| Mild or moderate claudication vs asymptomatic | <1.0 | 1.12 (0.89-1.40) | .33 | 1.5 | 1.20 (0.90-1.61) | .22 | 1.0 | 1.21 (0.84-1.74) | .31 |
| Former tobacco use vs never | <1.0 | 1.05 (0.83-1.32) | .68 | <1.0 | 1.02 (0.76-1.36) | .91 | NA | NA | NA |
| Asia vs Europe | <1.0 | 1.04 (0.78-1.38) | .79 | 1.3 | 1.22 (0.87-1.71) | .25 | <1.0 | 0.91 (0.54-1.53) | .73 |
| Prior CHF | NA | NA | NA | NA | NA | NA | 10.4 | 1.65 (1.22-2.24) | .001 |
| Weightb | |||||||||
| <80 kg | NA | NA | NA | NA | NA | NA | 4.9 | 0.91 (0.83-0.99) | .03 |
| >80 kg | NA | NA | NA | NA | NA | NA | <1.0 | 1.01 (0.94-1.08) | .79 |
Abbreviations: ABI, ankle-brachial index; CABG, coronary artery bypass graft; CHF, congestive heart failure; HR, hazard ratio; NA, not applicable; PCI, percutaneous coronary intervention; TBI, toe-brachial index; TIA, transient ischemic attack.
Ordered by decreasing χ2 value for MI. For variables not selected as a predictor, no value is provided.
Age and weight were included as continuous variables in the model and shown per 5-year or 5-kg increase, respectively.
Association With Outcome
Table 3 shows the association of MI, type 1 MI, and type 2 MI with cardiovascular death and ALI requiring hospitalization. Myocardial infarction, type 1 MI, and type 2 MI were associated with a 6-fold to 9-fold increased risk of cardiovascular death and a 1.5-fold to 5-fold increased risk of ALI requiring hospitalization.
Table 3. Risk of Cardiovascular Death and Acute Limb Ischemia (ALI) Requiring Hospitalization Following a Myocardial Infarction (MI) During Follow-up Period.
| Outcome | Events, No./Total No. (%) | Adjusted HR (95% CI) | P Value | |
|---|---|---|---|---|
| With MIa | Without MI | |||
| Cardiovascular death | ||||
| MI | 122/683 (17.3) | 584/13 202 (1.8) | 9.0 (7.3-11.2) | <.001 |
| Type 1 MI | 78/415 (17.1) | 628/13 470 (1.9) | 8.1 (6.2-10.4) | <.001 |
| Type 2 MI | 41/267 (17.1) | 665/13 618 (2.0) | 6.3 (4.5-8.8) | <.001 |
| ALI requiring hospitalization | ||||
| MI | 11/669 (1.6) | 221/13 216 (0.7) | 2.5 (1.3-5.0) | .008 |
| Type 1 MI | 4/411 (0.9) | 228/13 474 (0.7) | 1.7 (0.6-4.6) | .32 |
| Type 2 MI | 8/259 (3.6) | 224/13 626 (0.7) | 5.1 (2.2-11.8) | <.001 |
Abbreviation: HR, hazard ratio.
The index event is the first MI of each category that occurred during the follow-up period.
Discussion
This study characterized the types of MI in a large PAD population. The main findings are (1) 5% of patients with symptomatic PAD experienced MI during a median follow-up of 30 months; (2) most of the first MIs in these patients were type 1 MI (spontaneous) but more than one-third were type 2 MI (secondary), and peak troponin values tended to be higher in type 1 MI vs type 2 MI; (3) region, age, diabetes, atherosclerotic disease, and prior procedures were independently associated with MI in this patient population, and advanced limb symptoms appeared to be associated more strongly with type 2 MI than with type 1 MI; and (4) MIs were independently associated with an increased risk for cardiovascular death and ALI requiring hospitalization.
Risk of MI in Patients With PAD
Patients with PAD have high rates of comorbidities and atherosclerosis in other vascular beds.1,3 In registry data, 40% of patients with PAD had no other vascular bed affected.10 In the EUCLID trial,8 55% of patients had PAD only. One-third of patients had a total of 2 vascular beds affected, and 10% of patients had 3 vascular beds affected. Despite these lower rates of atherosclerotic disease in more than 1 vascular bed and the frequent use of drugs for secondary prevention (eg, 75% of patients were receiving statin therapy) in the EUCLID trial, 5% of patients experienced an MI during a median follow-up of 30 months. This is similar to previously described MI rates.11 It has been described before that death from cardiovascular causes for patients with PAD (even without history of MI) is similar to patients with a prior cardiac event.3,12 However, many patients with PAD are undertreated.3 This study confirms the high risk of MI in a large PAD population.
Types of MI
Risk factors and diagnostic assessment, treatment strategies, and prognostic information are mostly suited for type 1 MI, the typical clinical picture of MI. As expected, most first MIs during follow-up in our study were type 1 MI; however, one-third of first MIs were classified as type 2 MI. This rate seems high in comparison with reports in the literature from other MI populations. In acute coronary syndrome trials with MI adjudication, the proportion of type 4a MI is higher, and the prevalence of type 2 MI can be less than 5%.13,14 In cohort studies of a high-risk population, the incidence might even be higher than the rates observed here.15 In a trial subanalysis of more than 20 000 patients with stable atherosclerotic disease (prior MI, 86%; symptomatic PAD, 14%), Kidd et al16 found that 10% of the MIs were type 2 MI. Taken together, the incidence of type 2 MI varies among different populations and seems high among patients with PAD. The higher rate in patients with PAD might be explained by the frequent comorbidities among patients with PAD contributing to the occurrence of type 2 MI.17 The high rate of type 2 MI during follow-up in this study of a PAD population highlights the ongoing need for evidence-based management of type 2 MI.
The optimal interpretation of type 2 MI has been somewhat controversial.18,19 Unfortunately, we had little information about the clinical context of these type 2 MIs that could add to the discussion regarding the differentiation between myocardial injury and MI.
Electrocardiography Criteria, Biomarker, and Size
Overall, the electrocardiography characteristics for first MIs in patients with PAD were similar to reports from general populations.13,16,20 Most first MIs in our study were non-STEMI and were in the highest UDMI category of peak biomarker elevation (greater than 10-fold the upper limit of normal). These observations are similar to results from a mixed population of patients with stable atherosclerosis (symptomatic PAD, 14%).16 Similar to our observations, type 1 MI tended to be larger than type 2 MI in an acute coronary syndrome trial population.21
Factors Associated With MI
Age, diabetes, atherosclerotic disease, prior procedures, and chronic kidney disease were identified as major factors associated with MI in this patient population and represent known risk factors comparable with those in a general population.22,23 We speculate that the reason region is a major factor associated with MI relies on the differences in ascertainment, treatment paradigms, or diagnostic evaluations.
We identified differences in the multivariate models for type 1 MI vs type 2 MI. First, in contrast to the model for type 1 MI, the model for type 2 MI did not include history of MI or PCI. History of MI and history of revascularization have been identified as effect modifiers for antiplatelet agents for secondary prevention of thrombotic events, including MI, in trials with patients with atherosclerosis.24,25,26 To our knowledge, the manner in which this effect modification relates to MI type has not been evaluated. Second, there has been discussion about whether the definition of type 2 MI should require CAD.27,28,29 Saaby et al30 reported that up to half of patients with type 2 MI do not have CAD. Our results affirm that known CAD, prior MI, or prior PCI are not present in many patients with type 2 MI. However, since the primary condition in the EUCLID trial was PAD, CAD status was not assessed angiographically in many patients. Third, age and heart failure were independently associated with type 2 MI but not with type 1 MI, indicating the relevance of comorbidities for type 2 MI and a potential benefit from multidisciplinary care for patients at risk.15,31 Fourth, advanced limb symptoms were major factors independently associated with type 2 MI and appeared to be associated more strongly with type 2 MI than with type 1 MI. This may be of relevance to identify patients at risk for type 2 MI. Studies are needed to test whether further diagnostic assessments and treatment strategies can help to prevent these events.
Outcome
Most treatment strategies are optimally suited for type 1 MI. It has been shown that both type 1 MI and type 2 MI are independently associated with cardiovascular death.15,16,21 We confirmed these associations in a large PAD population over a median follow-up of 30 months. The risk of cardiovascular death was high for patients with type 2 MI but appeared to be even higher for those with type 1 MI. Other studies reported opposite trends16,21 but relied on fewer type 2 MI events, and most patients had prior CAD. Our results highlight the need for further studies to identify and test treatment strategies for type 2 MI to prevent cardiovascular death.
Type 2 MI was independently associated with ALI requiring hospitalization. This information may be helpful for clinicians to identify patients at increased risk for these events. Whether this risk simply reflects advanced atherosclerosis or if MI has a causal relationship is unknown. However, there were only 4 ALI events requiring hospitalization after a first type 1 MI and only 8 after a first type 2 MI. The large confidence intervals indicate that these numbers should be interpreted with caution. Although MI treatment is mostly suited for type 1 MI, it seems unlikely that the infarction itself increases the risk for ALI (eg, through heart failure or emboli). Common risk factors or comorbidities not included in our model might explain this increased risk. Whether multidisciplinary care can avoid ALI in these comorbid patients at risk or whether this increased risk would justify revascularization despite low event rates deserves further studies.
Limitations
Our study had limitations. The generalizability of results from randomized clinical trials can be limited.32For example, patients receiving long-term anticoagulation and those requiring dialysis were excluded from the EUCLID trial, and our observations might underestimate or overestimate the occurrence of MI in a general PAD population. The members of the clinical events committee responsible for MI adjudication were highly trained. However, adjudication of MI as type 2 MI often relies on physician judgment. The etiology of type 2 MI was not specified but would have been helpful to add important insights to the discussion around myocardial injury vs MI. For this trial, contemporary troponin assays were used. High-sensitivity troponin might identify more MI events, particularly type 2 MI. Since ticagrelor was not shown to reduce cardiovascular events compared with clopidogrel in the EUCLID trial, we did not look at treatment effects on MI for this analysis. The association of MI with subsequent events relies on observational data and does not imply causation.
Conclusions
Approximately 5% of patients with symptomatic PAD experienced MI during a median follow-up of 30 months. Type 1 MI was the most common MI type, but one-third of MIs were type 2 MI. Non–ST-segment elevation MI occurred more frequently than STEMI. Peak troponin values tended to be higher in type 1 MI vs type 2 MI. A multivariate model identified region, age, diabetes, chronic kidney disease, atherosclerotic disease, and prior procedures as major predictors of MI in this patient population. Advanced limb symptoms appeared to be stronger predictors of type 2 MI than of type 1 MI. Myocardial infarction in patients with PAD was associated with an increased risk for cardiovascular death and ALI events requiring hospitalization. More research is needed to identify therapies to reduce the risk of MI in patients with PAD and to improve the management of type 2 MI.
eMethods. Definition of myocardial infarction (MI).
eTable 1. Adjustment variables for risk of cardiovascular death and acute limb ischemia following myocardial infarction.
eTable 2. Baseline characteristics of patients included by region.
eTable 3. Incidence rates of myocardial infarction by region.
eTable 4. Electrocardiography characteristics of the first myocardial infarction during follow-up.
References
- 1.Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135(12):e686-e725. doi: 10.1161/CIR.0000000000000470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saw J, Bhatt DL, Moliterno DJ, et al. The influence of peripheral arterial disease on outcomes: a pooled analysis of mortality in eight large randomized percutaneous coronary intervention trials. J Am Coll Cardiol. 2006;48(8):1567-1572. doi: 10.1016/j.jacc.2006.03.067 [DOI] [PubMed] [Google Scholar]
- 3.Hiatt WR. Medical treatment of peripheral arterial disease and claudication. N Engl J Med. 2001;344(21):1608-1621. doi: 10.1056/NEJM200105243442108 [DOI] [PubMed] [Google Scholar]
- 4.Patel MR, Becker RC, Wojdyla DM, et al. Cardiovascular events in acute coronary syndrome patients with peripheral arterial disease treated with ticagrelor compared with clopidogrel: data from the PLATO trial. Eur J Prev Cardiol. 2015;22(6):734-742. doi: 10.1177/2047487314533215 [DOI] [PubMed] [Google Scholar]
- 5.Fowkes FG, Murray GD, Butcher I, et al. ; Ankle Brachial Index Collaboration . Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality: a meta-analysis. JAMA. 2008;300(2):197-208. doi: 10.1001/jama.300.2.197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thygesen K, Alpert JS, Jaffe AS, et al. ; Writing Group on the Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction; ESC Committee for Practice Guidelines (CPG) . Third universal definition of myocardial infarction. Eur Heart J. 2012;33(20):2551-2567. doi: 10.1093/eurheartj/ehs184 [DOI] [PubMed] [Google Scholar]
- 7.Thygesen K, Alpert JS, Jaffe AS, et al. ; Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction . Fourth universal definition of myocardial infarction (2018). J Am Coll Cardiol. 2018;72(18):2231-2264. doi: 10.1016/j.jacc.2018.08.1038 [DOI] [PubMed] [Google Scholar]
- 8.Hiatt WR, Fowkes FGR, Heizer G, et al. ; EUCLID Trial Steering Committee and Investigators . Ticagrelor versus clopidogrel in symptomatic peripheral artery disease. N Engl J Med. 2017;376(1):32-40. doi: 10.1056/NEJMoa1611688 [DOI] [PubMed] [Google Scholar]
- 9.Berger JS, Katona BG, Jones WS, et al. Design and rationale for the Effects of Ticagrelor and Clopidogrel in Patients with Peripheral Artery Disease (EUCLID) trial. Am Heart J. 2016;175(suppl C):86-93. doi: 10.1016/j.ahj.2016.01.018 [DOI] [PubMed] [Google Scholar]
- 10.Bhatt DL, Steg PG, Ohman EM, et al. ; REACH Registry Investigators . International prevalence, recognition, and treatment of cardiovascular risk factors in outpatients with atherothrombosis. JAMA. 2006;295(2):180-189. doi: 10.1001/jama.295.2.180 [DOI] [PubMed] [Google Scholar]
- 11.Bonaca MP, Scirica BM, Creager MA, et al. Vorapaxar in patients with peripheral artery disease: results from TRA2degreesP-TIMI 50. Circulation. 2013;127(14):1522-1529, 1529e1-e6. doi: 10.1161/CIRCULATIONAHA.112.000679 [DOI] [PubMed] [Google Scholar]
- 12.Bhatt DL, Eagle KA, Ohman EM, et al. ; REACH Registry Investigators . Comparative determinants of 4-year cardiovascular event rates in stable outpatients at risk of or with atherothrombosis. JAMA. 2010;304(12):1350-1357. doi: 10.1001/jama.2010.1322 [DOI] [PubMed] [Google Scholar]
- 13.Morrow DA, Wiviott SD, White HD, et al. Effect of the novel thienopyridine prasugrel compared with clopidogrel on spontaneous and procedural myocardial infarction in the Trial to Assess Improvement in Therapeutic Outcomes By Optimizing Platelet Inhibition With Prasugrel-Thrombolysis in Myocardial Infarction 38: an application of the classification system from the universal definition of myocardial infarction. Circulation. 2009;119(21):2758-2764. doi: 10.1161/CIRCULATIONAHA.108.833665 [DOI] [PubMed] [Google Scholar]
- 14.Tricoci P, Huang Z, Held C, et al. ; TRACER Investigators . Thrombin-receptor antagonist vorapaxar in acute coronary syndromes. N Engl J Med. 2012;366(1):20-33. doi: 10.1056/NEJMoa1109719 [DOI] [PubMed] [Google Scholar]
- 15.Gaggin HK, Liu Y, Lyass A, et al. Incident type 2 myocardial infarction in a cohort of patients undergoing coronary or peripheral arterial angiography. Circulation. 2017;135(2):116-127. doi: 10.1161/CIRCULATIONAHA.116.023052 [DOI] [PubMed] [Google Scholar]
- 16.Kidd SK, Bonaca MP, Braunwald E, et al. Universal classification system type of incident myocardial infarction in patients with stable atherosclerosis: observations from Thrombin Receptor Antagonist in Secondary Prevention of Atherothrombotic Ischemic Events (TRA 2°P)-TIMI 50. J Am Heart Assoc. 2016;5(7):e003237. doi: 10.1161/JAHA.116.003237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta S, Vaidya SR, Arora S, Bahekar A, Devarapally SR. Type 2 versus type 1 myocardial infarction: a comparison of clinical characteristics and outcomes with a meta-analysis of observational studies. Cardiovasc Diagn Ther. 2017;7(4):348-358. doi: 10.21037/cdt.2017.03.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alpert JS, Thygesen KA. The case for a revised definition of myocardial infarction: the ongoing conundrum of type 2 myocardial infarction vs myocardial injury. JAMA Cardiol. 2016;1(3):249-250. doi: 10.1001/jamacardio.2016.0543 [DOI] [PubMed] [Google Scholar]
- 19.Nagele P. The case for a revised definition of myocardial infarction: resolving the ambiguity of type 2 myocardial infarction. JAMA Cardiol. 2016;1(3):247-248. doi: 10.1001/jamacardio.2016.0511 [DOI] [PubMed] [Google Scholar]
- 20.Ramos R, Albert X, Sala J, et al. ; REGICOR Investigators . Prevalence and incidence of Q-wave unrecognized myocardial infarction in general population: diagnostic value of the electrocardiogram: the REGICOR study. Int J Cardiol. 2016;225(suppl C):300-305. doi: 10.1016/j.ijcard.2016.10.005 [DOI] [PubMed] [Google Scholar]
- 21.Guimarães PO, Leonardi S, Huang Z, et al. Clinical features and outcomes of patients with type 2 myocardial infarction: insights from the Thrombin Receptor Antagonist for Clinical Event Reduction in Acute Coronary Syndrome (TRACER) trial. Am Heart J. 2018;196:28-35. doi: 10.1016/j.ahj.2017.10.007 [DOI] [PubMed] [Google Scholar]
- 22.Goff DC Jr, Lloyd-Jones DM, Bennett G, et al. ; American College of Cardiology/American Heart Association Task Force on Practice Guidelines . 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25, suppl 2):S49-S73. doi: 10.1161/01.cir.0000437741.48606.98 [DOI] [PubMed] [Google Scholar]
- 23.Meisinger C, Döring A, Löwel H; KORA Study Group . Chronic kidney disease and risk of incident myocardial infarction and all-cause and cardiovascular disease mortality in middle-aged men and women from the general population. Eur Heart J. 2006;27(10):1245-1250. doi: 10.1093/eurheartj/ehi880 [DOI] [PubMed] [Google Scholar]
- 24.Scirica BM, Bonaca MP, Braunwald E, et al. ; TRA 2°P-TIMI 50 Steering Committee Investigators . Vorapaxar for secondary prevention of thrombotic events for patients with previous myocardial infarction: a prespecified subgroup analysis of the TRA 2°P-TIMI 50 trial. Lancet. 2012;380(9850):1317-1324. doi: 10.1016/S0140-6736(12)61269-0 [DOI] [PubMed] [Google Scholar]
- 25.Bhatt DL, Flather MD, Hacke W, et al. ; CHARISMA Investigators . Patients with prior myocardial infarction, stroke, or symptomatic peripheral arterial disease in the CHARISMA trial. J Am Coll Cardiol. 2007;49(19):1982-1988. doi: 10.1016/j.jacc.2007.03.025 [DOI] [PubMed] [Google Scholar]
- 26.Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK; Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial Investigators . Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001;345(7):494-502. doi: 10.1056/NEJMoa010746 [DOI] [PubMed] [Google Scholar]
- 27.Nestelberger T, Boeddinghaus J, Badertscher P, et al. ; APACE Investigators . Effect of definition on incidence and prognosis of type 2 myocardial infarction. J Am Coll Cardiol. 2017;70(13):1558-1568. doi: 10.1016/j.jacc.2017.07.774 [DOI] [PubMed] [Google Scholar]
- 28.Chapman AR, Adamson PD, Mills NL. Assessment and classification of patients with myocardial injury and infarction in clinical practice. Heart. 2017;103(1):10-18. doi: 10.1136/heartjnl-2016-309530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sandoval Y, Thygesen K. Myocardial infarction type 2 and myocardial injury. Clin Chem. 2017;63(1):101-107. doi: 10.1373/clinchem.2016.255521 [DOI] [PubMed] [Google Scholar]
- 30.Saaby L, Poulsen TS, Hosbond S, et al. Classification of myocardial infarction: frequency and features of type 2 myocardial infarction. Am J Med. 2013;126(9):789-797. doi: 10.1016/j.amjmed.2013.02.029 [DOI] [PubMed] [Google Scholar]
- 31.Saaby L, Poulsen TS, Diederichsen ACP, et al. Mortality rate in type 2 myocardial infarction: observations from an unselected hospital cohort. Am J Med. 2014;127(4):295-302. doi: 10.1016/j.amjmed.2013.12.020 [DOI] [PubMed] [Google Scholar]
- 32.Rothwell PM. External validity of randomised controlled trials: “to whom do the results of this trial apply?” Lancet. 2005;365(9453):82-93. doi: 10.1016/S0140-6736(04)17670-8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Definition of myocardial infarction (MI).
eTable 1. Adjustment variables for risk of cardiovascular death and acute limb ischemia following myocardial infarction.
eTable 2. Baseline characteristics of patients included by region.
eTable 3. Incidence rates of myocardial infarction by region.
eTable 4. Electrocardiography characteristics of the first myocardial infarction during follow-up.