Key Points
Question
Has survival after extremely preterm birth changed in Sweden from 2004-2007 to 2014-2016?
Findings
In this comparison of 2 birth cohorts in Sweden that included 2205 births at 22-26 weeks’ gestational age, 1-year survival among those born alive in 2004-2007 was 70% compared with 77% for those born alive in 2014-2016 and the difference was statistically significant.
Meaning
In Sweden, 1-year survival after extremely preterm birth improved between 2004-2007 and 2014-2016.
Abstract
Importance
Since 2004-2007, national guidelines and recommendations have been developed for the management of extremely preterm births in Sweden. If and how more uniform management has affected infant survival is unknown.
Objective
To compare survival of extremely preterm infants born during 2004-2007 with survival of infants born during 2014-2016.
Design, Setting and Participants
All births at 22-26 weeks’ gestational age (n = 2205) between April 1, 2004, and March 31, 2007, and between January 1, 2014, and December 31, 2016, in Sweden were studied. Prospective data collection was used during 2004-2007. Data were obtained from the Swedish pregnancy, medical birth, and neonatal quality registries during 2014-2016.
Exposures
Delivery at 22-26 weeks’ gestational age.
Main Outcomes and Measures
The primary outcome was infant survival to the age of 1 year. The secondary outcome was 1-year survival among live-born infants who did not have any major neonatal morbidity (specifically, without intraventricular hemorrhage grade 3-4, cystic periventricular leukomalacia, necrotizing enterocolitis, retinopathy of prematurity stage 3-5, or severe bronchopulmonary dysplasia).
Results
During 2004-2007, 1009 births (3.3/1000 of all births) occurred at 22-26 weeks’ gestational age compared with 1196 births (3.4/1000 of all births) during 2014-2016 (P = .61). One-year survival among live-born infants at 22-26 weeks’ gestational age was significantly lower during 2004-2007 (497 of 705 infants [70%]) than during 2014-2016 (711 of 923 infants [77%]) (difference, −7% [95% CI, −11% to −2.2%], P = .003). One-year survival among live-born infants at 22-26 weeks’ gestational age and without any major neonatal morbidity was significantly lower during 2004-2007 (226 of 705 infants [32%]) than during 2014-2016 (355 of 923 infants [38%]) (difference, −6% [95% CI, −11% to −1.7%], P = .008).
Conclusions and Relevance
Among live births at 22-26 weeks’ gestational age in Sweden, 1-year survival improved between 2004-2007 and 2014-2016.
This population epidemiology study uses Swedish national registry data to compare 1-year survival of extremely preterm infants born at 22-26 weeks’ gestational age during 2004-2007 vs those born during 2014-2016 after the release of a national consensus guideline on perinatal management.
Introduction
Preterm birth is difficult to predict and prevent. Although the long-term health outcomes for preterm infants have improved over time, extremely preterm birth has continued to be an issue in terms of optimal antenatal and postnatal management,1,2,3,4,5 resource allocation and costs,6 quality of care,7 and long-term health outcomes.8,9 Given these uncertainties, and compounded by changes in reproductive epidemiology,10,11 it is important to evaluate international variations and time trends for the management and outcomes of extremely preterm birth.
To create a more equal and evidence-based health care system, new laws, regulations, and recommendations on extremely preterm births have been issued in Sweden. Civil registration of all stillbirths at 22-27 weeks’ gestational age became mandatory12 in 2008 to ensure the existence of vital statistics for all extremely preterm births (not only live born) and to strengthen the legal status of the extremely preterm patient. New regulations on withholding or withdrawing advanced life support for patients were issued in 2011 by Socialstyrelsen (the Swedish national board of health and welfare),13 focusing on the needs for informed consent and improved documentation. The Swedish national board of health and welfare recommended centralization of care for all extremely preterm births at university hospitals in 2014.14 The first national consensus guideline on perinatal management was issued in 2016 by the Swedish society of perinatal medicine15 and provided specific recommendations on centralization of care, antenatal corticosteroid treatment, mode of delivery, a neonatologist attending at the birth, and resuscitation of infants delivered at 22, 23 and 24 weeks’ gestational age.
The significance of these new recommendations and guidelines, as well as knowledge on short- and long-term outcomes among extremely preterm infants, is not known in Sweden. The aim of this study was to compare perinatal practice and survival rates of extremely preterm infants born in 2004-2007 with those born in 2014-2016.
Methods
This study was approved by the Swedish regional ethical review board in Lund during the first study period and by the ethical review board in Stockholm during the second study period. During the first study period, oral informed consent from the parents or caregivers was obtained. During the second study period, all parents were informed (a waiver for consent was granted by the ethical review board) that perinatal data was to be recorded in the Swedish pregnancy and neonatal quality registries, with the possibility to opt out at any time. Data collection on the inhabitants of Sweden (including civil registration of births and deaths) was mandatory during both study periods to the national population registries that are kept by Statistiska centralbyrån (statistics Sweden), the Socialstyrelsen (Swedish national board of health and welfare), and Skatteverket (the Swedish tax agency).
Participants
During the first study period, the mother-infant dyads participated in the Extremely Preterm Infants in Sweden Study (EXPRESS).16,17 The first EXPRESS study was a national, population-based prospective cohort study including all births at 22-26 weeks’ gestational age occurring in Sweden between April 1, 2004, and March 31, 2007. Infants born outside Sweden and transferred to Sweden for neonatal care were excluded (total of 3 infants for both study periods). Termination of pregnancy after gestational week 21 plus 6 days was not permitted unless malformations incompatible with postnatal life (eg, acrania) were diagnosed. Such conditions were considered exclusion criteria a priori.
Using the same criteria as in EXPRESS, all births in Sweden at 22-26 weeks’ gestational age were included in EXPRESS 2 during a second 3-year period between January 1, 2014, and December 31, 2016. The study plan appears in the Supplement. Descriptive information also was collected on live-born infants who were younger than 22 weeks’ gestational age.
Data Sources
During 2004-2007, all obstetric and pediatric departments participated in prospective data collection. At that time, records with missing or obviously erroneous information were returned to the regional study coordinators for completeness or correction (there were 3 coordinators for each region representing obstetrics, neonatology, and ophthalmology). If there were missing data, the coordinators made personal contact and tracking attempts until they concluded the data were unobtainable. Two of the researchers performed internal data control at site visits to all 7 regional centers and found satisfactory agreement between 20 randomly selected medical records per site and the database information. To ensure accuracy, all variables regarding mortality and major morbidity in the database were cross-checked against the original information in the medical records and against the Swedish medical birth registry (SMBR; a national population registry).18
Information on the total number of births in Sweden was obtained from the SMBR during both 2004-2007 and 2014-2016. The number of stillbirths was prospectively collected during 2004-2007, whereas this information was retrieved from the SMBR during 2014-2016. The Swedish neonatal quality (SNQ) registry was used as the main data source for live-born infants during 2014-2016. The SNQ registry collects data on the outcomes for all live-born infants and on neonatal care (including admissions within 28 days after birth). Data were collected on a daily basis from electronic medical records or entered at the time of death or discharge. Data obtained from the SNQ registry for any major neonatal morbidity were validated against medical records for 96% of infants. Diagnoses of retinopathy of prematurity were provided by the Swedish national registry for retinopathy of prematurity.19
The majority of data on the number of live-born infants during 2014-2016 was collected from the SNQ registry. However, because of limited completeness in the SNQ registry, number of births at 22 weeks’ gestational age were obtained from the SMBR. Information on deaths occurring after hospital discharge but before the age of 1 year was obtained from the Swedish cause of death registry because deaths after hospital discharge were not recorded in the SMBR or in the SNQ registry.
Exposures and Covariates
The main exposure during both studies was delivery at 22-26 weeks’ gestational age. Gestational age was recorded in completed weeks and days, and a majority (95% in 2004-2007 and 97% in 2014-2016) of infants during both study periods had gestational age determined by routine antenatal ultrasonography early during the second trimester, or by date of embryo transfer if in vitro fertilization was performed. For the remaining pregnancies, gestational age was based on the date of the last menstrual period, clinical maturity rating, or the dating method was unspecified. Information was collected regarding the presence of preeclampsia, preterm prelabor rupture of membranes, abruption of the placenta or placenta previa, chorioamnionitis, multiple pregnancy, Apgar score of less than 4 measured at 1 and 5 minutes after birth, birth weight, infant sex, small for gestational age (defined as infants with a birth weight >2 SDs below the sex-specific expected weight), and selected perinatal interventions.
Infants with congenital anomalies were included. The following were not classified as anomalies during either study period: luxations of the hip (International Statistical Classification of Diseases and Related Health Problems, Tenth Revision [ICD-10] codes Q65.0-Q65.5), balanic hypospadias (ICD-10 code Q54.0), and patent ductus arteriosus (ICD-10 code Q25.0).
Outcomes
The prespecified primary outcome was survival among live-born infants to the age of 1 year. The secondary outcome was infant survival among live-born infants to the age of 1 year and without any major neonatal morbidity (specifically, without intraventricular hemorrhage grade 3-4,20 cystic periventricular leukomalacia,21 necrotizing enterocolitis,22 retinopathy of prematurity stage 3-5,23 or severe bronchopulmonary dysplasia [defined as treatment with ≥30% oxygen at 36 weeks’ postmenstrual age]).
The other outcomes were stillbirth (defined as without any vital signs at birth), live birth (defined as any breathing, any other movements, or any other signs of life at birth), delivery room death, survival to 1 year among hospital-admitted infants, the number and proportions of infants treated surgically for necrotizing enterocolitis,7,24 the number and proportions of infants treated with a laser for retinopathy of prematurity,25 and any bronchopulmonary dysplasia (defined as treatment with any level of oxygen at 36 weeks’ postmenstrual age).16
End points for active obstetric management included use of any antenatal corticosteroids, cesarean delivery, and delivery in a hospital with a level III neonatal intensive care unit (NICU). The standard protocol for antenatal corticosteroids used during both study periods was 1 course consisting of two 12-mg injections of betamethasone given 12 to 24 hours apart. Rescue or repeat doses were not recommended.
Neonatal activity end points included the neonatologist attending at the birth, intubation at the birth, surfactant administration less than 2 hours after the birth, admission to the NICU, and postnatal transport to a level III NICU when born at another center. The protocols used for data collection and the definitions used for the exposures and the outcomes during the first study period16 also were used during the second study period. Both EXPRESS and EXPRESS 2 were observational in design and no attempts were made to standardize management in the study protocols.
Completeness, Validity, and Missing Data
Using unique personal identification numbers for cross-linkage of EXPRESS data with those of national population registries (the SMBR and the cause of death registry), completeness of data for infants born alive during the first study period was estimated to be greater than 98% at all weeks of gestational age (22-26 weeks).
Completeness of data during the second study period was determined as a ratio with the numerator defined as the number of live-born infants registered in the SNQ registry. The denominator was defined as the numerator plus the remaining number of live-born infants exclusively registered in the SMBR, in the inpatient registry, or in the cause of death registry (all national population registries) during the study period. Completeness of the SNQ registry data during 2014-2016 (as validated against the 3 national population registries: SMBR, inpatient registry, and cause of death registry) was 99% for infants born alive at 24-26 weeks’ gestational age, 92% at 23 weeks’ gestational age, and 70% at 22 weeks’ gestational age. Conversely, completeness of the data from the national population registries validated against the SNQ registry was 95% at 24-26 weeks’ gestational age, 96% at 23 weeks’ gestational age, and 99% at 22 weeks’ gestational age.
Given the prospective data collection and cross-linkage of reported mortality in the cause of death registry, information on 1-year survival was considered as complete and comparable during both study periods. Among infant survivors, there were no missing data on periventricular leukomalacia and necrotizing enterocolitis (any or surgical). There were missing data for 0.3% cases of intraventricular hemorrhage grade 3-4, 1.7% for retinopathy of prematurity stage 3-5, 2.0% for laser-treated retinopathy of prematurity, 3.4% for any bronchopulmonary dysplasia, and 4.8% for severe bronchopulmonary dysplasia. Imputation of missing data was not performed because the study was descriptive (not analytical) by design.
Statistical Methods
The numbers and proportions (%), means (95% CIs), and median (range) values were stratified by weeks of gestational age for both study periods. The 2-tailed Fisher exact test was used to compare rates and proportions between the study periods. Overall survival was determined using Kaplan-Meier survival analysis. Differences (95% CIs) were calculated as rates and proportions for the 2004-2007 cohort minus the rates and proportions for the 2014-2016 cohort.
P < .05 was considered statistically significant. Because of the potential for type I error due to multiple comparisons, findings for additional analyses beyond the predefined primary and secondary end points should be interpreted as exploratory. Interactive statistical pages (http://statpages.info/ctab2x2) and Excel 2013 version (Microsoft) were used for the calculations.
Results
The total number of all births in Sweden was 305 318 during 2004-2007 and 353 888 during 2014-2016. During 2004-2007, 1009 births (3.3/1000 of all births) occurred at 22-26 weeks’ gestational age compared with 1196 births (3.4/1000 of births) during 2014-2016 (P = .61). There was no termination of any pregnancies at 22-26 weeks’ gestational age.
Primary and Secondary Outcomes
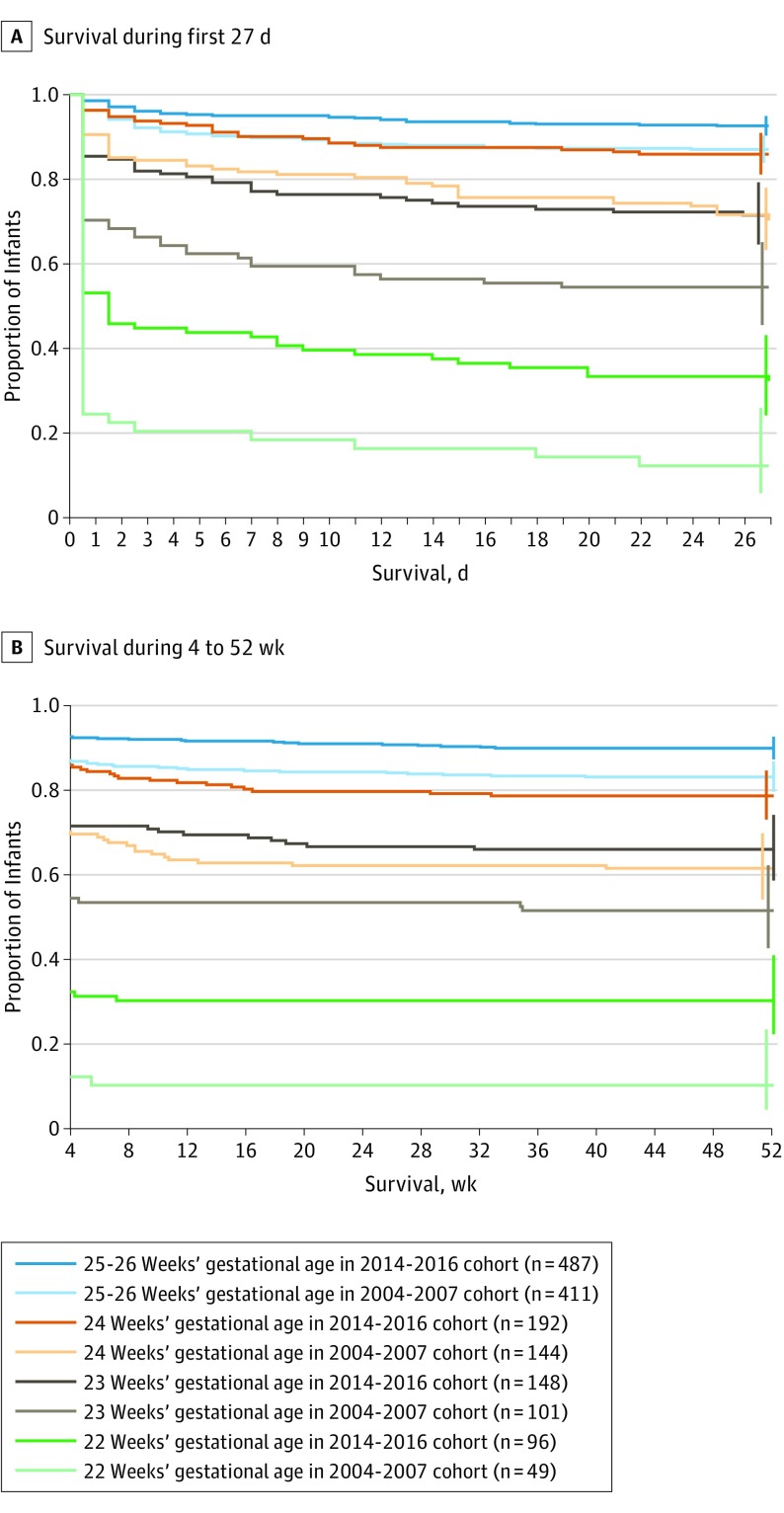
One-year survival among live-born infants at 22-26 weeks’ gestational age was significantly lower during 2004-2007 (497 of 705 infants [70%]) than during 2014-2016 (711 of 923 infants [77%]) (difference, −7% [95% CI, −11% to −2.2%], P = .003). One-year survival among live-born infants at 22-26 weeks’ gestational age without any major neonatal morbidity was significantly lower during 2004-2007 (226 of 705 infants [32%]) than during 2014-2016 (355 of 923 infants [38%]) (difference, −6% [95% CI, −11% to −1.7%], P = .008; Table 1 and the Figure).
Table 1. One-Year Survival Among Infants Delivered at 22-26 Weeks’ Gestational Age During 2004-2007 or 2014-2016 in Sweden.
| 22 Weeks’ Gestational Age | 23 Weeks’ Gestational Age | 24 Weeks’ Gestational Age | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No./Total (%) | Difference, % (95% CI)b |
P Value | No./Total (%) | Difference, % (95% CI)b |
P Value | No./Total (%) | Difference, % (95% CI)b |
P Value | ||||
| 2004-2007a | 2014-2016 | 2004-2007a | 2014-2016 | 2004-2007a | 2014-2016 | |||||||
| All Deliveriesc | ||||||||||||
| Stillbirths | 91/140 (65) |
52/148 (35) |
30 (19 to 41) |
<.001 | 82/183 (45) |
59/207 (29) |
16 (6.8 to 26) |
.001 | 47/191 (25) |
68/260 (26) |
−1 (−9.7 to 6.6) |
.79 |
| Live-born infants | 49/140 (35) |
96/148 (65) |
−30 (−41 to −19) |
<.001 | 101/183 (55) |
148/207 (71) |
−16 (−26 to −6.8) |
.001 | 144/191 (75) |
192/260 (74) |
1 (−6.6 to 9.7) |
.79 |
| 1-y Survival | ||||||||||||
| Infants admitted to NICU | 5/17 (29) |
29/50 (58) |
−29 (−54 to −3.0) |
.08 | 53/81 (65) |
91/138 (66) |
0 (−14 to 13) |
>.99 | 96/132 (73) |
151/191 (79) |
−6 (−16 to 3.2) |
.24 |
| Live-born infantsd | 5/49 (10) |
29/96 (30) |
−20 (−33 to −7.5) |
.01 | 53/101 (52) |
91/148 (61) |
−9 (−22 to 3.5) |
.20 | 96/144 (67) |
151/192 (79) |
−12 (−22 to −2.3) |
.02 |
| All deliveriese | 5/140 (3.6) |
29/148 (20) |
−16 (−23 to −8.9) |
<.001 | 53/183 (29) |
91/207 (44) |
−15 (−24 to −5.6) |
.003 | 96/191 (50) |
151/260 (58) |
−8 (−17 to 1.9) |
.10 |
| 1-y Survival Without Any Major Neonatal Morbidityf | ||||||||||||
| Infants admitted to NICU | 1/5 (20) |
5/29 (17) |
3 (−35 to 40) |
>.99 | 9/53 (17) |
25/91 (28) |
−10 (−24 to 3.2) |
.22 | 30/96 (31) |
60/151 (40) |
−9 (−21 to 3.6) |
.22 |
| Live-born infantsg | 1/49 (2.0) |
5/96 (5.2) |
−3 (−9.1 to 2.8) |
.66 | 9/101 (8.9) |
25/148 (17) |
−8 (−16 to 0.2) |
.09 | 30/144 (21) |
60/192 (31) |
−10 (−20 to −1.1) |
.04 |
| 25 Weeks’ Gestational Age | 26 Weeks’ Gestational Age | 22-26 Weeks’ Gestational Age (All Infants) | ||||||||||
| All Deliveriesc | ||||||||||||
| Stillbirths | 45/250 (18) |
58/277 (21) |
−3 (−9.7 to 3.8) |
.46 | 39/245 (16) |
36/304 (12) |
4 (−1.8 to 9.9) |
.21 | 304/1009 (30) |
273/1196 (23) |
7 (3.6 to 11) |
<.001 |
| Live-born infants | 205/250 (82) |
219/277 (79) |
3 (−3.8 to 9.7) |
.46 | 206/245 (84) |
268/304 (88) |
−4 (−9.9 to 1.8) |
.21 | 705/1009 (70) |
923/1196 (77) |
−7 (−11 to −3.6) |
<.001 |
| 1-y Survival | ||||||||||||
| Infants admitted to NICU | 167/200 (84) |
193/219 (88) |
−5 (−11 to 2.1) |
.22 | 176/204 (86) |
247/267 (93) |
−6 (−12 to −0.5) |
.04 | 497/634 (78) |
711/865 (82) |
−4 (−7.9 to 0.3) |
.07 |
| Live-born infantsd | 167/205 (81) |
193/219 (88) |
−7 (−13 to 0.2) |
.07 | 176/206 (85) |
247/268 (92) |
−7 (−13 to −0.9) |
.03 | 497/705 (70) |
711/923 (77) |
−7 (−11 to −2.2) |
.003 |
| All deliveriese | 167/250 (67) |
193/277 (70) |
−3 (−11 to 5.1) |
.54 | 176/245 (72) |
247/304 (81) |
−9 (−17 to −2.3) |
.01 | 497/1009 (49) |
711/1196 (59) |
−10 (−14 to −6.0) |
<.001 |
| 1-y Survival Without Any Major Neonatal Morbidityf | ||||||||||||
| Infants admitted to NICU | 75/167 (45) |
104/193 (54) |
−9 (−19 to 1.3) |
.09 | 111/176 (63) |
161/247 (65) |
−2 (−11 to 7.2) |
.68 | 226/497 (45) |
355/711 (50) |
−5 (−10 to 1.3) |
.14 |
| Live-born infantsg | 75/205 (37) |
104/219 (47) |
−11 (−20 to −1.6) |
.03 | 111/206 (54) |
161/268 (60) |
−6 (−15 to 2.8) |
.19 | 226/705 (32) |
355/923 (38) |
−6 (−11 to −1.7) |
.008 |
Abbreviation: NICU, neonatal intensive care unit.
Data are from Fellman et al.16
Difference in the prevalence for the 2004-2007 cohort minus the prevalence for the 2014-2016 cohort.
Provided by the Swedish medical birth registry for 22 weeks’ gestational age in 2014-2016. The remaining data for 2014-2016 were from the Swedish neonatal quality registry.
Primary outcome.
Includes stillbirths.
Major neonatal morbidity defined as intraventricular hemorrhage grade 3 or 4; periventricular leukomalacia; necrotizing enterocolitis; retinopathy of prematurity stage 3, 4, or 5; or severe bronchopulmonary dysplasia.
Secondary outcome.
Figure. One-Year Survival Among Infants Delivered at 22-26 Weeks’ Gestational Age in Sweden.
Infants were born between April 1, 2004, and March 31, 2007, in the Extremely Preterm Infants in Sweden Study (EXPRESS) and between January 1, 2014, and December 31, 2016, in EXPRESS 2. The observation time was 1 year for all infants during both studies. The vertical ticks at the ends of the lines are 95% CIs. The lines are staggered to avoid superimposition.
Exploratory Outcomes
Stillbirths and Live Births
The stillbirth rate was significantly higher at 22-26 weeks’ gestational age during 2004-2007 (304 of 1009 births [30%]; 1.0/1000 among all births) than during 2014-2016 (273 of 1196 births [23%]; 0.77/1000 among all births) (difference, 7% [95% CI, 3.6% to 11%], P < .001). Conversely, the proportion of live-born infants was significantly lower during 2004-2007 (705 of 1009 births [70%]; 2.3/1000 among all births) than during 2014-2016 (923 of 1196 births [77%]; 2.6/1000 among all births) (difference, −7% [95% CI, −11% to −3.6%], P < .001; Table 1).
Delivery Room Deaths
The proportion of live-born infants that died before admission to the NICU was significantly higher during 2004-2007 (71 of 705 infants or 10%) than during 2014-2016 (58 of 923 infants or 6.3%) (difference, 4% [95% CI, 1.0%-6.5%], P = .005). During 2004-2007, 32 of 49 (65%) live births at 22 weeks’ gestational age died before admission to the NICU; 20 of 101 (20%) live births at 23 weeks’ gestational age died before admission to the NICU; and 12 of 144 (8.3%) live births at 24 weeks’ gestational age died before admission to the NICU.
During 2014-2016, 46 of 96 births (48%) live births at 22 weeks’ gestational age died before admission to the NICU. Twenty-two of these could only be retrieved from civil registration. For the remaining 24 live births reported to the SNQ registry, 16 infants (67%) were not resuscitated (6 in 2014, 9 in 2015, and 1 in 2016). At 23 weeks’ gestational age, delivery room deaths occurred among 10 of 148 infants (6.8%); 4 of these infants were not resuscitated. Among live-born infants at 24 weeks’ gestational age, 1 of 192 infants (0.5%) died in the delivery room despite resuscitation efforts during 2014-2016.
Survival Among Infants Admitted to the NICU
One-year survival among infants admitted to the NICU was not significantly different during 2004-2007 (497 of 634 infants or 78%) compared with during 2014-2016 (711 of 865 infants or 82%) (difference, −4% [95% CI, −7.9% to 0.3%], P = .07). One-year survival without any major neonatal morbidity among infants admitted to the NICU was not significantly different between the study periods (226/497 [45%] during 2004-2007 vs 355/711 [50%] during 2014-2016; difference, −5% [95% CI, −10% to 1.3%], P = .14; Table 1).
Neonatal Morbidity
Among 1-year infant survivors, the proportions were significantly higher during 2004-2007 than during 2014-2016 for those with cystic periventricular leukomalacia (28/497 [5.6%] vs 12/711 [1.7%], respectively; difference, 4% [95% CI, 1.7% to 5.7%], P < .001), with any degree of bronchopulmonary dysplasia (338/461 [73%] vs 439/706 [62%]; difference, 11% [95% CI, 5.5% to 17%], P < .001), and with severe bronchopulmonary dysplasia (113/452 [25%] vs 95/698 [14%]; difference, 11% [95% CI, 6.6% to 16%], P < .001).
The proportions did not differ significantly between 2004-2007 and 2014-2016 for intraventricular hemorrhage grade 3-4 (50/493 [10%] vs 73/711 [10%], respectively; difference, 0% [95% CI, −3.6% to 3.6%], P > .99), for retinopathy of prematurity stage 3-5 (169/493 [34%] vs 243/694 [35%]; difference −1% [95% CI, −6.3% to 5.0%], P = .80), or for laser-treated retinopathy of prematurity (99/497 [20%] vs 134/687 [20%]; difference 0% [95% CI, −4.3% to 5.2%], P = .88).
The proportion with any necrotizing enterocolitis was significantly lower during 2004-2007 than during 2014-2016 (29/497 [6%] vs 69/711 [10%], respectively; difference −4% [95% CI, −6.7% to −0.6%], P = .02). In contrast, the proportion with surgically treated necrotizing enterocolitis did not differ significantly between 2004-2007 and 2014-2016 (20/497 [4%] vs 40/711 [6%], respectively; difference −2% [95% CI, −3.9% to 1.1%], P = .23; Table 2).
Table 2. Categories of Major Neonatal Morbidity Among 1-Year Survivors Delivered at 22-26 Weeks’ Gestational Age During 2004-2007 or 2014-2016 in Sweden.
| Categories of Major Neonatal Morbidity |
22 Weeks’ Gestational Age | 23 Weeks’ Gestational Age | 24 Weeks’ Gestational Age | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No./Total (%) | Difference, % (95% CI)b |
P Value | No./Total (%) | Difference, % (95% CI)b |
P Value | No./Total (%) | Difference, % (95% CI)b |
P Value | ||||
| 2004-2007a | 2014-2016 | 2004-2007a | 2014-2016 | 2004-2007a | 2014-2016 | |||||||
| Intraventricular hemorrhage grade 3-4 | 1/5 (20) |
4/29 (14) |
6 (−16 to 56) |
>.99 | 10/53 (19) |
11/91 (12) |
7 (−5.9 to 20) |
.33 | 10/96 (10) |
14/151 (9.3) |
1 (−6.4 to 9.5) |
.83 |
| Cystic periventricular leukomalacia | 0/5 | 0/29 | 0 | >.99 | 5/53 (9.4) |
1/91 (1.1) |
8 (0.1 to 11) |
.02 | 6/96 (6.2) |
1/151 (0.7) |
6 (0.4 to 7.2) |
.02 |
| Necrotizing enterocolitis | ||||||||||||
| Any | 0/5 | 4/29 (14) |
−14 (−14 to 37) |
>.99 | 1/53 (1.9) |
9/91 (9.9) |
−8 (−11 to 2.3) |
.09 | 9/96 (9.4) |
15/151 (9.9) |
0 (−7.7 to 7.9) |
>.99 |
| Surgically treated | Unknownc | 2/29 (6.9) |
Unknownc | 8/91 (8.8) |
Unknownc | 9/151 (6.0) |
||||||
| Bronchopulmonary dysplasia | ||||||||||||
| Any degree | 5/5 (100) |
28/29 (97) |
3 (−19 to 3) |
>.99 | 45/51 (88) |
72/91 (79) |
9 (−5.8 to 20) |
.25 | 71/88 (81) |
105/151 (70) |
9 (−1.4 to 22) |
.07 |
| Severe | 2/5 (40) |
10/29 (34) |
6 (−33 to 54) |
>.99 | 13/49 (26) |
21/88 (24) |
3 (−13 to 20) |
.84 | 27/87 (31) |
21/150 (14) |
17 (5.3 to 28) |
.002 |
| Retinopathy of prematurity | ||||||||||||
| Stage 3-5 | 4/5 (80) |
20/28 (71) |
9 (−47 to 31) |
>.99 | 33/53 (62) |
51/90 (57) |
6 (−12 to 23) |
.49 | 45/94 (48) |
74/149 (50) |
−2 (−15 to 12) |
.79 |
| Treated with laserd | 4/5 (80) |
13/28 (46) |
34 (−23 to 56) |
.34 | 23/53 (43) |
30/89 (34) |
10 (−8 to 27) |
.28 | 31/96 (32) |
45/149 (30) |
2 (−10 to 15) |
.78 |
| 25 Weeks’ Gestational Age | 26 Weeks’ Gestational Age | 22-26 Weeks’ Gestational Age (All Infants) | ||||||||||
| Intraventricular hemorrhage grade 3-4 | 20/166 (12) |
28/193 (15) |
−2 (−9.5 to 5.1) |
.54 | 9/173 (5.2) |
16/247 (6.5) |
−1 (−5.5 to 3.8) |
.68 | 50/493 (10) |
73/711 (10) |
0 (−3.6 to 3.6) |
>.99 |
| Cystic periventricular leukomalacia | 9/167 (5.4) |
5/193 (2.6) |
3 (−1.6 to 6.2) |
.18 | 8/176 (4.5) |
5/247 (2.0) |
2 (−1.1 to 5.5) |
.16 | 28/497 (5.6) |
12/711 (1.7) |
4 (1.7 to 5.7) |
<.001 |
| Necrotizing enterocolitis | ||||||||||||
| Any | 10/167 (6.0) |
22/193 (11) |
−5 (−11 to 1.1) |
.09 | 9/176 (5.1) |
19/247 (7.7) |
−3 (−6.8 to 2.8) |
.33 | 29/497 (5.8) |
69/711 (9.7) |
−4 (−6.7 to −0.6) |
.02 |
| Surgically treated | Unknownc | 12/193 (6.2) |
Unknownc | 9/247 (3.6) |
20/497 (4.0)e |
40/711 (5.6) |
−2 (−3.9 to 1.1) |
.23 | ||||
| Bronchopulmonary dysplasia | ||||||||||||
| Any degree | 122/162 (75) |
115/191 (60) |
15 (4.7 to 25) |
.003 | 95/155 (61) |
119/244 (49) |
12 (2.0 to 23) |
.02 | 338/461 (73) |
439/706 (62) |
11 (5.5 to 17) |
<.001 |
| Severe | 45/153 (29) |
24/189 (12) |
17 (7.6 to 25) |
<.001 | 26/158 (16) |
19/242 (7.9) |
8 (1.7 to 15) |
.009 | 113/452 (25) |
95/698 (14) |
11 (6.6 to 16) |
<.001 |
| Retinopathy of prematurity | ||||||||||||
| Stage 3-5 | 54/167 (32) |
48/191 (25) |
7 (−2.7 to 17) |
.16 | 33/174 (19) |
50/236 (21) |
−2 (−10 to 6.2) |
.62 | 169/493 (34) |
243/694 (35) |
−1 (−6.3 to 5.0) |
.80 |
| Treated with laserd | 28/167 (17) |
24/190 (13) |
4 (−3.7 to 12) |
.30 | 13/176 (7.4) |
22/231 (9.5) |
−2 (−7.3 to 3.9) |
.48 | 99/497 (20) |
134/687 (20) |
0 (−4.3 to 5.2) |
.88 |
Management of Perinatal Care
The proportion of live-born infants at 22-26 weeks’ gestational age who were exposed to antenatal steroids did not differ significantly between 2004-2007 and 2014-2016. The proportion of live-born infants who were cesarean deliveries, delivered in a university hospital with a level III NICU, intubated at birth, and admitted to the NICU were significantly lower during 2004-2007 than during 2014-2016.
The proportion of deliveries with an attending neonatologist did not differ significantly between the study periods. However, the proportion of infants born outside university hospitals and transported to a level III NICU after birth was significantly higher during 2004-2007 than during 2014-2016. Surfactant administration within 2 hours after birth was significantly more common during 2004-2007 than during 2014-2016 (Table 3 and Table 4).
Table 3. Exploratory Analysis of Live-Born Infants at 22, 23, or 24 Weeks’ Gestational Age During 2004-2007 or 2014-2016 in Swedena.
| 22 Weeks’ Gestational Ageb | 23 Weeks’ Gestational Age | 24 Weeks’ Gestational Age | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. With Event/Total (%) | Difference, % (95% CI)d |
P Value | No. With Event/Total (%) | Difference, % (95% CI)d |
P Value | No. With Event/Total (%) | Difference, % (95% CI)d |
P Value | ||||
| 2004-2007c | 2014-2016 | 2004-2007c | 2014-2016 | 2004-2007c | 2014-2016 | |||||||
| Infant Characteristics | ||||||||||||
| Male sex | 25/49 (51) |
39/74 (53) |
−2 (−20 to 16) |
>.99 | 55/101 (55) |
82/148 (55) |
−1 (−14 to 12) |
.90 | 78/144 (54) |
119/192 (62) |
−8 (−18 to 2.8) |
.18 |
| Female sex | 24/49 (49) |
35/74 (47) |
2 (−16 to 20) |
>.99 | 46/101 (45) |
66/148 (45) |
1 (−12 to 14) |
.90 | 66/144 (46) |
73/192 (38) |
8 (−2.8 to 18) |
.18 |
| Singleton | 29/49 (59) |
54/74 (73) |
−14 (−31 to 3.3) |
.16 | 85/101 (84) |
115/148 (78) |
6 (−3.3 to 16) |
.26 | 112/144 (78) |
165/192 (86) |
−8 (−17 to 0.7) |
.06 |
| Twin or triplet | 20/49 (41) |
20/74 (27) |
14 (−3.3 to 31) |
.16 | 16/101 (16) |
33/148 (22) |
−6 (−16 to 3.3) |
.26 | 32/144 (22) |
27/192 (14) |
8 (−0.7 to 17) |
.06 |
| Apgar score <4e | ||||||||||||
| At 1 min after birth | 34/49 (69) |
49/73 (67) |
2 (−15 to 19) |
.94 | 53/101 (53) |
77/145 (53) |
−0.6 (−13 to 12) |
>.99 | 63/144 (44) |
76/188 (40) |
3 (−7.4 to 14) |
.58 |
| At 5 min after birth | 38/49 (71) |
35/71 (49) |
28 (12 to 44) |
.003 | 47/101 (47) |
42/146 (29) |
18 (5.6 to 30) |
.005 | 47/144 (33) |
44/188 (23) |
9 (−0.5 to 19) |
.06 |
| Small for gestational agef | 3/49 (6) |
4/73 (5.5) |
0.6 (−7.9 to 9.1) |
>.99 | 7/100 (7.0) |
14/146 (9.6) |
−3 (−0.5 to 4.3) |
.64 | 16/142 (11) |
31/187 (17) |
−5 (−13 to 2.1) |
.20 |
| Birth weight, median (range), g | 519 (290 to 730) |
489 (345 to 670) |
24 (−195 to 310) |
.12 | 590 (320 to 808) |
592 (300 to 1615) |
−1 (−295 to 270) |
.82 | 674 (374 to 1070) |
680 (380 to 930) |
−5 (−300 to 363) |
.46 |
| Obstetric Care Management | ||||||||||||
| Any antenatal steroids | 19/47 (40) |
47/73 (64) |
−24 (−42 to −6.1) |
.02 | 85/99 (86) |
132/144 (92) |
−6 (−14 to 2.4) |
.20 | 130/137 (95) |
169/187 (90) |
5 (−1.1 to 10) |
.15 |
| Cesarean delivery | 2/49 (4.1) |
4/74 (5.4) |
1 (−8.9 to 6.2) |
>.99 | 17/101 (17) |
50/148 (34) |
−17 (−28 to −6.4) |
.003 | 67/144 (46) |
110/191 (58) |
−11 (−22 to −0.3) |
.05 |
| Delivery at hospital with level III NICU | 22/49 (45) |
66/74 (89) |
−44 (−60 to −29) |
<.001 | 79/101 (78) |
127/148 (86) |
−8 (−17 to 2.2) |
.13 | 129/144 (90) |
162/192 (84) |
5 (−2.0 to 12) |
.20 |
| Delivery at hospital with level I-II NICU | 27/49 (55) |
8/74 (11) |
44 (29 to 60) |
<.001 | 22/101 (22) |
21/148 (14) |
8 (−2.7 to 18) |
.13 | 15/144 (10) |
30/192 (16) |
−5 (−12 to 2.0) |
.20 |
| Neonatal Care Management | ||||||||||||
| Neonatologist attending the birth | 22/48 (46) |
66/73 (90) |
−45 (−60 to −29) |
<.001 | 83/100 (83) |
132/147 (90) |
−7 (−16 to 2.0) |
.13 | 130/142 (92) |
168/192 (88) |
4 (−2.5 to 11) |
.29 |
| Intubation at birth | 12/21 (57) |
52/74 (70) |
−13 (−37 to 10) |
.38 | 68/84 (81) |
129/148 (87) |
−6 (−16 to 3.8) |
.25 | 113/142 (80) |
160/192 (83) |
−4 (−12 to 4.7) |
.39 |
| Surfactant administration <2 h after birth | 13/42 (31) |
50/74 (68) |
−37 (−54 to −19) |
<.001 | 72/95 (76) |
116/140 (83) |
−7 (−18 to 3.6) |
.19 | 120/140 (86) |
150/185 (81) |
5 (−3.5 to 13) |
.30 |
| Admission to NICUg | 18/48 (38) |
50/74 (68) |
−30 (−47 to −13) |
<.001 | 81/100 (81) |
138/148 (93) |
−12 (−21 to −3.6) |
.004 | 132/142 (93) |
191/192 (99) |
−7 (−11 to −2.2) |
.001 |
| Transportation of infant to level III NICU | 6/49 (12) |
6/74 (8.1) |
4 (−7.0 to 15) |
.65 | 13/101 (13) |
16/148 (11) |
2 (−6.2 to 10) |
.62 | 15/144 (10) |
24/192 (13) |
−2 (−8.9 to 4.8) |
.56 |
Abbreviation: NICU, neonatal intensive care unit.
Data for live-born infants at 25 or 26 weeks’ gestational age appear in Table 4.
Data for 22 infants identified in the Swedish medical birth registry but who were dead before admission were excluded from the data presented in the majority of rows.
Data are from Fellman et al.16
Difference in the prevalence for the 2004-2007 cohort minus the prevalence for the 2014-2016 cohort.
A score of 10 represents the best possible condition.
Some birth weights were missing because infants were not weighed until after birth.
Data for the 22 infants were included in this row (identified in the Swedish medical birth registry but who were dead before admission).
Table 4. Exploratory Analysis of Live-Born Infants at 25 or 26 Weeks’ Gestational Age and Summary Data for 22-26’ Weeks Gestational Age (All Infants) During 2004-2007 or 2014-2016 in Sweden.
| 25 Weeks’ Gestational Age | 26 Weeks’ Gestational Age | 22-26 Weeks’ Gestational Age (All Infants) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. With Event/Total (%) | Difference, % (95% CI)b |
P Value | No. With Event/Total (%) | Difference, % (95% CI)b |
P Value | No. With Event/Total (%) | Difference, % (95% CI)b |
P Value | ||||
| 2004-2007a | 2014-2016 | 2004-2007a | 2014-2016 | 2004-2007a | 2014-2016 | |||||||
| Infant Characteristics | ||||||||||||
| Male sex | 120/205 (59) |
123/219 (56) |
2 (−7.0 to 12) |
.63 | 108/206 (52) |
146/268 (54) |
−2 (−11 to 7.0) |
.71 | 386/705 (55) |
509/901 (56) |
−1 (−6.6 to 3.2) |
.52 |
| Female sex | 85/205 (41) |
96/219 (44) |
−2 (−12 to 7.0) |
.63 | 98/206 (48) |
122/268 (46) |
2 (−7.0 to 11) |
.71 | 319/705 (45) |
392/901 (44) |
1 (−3.1 to 6.6) |
.52 |
| Singleton | 167/205 (82) |
162/219 (74) |
7 (−0.4 to 15) |
.08 | 155/206 (75) |
193/268 (72) |
3 (−4.8 to 11) |
.46 | 548/705 (78) |
689/901 (76) |
2 (−2.9 to 5.4) |
.59 |
| Twin or triplet | 38/205 (18) |
57/219 (26) |
−7 (−15 to 0.4) |
.08 | 51/206 (25) |
75/268 (28) |
−3 (−11 to 4.8) |
.46 | 157/705 (22) |
212/901 (24) |
−2 (−5.4 to 2.9) |
.59 |
| Apgar score <4c | ||||||||||||
| At 1 min after birth | 59/205 (29) |
61/211 (29) |
0 (−8.8 to 8.6) |
.98 | 34/206 (17) |
79/263 (30) |
−14 (−21 to −6.0) |
.001 | 243/705 (34) |
342/880 (39) |
−4 (−9.2 to 0.4) |
.08 |
| At 5 min after birth | 42/205 (21) |
28/215 (13) |
8 (0.3 to 15) |
.05 | 27/206 (13) |
27/264 (10) |
3 (−3.0 to 8.8) |
.38 | 201/705 (29) |
176/884 (20) |
9 (4.4 to 13) |
<.001 |
| Small for gestational aged | 39/205 (19) |
50/217 (23) |
−4 (−12 to 3.8) |
.34 | 48/206 (23) |
61/263 (23) |
0 (−7.6 to 7.8) |
>.99 | 113/701 (16) |
160/886 (18) |
−2 (−5.7 to 1.8) |
.34 |
| Birth weight, median (range), g | 784 (266 to 1235) |
777 (340 to 1220) |
17 (−397 to 415) |
.20 | 920 (430 to 1500) |
887 (450 to 1265) |
26 (−445 to 474) |
.07 | 730 (266 to 1500) |
719 (30 to 1615) |
22 (−470 to 538) |
.03 |
| Obstetric Care Management | ||||||||||||
| Any antenatal steroids | 176/198 (89) |
204/215 (95) |
−6 (−11 to −0.7) |
.03 | 180/193 (93) |
233/248 (94) |
−1 (−5.3 to 3.9) |
.85 | 590/674 (88) |
785/867 (91) |
−3 (−6.2 to 0.2) |
.07 |
| Cesarean delivery | 128/205 (62) |
144/218 (66) |
−4 (−13 to 5.5) |
.48 | 141/206 (68) |
189/265 (71) |
−3 (−11 to 5.5) |
.54 | 355/705 (50) |
497/896 (55) |
−5 (−10 to −0.2) |
.047 |
| Delivery at hospital with level III NICU | 166/205 (81) |
199/219 (91) |
−10 (−16 to −3.3) |
.005 | 160/206 (78) |
235/268 (88) |
−10 (−17 to −3.1) |
.004 | 556/705 (79) |
789/901 (88) |
−9 (−12 to −5.0) |
<.001 |
| Delivery at hospital with level I-II NICU | 39/205 (19) |
20/219 (9.1) |
10 (3.3 to 16) |
.005 | 46/206 (22) |
33/268 (12) |
10 (3.1 to 17) |
.004 | 117/705 (17) |
112/901 (12) |
4 (0.6 to 7.7) |
.02 |
| Neonatal Care Management | ||||||||||||
| Neonatologist attending the birth | 180/205 (88) |
186/218 (85) |
3 (−4.0 to 9.0) |
.48 | 170/206 (83) |
201/264 (76) |
7 (−0.9 to 14) |
.11 | 585/701 (83) |
753/894 (84) |
−1 (−4.4 to 2.9) |
.73 |
| Intubation at birth | 126/202 (62) |
147/218 (67) |
−5 (−14 to 4.1) |
.31 | 80/206 (39) |
130/266 (49) |
−10 (−19 to −1.0) |
.03 | 399/655 (61) |
618/898 (69) |
−8 (−13 to −3.1) |
<.001 |
| Surfactant administration <2 h after birth | 153/203 (75) |
141/213 (66) |
9 (−0.5 to 18) |
.07 | 138/205 (67) |
123/257 (48) |
19 (11 to 28) |
<.001 | 495/684 (72) |
580/869 (67) |
6 (1.0 to 10) |
.02 |
| Admission to NICU | 200/205 (98) |
219/219 (100) |
−2 (−4.5 to −0.3) |
.03 | 206/206 (100) |
267/268 (99) |
0 (−0.4 to 1.1) |
>.99 | 637/701 (91) |
865/923 (94)e |
−3 (−5.5 to −0.2) |
.04 |
| Transportation of infant to level III NICU | 37/200 (19) |
19/219 (8.7) |
10 (3.3 to 16) |
.004 | 46/206 (22) |
21/268 (7.8) |
14 (8.0 to 21) |
<.001 | 117/705 (17) |
86/901 (9.5) |
7 (3.7 to 10) |
<.001 |
Abbreviation: NICU, neonatal intensive care unit.
Data are from Fellman et al.16
Difference in the prevalence for the 2004-2007 cohort minus the prevalence for the 2014-2016 cohort.
A score of 10 represents the best possible condition.
Some birth weights were missing because infants were not weighed until after birth.
Data for 22 infants identified in the Swedish medical birth registry but who were dead before admission were included in the denominator.
Perinatal Characteristics
Among live-born infants, those exposed to maternal preeclampsia during pregnancy did not differ significantly between the 2 periods (85 of 705 infants [12%] during 2004-2007 vs 84 of 899 infants [9.3%] during 2014-2016; difference, 3% [95% CI, −0.6% to 5.8%], P = .10). Similarly there was no significant difference for preterm prelabor rupture of membranes during 2004-2007 compared with during 2014-2016 (107/705 [15%] vs 157/899 [17%], respectively; difference, −2% [95% CI, −6.0% to 1.5%], P = .22).
Among live-born infants exposed to chorioamnionitis during fetal life, the proportion was higher during 2004-2007 than during 2014-2016 (114/705 [16%] vs 90/899 [10%], respectively; difference, 6% [95% CI, 2.7% to 9.5%], P < .001) and was significantly lower for abruption of the placenta or bleeding (96/705 [14%] vs 176/899 [20%]; difference, −6% [95% CI, −9.6% to −2.2%], P = .002).
Congenital anomalies were reported in 85 of 705 live-born infants (12%) during 2004-2007 compared with 129 of 910 live-born infants (14%) during 2014-2016 (difference, −2% [95% CI, −5.4% to 1.4%], P = .20). Among live-born infants, the mean gestational age was higher at 25.0 weeks (95% CI, 24.9-25.1 weeks) during 2004-2007 compared with the mean gestational age of 24.4 weeks (95% CI, 24.3-24.5 weeks) during 2014-2016 (P < .001).
Distributions did not differ significantly between the study periods for multiple pregnancies, sex, small for gestational age, and Apgar score less than 4 at 1 minute after birth. The proportion of live-born infants with Apgar scores less than 4 at 5 minutes after birth was significantly higher during 2004-2007 (201 of 705 infants or 29%) compared with during 2014-2016 (176 of 884 infants or 20%) (difference, 9% [95% CI, 4.4%-13%], P < .001; Table 4).
Live Births Before 22 Weeks’ Gestational Age
Births before 22 weeks’ gestational age were not included in the civil registrations. During 2004-2007, 2 live-born infants were reported with a gestational age of 21 weeks. Both were admitted for care but died within hours. During 2014-2016, 9 infants were reported to the SNQ registry as live-born infants at 21 weeks’ gestational age, all of whom were dated by antenatal ultrasound. All except 2 (twins delivered at home who were both alive at hospital arrival) were delivered at the same hospital (2 at 21 weeks’ gestational age plus 5 days and 5 infants at 21 weeks’ gestational age plus 6 days). The birth weights varied between 349 and 500 g. All 9 infants were resuscitated and admitted to the NICU. Two survived to the age of 1 year and 7 died after 3 to 12 days in the NICU.
Discussion
One-year survival among infants born at 22-26 weeks’ gestational age in Sweden increased between 2004-2007 and 2014-2016, as well as 1-year survival without any major neonatal morbidity. The findings may be the result of Swedish recommendations for more active management of perinatal care that were issued between the study periods.
The hospital discharge survival rate among live-born infants at 22-26 weeks’ gestational age was 51% in the United Kingdom26 in 2006 and 52% in France27 in 2011. In the United States, neonatal survival (during the first 28 days) among singleton live births at 20-27 weeks’ gestational age without malformations increased from 37% in 2006 to 50% in 2013.28 In parallel, rates of stillbirths increased.29 The present study showed a survival rate of 77% among all live-born infants at 22-26 weeks’ gestational age in 2014-2016, and the stillbirth rate was found to be significantly lower than the rate reported in the rest of Europe.30
Systematic survival among infants at 22 weeks’ gestational age has not been restricted to Swedish hospitals. For example, the Japanese Neonatal Network (including tertiary centers only) reported a survival rate of 36% among live-born infants at 22 weeks’ gestational age during 2003-2005.31 Researchers at the University of Iowa Hospitals and Clinics reported a survival rate of 33% among infants born without any major congenital anomalies at 22 weeks’ gestational age during 2000-2009,32 and the University of Cologne (in Germany) reported a survival rate of 67% to hospital discharge among infants born at 22-23 weeks’ gestational age receiving active care (instead of only comfort care) during 2010-2014.33 At 24 US hospitals, the survival rate was 23% among infants born at 22 weeks’ gestational age if active care was offered.1 A study of US tertiary centers reported a survival rate of 28% at 22 weeks’ gestation when infants were resuscitated, which increased to 38% in the subgroup that also received antenatal corticosteroids.34
Recommendations on the management of extremely preterm births vary and international consensus is lacking. Most guidelines still advocate comfort care at 22 weeks’ gestational age and active care at 25 weeks’ gestational age, whereas there is a wide variation in recommendations regarding deliveries at 23 and 24 weeks’ gestational age.2 The diversity in the recommendations is likely to contribute to variability in survival rates. However, even among infants admitted with the intention to treat, survival rates can vary markedly.35
The perinatal interventions studied herein should primarily be looked upon as markers of active management. Some of them (eg, antenatal corticosteroids and centralization of care) rest on a solid base of evidence,7 whereas others (eg, cesarean delivery and intubation at birth) were chosen to capture the intention to treat among physicians rather than as markers of evidence-based care.
Among live-born infants, a larger proportion of survivors was free of major neonatal morbidities during 2014-2016 than during 2004-2007 with necrotizing enterocolitis as the 1 important exception. Diagnosing necrotizing enterocolitis can be difficult and the rates reported in EXPRESS16,17 were recently described as overestimated when validated against medical records and radiographic images.24 Even if this validation had some limitations because it was performed 10 years after the end of the study by 1 resident and 1 neonatologist but no radiologist, it indicated a potential problem comparing necrotizing enterocolitis rates during the study periods. The percentage of verified cases of necrotizing enterocolitis during the first study period was 80% among those who had been surgically treated. To increase comparability of necrotizing enterocolitis data between the study periods, a comparison limited to surgical rates for necrotizing enterocolitis was added that demonstrated no statistically significant difference between study periods.
The most important strength of this study was the national, population-based design and the inclusion of stillbirths and live-born infants. Ultrasonographic dating of gestational age was used in 95% to 97% of pregnancies. To avoid a biased comparison of infant survival and morbidity, the study was designed with equivalent definitions of end points and cross-linkage of data between different sources of information that ensured high data completeness and high validity for survival during both study periods.
Information on neonatal morbidity was validated against medical records. Survival (both total survival and survival without any major neonatal morbidity) up to the age of 1 year could be provided by each gestational week. For both periods, reports on the use of perinatal interventions provided valuable information on the willingness of obstetricians and neonatologists to actively intervene. The proportion of missing data was low during both study periods. Both cohorts fulfilled international recommendations on reporting outcomes for extremely preterm births.36
Limitations
This study has several limitations. First, although data collection was prospective, EXPRESS 2 had a retrospective design. Second, stillbirths occurring before admission to the hospital could not be differentiated from those occurring intrapartum during the second study period, and fetal losses before 22 weeks’ gestational age were not accounted for during either study period.
Third, information on the causes of fetal and infant deaths was lacking. During the second study period, there was no information on pregnancy complications for women delivering stillborn infants. Fourth, this was an observational study and it cannot be excluded that the association between study period and infant survival resulted from other risk factors than changes in active perinatal care, and unmeasured or unknown confounding may have occurred.
Fifth, single families may have opted-out from registration in the SNQ registry. The SNQ registry was not allowed to independently register these families. However, data collection to the national population registries on the number of births and survival was mandatory, excluding opt-outs from being a limitation for data completeness and survival.
Sixth, some sample sizes were small, especially in the youngest gestational age group for the earlier cohort. Seventh, long-term outcome data for infant survivors is still lacking for the second study period. EXPRESS suggested that active obstetric and neonatal care management could reduce stillbirth and infant mortality without increasing later neurodevelopmental disability.5 Nevertheless, 34% of the survivors showed moderate to severe disability at the age of 6 years.37 Recent research indicates that effects on a child’s neurodevelopment associated with very preterm birth could persist up to adulthood,38 and that no improvement in this relationship has been observed during the last 1 or 2 decades.8,39
Conclusions
Among live births at 22-26 weeks’ gestational age in Sweden, 1-year survival improved between 2004-2007 and 2014-2016.
Study plan
References
- 1.Rysavy MA, Li L, Bell EF, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network . Between-hospital variation in treatment and outcomes in extremely preterm infants. N Engl J Med. 2015;372(19):1801-1811. doi: 10.1056/NEJMoa1410689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guillén Ú, Weiss EM, Munson D, et al. Guidelines for the management of extremely premature deliveries: a systematic review. Pediatrics. 2015;136(2):343-350. doi: 10.1542/peds.2015-0542 [DOI] [PubMed] [Google Scholar]
- 3.Janvier A, Baardsnes J, Hebert M, Newell S, Marlow N. Variation of practice and poor outcomes for extremely low gestation births: ordained before birth? Arch Dis Child Fetal Neonatal Ed. 2017;102(6):F470-F471. doi: 10.1136/archdischild-2017-313332 [DOI] [PubMed] [Google Scholar]
- 4.Backes CH, Söderström F, Ågren J, et al. Outcomes following a comprehensive versus a selective approach for infants born at 22 weeks of gestation. J Perinatol. 2019;39(1):39-47. doi: 10.1038/s41372-018-0248-y [DOI] [PubMed] [Google Scholar]
- 5.Serenius F, Blennow M, Maršál K, Sjörs G, Källen K; EXPRESS Study Group . Intensity of perinatal care for extremely preterm infants: outcomes at 2.5 years. Pediatrics. 2015;135(5):e1163-e1172. doi: 10.1542/peds.2014-2988 [DOI] [PubMed] [Google Scholar]
- 6.Petrou S, Abangma G, Johnson S, Wolke D, Marlow N. Costs and health utilities associated with extremely preterm birth: evidence from the EPICure study. Value Health. 2009;12(8):1124-1134. doi: 10.1111/j.1524-4733.2009.00580.x [DOI] [PubMed] [Google Scholar]
- 7.Zeitlin J, Manktelow BN, Piedvache A, et al. ; EPICE Research Group . Use of evidence based practices to improve survival without severe morbidity for very preterm infants: results from the EPICE population based cohort. BMJ. 2016;354:i2976. doi: 10.1136/bmj.i2976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Twilhaar ES, Wade RM, de Kieviet JF, van Goudoever JB, van Elburg RM, Oosterlaan J. Cognitive outcomes of children born extremely or very preterm since the 1990s and associated risk factors: a meta-analysis and meta-regression. JAMA Pediatr. 2018;172(4):361-367. doi: 10.1001/jamapediatrics.2017.5323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brydges CR, Landes JK, Reid CL, Campbell C, French N, Anderson M. Cognitive outcomes in children and adolescents born very preterm: a meta-analysis. Dev Med Child Neurol. 2018;60(5):452-468. doi: 10.1111/dmcn.13685 [DOI] [PubMed] [Google Scholar]
- 10.Cnattingius S, Villamor E, Johansson S, et al. Maternal obesity and risk of preterm delivery. JAMA. 2013;309(22):2362-2370. doi: 10.1001/jama.2013.6295 [DOI] [PubMed] [Google Scholar]
- 11.Waldenström U, Cnattingius S, Vixner L, Norman M. Advanced maternal age increases the risk of very preterm birth, irrespective of parity: a population-based register study. BJOG. 2017;124(8):1235-1244. doi: 10.1111/1471-0528.14368 [DOI] [PubMed] [Google Scholar]
- 12.Swedish Ministry of Justice SFS 2008:207: lag om ändring i folkbokföringslagen [law on changes in the law on civil registration SFS 1991:481]. https://www.lagboken.se/views/pages/getfile.ashx?portalId=56&docId=181769&propId=5. Accessed February 4, 2019.
- 13.National Board of Health and Welfare of Sweden SOSFS 2011:7 (M): socialstyrelsens föreskrifter och allmänna råd om livsuppehållande behandling [regulations and recommendations on life supporting treatment]. https://www.socialstyrelsen.se/Lists/Artikelkatalog/Attachments/18374/2011-6-26.pdf. Accessed February 4 2019.
- 14.National Board of Health and Welfare of Sweden Vård av extremt för tidigt födda barn [care of extremely preterm infants]. Stockholm, Sweden: National Board of Health and Welfare of Sweden; 2014. [Google Scholar]
- 15.Domellöf M, Pettersson K. Riktlinjer vid hotande förtidsbörd ska ge bättre och mer jämlik vård - Konsensusdokument för enhetligt omhändertagande av gravida och extremt för tidigt födda barn [in Swedish]. Lakartidningen. 2017;114:114. [PubMed] [Google Scholar]
- 16.Fellman V, Hellström-Westas L, Norman M, et al. ; EXPRESS Group . One-year survival of extremely preterm infants after active perinatal care in Sweden. JAMA. 2009;301(21):2225-2233. doi: 10.1001/jama.2009.771 [DOI] [PubMed] [Google Scholar]
- 17.Austeng D, Blennow M, Ewald U, et al. ; EXPRESS Group . Incidence of and risk factors for neonatal morbidity after active perinatal care: Extremely Preterm Infants Study in Sweden (EXPRESS). Acta Paediatr. 2010;99(7):978-992. doi: 10.1111/j.1651-2227.2010.01846.x [DOI] [PubMed] [Google Scholar]
- 18.Cnattingius S, Ericson A, Gunnarskog J, Källén B. A quality study of a medical birth registry. Scand J Soc Med. 1990;18(2):143-148. doi: 10.1177/140349489001800209 [DOI] [PubMed] [Google Scholar]
- 19.Holmström GE, Hellström A, Jakobsson PG, Lundgren P, Tornqvist K, Wallin A. Swedish national register for retinopathy of prematurity (SWEDROP) and the evaluation of screening in Sweden. Arch Ophthalmol. 2012;130(11):1418-1424. doi: 10.1001/archophthalmol.2012.2357 [DOI] [PubMed] [Google Scholar]
- 20.Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92(4):529-534. doi: 10.1016/S0022-3476(78)80282-0 [DOI] [PubMed] [Google Scholar]
- 21.de Vries LS, Eken P, Dubowitz LM. The spectrum of leukomalacia using cranial ultrasound. Behav Brain Res. 1992;49(1):1-6. doi: 10.1016/S0166-4328(05)80189-5 [DOI] [PubMed] [Google Scholar]
- 22.Bell MJ, Ternberg JL, Feigin RD, et al. Neonatal necrotizing enterocolitis: therapeutic decisions based upon clinical staging. Ann Surg. 1978;187(1):1-7. doi: 10.1097/00000658-197801000-00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.International Committee for the Classification of Retinopathy of Prematurity The international classification of retinopathy of prematurity revisited. Arch Ophthalmol. 2005;123(7):991-999. doi: 10.1001/archopht.123.7.991 [DOI] [PubMed] [Google Scholar]
- 24.Challis P, Larsson L, Stoltz Sjöström E, Serenius F, Domellöf M, Elfvin A. Validation of the diagnosis of necrotising enterocolitis in a Swedish population-based observational study. Acta Paediatr. 2018. doi: 10.1111/apa.14585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Austeng D, Källen KB, Ewald UW, Wallin A, Holmström GE. Treatment for retinopathy of prematurity in infants born before 27 weeks of gestation in Sweden. Br J Ophthalmol. 2010;94(9):1136-1139. doi: 10.1136/bjo.2009.170704 [DOI] [PubMed] [Google Scholar]
- 26.Costeloe KL, Hennessy EM, Haider S, Stacey F, Marlow N, Draper ES. Short term outcomes after extreme preterm birth in England: comparison of two birth cohorts in 1995 and 2006 (the EPICure studies). BMJ. 2012;345:e7976. doi: 10.1136/bmj.e7976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ancel PY, Goffinet F, Kuhn P, et al. ; EPIPAGE-2 Writing Group . Survival and morbidity of preterm children born at 22 through 34 weeks’ gestation in France in 2011: results of the EPIPAGE-2 cohort study [published correction appears in JAMA Pediatr. 2015;169(4):323]. JAMA Pediatr. 2015;169(3):230-238. doi: 10.1001/jamapediatrics.2014.3351 [DOI] [PubMed] [Google Scholar]
- 28.Ananth CV, Friedman AM, Goldenberg RL, Wright JD, Vintzileos AM. Association between temporal changes in neonatal mortality and spontaneous and clinician-initiated deliveries in the United States, 2006-2013. JAMA Pediatr. 2018;172(10):949-957. doi: 10.1001/jamapediatrics.2018.1792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ananth CV, Goldenberg RL, Friedman AM, Vintzileos AM. Association of temporal changes in gestational age with perinatal mortality in the United States, 2007-2015. JAMA Pediatr. 2018;172(7):627-634. doi: 10.1001/jamapediatrics.2018.0249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith LK, Hindori-Mohangoo AD, Delnord M, et al. ; Euro-Peristat Scientific Committee . Quantifying the burden of stillbirths before 28 weeks of completed gestational age in high-income countries: a population-based study of 19 European countries. Lancet. 2018;392(10158):1639-1646. doi: 10.1016/S0140-6736(18)31651-9 [DOI] [PubMed] [Google Scholar]
- 31.Ishii N, Kono Y, Yonemoto N, Kusuda S, Fujimura M; Neonatal Research Network, Japan . Outcomes of infants born at 22 and 23 weeks’ gestation. Pediatrics. 2013;132(1):62-71. doi: 10.1542/peds.2012-2857 [DOI] [PubMed] [Google Scholar]
- 32.Kyser KL, Morriss FH Jr, Bell EF, Klein JM, Dagle JM. Improving survival of extremely preterm infants born between 22 and 25 weeks of gestation. Obstet Gynecol. 2012;119(4):795-800. doi: 10.1097/AOG.0b013e31824b1a03 [DOI] [PubMed] [Google Scholar]
- 33.Mehler K, Oberthuer A, Keller T, et al. Survival among infants born at 22 or 23 weeks’ gestation following active prenatal and postnatal care. JAMA Pediatr. 2016;170(7):671-677. doi: 10.1001/jamapediatrics.2016.0207 [DOI] [PubMed] [Google Scholar]
- 34.Ehret DEY, Edwards EM, Greenberg LT, et al. Association of antenatal steroid exposure with survival among infants receiving postnatal life support at 22 to 25 weeks’ gestation. JAMA Netw Open. 2018;1(6):e183235. doi: 10.1001/jamanetworkopen.2018.3235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Helenius K, Sjörs G, Shah PS, et al. ; International Network for Evaluating Outcomes (iNeo) of Neonates . Survival in very preterm infants: an international comparison of 10 national neonatal networks. Pediatrics. 2017;140(6):e20171264. doi: 10.1542/peds.2017-1264 [DOI] [PubMed] [Google Scholar]
- 36.Rysavy MA, Marlow N, Doyle LW, et al. Reporting outcomes of extremely preterm births. Pediatrics. 2016;138(3):e20160689. doi: 10.1542/peds.2016-0689 [DOI] [PubMed] [Google Scholar]
- 37.Serenius F, Ewald U, Farooqi A, et al. ; Extremely Preterm Infants in Sweden Study Group . Neurodevelopmental outcomes among extremely preterm infants 6.5 years after active perinatal care in Sweden. JAMA Pediatr. 2016;170(10):954-963. doi: 10.1001/jamapediatrics.2016.1210 [DOI] [PubMed] [Google Scholar]
- 38.Allotey J, Zamora J, Cheong-See F, et al. Cognitive, motor, behavioural and academic performances of children born preterm: a meta-analysis and systematic review involving 64 061 children. BJOG. 2018;125(1):16-25. doi: 10.1111/1471-0528.14832 [DOI] [PubMed] [Google Scholar]
- 39.Myrhaug HT, Brurberg KG, Hov L, Markestad T. Survival and impairment of extremely premature infants: a meta-analysis. Pediatrics. 2019;e20180933. doi: 10.1542/peds.2018-0933 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Study plan