Key Points
Question
What are the patient and surgical characteristics affecting long-term transplant-free survival following surgical tetralogy of Fallot repair?
Findings
In this cohort study of 3283 patients with tetralogy of Fallot, survival following complete repair was 98.6%, 97.8%, 97.1%, 95.5%, and 94.5% for 1-year, 5-year, 10-year, 20-year, and 25-year survival, respectively, with an early peaking hazard of mortality shortly after repair. Statistically significant associations with decreased long-term survival included staged repair, non–valve-sparing operation, repair in earlier surgical era, and presence of a genetic abnormality.
Meaning
Overall long-term transplant-free survival in repaired tetralogy of Fallot is excellent, with several factors affecting survival, some of which may be modifiable such as planning of the surgical strategy.
Abstract
Importance
Tetralogy of Fallot (TOF) is a surgically repairable form of cyanotic congenital heart disease. Multicenter data for long-term survival following repair are sparse.
Objective
To evaluate the long-term transplant-free survival of TOF by surgical strategy adjusted for era and patient characteristics.
Design, Setting, and Participants
Retrospective cohort study enriched with data from the National Death Index and the Organ Procurement and Transplantation Network through 2014. Multicenter cohort from the Pediatric Cardiac Care Consortium (PCCC), a large, US-based clinical registry for interventions for congenital heart disease. The cohort included patients with adequate identifiers for linkage with the National Death Index and the Organ Procurement and Transplantation Network who were enrolled in the PCCC registry between 1982 and 2003 and survived surgical repair of simple TOF. Data were analyzed between September 2015 and April 2018.
Exposures
We examined patient-associated and surgery-associated risk factors affecting survival.
Main Outcomes and Measures
We analyzed the transplant-free survival during early (<6 years) and late (≥6 years) phase after TOF surgical repair.
Results
Of the 3283 patients who survived repair for simple TOF and met the study’s inclusion criteria, 56.4% were male and 43.6% were female. Twenty-five–year survival following TOF repair was 94.5%. Multivariable analysis demonstrated increased risk of early mortality with staged repair (HR, 2.68; 95% CI, 1.59-4.49) and non–valve-sparing operation (HR, 3.76; 95% CI, 1.53-9.19). Presence of a genetic abnormality was associated with increased risk of death both in the early (HR, 3.64; 95% CI, 2.05-6.47) and late postoperative phase (HR, 4.41; 95% CI, 2.62-7.44).
Conclusions and Relevance
Long-term survival after simple TOF repair is excellent. Staged repair and non–valve-sparing operations were negatively associated with survival in the early postrepair phase but not the late postrepair phase. These data are important for patients with repaired TOF and their caretakers and may guide surgical strategies for optimizing the long-term outcomes of this population.
This study evaluates the long-term transplant-free survival of tetralogy of Fallot by surgical strategy adjusted for era and patient characteristics.
Introduction
Tetralogy of Fallot (TOF) is the most common form of cyanotic heart disease, with an incidence of 4 of 10 000 live births.1 Surgical repair for TOF was first reported in 1954 for a cohort of 106 patients, with 30-year survival of 77%.2 Long-term outcome data for patients with repaired TOF have been derived from single-center series, typically from tertiary referral centers,3,4,5,6 with results largely influenced by the center’s surgical experience7 or from non-US cohorts leveraging national health registries.8,9,10 These reports are not representative of the survival experience of TOF patients in the United States owing to selection biases, differences in treatment strategies, techniques, or variation in population or health care systems.
The Pediatric Cardiac Care Consortium (PCCC) is the oldest and one of the largest clinical registries for interventions for congenital heart disease in the United States.11,12,13 Linkage of the PCCC to the registries of the National Death Index (NDI) and the Organ Procurement and Transplantation Network (OPTN) extends the follow-up time of PCCC-enrolled persons for major outcomes with nearly 90% sensitivity and almost 100% specificity for death and transplant events.14,15 In this study, we used the linked PCCC-NDI-OPTN data set to evaluate long-term transplant-free survival and causes of death (COD) of a large multicenter US-based cohort following TOF repair.
Methods
This is a retrospective cohort study enriched with prospective data from the NDI and OPTN. The OPTN includes data on all donor candidates, wait-listed candidates, and transplant recipients in the United States. The Health Resources and Services Administration and US Department of Health and Human Services provide oversight to OPTN contractors. The study was approved by the institutional review boards of Emory University, University of Minnesota, and the NDI and OPTN registries. Patient consent was not obtained owing to the retrospective nature of the study.
Cohort Selection
We queried the PCCC for patients who underwent repair for simple TOF (ie, excluding pulmonary atresia, absent pulmonary valve, or atrioventricular canal). Repair was defined as ventricular septal defect closure and right ventricular outflow tract (RVOT) reconstruction. We used PCCC surgical codes to determine the surgical treatment, dividing the cohort into 2 groups; those who underwent primary repair and those who underwent staged approach with initial palliative shunt. Other inclusion criteria included repair before age 21 years, US residency, surgery performed at a US center, and available full set of identifiers to allow reliable linkage with the NDI/OPTN registries. We included all patients with TOF repair after January 1, 1982, provided the first palliative procedure (shunt) was performed after January 1, 1980. Patients enrolled in the PCCC after April 15, 2003, lacked direct identifiers owing to implementation of stricter Health Insurance Portability and Accountability Act of 1996 rules and were excluded. Patients with in-hospital mortality after undergoing TOF repair as primary or staged approach were separated from the long-term survival cohort.
Data Collection
Variables for analysis included sex, age at repair (infancy, 1-5 years, and ≥5 years), era of TOF repair by decade (1982-1989, 1990-1999, and 2000-2006), surgical staging (primary vs staged repair), pulmonary valve approach (valve sparing vs non–valve sparing), and presence of a genetic condition such as chromosomal abnormality or DiGeorge syndrome (clinical criteria or documented 22q11.2 deletion). Prior shunt palliation was determined by querying the PCCC for surgery consistent with any systemic-to-pulmonary shunt (eg, Blalock-Taussig). Type of surgical RVOT reconstruction and valve approach were assigned by the respective PCCC surgical codes; manual review of operative notes was performed when ambiguity existed.
Ascertainment of Death or Transplant Events
Death or transplant events were ascertained from the PCCC and by matching to NDI and OPTN records through December 31, 2014.14 Records submitted to the NDI and OPTN included first name, middle initial, surname, date of birth, sex, state of last known residence, and state of birth. Those with inadequate identifiers could not be reliably linked and were excluded.
Causes of Death
The NDI defines underlying COD as the disease or injury that initiated the train of events leading directly to death or the circumstances of the incident of violence that produced fatal injury.16 Other conditions on the death certificate are considered contributing COD. Information about underlying and contributing COD for each confirmed death was supplied by NDI-Plus, as reported on death certificates, and was used to assign deaths into one of several categories.
Congenital heart disease was assigned as the underlying COD when TOF was listed as the primary condition in the sequence of events. Other cardiovascular disorders (CVD) were assigned as the COD when such conditions were reported without reference to underlying CHD. Additional categories included other congenital noncardiac malformations, respiratory diseases, infections, neoplasms, external causes (ie, injury, poisoning, or suicide), and miscellaneous causes.
Statistical Methods
Descriptive statistics were calculated for variables of interest and included means with standard deviations, medians with interquartile ranges (IQR), or counts and percentages. In a univariate analysis, hospital survivors and nonsurvivors were compared using χ2 tests (or a Fischer exact test when expected cell counts were <5) for categorical data and Wilcoxon rank-sum tests for continuous data. Multivariable logistic regression was used to identify risk factors associated with mortality following TOF repair.
Transplant-free survival following hospital discharge for TOF repair was treated as a time-dependent outcome and modeled parametrically. Parametric probability estimates for this time-dependent outcome used models based on multiple overlapping phases of risk using the PROC HAZARD function available with SAS (SAS Institute Inc). The HAZARD procedure uses maximum likelihood estimates to resolve risk distribution of time to event in up to 3 phases of risk (early decreasing or peaking hazard, constant hazard, and late increasing or decreasing hazard). An early steep hazard followed by a late increasing hazard was found for the primary outcome of transplant-free survival. As a result, a 2-phase (early and late) survival parametrization was used to estimate survival. Therefore, early and late phase survival was defined as less than 6 years and at least 6 years following repair, respectively.
In these models, maximum likelihood estimates were iteratively calculated using nonlinear optimization-based algorithms. Smoothed survival curves were generated using the HAZPRED procedure in SAS. The PROC HAZPRED computes predictions for the survivorship and hazard functions along with their confidence limits. To identify risk factors associated with death/transplant following TOF repair, parametric survival models were constructed using 1 risk factor at a time. Multivariable models used forward selection of variables significant at the 0.2 level in univariate analysis. Effects of covariates on the likelihood of death/transplant are presented as hazard ratios (HRs) with 95% confidence intervals. Risk factors are presented separately for the early-phase and late-phase survival periods. Statistical analyses were conducted using SAS, version 9.4 (SAS Institute). Statistical significance was assessed at the .05 level unless otherwise noted, and all P values were 2-sided.
Results
There were 3894 total patients with simple TOF who underwent repair and met inclusion criteria. Three thousand one hundred sixty-eight underwent primary repair, while 726 had a staged approach with initial shunt followed by repair. During the study period, there was a trend toward earlier primary repair (aged <1 year), but otherwise, there was no significant difference in the use of staged vs primary repair or between valve-sparing vs non–valve-sparing strategies (eTable 1 in the Supplement).
There were 161 in-hospital deaths following surgery: 47 in the staged group and 114 in the primary repair group. Following repair, 3733 were discharged alive. Of these, 3283 patients had direct identifiers available and compose the cohort submitted to the NDI/OPTN, while 450 had insufficient identifiers and were excluded. There were no significant differences in the clinical characteristics of patients with TOF with and without identifiers (data not shown).14 A STROBE-style diagram detailing cohort selection is included (eFigure 1 in the Supplement). Over a median follow-up period of 18.5 years (maximum, 33 years; IQR, 14.6-22.4 years), 145 deaths (3 following transplant) and 5 transplants occurred. The median age at death was 1.0 years (IQR, 0.6-2.1 years), with range 3 days to 19.7 years.
In-Hospital Mortality After TOF Repair
In-hospital mortality following TOF repair was 4.1% and occurred more commonly in infants, patients weighing less than 2.5 kg (proxy for prematurity) at repair, patients with prior shunt palliation, in earlier surgical eras (1982-1989 and 1999-2000), and concurrent genetic syndrome (Table 1). After adjustment covariates, these factors remained significantly associated with increased risk of perioperative death (eTable 2 in the Supplement).
Table 1. Summary of Characteristics of Patient Undergoing Repair for Simple TOF in PCCC.
| Characteristic | No. (%) | P Value | ||
|---|---|---|---|---|
| Overall (n = 3894) |
Alive at Discharge (n = 3707) |
In-Hospital Mortality (n = 161) |
||
| Median age at repair, (IQR), y | 0.9 (0.5-1.9) | 0.9 (0.5-1.9) | 0.7 (0.3-1.4) | <.001 |
| Age group, y | ||||
| Infant (<1) | 2120 (54.4) | 2019 (54.1) | 101 (62.7) | .04 |
| 1 to <5 | 1475 (37.9) | 1421 (38.2) | 54 (33.5) | |
| 5-21 | 299 (7.7) | 293 (7.9) | 6 (3.7) | |
| Sex | ||||
| Male | 2197 (56.4) | 2104 (56.4) | 93 (57.8) | .73 |
| Female | 1697 (43.6) | 1629 (43.6) | 68 (42.2) | |
| Median surgical weight, kga (IQR) | 8.0 (6.1-10.8) | 8.0 (6.1-10.9) | 6.6 (4.3-8.8) | <.001 |
| Weight <2.5 kg | 15 (0.4) | 9 (0.2) | 6 (3.7) | <.001 |
| Staged strategy | ||||
| No | 3168 (81.4) | 3054 (81.8) | 114 (70.8) | <.001 |
| Yes | 726 (18.6) | 679 (18.2) | 47 (29.2) | |
| Repair approachb | ||||
| Valve sparing | 1179 (31.6) | 1157 (32.3) | 22 (15.0) | <.001 |
| Non–valve sparing | 2549 (68.4) | 2424 (67.7) | 125 (85.0) | |
| Era | ||||
| 1982-1989 | 653 (16.8) | 606 (16.2) | 47 (29.2) | <.001 |
| 1990-1999 | 2287 (58.7) | 2192 (58.7) | 95 (59.0) | |
| 2000-2006 | 954 (24.5) | 935 (25.1) | 19 (11.8) | |
| Genetic condition | ||||
| No | 3466 (89.0) | 3335 (89.3) | 131 (81.4) | .002 |
| Yes | 428 (11.0) | 398 (10.7) | 30 (18.6) | |
| Trisomies | 245 (6.3) | 231 (6.2) | 14 (8.7) | .24 |
| DGSc | 86 (2.2) | 83 (2.2) | 3 (1.9) | .80 |
| Other conditionsc | 101 (2.6) | 87 (2.3) | 14 (8.7) | <.001 |
Abbreviations: DGS, DiGeorge syndrome; PCCC, Pediatric Cardiac Care Consortium; TOF, tetralogy of Fallot.
n = 3862 (missing 32 surgical weights).
n = 3728 (repair approach was indeterminate in 166 patients).
Four patients had more than 1 genetic abnormality: (1) trisomy and translocation, (2) trisomy and DGS, and (3) sex or other chromosomal abnormality and DGS.
Long-term Survival After Complete TOF Repair
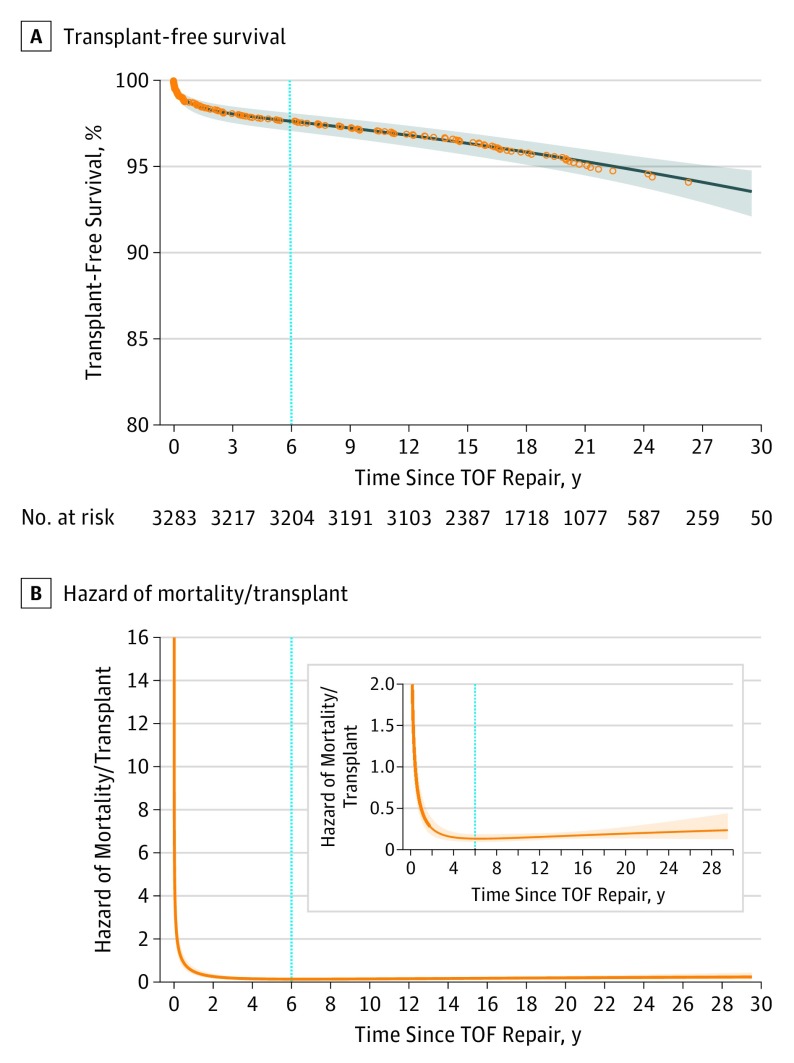
Overall long-term transplant-free survival following discharge alive after TOF repair was 98.6%, 97.8%, 97.1%, 95.5%, and 94.5% at 1, 5, 10, 20, and 25 years after repair (Figure 1A). The overall hazard of mortality or transplant in patients with simple TOF following repair showed an early steep hazard followed by a late, increasing hazard. As a result, a 2-phase survival parametrization was used to estimate transplant-free survival (Figure 1B): (1) an early postdischarge period with high hazard that decreases rapidly before reaching a minimum at 6 years following discharge and (2) a late period with low but steadily increasing hazard beyond 6 years following repair and with acceleration around 20 years postoperatively.
Figure 1. Kaplan-Meier Survival Curve and Hazard of Mortality/Transplant Curve Following Discharge From Definitive TOF Repair .
A, Transplant-free survival following TOF repair. Red dots represent the nonparametric Kaplan-Meier survival estimates. The solid black line is the estimated parametric survival model. Dashed lines represent the 95% confidence limits for the parametric survival estimates. B, Hazard of mortality/transplant following discharge from definitive TOF repair. Hazard function shows an early peaking hazard that rapidly decreases during the first year and reaches a minimum at 4 years following discharge followed by an increasing late hazard period through 30 years following TOF repair. Insert: zoomed view of the hazard function that shows the trough of the hazard around 6 years followed by a slow steady increase throughout the follow-up period. The dotted line at 6 years for both Figures represents division between early and late deaths.
Factors Associated With Long-term Mortality
Stratified transplant-free survival patterns (Figure 2A-F) demonstrated a rapid decrease in survival in the first 2 years following repair for those undergoing staged repair and non–valve-sparing repair, resulting in a long-term survival advantage over single-stage and valve-sparing approaches throughout the subsequent 20 years (Figure 2C and D, respectively). In addition, the survival of those with a genetic abnormality was significantly disadvantaged compared with those without during the whole postoperative study period (Figure 2E). A more rapid early mortality was noted for those who underwent repair in the earliest era, but long-term survival was similar across all eras (Figure 2F).
Figure 2. Stratified Transplant-Free Survival Plots of Survivors of TOF Repair by Variables of Interest.
A, Sex (m = male; f = female); B, Age at time of TOF repair; C, Primary repair vs staged repair strategy; D, Valve-sparing vs non–valve-sparing approach; E, Genetic condition; F, Era of repair. Shaded area illustrates the 95% confidence intervals.
A unique group of patients included within the non–valve-sparing group were those repaired with a right ventricle–pulmonary artery conduit (n = 88). This group experienced a higher number of deaths 2 to 4 years after TOF repair compared with the other non–valve-sparing patients, but the number of events is too small to detect a significant difference in long-term survival (eFigure 2 in the Supplement).
Univariate analysis revealed several factors associated with increased late mortality following TOF repair (eTable 3 in the Supplement). In the early postoperative phase (<6 years), these factors included staged vs primary repair (HR, 3.45; 95% CI, 2.02-5.89), non–valve-sparing vs valve-sparing operation (HR, 4.56; 95% CI, 1.86-11.20), and presence of a coexisting genetic condition (HR, 3.81; 95% CI, 2.14-6.79). Genetic abnormality was the only factor associated with higher risk for premature mortality in the later phase (>6 years) (HR, 4.41; 95% CI, 2.59-7.52).
After covariate adjustment, staged surgical strategy (HR, 2.68; 95% CI, 1.59-4.49), non–valve-sparing approach (HR, 3.76; 95% CI, 1.53-9.91), repair in the earliest surgical era (HR, 2.31; 95% CI, 1.06-5.03), and coexisting genetic condition (HR, 3.64; 95% CI, 2.05-6.47) were all linked to increased mortality in the early period (Table 2). In the later phase, presence of a genetic abnormality was the only association with increased risk of death (HR, 4.41; 95% CI, 2.62-7.44).
Table 2. Adjusted Analysis of Risk Factors Associated With Mortality or Transplant Following TOF Repair.
| Risk Factors | Hazard Ratio (95% CI) | P Value |
|---|---|---|
| Risk factors for early death/transplant | ||
| Staged strategy | 2.68 (1.59-4.49) | <.001 |
| Non–valve-sparing operation | 3.76 (1.53-9.19) | .004 |
| Era | ||
| 1982-1989 | 2.31 (1.06-5.03) | .04 |
| 1990-1999 | 1.27 (0.65-2.45) | .49 |
| 2000 and beyond | 1 [Reference] | NA |
| Genetic condition | 3.64 (2.05-6.47) | <.001 |
| Risk factors for late death/transplant | ||
| Male | 1.56 (0.96-2.56) | .08 |
| Genetic condition | 4.41 (2.62-7.44) | <.001 |
Abbreviations: NA, not applicable, TOF, tetralogy of Fallot.
Causes of Death
Most deaths among those surviving TOF repair were directly (n = 63 [43.5%]) or indirectly (n = 76 [52.4%]) attributed to TOF (Table 3), with a variety of contributing CVDs including arrhythmia (n = 12 [8.3%]), cardiac arrest (n = 34 [23.5%]), and congestive heart failure (n = 26 [17.9%]) (eTable 4 in the Supplement). Other common CODs include other CVD such as myocardial ischemia and stroke, other congenital anomalies, and external causes (death from unintentional injury or suicide).
Table 3. Underlying Late Cause of Death Following TOF Repair.
| Underlying Cause of Death | No. (%) |
|---|---|
| Congenital heart disease | 63 (43.45) |
| Disease of the circulatory system | 22 (15.17) |
| Miscellaneous | 19 (13.1) |
| External causes of injury and poisoning | 16 (11.03) |
| Other congenital malformations | 10 (6.9) |
| Respiratory diseases | 8 (5.52) |
| Infections | 2 (1.38) |
| Neoplasms | 5 (3.45) |
Abbreviation: TOF, tetralogy of Fallot.
Cardiovascular disease, without reference to TOF, accounted for 15.2% of deaths (n = 22), while 41.4% (n = 60) were assigned to a non-CHD/non-CVD condition. The most frequent CODs outside CHD/CVD were external causes (n = 16 [11%]), other medical causes (n = 19 [13.1%]), other congenital anomalies (n = 10 [6.9%]), and neoplasms (n = 5 [3.5%]). Review of COD by surgical era did not reveal any significant differences (data not shown).
Discussion
Tetralogy of Fallot is a common form of cyanotic CHD that has been repaired for the past 60 years with evolving treatment approaches.2 The number of adult survivors of TOF repair is rapidly increasing.8,17 While there have been many studies evaluating short-term and midterm outcomes in TOF, data about factors putting patients at risk for remote death are scarce, primarily limited to single-center or international registry cohorts.3,4,5,6,7,8,9,10,15,18 To our knowledge, this study describes the largest multicenter cohort of patients with repaired TOF in the United States, with more than 25 years of follow-up after surgical repair. The study of long-term outcomes by various surgical strategies in relation to era and patient-level factors provides opportunities to assess treatment decisions affecting the long-term outcomes of patients with repaired TOF.
Survival in the PCCC cohort exceeds 94% at 25 years after TOF repair, which is comparable with single-center US and international registry studies reporting 30-year survival between 80% and 98%.7,8,9,17 The large size of the PCCC cohort coupled with a long period of follow-up after linkage with national registries allowed us to provide a nuanced report on the factors associated with long-term survival.
Patterns of Long-term Survival After TOF Repair
Patients with repaired TOF have a biphasic hazard for mortality, an early steep hazard during the first 4 years after TOF repair, and a late hazard with a slow increase in mortality after 6 years and acceleration 20 years later. Earlier surgical eras had an inverse association with the chances of survival in the early postoperative phase, indicating higher residual postoperative morbidity, which is consistent with previous reports.8,9,17,19 In the PCCC cohort, the late-phase survival and COD have not substantially changed over time. These findings suggest that residual morbidity remains a major factor affecting survival of these individuals despite improvements in acute and midterm treatment. Not surprisingly, coexistence of a genetic anomaly remains a risk factor for mortality in both early and late postoperative phases. This indicates that at least some of the residual morbidity can be explained by coexisting genetic abnormalities.
Staged repair and non–valve-sparing operation were both associated with increased early-phase hazard for death following repair, ultimately translating to worsened long-term survival. The choice between staged vs single-stage repair and between valve-sparing vs not may reflect nonmeasured covariables such as more severe underlying pathology or other comorbidities. Those undergoing initial palliation with a shunt may also have residual deleterious effects related to the initial palliative shunt (eg, pulmonary artery distortion, excessive pulmonary blood flow, and left heart overload). Patients with non–valve-sparing approach are exposed to higher likelihood for subsequent reoperation and perioperative complications. Potentially, pulmonary insufficiency associated with non–valve-sparing operation has an adverse association with early ventricular hemodynamics affecting survival.
There is also a trend toward increased mortality in the subgroup undergoing right ventricle–pulmonary artery conduit placement, at a time when procedures for an outgrown conduit are expected to take place, but the relatively small number of this group limits conclusions.
Mortality differences between staged and primary repair strategies have been inconsistent among multiple studies supporting each strategy.18,20,21,22 In regards to the use of valve vs non–valve-sparing approach, prior studies detected no significant difference in long-term survival between patients treated with transannular patch (TAP) vs those with valve-sparing operation despite less valve replacements in the valve-sparing group.23,24 Because most of these studies are single-center, they are not suitable to address the issue of center effect. The large number of participating centers in the PCCC allows us to leverage center variation in the TOF surgical strategy and compare the effectiveness of each strategy in a quasiexperimental design based on center and regional variation. In this regard, the lack of significant variation in the number of staged vs primary repairs and types of RVOT reconstruction between centers suggests these choices are less likely to be driven by center-related experience or level of expertise.
Modifications aiming to preserve the infundibulum with a mini-incision have been described, hoping to confer long-term survival advantages.25,26 Reported midterm survival (97% at 7 years of postoperative follow-up) with this strategy is similar to the outcomes in our study, and longer follow-up will be needed to determine whether a positive effect on morbidity or mortality exists with this modification.
Causes of Death After TOF Repair
Most deaths after TOF repair were directly or indirectly associated with TOF and were reported as sudden death, arrhythmia, and congestive heart failure. The rates of sudden death and heart failure in our cohort are similar to those reported in other studies.9,18 The CODs in our cohort were similar to those reported in a study from Taiwan,10 with the exception of the high incidence of deaths from unintentional injury and suicides, which were not confirmed in our cohort. This difference may be associated with local factors and the relatively small number of patients and death events in the Taiwanese study, limiting generalization for reported COD in categories with relatively rare events.10
Strengths and Limitations
The primary strengths of our study are the large number of patients and participating centers as well as the length of follow-up. However, our study does have limitations as well. The PCCC registry lacks information regarding potentially important contributors to long-term mortality such as presurgical variables affecting choice of surgical strategy, frequency and type of subsequent procedures, medications used, and socioeconomic variables. In addition, there is limited information regarding the status of the RVOT following surgery.
There are also important limitations in the use of NDI for ascertainment of COD as highlighted by some comparison studies of assigned COD between NDI-based vs direct review methods. For example, such a study27 in a cohort of patients with coronary heart disease concluded that sensitivity and positive predictive value for the NDI-assigned coronary heart disease COD were relatively weak, while specificity and negative predictive value were strong. More specifically, NDI data tended to overestimate coronary disease as the COD, especially in older patients with multiple comorbidities.27 Extrapolating to our study, it is possible that some of the deaths in the TOF population may have been misclassified. Nonetheless, attributed COD based on NDI data have been shown to correlate well to other methods of death ascertainment including case-by-case review by nosologists or review with state-level death records with discordance rates less than 10%.28,29
Conclusions
Our study provides important information about the long-term outcomes of patients with repaired TOF in the United States. The data highlight the shortcomings of early repair, staged, and non–valve-sparing approaches in the early period following repair. Long-term transplant-free survival beyond the first 6 years following repair is excellent and influenced primarily by the coexistence of an associated genetic condition. Within the 25-year follow-up period, most deaths were associated with the underlying diagnosis of TOF and mediated by arrhythmias and congestive heart failure. Continuous surveillance of this cohort is important in identifying additional risks resulting from the interaction of underlying conditions with cardiovascular morbidities expected with aging.
eFigure 1. Flow Diagram Illustrating Selection of Long-Term Simple TOF Survivor Cohort.
eFigure 2. Stratified Transplant Free Survival Plot of Patients Undergoing RV-PA Conduit Replacement Compared With Other Forms of Non-Valve Sparing Procedure
eFigure 3. Causes of Death (COD) Distribution Among Survivors of Perioperative TOF Repair.
eTable 1. Trends in Management Strategy by Era
eTable 2. Adjusted Analysis of Risk Factors Associated With In-Hospital Mortality for TOF Repair
eTable 3. Univariable Analysis of Characteristics Associated With Mortality or Transplant Conditioning Discharge Alive After TOF Repair
eTable 4. Contributing Late Causes of Death Patients After TOF Repair
References
- 1.Hoffman JIE, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39(12):1890-1900. doi: 10.1016/S0735-1097(02)01886-7 [DOI] [PubMed] [Google Scholar]
- 2.Lillehei CW, Varco RL, Cohen M, et al. The first open heart corrections of tetralogy of Fallot: a 26-31 year follow-up of 106 patients. Ann Surg. 1986;204(4):490-502. doi: 10.1097/00000658-198610000-00017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bacha EA, Scheule AM, Zurakowski D, et al. Long-term results after early primary repair of tetralogy of Fallot. J Thorac Cardiovasc Surg. 2001;122(1):154-161. doi: 10.1067/mtc.2001.115156 [DOI] [PubMed] [Google Scholar]
- 4.Cobanoglu A, Schultz JM. Total correction of tetralogy of Fallot in the first year of life: late results. Ann Thorac Surg. 2002;74(1):133-138. doi: 10.1016/S0003-4975(02)03619-6 [DOI] [PubMed] [Google Scholar]
- 5.Horneffer PJ, Zahka KG, Rowe SA, et al. Long-term results of total repair of tetralogy of Fallot in childhood. Ann Thorac Surg. 1990;50(2):179-183. doi: 10.1016/0003-4975(90)90728-O [DOI] [PubMed] [Google Scholar]
- 6.Murphy JG, Gersh BJ, Mair DD, et al. Long-term outcome in patients undergoing surgical repair of tetralogy of Fallot. N Engl J Med. 1993;329(9):593-599. doi: 10.1056/NEJM199308263290901 [DOI] [PubMed] [Google Scholar]
- 7.Knott-Craig CJ, Elkins RC, Lane MM, Holz J, McCue C, Ward KE. A 26-year experience with surgical management of tetralogy of Fallot: risk analysis for mortality or late reintervention. Ann Thorac Surg. 1998;66(2):506-511. doi: 10.1016/S0003-4975(98)00493-7 [DOI] [PubMed] [Google Scholar]
- 8.Hickey EJ, Veldtman G, Bradley TJ, et al. Late risk of outcomes for adults with repaired tetralogy of Fallot from an inception cohort spanning four decades. Eur J Cardiothorac Surg. 2009;35(1):156-164. doi: 10.1016/j.ejcts.2008.06.050 [DOI] [PubMed] [Google Scholar]
- 9.Nollert G, Fischlein T, Bouterwek S, Böhmer C, Klinner W, Reichart B. Long-term survival in patients with repair of tetralogy of Fallot: 36-year follow-up of 490 survivors of the first year after surgical repair. J Am Coll Cardiol. 1997;30(5):1374-1383. doi: 10.1016/S0735-1097(97)00318-5 [DOI] [PubMed] [Google Scholar]
- 10.Chiu SN, Wang JK, Chen HC, et al. Long-term survival and unnatural deaths of patients with repaired tetralogy of Fallot in an Asian cohort. Circ Cardiovasc Qual Outcomes. 2012;5(1):120-125. doi: 10.1161/CIRCOUTCOMES.111.963603 [DOI] [PubMed] [Google Scholar]
- 11.Moller JH, Borbas C; The Pediatric Cardiac Care Consortium . The Pediatric Cardiac Care Consortium: a physician-managed clinical review program. QRB Qual Rev Bull. 1990;16(9):310-316. doi: 10.1016/S0097-5990(16)30386-4 [DOI] [PubMed] [Google Scholar]
- 12.Moller JH. A multi-center cardiac registry: a method to assess outcome of catheterization intervention or surgery. Prog Pediatr Cardiol. 2005;20:7-12. doi: 10.1016/j.ppedcard.2004.12.009 [DOI] [Google Scholar]
- 13.Vinocur JM, Moller JH, Kochilas LK. Putting the Pediatric Cardiac Care Consortium in context: evaluation of scope and case mix compared with other reported surgical datasets. Circ Cardiovasc Qual Outcomes. 2012;5(4):577-579. doi: 10.1161/CIRCOUTCOMES.111.964841 [DOI] [PubMed] [Google Scholar]
- 14.Spector LG, Menk JS, Vinocur JM, et al. ; In-Hospital Vital Status and Heart Transplants After Intervention for Congenital Heart Disease in the Pediatric Cardiac Care Consortium . In-hospital vital status and heart transplants after intervention for congenital heart disease in the pediatric cardiac care consortium: completeness of ascertainment using the National Death Index and United Network for Organ Sharing Datasets. J Am Heart Assoc. 2016;5(8):e003783. doi: 10.1161/JAHA.116.003783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spector LG, Menk JS, Knight JH, et al. Trends in long-term mortality after congenital heart surgery. J Am Coll Cardiol. 2018;71(21):2434-2446. doi: 10.1016/j.jacc.2018.03.491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bilgrad R. National Death Index Plus: Coded Causes of death: Supplement to the National Death Index user’s Manual. Hyattsville, MD: National Center for Health Statistics; 1999. [Google Scholar]
- 17.Cuypers JA, Menting ME, Konings EE, et al. Unnatural history of tetralogy of Fallot: prospective follow-up of 40 years after surgical correction. Circulation. 2014;130(22):1944-1953. doi: 10.1161/CIRCULATIONAHA.114.009454 [DOI] [PubMed] [Google Scholar]
- 18.Nørgaard MA, Lauridsen P, Helvind M, Pettersson G. Twenty-to-thirty-seven-year follow-up after repair for Tetralogy of Fallot. Eur J Cardiothorac Surg. 1999;16(2):125-130. doi: 10.1016/S1010-7940(99)00137-2 [DOI] [PubMed] [Google Scholar]
- 19.Kim H, Sung SC, Kim SH, et al. Early and late outcomes of total repair of tetralogy of Fallot: risk factors for late right ventricular dilatation. Interact Cardiovasc Thorac Surg. 2013;17(6):956-962. doi: 10.1093/icvts/ivt361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steiner MB, Tang X, Gossett JM, et al. Alternative repair strategies for ductal-dependent tetralogy of Fallot and short-term postoperative outcomes, a multicenter analysis. Pediatr Cardiol. 2015;36(1):177-189. doi: 10.1007/s00246-014-0983-6 [DOI] [PubMed] [Google Scholar]
- 21.Kanter KR, Kogon BE, Kirshbom PM, Carlock PR. Symptomatic neonatal tetralogy of Fallot: repair or shunt? Ann Thorac Surg. 2010;89(3):858-863. doi: 10.1016/j.athoracsur.2009.12.060 [DOI] [PubMed] [Google Scholar]
- 22.Steiner MB, Tang X, Gossett JM, Malik S, Prodhan P. Timing of complete repair of non-ductal-dependent tetralogy of Fallot and short-term postoperative outcomes, a multicenter analysis. J Thorac Cardiovasc Surg. 2014;147(4):1299-1305. doi: 10.1016/j.jtcvs.2013.06.019 [DOI] [PubMed] [Google Scholar]
- 23.Kim GS, Han S, Yun TJ. Pulmonary annulus preservation lowers the risk of late postoperative pulmonary valve implantation after the repair of tetralogy of Fallot. Pediatr Cardiol. 2015;36(2):402-408. doi: 10.1007/s00246-014-1021-4 [DOI] [PubMed] [Google Scholar]
- 24.Luijten LW, van den Bosch E, Duppen N, et al. Long-term outcomes of transatrial-transpulmonary repair of tetralogy of Fallot. Eur J Cardiothorac Surg. 2015;47(3):527-534. doi: 10.1093/ejcts/ezu182 [DOI] [PubMed] [Google Scholar]
- 25.McKenzie ED, Maskatia SA, Mery C. Surgical management of tetralogy of fallot: in defense of the infundibulum. Semin Thorac Cardiovasc Surg. 2013;25(3):206-212. doi: 10.1053/j.semtcvs.2013.10.003 [DOI] [PubMed] [Google Scholar]
- 26.Morales DL, Zafar F, Heinle JS, et al. Right ventricular infundibulum sparing (RVIS) tetralogy of fallot repair: a review of over 300 patients. Ann Surg. 2009;250(4):611-617. [DOI] [PubMed] [Google Scholar]
- 27.Olubowale OT, Safford MM, Brown TM, et al. Comparison of expert adjudicated coronary heart disease and cardiovascular disease mortality with the national death index: results from the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study. J Am Heart Assoc. 2017;6(5):e004966. doi: 10.1161/JAHA.116.004966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doody MM, Hayes HM, Bilgrad R. Comparability of national death index plus and standard procedures for determining causes of death in epidemiologic studies. Ann Epidemiol. 2001;11(1):46-50. doi: 10.1016/S1047-2797(00)00177-0 [DOI] [PubMed] [Google Scholar]
- 29.Sathiakumar N, Delzell E, Abdalla O. Using the National Death Index to obtain underlying cause of death codes. J Occup Environ Med. 1998;40(9):808-813. doi: 10.1097/00043764-199809000-00010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Flow Diagram Illustrating Selection of Long-Term Simple TOF Survivor Cohort.
eFigure 2. Stratified Transplant Free Survival Plot of Patients Undergoing RV-PA Conduit Replacement Compared With Other Forms of Non-Valve Sparing Procedure
eFigure 3. Causes of Death (COD) Distribution Among Survivors of Perioperative TOF Repair.
eTable 1. Trends in Management Strategy by Era
eTable 2. Adjusted Analysis of Risk Factors Associated With In-Hospital Mortality for TOF Repair
eTable 3. Univariable Analysis of Characteristics Associated With Mortality or Transplant Conditioning Discharge Alive After TOF Repair
eTable 4. Contributing Late Causes of Death Patients After TOF Repair