This phase 2 clinical trial investigates whether injecting the myocardium of patients with heart failure with a high dose of mesenchymal precursor cells derived from bone marrow stem cells, compared with a sham injection, repairs myocardial damage enough to facilitate weaning from left ventricular assist devices (LVADs).
Key Points
Question
Does injection of mesenchymal precursor cells (MPCs) into the myocardium of patients undergoing a left ventricular assist device (LVAD) implant improve frequency of temporary weaning from device support?
Findings
In this randomized clinical trial involving 159 patients with advanced heart failure, the mean proportion of successful temporary weaning from LVAD support was 61% among patients who received MPCs and 58% among controls, a difference that was not statistically significant. The probability that MPCs increased the likelihood of successful temporary weaning was 69%, which did not meet the predefined 80% threshold for success.
Meaning
These findings do not support the use of MPCs to promote cardiac recovery as measured by temporary weaning from device support in LVAD recipients.
Abstract
Importance
Left ventricular assist device (LVAD) therapy improves myocardial function, but few patients recover sufficiently for explant, which has focused attention on stem cells to augment cardiac recovery.
Objective
To assess efficacy and adverse effects of intramyocardial injections of mesenchymal precursor cells (MPCs) during LVAD implant.
Design, Setting, and Participants
A randomized phase 2 clinical trial involving patients with advanced heart failure, undergoing LVAD implant, at 19 North American centers (July 2015-August 2017). The 1-year follow-up ended August 2018.
Interventions
Intramyocardial injections of 150 million allogeneic MPCs or cryoprotective medium as a sham treatment in a 2:1 ratio (n = 106 vs n = 53).
Main Outcomes and Measures
The primary efficacy end point was the proportion of successful temporary weans (of 3 planned assessments) from LVAD support within 6 months of randomization. This end point was assessed using a Bayesian analysis with a predefined threshold of a posterior probability of 80% to indicate success. The 1-year primary safety end point was the incidence of intervention-related adverse events (myocarditis, myocardial rupture, neoplasm, hypersensitivity reactions, and immune sensitization). Secondary end points included readmissions and adverse events at 6 months and 1-year survival.
Results
Of 159 patients (mean age, 56 years; 11.3% women), 155 (97.5%) completed 1-year of follow-up. The posterior probability that MPCs increased the likelihood of successful weaning was 69%; below the predefined threshold for success. The mean proportion of successful temporary weaning from LVAD support over 6 months was 61% in the MPC group and 58% in the control group (rate ratio [RR], 1.08; 95% CI, 0.83-1.41; P = .55). No patient experienced a primary safety end point. Of 10 prespecified secondary end points reported, 9 did not reach statistical significance. One-year mortality was not significantly different between the MPC group and the control group (14.2% vs 15.1%; hazard ratio [HR], 0.89; 95%, CI, 0.38-2.11; P = .80). The rate of serious adverse events was not significantly different between groups (70.9 vs 78.7 per 100 patient-months; difference, −7.89; 95% CI, −39.95 to 24.17; P = .63) nor was the rate of readmissions (0.68 vs 0.75 per 100 patient-months; difference, −0.07; 95% CI, −0.41 to 0.27; P = .68).
Conclusions and Relevance
Among patients with advanced heart failure, intramyocardial injections of mesenchymal precursor cells, compared with injections of a cryoprotective medium as sham treatment, did not improve successful temporary weaning from left ventricular assist device support at 6 months. The findings do not support the use of intramyocardial mesenchymal stem cells to promote cardiac recovery as measured by temporary weaning from device support.
Trial Registration
clinicaltrials.gov Identifier: NCT02362646
Introduction
Implantable left ventricular assist devices (LVADs) are a mainstay of surgical therapy for patients with advanced heart failure refractory to medical therapy. Left ventricular assist device support can lead to improved survival and quality of life yet is associated with adverse events, including major bleeding, thrombosis, stroke, and infection. Although used for hemodynamic support, LVAD therapy has also been shown to enhance reverse remodeling and improve myocardial function in a minority of patients. Adjuvant therapies that might elicit greater degrees of myocardial recovery—or reduce adverse events—during LVAD support have been the focus of intensive research.
Mesenchymal precursor cells (MPCs) have been identified as a promising adjuvant therapy to promote cardiac recovery. Delivery of mesenchymal stem cells into failing hearts has been associated with improvement in the geometry and function of the failing left ventricle.1,2,3 In addition, MPCs may suppress inflammatory cytokines that incite the development of infections and predispose to bleeding and thrombosis.4 These findings led to an exploratory trial by the Cardiothoracic Surgical Trials Network investigating the use of MPCs in LVAD recipients.5 In this preliminary trial, a higher proportion of patients receiving MPCs achieved temporary weaning from full LVAD support at 90 days compared with patients receiving the sham treatment. In a Bayesian analysis, the posterior probability that MPCs increased the likelihood of successful temporary weaning was 93%.
This signal of efficacy in the absence of substantial concerns about adverse events led to this follow-up trial using a 6-fold higher dose (150 million) of MPCs at the time of LVAD implant. Support for the higher dose was provided by a phase 2 trial involving patients with chronic systolic heart failure that was being treated medically, in which trial 150 million cells were safe and potentially more efficacious than were lower doses.6 The aims of the current trial were to evaluate the efficacy and adverse effects of higher-dose MPCs in an LVAD population.
Methods
Study Design and Trial Oversight
The trial was conducted in 19 North American centers under an investigational new drug application (see the protocol and statistical analytical plan in Supplement 1). A coordinating center, independent adjudication committee, echocardiographic core lab, and data and safety monitoring board oversaw the trial’s conduct. Institutional review boards of participating centers and the coordinating center approved the protocol, and written informed consent was obtained for all patients.
Patients and Interventions
The target population comprised adults with end-stage heart failure (ischemic or nonischemic), who were hospitalized for a clinically indicated LVAD for a bridge to a heart transplant or to destination therapy for patients who are not candidates for heart transplants. All LVADs were required to be approved by either the US Food and Drug Administration or Health Canada and were required to be continuous-flow devices. Exclusion criteria included percutaneous LVAD or biventricular mechanical support, planned ablation or aortic valve intervention, recent cardiothoracic surgery or myocardial infarction, prior cardiac transplant, left ventricular reduction surgery, or cardiomyoplasty, more than 10% anti-HLA antibody titers specific to MPC-donor HLA antigens, and prior cell therapy for cardiac repair (eAppendix in Supplement 1). Self-reported race and ethnicity was collected in National Institutes of Health–defined categories to monitor diversity.
Patients were randomly assigned in a 2:1 ratio to an intramyocardial injection of 150 million MPCs (from 1 of 2 donors) or cryoprotective medium alone. All investigators and patients were masked to treatment assignment and outcomes data. Randomization in blocks of 6 was stratified by center. The randomization sequence was generated by a statistician and controlled centrally through a web-based data collection system.
Intramyocardial injections were performed at the time of LVAD implant. These allogeneic MPCs, obtained from healthy donors and expanded in a Good Manufacturing Practice facility (Mesoblast Ltd), were STRO-3 immunoselected, culture-expanded, immature subfraction of adult bone marrow–derived mononuclear cells.7 The injection procedures were defined in the protocol to increase standardization across sites and designed to maximize distribution of MPCs across the left ventricular myocardium (see Figure 1 in the protocol in Supplement 1). The protocol specified that long-term medical management should adhere to clinical practice guidelines with regard to blood pressure control and anticoagulation.
End Points
All patients were followed up for up to 12 months after randomization or until heart transplant, whichever came first, with end points evaluated at 2, 4, 6, 9, and 12 months. The ongoing 2-year follow-up assessment documents vital status, transplant, and LVAD explant or replacement.
Primary End Points
There were 2 primary end points. The primary efficacy end point was the proportion of successful temporary weans (≥30 minutes) out of 3 assessments (2, 4, and 6 months) from full to minimal LVAD support over 6 months after randomization. Weaning failures were defined as an inability to tolerate the wean for 30 minutes, inability to attempt weaning as determined by the patient’s cardiologist, or death. The primary safety end point was the incidence of study intervention–related adverse events (infectious myocarditis, myocardial rupture, neoplasm, hypersensitivity reaction, and immune sensitization syndrome) during the 1-year follow-up period.
Secondary End Points
Secondary end points included 3 echocardiographic assessments timed to 3 stages of the weaning process: (1) before full LVAD support was withdrawn, prior to initiating the wean, (2) 15 minutes into weaning, and (3) immediately following the 6-minute walk test, if tolerated. Echocardiographic measurements included LVEF, left ventricular dimensions, and right ventricular function. The 6-minute walk test was conducted 20 minutes into the weaning (plus or minus 10 minutes).
Other end points included survival, anti-HLA antibody sensitization and transplant, and serious adverse events, according to Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) definitions. The cumulative incidence and rate of serious gastrointestinal tract bleeding, epistaxis, or both by 6 months after randomization was specified midtrial as a separate secondary safety end point, while investigators remained blinded. The INTERMACS bleeding definition was operationalized such that if patients (1) had a series of transfusions over time and (2) experienced a hiatus for more than 48 hours between transfusions, then the next transfusion was attributed to a new bleeding event.
Length of stay of the index hospitalization and frequency and cause of readmissions were assessed. Quality of life was assessed by the 12-item Short Form Health Survey (SF-12) and the Kansas City Cardiomyopathy Questionnaire (KCCQ), both scaled from 0 (worst) to 100 (best).
This article reports on the primary efficacy end point over 6 months, along with 6-month echocardiographic measurements, 6-minute walk test, adverse events, hospitalizations, and quality of life. The primary safety end point (ie, myocarditis, myocardial rupture, neoplasm, hypersensitivity reactions, and immune sensitization), survival, and sensitization were reported over 12 months. Resource utilization, neurocognition (at 3 and 12 months), and temporary weaning, echocardiographic assessments and 6-minute walk test performed at 12 months are not reported. Also not reported are the mechanistic end points, which are defined as tertiary.
Statistical Analysis
Efficacy end points were analyzed according to treatment assignment. Superiority of MPCs compared with the sham treatment was assessed using a Bayesian approach based on the probability that the proportion of successful weans out of 3 planned was greater in the MPC group than in the control group. By consensus, the investigators selected a wean success ratio of 1.5 as clinically meaningful evidence of improvement. This probability of superiority was calculated based on observed proportions of successes in the 2 groups. Independent gamma distribution as priors with a mean of 0.18 (SD, 0.10) were assumed for each group. A sample size of 159 patients, based on simulations, provided a probability of 80% to detect a wean success rate ratio of 1.5, with a false-positive rate of 15%. This threshold probability was chosen to ensure the same level of certainty for ruling out a false-negative that is commonly used in a frequentist approach to sample size estimations. An additional analysis based on a frequentist approach was conducted. Negative binomial regression was used to compare the number of successful weans in the first 6 months after randomization between the groups, with an offset term to adjust for the total number of possible wean assessments.
Patients with at least 1 assessment of the primary efficacy end point were included in the analysis set. Missing data due to transplant, patient refusal, missed visits, or dropouts were not imputed and the total number of available weans was used. As a post hoc sensitivity analysis, a mixed-effect model analysis of the primary outcome with site as a random effect was used. Post hoc subgroup analyses, stratified by LVAD indication and heart failure subtype, included an interaction term between randomization assignment and subgroup specification using a negative binomial model.
All serious adverse events (excluding bleeding) were analyzed from the time of injection, and safety analyses were conducted by actual treatment received. Event rates were calculated as the ratio of the total number of events over 6 months divided by total patient-months at risk of the specific event from the index surgery. Time to first bleeding event was estimated by cumulative incidence curves and differences between groups were assessed via Gray test. A Poisson model with robust variance estimation was used to compare serious adverse events and readmissions between groups.
Random effects pattern mixture models with an interaction term of time × treatment group (or standard mixed models if no differential effect was observed across patterns) were used to determine the difference between groups in functional status (eg, echo parameters, 6-minute walk test) and quality-of-life measures over 6 months. Missing outcome values for secondary end points were not imputed. To accommodate for missingness due to death, transplant, or study dropout, pattern mixture models were used to analyze longitudinal data and intermittent missing data were assumed to be missing at random. Values of the 6-minute walk test that were missing because of inability to attempt weaning as determined by the patient’s cardiologist, inability to reach minimal support, or failure to start or complete the 6-minute walk test were assigned a value of zero.
One-year survival was described by Kaplan-Meier curves and differences assessed via the log-rank test. Time to heart transplant by 12 months was described by cumulative incidence curves and differences assessed via the Gray test. For all time-to-event analyses, the interaction of treatment with log time and Schoenfeld residuals against time and log time were used to evaluate the proportionality of hazards. The proportional hazards assumption was met for all Cox and competing risk models.
All hypothesis testing was carried out at the .05 (2-sided) significance level using SAS version 9.4 (SAS Institute Inc). Because of the potential for type I error due to multiple comparisons, findings for analyses of secondary end points should be considered exploratory.
Results
Patients
Between July 2015 and August 2017, 637 patients undergoing LVAD implants were screened (Figure 1), 159 of whom were randomized to receive either MPCs (n = 106) or sham injections (n = 53). The 2 groups had similar baseline characteristics (Table 1). Median left ventricular ejection fraction was 15% (interquartile range [IQR], 15%-20%) in the MPCs group and 15% (IQR, 12%-20%) in the control group. Most patients were New York Heart Association class IV and had an INTERMACS Profile ranging from 1 to 3. The cause of heart failure was ischemic in 43 (40.6%) of MPC patients vs 27 (50.9%) control patients. The indication for LVAD implant was similar in both groups, as was the type of LVAD. The 1-year follow-up ended in August 2018.
Figure 1. Enrollment, Randomization, and Follow-up of Patients in the Left Ventricular Assist Device and Mesenchymal Precursor Cells Trial.
ICD indicates implantable cardioverter-defibrillator; MPC, mesenchymal precursor cells.
aThe 3 most common reasons for not meeting inclusion criteria were not being an acceptable candidate for a left ventricular assist device, having more than 10% anti-HLA antibody titer specific to MPC donor HLA antigens, and having a history of cancer within 3 years prior to screening. Patients could be excluded for more than 1 reason.
bThe primary safety end point includes patients until the month-12 visit, transplant, withdrawal of consent, or death.
Table 1. Baseline Patient and Operative Characteristics.
| Baseline Characteristics | MPC (n = 106) | Control (n = 53) |
|---|---|---|
| Sex, No. (%) | ||
| Men | 94 (88.7) | 47 (88.7) |
| Women | 12 (11.3) | 6 (11.3) |
| Age, mean (SD), y | 55.5 (12.3) | 56.9 (11.7) |
| Race, No. (%) | ||
| White | 82 (77.4) | 40 (75.5) |
| Black or African American | 16 (15.1) | 7 (13.2) |
| Asian | 2 (1.9) | 5 (9.4) |
| American Indian or Alaska Native | 2 (1.9) | 0 |
| Native Hawaiian or other Pacific Islander | 1 (0.9) | 0 |
| Unknown or not reported | 3 (2.8) | 1 (1.9) |
| Medical and surgical history, No. (%) | ||
| Prior ventricular dysrhythmia | 61 (57.5) | 31 (58.5) |
| Cardiomyopathy | ||
| Nonischemic | 63 (59.4) | 26 (49.1) |
| Ischemic | 43 (40.6) | 27 (50.9) |
| Preoperative intraaortic balloon pump | 19 (17.9) | 11 (20.8) |
| Prior sternotomy | 16 (15.1) | 14 (26.4) |
| Permanent pacemaker | 13 (12.3) | 9 (17.0) |
| Prior stroke | 9 (8.5) | 6 (11.3) |
| Prior transient ischemic attack | 5 (4.7) | 5 (9.4) |
| Prior GI bleeding | 3 (2.8) | 2 (3.8) |
| LVEF, median (IQR), % | 15 (15-20) | 15 (12-20) |
| Cardiac index, mean (SD), L/min/m2 | 2.1 (0.6) | 2.0 (0.5) |
| Creatinine, mean (SD), mg/dL | 1.4 (0.5) | 1.5 (0.6) |
| INTERMACS profile, No. (%)a | ||
| 1-3 (>Severe) | 83 (78.3) | 46 (86.8) |
| 4-7 (<Severe) | 23 (21.7) | 7 (13.2) |
| NYHA class, No. (%)b | ||
| IIIa | 10 (9.4) | 1 (1.9) |
| IIIb | 21 (19.8) | 11 (20.8) |
| IV | 75 (70.8) | 41 (77.4) |
| Quality of life, mean (SD) | ||
| 12-Item Short-Form Health Survey scorec | ||
| Mental health component | 44.7 (12.4) | 44.3 (11.5) |
| Physical health component | 29.5 (9.9) | 27.9 (9.9) |
| KCCQ overall summary scored | 39.0 (21.1) | 37.1 (20) |
| Medications, No. (%) | ||
| Vasoactive therapy | 88 (83.0) | 45 (84.9) |
| Intropic therapy | 86 (81.1) | 46 (86.8) |
| Aldosterone receptor antagonist | 46 (43.4) | 23 (43.4) |
| β-Blocker | 47 (44.3) | 18 (34.0) |
| ACE inhibitor or ARB | 29 (27.4) | 11 (20.8) |
| Bridge to heart transplant, No. (%) | 66 (62.3) | 34 (64.2) |
| Destination therapy, No. (%)e | 40 (37.7) | 19 (35.8) |
| Operative Characteristics | ||
| Devices, No. (%) | ||
| HVAD | 67 (63.2) | 34 (64.2) |
| HeartMate II | 39 (36.8) | 19 (35.8) |
| Bypass time, mean (SD), min | 102.1 (39.8) | 101.7 (34.1) |
| Tricuspid valve intervention, No. (%) | 21 (19.8) | 11 (20.8) |
Abbreviations: ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blocker; GI, gastrointestinal tract; IQR, interquartile range; INTERMACS, Interagency Registry for Mechanically Assisted Circulatory Support Classification; LVEF, left ventricular ejection fraction; KCCQ, Kansas City Cardiomyopathy Questionnaire; MPCs, mesenchymal precursor cells; NYHA, New York Heart Association.
SI conversion factor: To convert creatinine from mg/dL to μmol/L, multiply by 88.4.
Grade 1 indicates critical cardiogenic shock; 2, progressive decline; 3, stable but inotrope dependent; 4, resting symptoms; 5, exertion intolerant; 6, exertion limited; 7, advanced NYHA III.
Class IIIa indicates moderate congestive heart failure with no symptoms in ordinary physical activity; IIIb, moderate congestive heart failure with mild symptoms in ordinary physical activity; IV, severe congestive heart failure.
A higher score indicates a better health state: 0 (worst) to 100 (best). Values are normed as t scores (mean, 50; SD, 10).
Scores range from 0 (worst) to 100 (best), with higher scores indicating better quality of life, fewer symptoms, and fewer physical limitations associated with heart failure. For example, a patient with a KCCQ summary score of 39 has a poor quality of life.8
Used as a long-term therapy for patients who are not candidates for a heart transplant.
Primary Efficacy and Safety End Points
The posterior probability that MPCs increased the likelihood of successful temporary weans from LVAD support of 3 planned assessments over 6 months was 69%, below the 80% predefined threshold for a positive signal (data were available for 157 patients [98.7%]; 105 in the MPC group and 52 in the control group, and there were no crossovers).
At 2 months, 58 of 99 patients (59%) in the MPC group vs 29 of 50 patients (58%) in the control group were successfully weaned; at 4 months, 63 of 95 patients (66%) in the MPC group vs 25 of 47 control patients (53%), and at 6 months, 58 of 93 patients (62%) in the MPC group vs 26 of 42 control patients (62%). The mean proportion of successful weans over 6 months was 61% in the MPC group and 58% in the control group (rate ratio [RR] for wean success rate, 1.08; 95% CI, 0.83-1.41; P = .55).
More than half of the weans classified as failure were due to patients deemed to be too unstable to attempt a given wean. The primary reasons for such failed weans were suspected or actual pump thrombus (31%), inadequate anticoagulation (14%), or bleeding (4%). In a post hoc sensitivity analysis of the primary outcome that accounted for the effect of site, the results were similar to the crude analysis (RR, 1.09; 95% CI, 0.84-1.42; P = .52). No patient experienced a primary safety end point, ie, infectious myocarditis, myocardial rupture, neoplasm, hypersensitivity reaction, and immune sensitization syndrome over the 1-year follow-up.
Functional Status and Quality of Life
Over 6 months, left ventricular ejection fraction (with full LVAD support) was not significantly different between groups (difference in slopes, 0.22; 95% CI, −0.26 to 0.70; P = .38; eTable 1 and eFigure 1 in Supplement 2). The adjusted mean (imputing zeros for those unable to exercise) 6-minute walk distance did not differ between groups (difference in slopes, 6.36; 95% CI, −47.45 to 60.16; P = .82).
Quality-of-life scores were not significantly different between groups. The mean (SD) change from baseline in the SF-12 mental health score was 9.5 (13.7) for the MPC group vs 7.2 (13.7) for the control group (difference in slopes, 0.32; 95% CI, −0.53 to 1.18; P = .46), and the mean (SD) change in the SF-12 physical health score was 6.9 (12.8) for the MPC group and 11.6 (13.3) for the control group (difference in slopes, −0.69; 95% CI, −1.47 to 0.09; P = .08). The mean (SD) change in the KCCQ overall summary score in the MPC group was 26.6 (28.4) vs 30.8 (22.9) in the control group (difference in slopes, −1.00; 95% CI, −2.60 to 0.61; P = .22; eTable 2 in Supplement 2).
Survival, Hospitalization, and Adverse Events
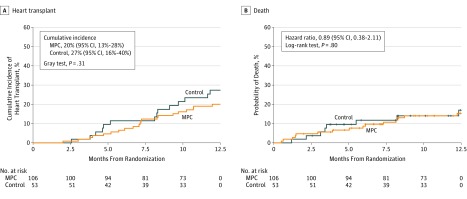
Within 1 year, 27 of 104 patients (26%) in the MPC group experienced sensitization to class I donor-specific HLA antigens (>5000 mean fluorescence intensity [MFI]) vs 5 of 52 patients (9.6%) in the control group (between-group difference, 16.35%; 95% CI, 4.72% to 27.97%). Sensitization to class II HLA system antigens (>1000 MFI scores) occurred in 7 of 104 patients (6.7%) in the MPC group and 6 of 52 patients (11.5%) in the control group (between-group difference, −4.81%; 95% CI, −14.74% to 5.12%). The increase in class I sensitization in the MPC group was not reflected in the median time to heart transplantation, which was 7.1 months (IQR, 5.2-9.3 months) for the MPC group and 8.2 months (IQR, 4.6-9.7 months) for the control group (Figure 2A).
Figure 2. Cumulative Incidence for Transplant and Hazard for Death.
A, Nonparametric estimates of the cumulative incidence functions for heart transplant with death as a competing risk. The P value was computed using the Gray test. The median observation time was 7.1 months (interquartile range [IQR], 5.2-9.3 months) for the mesenchymal precursor cells (MPC) treatment group and 8.2 months (IQR, 4.6-9.7 months) for the control group.
B. Kaplan-Meier estimates of the cumulative hazard for death. The hazard ratio was based on the Cox proportional hazards model and the P value was computed from log-rank test. Median observation time was 5.0 months (IQR, 1.4-8.2 months) for the MPC group and 3.5 months (IQR, 2.6-6.9 months) for the control group.
There was no significant difference in mortality between the MPC group and the control group at 6 months (9.4% vs 11.3%; HR, 0.81; 95% CI; 0.29 to 2.22; P = .68) nor at 12 months (14.2% vs 15.1%; HR, 0.89; 95% CI, 0.38-2.11; P = .80) (Figure 2B). Two patients in the MPC group had their devices removed for recovery at 94 and 141 days after randomization. Both patients were alive and had not undergone a heart transplant at 1 year. No control patients had their devices removed within 1 year.
The median length of stay for the index hospitalization was 20 days in both groups (IQR, 16-34 days for the MPC group; IQR, 16-37 days for the control group). The overall rate of readmissions was not significantly different in MPC and control groups (0.68 vs 0.75 per 100 patient-months; rate difference, −0.07; 95% CI, −0.41 to 0.27; P = .68; Table 2).
Table 2. Serious Adverse Events and Hospitalizations at 6 Months.
| MPC | Control | Rate Difference: MPC vs Control (95% CI)b | P Valueb | |||
|---|---|---|---|---|---|---|
| No. of Events (Rate per 100 Patient-Months)a | No. (%) of Patients | No. of Events (Rate per 100 Patient-Months)a | No. (%) of Patients | |||
| Serious Adverse Eventsc | ||||||
| Bleedingd | 153 (24.68) | 51 (48.1) | 96 (32.39) | 28 (52.8) | −7.72 (−25.41 to 9.97) | .39 |
| Cardiac dysrhythmia | 49 (7.72) | 36 (34) | 20 (6.59) | 17 (32.7) | 1.13 (−2.72 to 4.98) | .57 |
| Sustained ventricular dysrhythmia requiring defibrillation or cardioversion | 34 (5.35) | 26 (24.5) | 9 (2.97) | 8 (15.4) | 2.39 (−0.60 to 5.38) | .12 |
| Sustained supraventricular dysrhythmia requiring drug treatment or cardioversion | 15 (2.36) | 12 (11.3) | 11 (3.62) | 9 (17.3) | −1.26 (−3.97 to 1.44) | .36 |
| Major device malfunction | 22 (3.49) | 16 (15.1) | 9 (2.97) | 5 (9.6) | 0.53 (−2.71 to 3.77) | .75 |
| Pump thrombus confirmed | 11 (1.75) | 10 (9.4) | 5 (1.65) | 3 (5.8) | 0.10 (−2.09 to 2.29) | .93 |
| Pump thrombus suspected | 10 (1.59) | 9 (8.5) | 4 (1.32) | 3 (5.8) | 0.27 (−1.59 to 2.12) | .78 |
| Nonpump thrombus related | 1 (0.16) | 1 (0.9) | 0 | 0 | ||
| Minor device malfunction | 2 (0.32) | 2 (1.9) | 0 | 0 | ||
| Hemolysis | 5 (0.79) | 4 (3.8) | 2 (0.66) | 2 (3.8) | 0.13 (−1.07 to 1.33) | .83 |
| Major infection | 58 (9.13) | 31 (29.2) | 31 (10.21) | 19 (36.5) | −1.08 (−7.19 to 5.03) | .73 |
| Localized nondevice infection | 26 (4.09) | 20 (18.9) | 19 (6.26) | 14 (26.9) | −2.17 (−6.11 to 1.77) | .28 |
| Sepsis | 24 (3.78) | 14 (13.2) | 7 (2.31) | 6 (11.5) | 1.47 (−1.38 to 4.32) | .31 |
| Percutaneous site and/or pocket infection | 8 (1.26) | 6 (5.7) | 5 (1.65) | 5 (9.6) | −0.39 (−2.11 to 1.34) | .66 |
| Neurological dysfunction | 28 (4.41) | 24 (22.6) | 10 (3.29) | 8 (15.4) | 1.11 (−1.73 to 3.96) | .44 |
| Toxic metabolic encephalopathy | 5 (0.79) | 5 (4.7) | 5 (1.65) | 4 (7.7) | −0.86 (−2.64 to 0.92) | .34 |
| Ischemic stroke | 8 (1.26) | 6 (5.7) | 1 (0.33) | 1 (1.9) | 0.93 (−0.36 to 2.23) | .16 |
| Intracranial hemorrhagee | 6 (0.94) | 6 (5.7) | 0 | 0 | ||
| Transient ischemic attack | 4 (0.63) | 3 (2.8) | 1 (0.33) | 1 (1.9) | 0.30 (−0.68 to 1.28) | .55 |
| Other | 5 (0.79) | 5 (4.7) | 3 (0.99) | 3 (5.8) | −0.20 (−1.49 to 1.08) | .76 |
| Renal dysfunctionf | 11 (1.73) | 10 (9.4) | 5 (1.65) | 5 (9.6) | 0.08 (−1.69 to 1.86) | .93 |
| Right heart failure | 23 (3.62) | 19 (17.9) | 16 (5.27) | 10 (19.2) | −1.65 (−6.67 to 3.36)g | .52g |
| All serious adverse eventsg | 450 (70.85) | 88 (83.0) | 239 (78.75) | 44 (84.6) | −7.89 (−39.95 to 24.17)g | .63g |
| Hospitalizationsh | ||||||
| No. of patients | 99 | 49 | ||||
| All cause | 101 (0.68) | 56 (56.6) | 53 (0.75) | 25 (51.0) | −0.07 (−0.41 to 0.27)b | .68b |
| Cardiovascular | 64 (0.43) | 38 (38.4) | 28 (0.39) | 17 (34.7) | 0.03 (−0.21 to 0.28)b | .79b |
Abbreviation: GI, gastrointestinal tract; MPC, mesenchymal precursor cells.
Patient time for bleeding accounts for time at risk for transfusion (MPC patient-months, 620.1; control patient-months, 296.4); major or minor device malfunction patient time accounts for time at risk for patients after left ventricular assist device (LVAD) explant (MPC patient-months, 629.9; control patient-months, 303.5). All other serious adverse events include total patient time at risk within 6 months (MPC patient-months, 635.1; control patient-months, 303.5).
Based on the Poisson model with robust variance estimation for the comparison of rate between the MPC and control groups.
Nonbleeding analyses are based on the safety population (MPC, n = 106; control, n = 52) and bleeding analyses are based on the intention-to-treat population (MPC, n = 106; control, n = 53).
Bleeding includes the following: gastrointestinal tract bleeding or epistaxis, major bleeding, and intraoperative bleeding events.
Intracranial hemorrhage includes subarachnoid, intraventricular, parenchymal, and subdural hemorrhage.
Includes 2 categories of renal dysfunction. Acute renal dysfunction is abnormal kidney function requiring dialysis (including hemofiltration) in patients who did not require this procedure prior to LVAD implant or an increase in serum creatinine of greater than 3 times baseline or greater than 5 mg/dL sustained for over 48 hours. Chronic renal dysfunction is an increase in serum creatinine of 2 mg/dL (to convert creatinine from mg/dL to μmol/L, multiply by 88.4) or higher than the baseline or requirement for hemodialysis, either of which is sustained for at least 90 days.
All serious adverse events are based on the safety population and includes bleeding events.
Excludes patients who died during the index hospitalization or who were not discharged by month 6.
The overall 6-month rate of serious adverse events was not significantly different for the MPC group and the control group (70.9 vs 78.7 per 100 patient-months; rate difference, −7.89; 95% CI, −39.95 to 24.17; P = .63). The most frequent nonbleeding serious adverse events were major infection (13%), cardiac dysrhythmias (10%), neurological dysfunction (6%), and device malfunction (5%), which did not differ between groups (Table 2).
The rates of suspected (rate difference, 0.27; 95% CI, −1.59 to 2.12; P = .78) and confirmed (rate difference, 0.10; 95% CI, −2.09 to 2.29; P = .93) pump thrombosis were not significantly different between groups. The rate of LVAD replacement was 1.6 per 100 patient-months in the MPC group vs 1.0 per 100 patient-months in the control group (rate difference, 0.60; 95% CI, −1.27 to 2.47; P = .53); all LVAD replacements were due to pump thrombosis.
A total of 72 serious gastrointestinal tract bleeding events (n = 68) and epistaxis (4 events) occurred over 6 months. The 6-month cumulative incidence of serious gastrointestinal tract bleed, epistaxis, or both was 17.2% (95% CI, 10.66% to 25.04%) for the MPC group and 32.8% (95% CI, 20.39% to 45.84%) for the control group (P = .02; Figure 3). The rate of serious gastrointestinal tract bleeding, epistaxis, or both during this period was also lower in the MPC group than in the control group (3.8 per 100 patient-months vs 15.9 per 100 patient-months; rate difference, −12.14; 95% CI, −20.90 to −3.39; P = .007).
Figure 3. Cumulative Incidence for Gastrointestinal Tract Bleeding or Epistaxis.
Nonparametric estimates of the cumulative incidence functions for gastrointestinal (GI) tract bleeding or epistaxis with death as a competing risk and the P value was computed from the Gray test. Median observation time was 1.9 months (IQR, 0.9-2.6 months) for the mesenchymal precursor cells (MPC) treatment group and 1.0 months (IQR, 0.4-3.4 months) for the control group. Rate of GI tract bleed or epistaxis for MPC was 24 events (3.79 per 100 patient-months; total patient-months of 632.5) compared with 48 events (15.94 per 100 patient-months; total patient-months of 301.2) in the control (rate difference, −12.14; 95% CI, −20.90 to −3.39; P = .007). There were 18 patients (17%) in the MPC group and 17 patients (32.1%) in the control group who experienced a GI tract bleed or epistaxis.
Intraoperative bleeding was not different between the MPC group and the control group (10.4% vs 11.3%; between-group difference, −0.94%; 95% CI, −11.26% to 9.37%; P = .86). The proportion of patients experiencing major nongastrointestinal tract bleeding was 36% in the MPC group and 40% in the control group, and the event rate was 18.9 per 100 patient-months vs 13.9 per 100 patient-months, respectively (rate difference, 5.04; 95% CI, −8.29 to 18.37; P = .46).
Post Hoc Analyses
The mean proportion of successful weans in patients with ischemic heart failure (n = 69) was 64% in the MPC group vs 43% in the control group (RR, 1.55; 95% CI, 1.01-2.36), whereas in patients who did not have ischemic heart failure, it was 58% in the MPCs group vs 73% in the control group (RR, 0.82; 95% CI, 0.58-1.14; P = .02 for interaction). A subgroup analysis of those who received the implant as a bridge to receiving a heart transplant vs destination therapy patients showed no difference (P = .12 for interaction; eFigure 2 in Supplement 2).
Discussion
In this clinical trial of patients with heart failure, injection of 150 million MPCs did not result in improved left ventricular recovery as assessed by the proportion of successful LVAD weans over 6 months. There are several factors that may have contributed to the lack of a measurable effect in the overall trial population, in addition to the possibility that MPCs may not be an effective therapy for this condition. Cell delivery through direct transepicardial injection into the myocardium may result in significant cell loss and low rates of retention compared with alternative routes of administration.9 The injections of cells at the time of surgery, during a period of heightened inflammation, may also adversely affect cell survival and paracrine signaling. However, there was a signal of benefit in our pilot trial, using the same route and timing of administration.
The literature supports a small rate of spontaneous recovery, largely in patients with nonischemic heart failure, which is typically a younger population in which the underlying disease may be more inherently reversible.10 In an exploratory analysis, however, there was a greater improvement in successful weaning of patients with ischemic heart failure who were injected with MPCs. Given that this trial included patients with nonischemic heart failure etiology (56%), in whom no beneficial weaning effect of cells was seen, the ability to detect an efficacy signal may have been diluted.
No patient experienced a primary safety end point event. However, allosensitization to class I HLA system antigens was higher in patients receiving MPCs, which express only class I system antigens. The donor cells used in this study had HLA system antigens with a high prevalence in the US population. Nevertheless, time to heart transplant was not significantly different between groups. The design of future trials involving bridge-to-transplant patients should use MPC donors with less prevalent HLA system antigens, especially if they include repeat dosing. Overall survival and serious adverse event rates were similar to those reported from previous trials of LVADs used in the study.11,12
Mucosal bleeding is a frequent and morbid complication of LVAD therapy, observed in 30% to 70% of patients depending on age and presence of other important risk factors.13 Most bleeding events occur in the first 3 months after LVAD implant. In this trial, MPC injections were associated with a delay in the time to first serious gastrointestinal tract bleed and epistaxis and with a significant and clinically meaningful decrease in the rate of these events. These exploratory findings are consistent with the pilot trial, which showed a reduction in overall major bleeding rates.5 Although mucosal bleeding has been associated with increases in venous pressure, secondary hepatic dysfunction, or both, the reduction in mucosal bleeding seen with MPCs in our trial was not associated with a decrease in the rate of right heart failure or a qualitative improvement in right ventricular function.14 Mucosal bleeding in patients with LVAD relates to several other processes including anticoagulation, cytokine release, and acquired von Willebrand disease. There is excessive platelet release of angiopoietin-2 and reversal of the normal angiopoietin-1 to angiopoietin-2 ratio, which, in turn, promotes vessel instability, permeability, endothelial destabilization, and angiogenesis.15,16 Angiopoietin-1 functions in a counterregulatory fashion to maintain vessel stability, and MPCs are pericyte precursors, which produce high levels of angiopoietin-1.17
Limitations
This study has several limitations. First, it is well recognized that efficacy end points, such as functional status, echocardiographic parameters, and readmissions, as used in traditional heart failure trials, may not apply equally well to patients receiving mechanical circulatory support. The trial’s primary end point focused on the tolerance of temporary weaning from LVAD support. The observed high rate of suspected pump thrombosis affected the ability to wean patients, thus limiting the ability to interpret the primary end point.
Second, in an effort to increase generalizability, enrollment included a wide spectrum of patients (heart failure etiology, age range, LVAD indications) receiving 1 of 2 LVAD types (axial and centrifugal flow), which in a relatively small trial may have increased variability and reduced the likelihood of detecting a signal of treatment effect.
Third, although all bleeding events and transfusions were adjudicated, international normalized ratio values or platelet counts were not systematically collected nor were anticoagulation regimens. However, nongastrointestinal tract bleeding events did not differ between groups, which suggest that the lower rate of gastrointestinal tract bleeds or epistaxis was not due solely to reduced anticoagulation.
Conclusions
Among patients with advanced heart failure, intramyocardial injections of mesenchymal precursor cells, compared with injections of a cryoprotective medium as sham treatment, did not improve successful temporary weaning from left ventricular assist device support at 6 months. The findings do not support the use of intramyocardial mesenchymal stem cells to promote cardiac recovery as measured by temporary weaning from device support.
Trial protocol and statistical data plan
eTable 1. Echocardiographic assessments
eFigure 1. Echocardiographic parameters over time
eTable 2. Quality of life
eFigure 2. Wean success rate in subgroup analyses
Data Sharing Statement
References
- 1.Suncion VY, Ghersin E, Fishman JE, et al. Does transendocardial injection of mesenchymal stem cells improve myocardial function locally or globally? an analysis from the Percutaneous Stem Cell Injection Delivery Effects on Neomyogenesis (POSEIDON) randomized trial. Circ Res. 2014;114(8):1292-1301. doi: 10.1161/CIRCRESAHA.114.302854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu R, Ding S, Zhao Y, Pu J, He B. Autologous transplantation of bone marrow/blood-derived cells for chronic ischemic heart disease: a systematic review and meta-analysis. Can J Cardiol. 2014;30(11):1370-1377. doi: 10.1016/j.cjca.2014.01.013 [DOI] [PubMed] [Google Scholar]
- 3.Karantalis V, DiFede DL, Gerstenblith G, et al. Autologous mesenchymal stem cells produce concordant improvements in regional function, tissue perfusion, and fibrotic burden when administered to patients undergoing coronary artery bypass grafting: the Prospective Randomized Study of Mesenchymal Stem Cell Therapy in Patients Undergoing Cardiac Surgery (PROMETHEUS) trial. Circ Res. 2014;114(8):1302-1310. doi: 10.1161/CIRCRESAHA.114.303180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heldman AW, DiFede DL, Fishman JE, et al. Transendocardial mesenchymal stem cells and mononuclear bone marrow cells for ischemic cardiomyopathy: the TAC-HFT randomized trial. JAMA. 2014;311(1):62-73. doi: 10.1001/jama.2013.282909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ascheim DD, Gelijns AC, Goldstein D, et al. Mesenchymal precursor cells as adjunctive therapy in recipients of contemporary left ventricular assist devices. Circulation. 2014;129(22):2287-2296. doi: 10.1161/CIRCULATIONAHA.113.007412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.See F, Seki T, Psaltis PJ, et al. Therapeutic effects of human STRO-3-selected mesenchymal precursor cells and their soluble factors in experimental myocardial ischemia. J Cell Mol Med. 2011;15(10):2117-2129. doi: 10.1111/j.1582-4934.2010.01241.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perin EC, Borow KM, Silva GV, et al. A phase II dose-escalation study of allogeneic mesenchymal precursor cells in patients with ischemic or nonischemic heart failure. Circ Res. 2015;117(6):576-584. doi: 10.1161/CIRCRESAHA.115.306332 [DOI] [PubMed] [Google Scholar]
- 8.Arnold SV, Spertus JA, Vemulapalli S, et al. Quality-of-life outcomes after transcatheter aortic valve replacement in an unselected population: a report from the STS/ACC Transcatheter Valve Therapy Registry. JAMA Cardiol. 2017;2(4):409-416. doi: 10.1001/jamacardio.2016.5302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kanelidis AJ, Premer C, Lopez J, Balkan W, Hare JM. Route of delivery modulates the efficacy of mesenchymal stem cell therapy for myocardial infarction: a meta-analysis of preclinical studies and clinical trials. Circ Res. 2017;120(7):1139-1150. doi: 10.1161/CIRCRESAHA.116.309819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Birks EJ, George RS, Hedger M, et al. Reversal of severe heart failure with a continuous-flow left ventricular assist device and pharmacological therapy: a prospective study. Circulation. 2011;123(4):381-390. doi: 10.1161/CIRCULATIONAHA.109.933960 [DOI] [PubMed] [Google Scholar]
- 11.Mehra MR, Goldstein DJ, Uriel N, et al. ; MOMENTUM 3 Investigators . MOMENTUM 3 Investigators. Two-year outcomes with a magnetically levitated cardiac pump in heart failure. N Engl J Med. 2018;378(15):1386-1395. doi: 10.1056/NEJMoa1800866 [DOI] [PubMed] [Google Scholar]
- 12.Rogers JG, Pagani FD, Tatooles AJ, et al. Intrapericardial left ventricular assist device for advanced heart failure. N Engl J Med. 2017;376(5):451-460. doi: 10.1056/NEJMoa1602954 [DOI] [PubMed] [Google Scholar]
- 13.Kataria R, Jorde UP. GI bleeding during continuous-flow left ventricular assist device support: state of the field. Cardiol Rev. 2019;27(1):8-13. doi: 10.1097/CRD.0000000000000212 [DOI] [PubMed] [Google Scholar]
- 14.Balcioglu O, Kemal HS, Ertugay S, et al. Risk factors of gastrointestinal bleeding after continuous flow left ventricular assist device. ASAIO J. 2018;64(4):458-461. doi: 10.1097/MAT.0000000000000678 [DOI] [PubMed] [Google Scholar]
- 15.Tabit CE, Coplan MJ, Chen P, Jeevanandam V, Uriel N, Liao JK. Tumor necrosis factor-α levels and non-surgical bleeding in continuous-flow left ventricular assist devices. J Heart Lung Transplant. 2018;37(1):107-115. doi: 10.1016/j.healun.2017.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tabit CE, Chen P, Kim GH, et al. Elevated angiopoietin-2 level in patients with continuous-flow left ventricular assist devices leads to altered angiogenesis and is associated with higher nonsurgical bleeding. Circulation. 2016;134(2):141-152. doi: 10.1161/CIRCULATIONAHA.115.019692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fang X, Neyrinck AP, Matthay MA, Lee JW. Allogeneic human mesenchymal stem cells restore epithelial protein permeability in cultured human alveolar type II cells by secretion of angiopoietin-1. J Biol Chem. 2010;285(34):26211-26222. doi: 10.1074/jbc.M110.119917 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol and statistical data plan
eTable 1. Echocardiographic assessments
eFigure 1. Echocardiographic parameters over time
eTable 2. Quality of life
eFigure 2. Wean success rate in subgroup analyses
Data Sharing Statement