Key Points
Question
Does a ventilation strategy that involves sustained inflations, compared with standard intermittent positive pressure ventilation, reduce the risk of bronchopulmonary dysplasia or death among extremely preterm infants?
Findings
In this randomized clinical trial involving 426 infants that was stopped early due to suggestion of harm in the sustained inflation group, there was no significant difference in the rate of bronchopulmonary dysplasia or death at 36 weeks’ postmenstrual age for infants treated with sustained inflation vs standard resuscitation (63.7% vs 59.2%).
Meaning
These findings do not support the use of a ventilation strategy involving sustained inflations among extremely preterm infants, although early termination of the trial limits definitive conclusions.
Abstract
Importance
Preterm infants must establish regular respirations at delivery. Sustained inflations may establish lung volume faster than short inflations.
Objective
To determine whether a ventilation strategy including sustained inflations, compared with standard intermittent positive pressure ventilation, reduces bronchopulmonary dysplasia (BPD) or death at 36 weeks’ postmenstrual age without harm in extremely preterm infants.
Design, Setting, and Participants
Unmasked, randomized clinical trial (August 2014 to September 2017, with follow-up to February 15, 2018) conducted in 18 neonatal intensive care units in 9 countries. Preterm infants 23 to 26 weeks’ gestational age requiring resuscitation with inadequate respiratory effort or bradycardia were enrolled. Planned enrollment was 600 infants. The trial was stopped after enrolling 426 infants, following a prespecified review of adverse outcomes.
Interventions
The experimental intervention was up to 2 sustained inflations at maximal peak pressure of 25 cm H2O for 15 seconds using a T-piece and mask (n = 215); standard resuscitation was intermittent positive pressure ventilation (n = 211).
Main Outcome and Measures
The primary outcome was the rate of BPD or death at 36 weeks’ postmenstrual age. There were 27 prespecified secondary efficacy outcomes and 7 safety outcomes, including death at less than 48 hours.
Results
Among 460 infants randomized (mean [SD] gestational age, 25.30 [0.97] weeks; 50.2% female), 426 infants (92.6%) completed the trial. In the sustained inflation group, 137 infants (63.7%) died or survived with BPD vs 125 infants (59.2%) in the standard resuscitation group (adjusted risk difference [aRD], 4.7% [95% CI, −3.8% to 13.1%]; P = .29). Death at less than 48 hours of age occurred in 16 infants (7.4%) in the sustained inflation group vs 3 infants (1.4%) in the standard resuscitation group (aRD, 5.6% [95% CI, 2.1% to 9.1%]; P = .002). Blinded adjudication detected an imbalance of rates of early death possibly attributable to resuscitation (sustained inflation: 11/16; standard resuscitation: 1/3). Of 27 secondary efficacy outcomes assessed by 36 weeks’ postmenstrual age, 26 showed no significant difference between groups.
Conclusions and Relevance
Among extremely preterm infants requiring resuscitation at birth, a ventilation strategy involving 2 sustained inflations, compared with standard intermittent positive pressure ventilation, did not reduce the risk of BPD or death at 36 weeks’ postmenstrual age. These findings do not support the use of ventilation with sustained inflations among extremely preterm infants, although early termination of the trial limits definitive conclusions.
Trial Registration
clinicaltrials.gov Identifier: NCT02139800
This randomized trial compares the effects of 2 15-second sustained inflations vs standard intermittent positive pressure ventilation on the risk of bronchopulmonary dysplasia or death at 36 weeks’ postmenstrual age in extremely preterm infants.
Introduction
Preterm infants with weak respiratory muscles and liquid-filled lungs1,2 struggle to aerate their lungs. A meta-analysis of randomized trials comparing noninvasive respiratory support in the delivery room with continuous positive airway pressure (CPAP) against intubation and ventilation showed CPAP was associated with a reduced risk of bronchopulmonary dysplasia (BPD) or death.3 A longer inspiratory time during positive pressure ventilation, 5 seconds vs 1 second, facilitated spontaneous respiration in full-term infants.4 Establishing adequate lung volume quickly may reduce the risk of BPD. Sustained inflations appeared beneficial in animal models.5,6
Randomized trials of sustained inflations (>5 seconds’ duration) are limited, especially for preterm infants between 23 to 26 weeks’ gestational age. Pooled data of randomized trials totaling 564 infants showed sustained inflations were associated with reduced need for mechanical ventilation.7 Another meta-analysis of 941 infants found no benefit on mortality.8 In infants younger than 30 weeks’ gestation, sustained inflations are used frequently in Europe9 but uncommonly in the United States. The European Resuscitation Council recommends up to 5 inflation breaths of 2 to 3 seconds if an infant is apneic or gasping.10 However, the International Liaison Committee on Resuscitation concluded more data are needed.11
This study evaluated the hypothesis that sustained inflations, compared with standard intermittent positive pressure ventilation, reduces the risk of BPD or death at 36 weeks’ postmenstrual age without increasing harm in extremely preterm infants.
Methods
Study Design
The Sustained Aeration for Infant Lungs (SAIL) Trial was a pragmatic, unblinded, randomized parallel-group trial in 18 neonatal intensive care units in 9 countries (United States, Australia, the Netherlands, Canada, Germany, Italy, Austria, South Korea, and Singapore; August 2014-September 2017). The protocol has been published12 and is available in Supplement 1. The statistical analysis plan is in Supplement 2.
Institutional review boards (IRBs) at each center approved the study. Two methods were used to obtain written informed consent by the local study team. At 12 sites, women likely to deliver in the gestational age window were approached for antenatal consent, unless delivery was imminent. Institutional review boards at 6 sites endorsed a deferred consent process (Royal Women’s Hospital, Melbourne, Australia; Academic Teaching Hospital, Landeskrankenhaus Feldkirch, Feldkirch, Austria; Royal Alexandra Hospital, Edmonton, Canada; Leiden University Medical Center, Leiden, the Netherlands; Emma Children’s Hospital, Amsterdam, the Netherlands; and Seoul National University Medical Center, Seoul, South Korea). Four IRBs allowed this only when antenatal consent was impossible because of insufficient time or maternal condition; 2 sites only obtained deferred consent. Researchers and IRBs at these sites considered the deferred approach appropriate for 2 reasons. First, sustained inflation was standard care at some sites. Second, the generalizability of results obtained using antenatal consent is questioned.13 Regulations in these countries allowed deferred consent, and the Eunice Kennedy Shriver National Institute of Child Health and Human Development and data and safety monitoring committee (DSMC) reviewed consent procedures. Investigators and delegates obtained written consent and addressed any questions from the mother after delivery.
Participants
Infants between 23 weeks 0 days’ and 26 weeks 6 days’ gestational age were eligible if they required positive pressure resuscitation because of inadequate respiratory effort or a heart rate less than 100 beats per minute (bpm).10,11,14,15 Infants deemed nonviable or with major anomalies (including pulmonary hypoplasia with oligohydramnios) were excluded. Self-identified maternal race/ethnicity was recorded, as required by the National Institutes of Health.
Randomization
The trial used computer-generated permuted block randomization, with variable block sizes of 2, 4, or 6, stratified by site and gestational age (23 weeks-24weeks and 6 days; and 25weeks-26 weeks and 6 days). Sealed opaque envelopes, color coded by gestational age strata, were opened on confirming eligibility. Multiple births were randomized to the same group.
Delivery Room Intervention
eFigure 1 in Supplement 3 shows implementation of study interventions. Site education, including online videos, encouraged predelivery huddles to allocate roles of team members. Cord clamping followed local practice. Placement of eligible infants on a resuscitation bed became the reference time point, timed by a study team member or a caregiver. Ensuing routine care followed international guidelines,14 including those of the Neonatal Resuscitation Program,15 advising plastic wrapping, stimulation, and oxygen saturation monitoring. Determination of heart rate was mandated and ascertained using at least 2 of the following methods: pulse oximetry, electrocardiogram, palpation, or auscultation. Airway assessment and clearing was followed by initial CPAP of 5 to 7 cm H2O using locally preferred interfaces. Within 30 seconds, if caregivers assessed the infant as meeting criteria for further intervention, the randomization envelope was opened.
In the sustained inflation group, a sustained inflation lasting 15 seconds at a peak pressure of 20 cm H2O was given, followed if necessary by a second sustained inflation of 15 seconds at a peak of 25 cm H2O; both were delivered noninvasively by either a face mask or a nasopharyngeal tube (as unit protocol dictated) attached to a T-piece resuscitator. Investigators and external experts determined the parameters for the sustained inflations because optimal duration and pressure of sustained inflations are uncertain.16 The standard resuscitation group received intermittent positive pressure ventilation with positive end-expiratory pressure.
Otherwise, resuscitation was conducted according to local unit protocol. In both groups, standard ventilation corrective steps were undertaken if the infant did not respond. Following the experimental procedure, care reverted to standard pathways.
Respiratory Management After Delivery
Criteria for intubation in the delivery room followed recommendations.10,11,14,15 Extubation was recommended within 24 hours of meeting all the following criteria: Pco2 of 55 mm Hg or less, pH of 7.25 or greater, fraction of inspired oxygen (Fio2) of 0.40 or less with oxygen saturation as measured by pulse oximetry (Spo2) of 88% or greater, mean airway pressure of 8 cm H2O or less with hemodynamic stability, and receiving caffeine. Intubation criteria included any of Fio2 of 0.50 or greater to maintain Spo2 of 88% or greater, pH of 7.22 or less, Pco2 of 70 mm Hg or greater, more than 1 apneic event requiring intermittent positive pressure ventilation within 6 hours, 6 or more apneic events requiring stimulation within 6 hours, cardiovascular instability, or need for surgery.
Primary Outcome
The primary outcome was a composite of death or survival with BPD by 36 weeks’ postmenstrual age. On-site investigators provided best estimates of causes of death. Bronchopulmonary dysplasia was defined as the receipt of any form of positive airway pressure support or supplemental oxygen at 36 weeks.17 Supplemental oxygen requirement was defined as an Fio2 of 0.30 or greater, or by an oxygen reduction test18 if the Fio2 was 0.22 to 0.29, performed by nonstudy personnel. Infants were classified as having BPD if during stepwise reductions in the inspired Fio2, they displayed symptoms (apnea >20 seconds or heart rate <80 bpm for >10 seconds) or an oxygen saturation of less than 88. For infants missing oxygen reduction tests (eg, transferred prior to 36 weeks to a facility outside of the trial sites or oversight), BPD was defined as any supplemental oxygen use at 36 weeks’ postmenstrual age.
Secondary Outcomes
Prespecified secondary outcomes covered efficacy (n = 27) and safety (n = 7), and 5 DSMC-requested adverse events. These are listed in eMethods 1 in Supplement 3. Efficacy outcomes included the 2 individual components of the primary outcome at 36 weeks’ postmenstrual age; respiratory outcomes following delivery; outcomes reflecting intensity of support by 48 hours’ age, 7 days, and 10 days; retinopathy of prematurity stage 3 or greater; death in hospital; survival without morbidity; length of stay; use of postnatal steroids; and duration of respiratory support at discharge. Safety outcomes included death within 48 hours; oxygen requirements of 40% or more within 48 hours; rates of pneumothorax, pulmonary interstitial emphysema (air leaks), or pneumopericardium in the first 10 days; grade 3 or 4 intraventricular hemorrhage within the first 10 days; and any other serious adverse events. Exploratory outcomes included surfactant, need for mechanical ventilation over the first 14 days after birth, necrotizing enterocolitis, retinopathy of prematurity of any grade, intraventricular hemorrhage of any grade,19 pulmonary hemorrhage, and patent ductus arteriosus. Neurodevelopmental and respiratory outcomes at 22 to 26 months’ age are still being collected and are not reported here.
Statistical Analysis
Recent data of 2 randomized trials evaluating CPAP in the delivery room targeted absolute reductions in primary outcomes of 10%.20,21 However, because CPAP is less technically demanding than sustained aeration, we targeted an absolute reduction of 12.5%. Estimating event rates in the standard resuscitation group at 65%, to detect a 12.5% absolute reduction in the rate of death or BPD between the 2 groups at 80% power and 2-sided α = .038 (with 2 interim analyses), 263 infants per group were required. This was inflated by 1.12 for clustering of multiple births with a final number of 592; recruitment targeted 300 infants per group. Preplanned DSMC adverse event review when 10 infants were enrolled was to be followed by further review at 100, 200, and 400 infants for harm and 200 and 400 infants for efficacy. O’Brien-Fleming boundaries for the primary outcome were specified as α = .0002 at 200 infants, α = .012 at 400 infants, and α = .038 at 600 infants for 2-sided tests.
The primary outcome was analyzed as randomized in all consented and randomized infants, blinded to allocation. Generalized estimating equations (GEEs) compared the 2 groups to control for clustering within multiple births. Analyses were planned to be adjusted for gestational age, site, infant sex, small for gestational age, initial heart rate, maternal corticosteroid use, and consent type used. The marginal probability of death or BPD in each group was computed from the GEE model and compared using adjusted risk differences (aRDs) and relative risks (RRs).22
Secondary outcomes, including adverse events, were also analyzed using GEE models and aRDs with 95% CIs. Event rates per 100 infant days were calculated based on the number of days an infant was at risk for each outcome. GEE-based Poisson regressions computed incidence rate ratios with 95% CIs and P values.
A post hoc sensitivity analysis for the primary outcome was performed excluding infants not receiving an oxygen reduction test. Post hoc subgroup analyses of prognostic factors (infant sex, gestational age, maternal corticosteroid use, consent type used, and continent of site [because the intervention was more frequently used in Europe]) used tests for interactions to determine effect modification on observed treatment differences. Post hoc Kaplan-Meier survival curves were generated for the secondary outcome of death and compared between groups using log-rank tests.
After early stopping, the DSMC requested a post hoc Bayesian analysis to assess outcomes as if full recruitment had been completed. We used the predictive probabilities software for this purpose.23 Interim results from the study as of January 2018, with hypothetical prior estimates of mortality risk in each group (estimated from varying hypothetical sample sizes), estimated the probability distribution of possible outcomes (favor sustained inflation, favor standard resuscitation, or indeterminant) under full recruitment. (For further analytical methods, see eMethods 2 in Supplement 3.)
Analyses, except post hoc Bayesian ones, were conducted using Stata version 15.1 (StataCorp). A 2-sided P < .05 defined statistical significance. No adjustments for multiple comparisons were made so secondary outcome results should be interpreted as exploratory.
Data and Safety Monitoring Committee
Prior to trial commencement, a DSMC was appointed and consisted of 3 neonatologists with expertise in resuscitation and/or ethics, a trial statistician, and a representative of the National Institute of Child Health and Human Development. A priori stopping rules for adverse events and efficacy were established. To detect early harm, events in the first 10 days of life were monitored, including early death defined as occurring in the first 48 hours and related events within the first few days of delivery (pneumothorax, pulmonary interstitial emphysema [air leaks], chest compressions, need for epinephrine, pulmonary hemorrhage, and intraventricular hemorrhage). The DSMC blindly reviewed all early deaths to assess any possible relationship to the allocation group.
The study was temporarily halted in September 2017 after the third DSMC review. In January 2018, after all randomized infants had reached 36 weeks’ postmenstrual age, the DSMC reconvened. The trial was closed to recruitment due to harm as detected by a preplanned analysis of early deaths.
Results
Participants
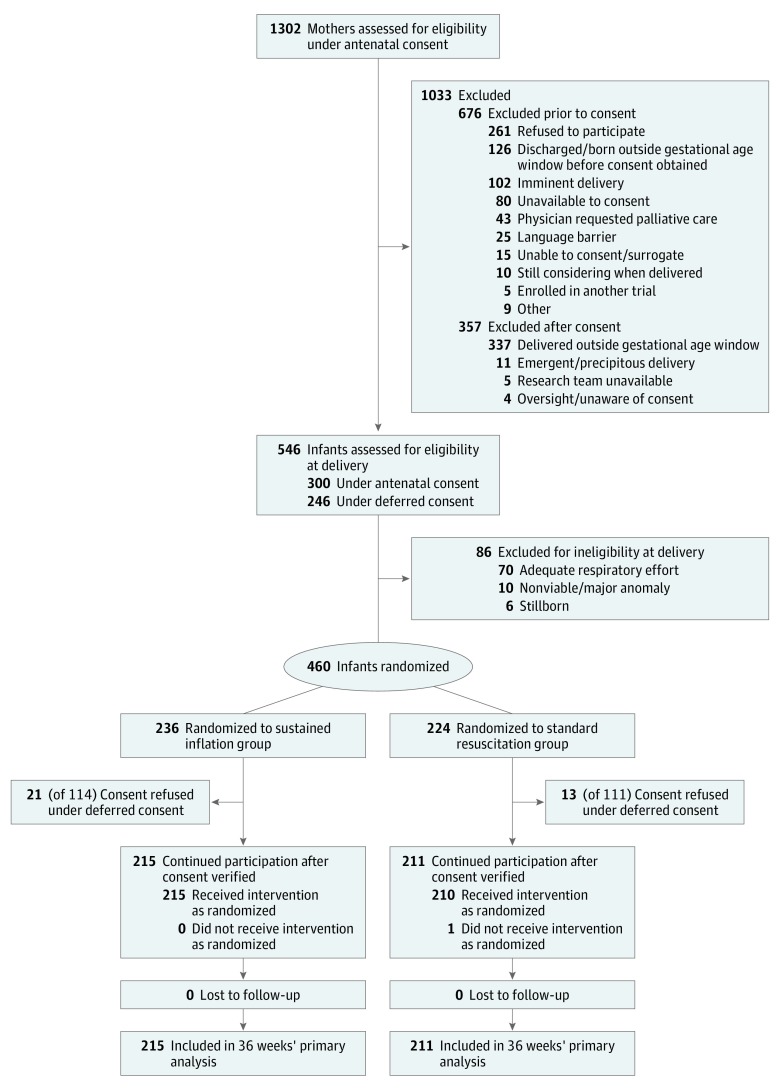
The determination of the primary outcome in the last infant occurred on November 26, 2017, and the last infant was discharged from a study site on February 15, 2018. A total of 215 infants were randomized to the sustained inflation group and 211 to the standard group. Of 426 enrolled infants, antenatal consent was obtained for 235 and deferred consent for 191 (Figure 1). Of 300 antenatally consented infants assessed for eligibility, 65 were ineligible at delivery, while in the deferred consent process, of 225 eligible infants randomized, 34 infants were excluded following refusal of consent. Proportions of consent types were similar by group. Demographic and clinical characteristics of the mothers and infants were similar in the 2 groups (Table 1). Exposure to any antenatal corticosteroids was high—more than 96% in both groups; however, fewer mothers in both groups received full courses (78.1% in the sustained inflation vs 78.2% in the standard resuscitation group). Median birth weight was similar between groups. Most infants were in the 25- to 26-week gestational age stratum (64.7% in the sustained inflation and 64.5% in the standard resuscitation group), and the overall mean (SD) gestational age was 25.30 (0.97) weeks. In the sustained inflation group (n = 215), 27 infants received 1 sustained inflation and 188 received 2 sustained inflations.
Figure 1. Patient Recruitment, Randomization, and Retention.
In the antenatal consent procedure, the informed-consent rate was 48% of women assessed for potential eligibility (626 of 1302 screened). Three hundred infants from 269 mothers for whom consent was given antenatally were assessed for eligibility. Of the infants delivered within the gestational age inclusion dates, but who could not be randomized (n = 65), most being infants with adequate respiration (n = 52). In centers undergoing a deferred-consent procedure, of 246 infants assessed for eligibility having met inclusion criteria, 18 had adequate respiration at birth and were not randomized.
Table 1. Maternal and Infant Characteristics by Randomized Group.
| Resuscitation, No. (%) | ||
|---|---|---|
| Sustained Inflation (n = 215) | Standard (n = 211) |
|
| Consent type | ||
| Antenatal | 122 (56.7) | 113 (53.6) |
| Deferred | 93 (43.3) | 98 (46.4) |
| Maternal age, median (IQR), y | 31.1 (26.5-35.4) | 30.5 (26.0-34.9) |
| Maternal race/ethnicity | ||
| White | 148 (68.8) | 110 (52.1) |
| Black | 29 (13.5) | 50 (23.7) |
| Asian | 18 (8.4) | 23 (10.9) |
| Othera | 20 (9.3) | 28 (13.3) |
| Receipt of antenatal corticosteroids | ||
| Any | 208 (96.7) | 205 (97.2) |
| Full course | 168 (78.1) | 165 (78.2) |
| Placental abruption | 33 (15.3) | 28 (13.3) |
| Chorioamnionitis (defined clinically) | 81 (37.7) | 68 (32.2) |
| Mode of delivery | ||
| Vaginal vertex | 68 (31.7) | 62 (29.4) |
| Vaginal breech | 13 (6.0) | 15 (7.1) |
| Cesarean delivery | 134 (62.3) | 134 (63.5) |
| Infant hemoglobin, median (IQR), g/dLb | 13.7 (12.2-15.0) | 13.9 (12.4-15.4) |
| Infant sex | ||
| Male | 119 (55.3) | 103 (48.8) |
| Female | 96 (44.7) | 108 (51.2) |
| Gestational age | ||
| 23- to 24-wk stratum | 76 (35.3) | 75 (35.5) |
| 25- to 26-wk stratum | 139 (64.7) | 136 (64.5) |
| Birth weight, median (IQR), g | 725 (620-855) | 731 (630-845) |
| Proportion <10th centile birth weightc | 28 (13.0) | 25 (11.8) |
| Time of cord clamping, sd | ||
| Immediate: 0-15 | 138 (64.2) | 138 (65.4) |
| Intermediate: >15-30 | 42 (19.5) | 30 (14.2) |
| Delayed: >30 | 35 (16.3) | 43 (20.4) |
| Multiple birth status | ||
| Single | 158 (73.5) | 153 (72.5) |
| Twin | 54 (25.1) | 54 (25.6) |
| Triplet | 3 (1.4) | 4 (1.9) |
| Sitee | ||
| 1 | 35 (16.3) | 32 (15.2) |
| 2 | 30 (14.0) | 35 (16.6) |
| 3 | 24 (11.2) | 24 (11.4) |
| 4 | 20 (9.3) | 23 (10.9) |
| 5 | 21 (9.8) | 18 (8.5) |
| 6 | 16 (7.4) | 17 (8.1) |
| 7 | 15 (7.0) | 13 (6.2) |
| 8 | 13 (6.0) | 11 (5.2) |
| 9 | 11 (5.1) | 10 (4.7) |
| 10 | 7 (3.3) | 7 (3.3) |
| 11 | 6 (2.8) | 7 (3.3) |
| 12-18 | 17 (7.9) | 14 (6.6) |
Abbreviation: IQR, interquartile range.
Other race/ethnicity consisted of mixed (n = 7), North American Indian (n = 6), Hawaiian/Pacific Islander (n = 3), or not specified/unknown (n = 32).
Infant hemoglobin levels were measured via blood gas in the delivery room.
Centile weights were adjusted for gestational age and sex, as per Kramer et al.24
Timing of all study interventions was relative to the time the infant was placed on the resuscitation bed. The timing of umbilical cord clamping after birth was performed per local clinical practice and recorded by the clinical team.
Site ordered by recruitment numbers. Sites 12-18 contributed less than 3% of patients in each group. Sites 19-22 did not recruit any eligible infants.
Primary Outcomes
All infants had the outcome of death available, and 11 of 348 infants (3.2%) alive at 36 weeks had missing oxygen reduction tests needed for BPD determination. The rate of the primary outcome consisting of death or BPD at 36 weeks’ postmenstrual age in the sustained inflation group (63.7%) did not significantly differ from the standard resuscitation group (59.2%) (aRD, 4.7% [95% CI, −3.8% to 13.1%]; P = .29) (Table 2).
Table 2. Primary Composite Outcome and Component Secondary Outcomes at 36 Weeks’ Postmenstrual Age.
| Outcome | Resuscitation, No. (%) | Adjusted Risk Difference, % (95% CI)a |
Adjusted Relative Risk (95% CI) | P Valueb | |
|---|---|---|---|---|---|
| Sustained Inflation (n = 215) | Standard (n = 211) | ||||
| Death or bronchopulmonary dysplasia | 137 (63.7) | 125 (59.2) | 4.7 (−3.8 to 13.1) | 1.1 (0.9 to 1.2) | .29 |
| Death | 45 (20.9) | 33 (15.6) | 5.2 (−2.3 to 12.7) | 1.3 (0.9 to 1.9) | .17 |
| Bronchopulmonary dysplasia | 92 (42.8) | 92 (43.6) | 0.5 (−8.5 to 9.4) | 1.0 (0.8 to 1.2) | .92 |
Risk difference of sustained inflation − standard resuscitation calculated using marginal probabilities from adjusted generalized estimating equation models. Covariates adjusted for in all models were gestational age, site, infant sex, maternal corticosteroid use, initial heart rate, small for gestational age, and consent type used. Also adjusted for the correlation between infants from multiple births.
P value from comparison of adjusted risk difference.
Secondary Outcomes
The secondary outcomes of the components of the primary outcome were not statistically significantly different between groups: death at 36 weeks’ postmenstrual age (20.9% in the sustained inflation vs 15.6% in the standard resuscitation group; aRD, 5.2% [95% CI, −2.3% to 12.7%]) and BPD (42.8% in the sustained inflation vs 43.6% in the standard resuscitation group; aRD, 0.5% [95% CI, −8.5% to 9.4%]) (Table 2). Of the other prespecified secondary efficacy outcomes (Table 3), only heart rate less than 60 bpm after the first resuscitation maneuver was statistically significantly different between groups (23.4% in the sustained inflation vs 11.4% in the standard resuscitation group; aRD 24.7% [95% CI, 12.0%-37.5%]; P < .001).
Table 3. Adjusted Comparisons of Prespecified Secondary Efficacy Outcomes by Group.
| Outcome | Resuscitation, No. (%) | Adjusted Risk Difference, % (95% CI)a | P Valueb | |
|---|---|---|---|---|
| Sustained Inflation (n = 215) | Standard (n = 211) | |||
| Delivery Room | ||||
| Heart rate after first resuscitation maneuver, bpm | ||||
| <60 | 50 (23.4) | 24 (11.4) | 24.7 (12.0 to 37.5) | <.001 |
| 60-100 | 110 (51.4) | 99 (47.1) | 12.6 (2.7 to 22.6) | |
| >100 | 54 (25.2) | 87 (41.4) | [Reference] | |
| Intubation in delivery room | 111 (51.6) | 119 (56.4) | −4.3 (−12.0 to 3.5) | .28 |
| Reason for intubation in delivery room | ||||
| Respiratory distress | 43 (38.7) | 45 (37.8) | ||
| Resuscitation | 52 (46.8) | 51 (42.9) | ||
| Persistent apnea after stabilization | 7 (6.3) | 17 (14.3) | ||
| Surfactant administration | 7 (6.3) | 4 (3.4) | ||
| Other | 2 (1.8) | 2 (1.7) | ||
| Final respiratory supportc | ||||
| CPAP | 88 (41.7) | 88 (41.9) | −1.7 (−9.8 to 6.4) | .68 |
| PPV | 116 (55.0) | 114 (54.3) | [Reference] | |
| Other | 7 (3.3) | 8 (3.8) | NA | |
| Final pressure-volume characteristics, mean (SD)c | ||||
| CPAP, cm H2O | 6.4 (1.0) | 6.4 (1.0) | ||
| PPV PIP | 20.1 (5.5) | 19.2 (4.9) | ||
| PPV PEEP | 5.7 (0.8) | 5.4 (0.7) | ||
| PPV frequency | 48.7 (11.5) | 47.2 (11.0) | ||
| Final Fio2 ≥0.4c,d | 69 (32.7) | 62 (29.5) | 0.8 (−8.4 to 10.0) | .87 |
| First 48 h of Life | ||||
| Inotrope administered on DOL 1 | 36 (16.7) | 24 (11.4) | 5.3 (−1.3 to 11.9) | .12 |
| Pneumothoraxe | 10 (4.7) | 11 (5.2) | −1.5 (−7.6 to 4.6) | .63 |
| Need for new chest drains | 6 (2.8) | 7 (3.3) | −1.8 (−6.7 to 3.2)f | .49 |
| Oxygen requirement of Fio2 ≥0.4 for ≥2 h, No./total No. (%)d,g | 64/196 (32.7) | 67/192 (34.9) | −2.3 (−11.5 to 7.0) | .63 |
| Highest Fio2 level recorded after deliveryd | ||||
| No. | 198 | 193 | ||
| Median (range), fraction | 0.42 (0.21 to 1.00) | 0.45 (0.21 to 1.00) | ||
| Intubation in delivery room or during first 48 h of life | 153 (71.2) | 154 (73.0) | −2.1 (−10.4 to 6.1) | .61 |
| First 7 d of Life | ||||
| Death or ventilation on DOL 7, No./total No. (%)g | 108/198 (54.5) | 105 (53.3) | 0.5 (−8.0 to 9.1) | .90 |
| First 10 d of Life | ||||
| Need for new chest drains | 7 (3.3) | 14 (6.6) | −4.8 (−10.9 to 1.4)f | .13 |
| Time with any chest drains, mean (SD), dh | 4.7 (2.9) | 4.7 (2.8) | ||
| Highest Fio2 level recorded from 48 h to 10 d of lifed,g | ||||
| No. | 179 | 183 | ||
| Median (range), fraction | 0.49 (0.22 to 1.00) | 0.45 (0.23 to 1.00) | ||
| Air leaki | 16 (7.4) | 22 (10.4) | −4.2 (−11.2 to 2.7) | .23 |
| Hospital Discharge | ||||
| Retinopathy of prematurity (grades 3 and 4), No./total No. (%) | 39/196 (19.9) | 41/182 (22.5) | −3.6 (−11.9 to 4.7) | .40 |
| Death in hospitalg | 48 (22.3) | 35 (16.6) | 5.4 (−2.4 to 13.1) | .17 |
| Survival to discharge home without BPD, retinopathy of prematurity (grades 3 and 4), or significant brain abnormalities on head ultrasound | 71 (33.0) | 73 (34.6) | −3.6 (−11.7 to 4.4) | .37 |
| Length of hospital stay in infants discharged home, wkj | ||||
| No. | 90 | 101 | ||
| Median (range) | 15 (7.6 to 31) | 15.3 (9.4 to 29) | ||
| Use of postnatal steroids for treatment of BPD, No./total No. (%)g | 75/196 (38.3) | 72/185 (38.9) | −0.2 (−9.3 to 8.9) | .96 |
| Only inhaled corticosteroids administered, No./total No. (%)g | 4/75 (5.4) | 3/72 (4.2) | ||
Abbreviations: BPD, bronchopulmonary dysplasia; bpm, beats per minute; CPAP, continuous positive airway pressure; DOL, days of life; Fio2, fraction of inspired oxygen; NA, not applicable; PEEP, positive end-expiratory pressure; PIP, peak inspiratory pressure; PPV, positive pressure ventilation.
Risk difference of sustained inflation − standard resuscitation calculated using marginal probabilities from adjusted generalized estimating equation models. Covariates adjusted for were gestational age, site, infant sex, maternal corticosteroid use, initial heart rate, small for gestational age (except where indicated with tablenote “f”), and consent type used. Also adjusted for the correlation between infants from multiple births.
P value calculated from adjusted generalized estimating equation models and are not adjusted for multiple outcomes.
Only includes infants who survived resuscitation (sustained inflation group: n = 211; standard group: n = 210). “Final” refers to the last measurement taken in the delivery room.
Secondary outcome of area under hourly Fio2 curve not assessed because sites found this too costly and complex to record. To substitute for this, the simpler Fio2 was substituted because this was possible to collect and conveys similar information.
Was also a prespecified safety outcome at 10 days of life. See Figure 2 and eFigure 2 in Supplement 3 for more information.
Not small for gestational age.
Outcome with missing data due to transfer or death prior to assessment. Both the number of infants with the outcome and the number assessed are shown.
Only reported for the 21 infants with chest drains within the first 10 days of life.
Air leak was defined as radiographic evidence of pneumothorax, pulmonary interstitial emphysema, or pneumopericardium. The individual components were also prespecified safety outcomes at 10 days of life. See eFigure 2 in Supplement 3 for more information.
Excludes infants who died (n = 83), were transferred to another hospital with no known discharge date from that facility (n = 109), or were still hospitalized at 44 weeks’ postmenstrual age (n = 43).
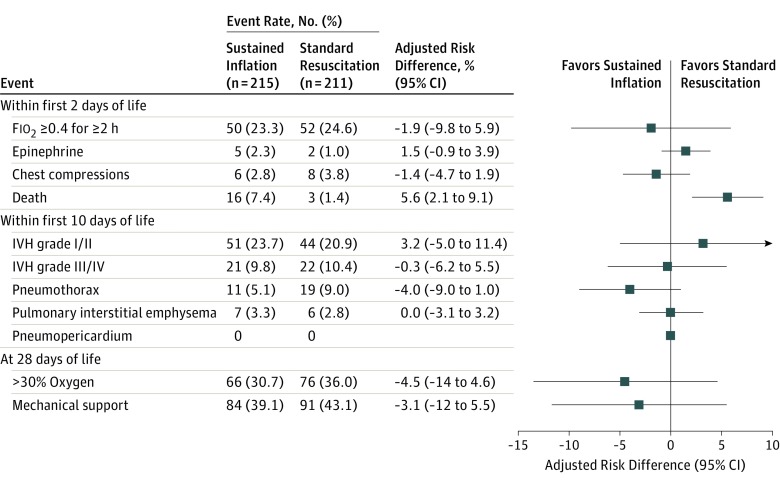
The only statistically significant difference in prespecified secondary safety outcomes by infant (Figure 2) was in the rate of early death, 16 (7.4%) in the sustained inflation group (11 of these were in the 23- to 24-week stratum) and 3 (1.4%) in the standard resuscitation group (2 in the 23- to 24-week stratum) (aRD, 5.6% [95% CI, 2.1%-9.1%]; P = .002; aRR, 4.7 [95% CI, 1.4-16.2]; P = .01). Other prespecified safety outcomes, at varying time epochs (in the delivery room, within the first 48 hours of life, or up to the first 10 days), were not significantly different between groups by infant or by incidence rates by proportion of infants (eFigure 2 in Supplement 3).
Figure 2. Adjusted Comparisons of Prespecified Secondary Safety Outcomes and Adverse Events by Group.
These prespecified markers of harm were chosen a priori by the executive committee and the data and safety monitoring committee together. Results expressed as adjusted risk differences (95% CIs), calculated using marginal probabilities from the adjusted generalized estimating equation models. Covariates adjusted for were gestational age, site, infant sex, maternal corticosteroid use, initial heart rate, small for gestational age, and consent type used, as well as for the correlation between infants from multiple births. Fio2 indicates fraction of inspired oxygen; and IVH, intraventricular hemorrhage.
Exploratory Outcomes
Exploratory secondary outcomes included surfactant, epinephrine, and chest comparisons in the delivery room, and showed no statistically significant differences between groups (eTable 1 in Supplement 3).
Post Hoc Analyses
In a post hoc sensitivity analysis, exclusion of the 11 infants without an oxygen reduction test did not substantially change the results for the primary outcome (eTable 2 in Supplement 3). Rates of the primary outcome by continental site compared by post hoc exact Mantel-Haenszel relative risks to test for homogeneity25 showed no differences (P = .78). Examination of subgroups found no statistically significant interactions in any subgroup analysis (eFigure 3 in Supplement 3). Post hoc Kaplan-Meier analysis (eFigure 4 in Supplement 3) showed excess mortality in the first week of life (log-rank test: first week: P = .001; entire curve: P = .11).
After the DSMC blindly reviewed early deaths, 11 of 16 early deaths in the sustained inflation group vs 1 of 3 in the standard resuscitation group were considered possibly related to allocation group. Of the 19 early deaths, 13 were in the lower gestational age stratum, with the predominant cause being assigned as cardiorespiratory failure (respiratory failure, 5; asphyxia or failed transition, 4; pulmonary hypertension, 2; hemorrhagic shock, 1; and pneumothorax, 1). Only 8 of 19 (42.1%) of those who had an early death survived long enough for a head ultrasound. Of these 3 (37.5%) had an intraventricular hemorrhage, 1 of whom also had a catastrophic gastrointestinal perforation. There were 3 cases of sepsis. eTable 3 in Supplement 3 compares early with late deaths, but does not reveal differences in selected risk factors.
To assess whether a significant mortality effect at 36 weeks was possible at full recruitment, a post hoc Bayesian analysis was performed at the request of the DSMC. For each scenario, hypothetical prior estimates of mortality risk in each group were set at 0.15 or 0.20, corresponding to noninformative priors (equal mortality in the 2 groups) and informative prior probabilities (different event rates per group). Hypothetical prior sample sizes per group ranged from 10 to 200. All scenarios tested showed the most likely outcome was indeterminate26 (favoring neither group), with probabilities ranging from 64% to 99.9% (eTable 4 in Supplement 3).
Discussion
In extremely preterm infants requiring resuscitation at birth, sustained inflations, compared with standard care with intermittent positive pressure ventilation, did not reduce the rate of death or BPD at 36 weeks’ postmenstrual age. An unexpected excess mortality rate with sustained inflation in the first 48 hours of life led to early trial closure, although mortality at 36 weeks’ postmenstrual age was not different and Bayesian analysis suggested a beneficial effect was unlikely even with full recruitment. Sustained inflation is currently standard practice in parts of Europe9 based on studies in animals, asphyxiated term infants,4 and smaller human randomized clinical trials in both preterm and near-term infants.7,8,27,28,29,30,31,32,33,34,35 Previous studies showed a reduction in mechanical ventilation within the first 72 hours of life27,28 in infants receiving sustained inflation. Two meta-analyses7,8 found a short-term benefit of sustained inflation, but a Cochrane review8 suggested caution in interpretation of pooled analyses, noting marked interstudy heterogeneity.
Prior smaller studies included more mature infants,27,31,32,33,34,35 involved shorter inspiratory durations,29 reported surrogate outcomes,29,35 had important co-interventions,27 or were stopped early for futility.30 This trial used sustained inflations with peak pressures that were similar to prior studies27,28,30 and durations similar to some.27,30
This study used a rescue approach in which eligible infants had demonstrated failure of transition, with gasping or apnea and/or bradycardia below 100 bpm, thus selecting the highest-risk infants. The study by Lista et al28 enrolled infants between 25 weeks’ and 28 weeks 6 days’ gestational age, including a subgroup (25 to 26 weeks’ gestation) similar to one stratum in the present study. They used 2 sustained inflations (25 cm H2O for 15 seconds), followed by CPAP of 5 cm H2O, in 148 infants, finding an adjusted RR for death of 1.39 (95% CI, 0.66-2.93). In contrast to Lista et al,28 a higher incidence of air leaks was not seen in this study. Moreover, no evidence of short-term benefits in delivery room outcomes or in the proportion of infants unintubated at day 3 was observed. Differences between the results may reflect differences in the sustained inflation approaches. Lista et al28 used a prophylactic approach in all extremely preterm infants. The present study may have preferentially selected infants with apnea, who are more likely to have a closed glottis and be unresponsive to the intervention.36 Prior studies included infants likely to be breathing spontaneously.27,28,33,34,35
The early deaths were predominantly in the smallest, most vulnerable infants. Supporting transition at birth in the most immature infants may require gentler support than the sustained inflations used. Effects of sustained inflation may vary depending on the amount of lung liquid present and the maturity of the lung. In some infants, sustained inflation could overdistend the lung, causing air leaks and predisposing to intraventricular hemorrhage. However, in this trial, the difference in early deaths could not be attributed to air leak, and only 3 infants had a confirmed intraventricular hemorrhage, although only 8 of 19 received a head ultrasound investigation. While the imbalance of early death by randomized group is evident, no specific cause was identifiable. This harm may be a chance finding.
Some non-US sites used a deferred consent process, which is uncommon in the United States but legal in other countries with strict provisions.37,38,39 Restriction of the consent process to an antenatal approach may result in bias,13,37,38,39 as women presenting with apparent preterm labor do not predictably deliver preterm, while mothers who precipitously deliver preterm are often unable to provide consent before delivery. At all sites, investigators pursued an open dialogue with parents,40 overseen by site principal investigators and their IRB.
Limitations
This study has several limitations. First, details of the delivery of the sustained inflations were not monitored or recorded. However, detailed training and video resources were used and there were no statistically significant differences in treatment effect between continents despite varying prior experience with sustained inflations. Second, the trial was stopped early and may have been underpowered.
Conclusions
Among extremely preterm infants requiring resuscitation at birth, a ventilation strategy involving 2 sustained inflations, compared with standard intermittent positive pressure ventilation, did not reduce the risk of BPD or death at 36 weeks’ postmenstrual age. These findings do not support the use of ventilation with sustained inflations among extremely preterm infants, although early termination of the trial limits definitive conclusions.
Trial Protocol
Statistical Analysis Plan
eMethods 1. All Study End Points
eMethods 2. Methods of the Post Hoc Bayesian Futility Analysis
eFigure 1. Assessment of Eligibility, Randomization, and Delivery of Sustained Inflation
eFigure 2. Incidence Rates of Prespecified Secondary Safety Outcomes and Adverse Events, Accounting for Days at Risk
eFigure 3. Primary Outcome and Interaction by Subgroup
eFigure 4. Post Hoc Kaplan-Meier Survival Curves by Group
eTable 1. Adjusted Comparisons of Exploratory Secondary Outcomes by Group
eTable 2. Sensitivity Analysis for Primary Outcome and Components Using Only Oxygen Reduction Test Ascertained Component
eTable 3. Characteristics of Three Groups of Infants: Early Death, Late Death (to 36 Weeks’ Gestational Age), and Survival at 36 Weeks’ Gestational Age
eTable 4. Bayesian Predictions for Final Study Outcomes
Data Sharing Statement
References
- 1.Dawes GS. The first breath. Proc R Soc Med. 1966;59(6):508. [PMC free article] [PubMed] [Google Scholar]
- 2.Smith CA. The first breath. Sci Am. 1963;209:27-35. doi: 10.1038/scientificamerican1063-27 [DOI] [PubMed] [Google Scholar]
- 3.Subramaniam P, Ho JJ, Davis PG. Prophylactic nasal continuous positive airway pressure for preventing morbidity and mortality in very preterm infants. Cochrane Database Syst Rev. 2016;(6):CD001243. [DOI] [PubMed] [Google Scholar]
- 4.Vyas H, Milner AD, Hopkin IE, Boon AW. Physiologic responses to prolonged and slow-rise inflation in the resuscitation of the asphyxiated newborn infant. J Pediatr. 1981;99(4):635-639. doi: 10.1016/S0022-3476(81)80279-X [DOI] [PubMed] [Google Scholar]
- 5.te Pas AB, Siew M, Wallace MJ, et al. Establishing functional residual capacity at birth: the effect of sustained inflation and positive end-expiratory pressure in a preterm rabbit model. Pediatr Res. 2009;65(5):537-541. doi: 10.1203/PDR.0b013e31819da21b [DOI] [PubMed] [Google Scholar]
- 6.Sobotka KS, Hooper SB, Allison BJ, et al. An initial sustained inflation improves the respiratory and cardiovascular transition at birth in preterm lambs. Pediatr Res. 2011;70(1):56-60. doi: 10.1203/PDR.0b013e31821d06a1 [DOI] [PubMed] [Google Scholar]
- 7.Schmölzer GM, Kumar M, Aziz K, et al. Sustained inflation versus positive pressure ventilation at birth: a systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed. 2015;100(4):F361-F368. doi: 10.1136/archdischild-2014-306836 [DOI] [PubMed] [Google Scholar]
- 8.Bruschettini M, O’Donnell CP, Davis PG, et al. Sustained versus standard inflations during neonatal resuscitation to prevent mortality and improve respiratory outcomes. Cochrane Database Syst Rev. 2017;7:CD004953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roehr CC, Gröbe S, Rüdiger M, et al. Delivery room management of very low birth weight infants in Germany, Austria and Switzerland: a comparison of protocols. Eur J Med Res. 2010;15(11):493-503. doi: 10.1186/2047-783X-15-11-493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wyllie J, Bruinenberg J, Roehr CC, Rüdiger M, Trevisanuto D, Urlesberger B. European Resuscitation Council Guidelines for Resuscitation 2015, section 7: resuscitation and support of transition of babies at birth. Resuscitation. 2015;95:249-263. doi: 10.1016/j.resuscitation.2015.07.029 [DOI] [PubMed] [Google Scholar]
- 11.Wyckoff MH, Aziz K, Escobedo MB, et al. Part 13: neonatal resuscitation: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2015;132(18)(suppl 2):S543-S560. doi: 10.1161/CIR.0000000000000267 [DOI] [PubMed] [Google Scholar]
- 12.Foglia EE, Owen LS, Thio M, et al. Sustained Aeration of Infant Lungs (SAIL) Trial: study protocol for a randomized controlled trial. Trials. 2015;16:95. doi: 10.1186/s13063-015-0601-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rich W, Finer NN, Gantz MG, et al. ; SUPPORT and Generic Database Subcommittees of the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network . Enrollment of extremely low birth weight infants in a clinical research study may not be representative. Pediatrics. 2012;129(3):480-484. doi: 10.1542/peds.2011-2121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perlman JM, Wyllie J, Kattwinkel J, et al. ; Neonatal Resuscitation Chapter Collaborators . Part 7: neonatal resuscitation, 2015 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations (reprint). Pediatrics. 2015;136(suppl 2):S120-S166. doi: 10.1542/peds.2015-3373D [DOI] [PubMed] [Google Scholar]
- 15.Neonatal Resuscitation Program (NRP); American Academy of Pediatrics and the American Heart Association . Neonatal Resuscitation Textbook. 6th ed Itasca, IL: American Academy of Pediatrics; 2011. [Google Scholar]
- 16.Foglia EE, Te Pas AB. Sustained lung inflation: physiology and practice. Clin Perinatol. 2016;43(4):633-646. doi: 10.1016/j.clp.2016.07.002 [DOI] [PubMed] [Google Scholar]
- 17.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163(7):1723-1729. doi: 10.1164/ajrccm.163.7.2011060 [DOI] [PubMed] [Google Scholar]
- 18.Walsh MC, Wilson-Costello D, Zadell A, Newman N, Fanaroff A. Safety, reliability, and validity of a physiologic definition of bronchopulmonary dysplasia. J Perinatol. 2003;23(6):451-456. doi: 10.1038/sj.jp.7210963 [DOI] [PubMed] [Google Scholar]
- 19.Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92(4):529-534. doi: 10.1016/S0022-3476(78)80282-0 [DOI] [PubMed] [Google Scholar]
- 20.Morley CJ, Davis PG, Doyle LW, Brion LP, Hascoet JM, Carlin JB; COIN Trial Investigators . Nasal CPAP or intubation at birth for very preterm infants. N Engl J Med. 2008;358(7):700-708. doi: 10.1056/NEJMoa072788 [DOI] [PubMed] [Google Scholar]
- 21.Finer NN, Carlo WA, Walsh MC, et al. ; SUPPORT Study Group of the Eunice Kennedy Shriver NICHD Neonatal Research Network . Early CPAP versus surfactant in extremely preterm infants. N Engl J Med. 2010;362(21):1970-1979. doi: 10.1056/NEJMoa0911783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Norton ED, Miller MM, Kleinman LC. Computing adjusted risk ratios and risk differences in Stata. Stata J. 2003;13(3):1-17. doi: 10.1177/1536867X1301300304 [DOI] [Google Scholar]
- 23.Wathen JK, et al. Predictive probabilities user's guide version 1.5. https://biostatistics.mdanderson.org/softwaredownload/ProductSupportFiles/PredictiveProbabilit/PredictiveProbabilitiesUsersGuide.pdf. Published March 19, 2014. Accessed December 28, 2017.
- 24.Kramer MS, Platt RW, Wen SW, et al. ; Fetal/Infant Health Study Group of the Canadian Perinatal Surveillance System . A new and improved population-based Canadian reference for birth weight for gestational age. Pediatrics. 2001;108(2):E35. doi: 10.1542/peds.108.2.e35 [DOI] [PubMed] [Google Scholar]
- 25.Luta G, Koch GG, Cascio WE, Smith WT. An application of methods for clustered binary responses to a cardiovascular study with small sample size. J Biopharm Stat. 1998;8(1):87-102. doi: 10.1080/10543409808835224 [DOI] [PubMed] [Google Scholar]
- 26.Sackett DL. Superiority trials, noninferiority trials, and prisoners of the 2-sided null hypothesis. ACP J Club. 2004;140(2):A11. [PubMed] [Google Scholar]
- 27.te Pas AB, Walther FJ. A randomized, controlled trial of delivery-room respiratory management in very preterm infants. Pediatrics. 2007;120(2):322-329. doi: 10.1542/peds.2007-0114 [DOI] [PubMed] [Google Scholar]
- 28.Lista G, Boni L, Scopesi F, et al. ; SLI Trial Investigators . Sustained lung inflation at birth for preterm infants: a randomized clinical trial. Pediatrics. 2015;135(2):e457-e464. doi: 10.1542/peds.2014-1692 [DOI] [PubMed] [Google Scholar]
- 29.Harling AE, Beresford MW, Vince GS, Bates M, Yoxall CW. Does sustained lung inflation at resuscitation reduce lung injury in the preterm infant? Arch Dis Child Fetal Neonatal Ed. 2005;90(5):F406-F410. doi: 10.1136/adc.2004.059303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lindner W, Högel J, Pohlandt F. Sustained pressure-controlled inflation or intermittent mandatory ventilation in preterm infants in the delivery room? a randomized, controlled trial on initial respiratory support via nasopharyngeal tube. Acta Paediatr. 2005;94(3):303-309. [DOI] [PubMed] [Google Scholar]
- 31.Jiravisitkul P, Rattanasiri S, Nuntnarumit P. Randomised controlled trial of sustained lung inflation for resuscitation of preterm infants in the delivery room. Resuscitation. 2017;111:68-73. doi: 10.1016/j.resuscitation.2016.12.003 [DOI] [PubMed] [Google Scholar]
- 32.Ngan AY, Cheung PY, Hudson-Mason A, et al. Using exhaled CO2 to guide initial respiratory support at birth: a randomised controlled trial. Arch Dis Child Fetal Neonatal Ed. 2017;102(6):F525-F531. doi: 10.1136/archdischild-2016-312286 [DOI] [PubMed] [Google Scholar]
- 33.El-Chimi MS, Awad HA, El-Gammasy TM, El-Farghali OG, Sallam MT, Shinkar DM. Sustained versus intermittent lung inflation for resuscitation of preterm infants: a randomized controlled trial. J Matern Fetal Neonatal Med. 2017;30(11):1273-1278. doi: 10.1080/14767058.2016.1210598 [DOI] [PubMed] [Google Scholar]
- 34.Mercadante D, Colnaghi M, Polimeni V, et al. Sustained lung inflation in late preterm infants: a randomized controlled trial. J Perinatol. 2016;36(6):443-447. doi: 10.1038/jp.2015.222 [DOI] [PubMed] [Google Scholar]
- 35.Schwaberger B, Pichler G, Avian A, Binder-Heschl C, Baik N, Urlesberger B. Do sustained lung inflations during neonatal resuscitation affect cerebral blood volume in preterm infants? a randomized controlled pilot study. PLoS One. 2015;10(9):e0138964. doi: 10.1371/journal.pone.0138964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Vonderen JJ, Hooper SB, Hummler HD, Lopriore E, te Pas AB. Effects of a sustained inflation in preterm infants at birth. J Pediatr. 2014;165(5):903-908.e1. doi: 10.1016/j.jpeds.2014.06.007 [DOI] [PubMed] [Google Scholar]
- 37.Songstad NT, Roberts CT, Manley BJ, Owen LS, Davis PG; HIPSTER trial investigators . Retrospective consent in a neonatal randomized controlled trial. Pediatrics. 2018;141(1):e20172092. doi: 10.1542/peds.2017-2092 [DOI] [PubMed] [Google Scholar]
- 38.Rich WD, Katheria AC. Waiver of consent in a trial intervention occurring at birth-how do parents feel? Front Pediatr. 2017;5:56. doi: 10.3389/fped.2017.00056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Foglia EE, Owen LS, Keszler M, Davis PG, Kirpalani H. Obtaining informed consent for delivery room research: the investigators’ perspective. Arch Dis Child Fetal Neonatal Ed. 2017;102(1):F90-F91. doi: 10.1136/archdischild-2016-310934 [DOI] [PubMed] [Google Scholar]
- 40.DeMauro SB, Cairnie J, D’Ilario J, Kirpalani H, Schmidt B. Honesty, trust, and respect during consent discussions in neonatal clinical trials. Pediatrics. 2014;134(1):e1-e3. doi: 10.1542/peds.2013-3720 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Statistical Analysis Plan
eMethods 1. All Study End Points
eMethods 2. Methods of the Post Hoc Bayesian Futility Analysis
eFigure 1. Assessment of Eligibility, Randomization, and Delivery of Sustained Inflation
eFigure 2. Incidence Rates of Prespecified Secondary Safety Outcomes and Adverse Events, Accounting for Days at Risk
eFigure 3. Primary Outcome and Interaction by Subgroup
eFigure 4. Post Hoc Kaplan-Meier Survival Curves by Group
eTable 1. Adjusted Comparisons of Exploratory Secondary Outcomes by Group
eTable 2. Sensitivity Analysis for Primary Outcome and Components Using Only Oxygen Reduction Test Ascertained Component
eTable 3. Characteristics of Three Groups of Infants: Early Death, Late Death (to 36 Weeks’ Gestational Age), and Survival at 36 Weeks’ Gestational Age
eTable 4. Bayesian Predictions for Final Study Outcomes
Data Sharing Statement