Key Points
Question
What is the association between prescribed opioids and community-acquired pneumonia requiring hospitalization among patients living with and without HIV?
Findings
In this nested case-control study of 4246 cases and 21 146 controls (25 392 participants), prescribed opioids were independently associated with community-acquired pneumonia in patients living with and without HIV. In addition, the risk for community-acquired pneumonia increased with higher opioid doses and opioids with known immunosuppressive properties.
Meaning
These findings suggest that prescribed opioids independently contribute to community-acquired pneumonia risk among patients living with and without HIV; efforts to minimize prescribed opioid use, as well as to minimize use of higher doses and immunosuppressive opioids, may help mitigate this risk.
This nested case-control study assesses the association of prescribed opioids with the risk for community-acquired pneumonia among patients with and without HIV in the Veterans Aging Cohort Study.
Abstract
Importance
Some opioids are known immunosuppressants; however, the association of prescribed opioids with clinically relevant immune-related outcomes is understudied, especially among people living with HIV.
Objective
To assess the association of prescribed opioids with community-acquired pneumonia (CAP) by opioid properties and HIV status.
Design, Setting, and Participants
This nested case-control study used data from patients in the Veterans Aging Cohort Study (VACS) from January 1, 2000, through December 31, 2012. Participants in VACS included patients living with and without HIV who received care in Veterans Health Administration (VA) medical centers across the United States. Patients with CAP requiring hospitalization (n = 4246) were matched 1:5 with control individuals without CAP (n = 21 146) by age, sex, race/ethnicity, length of observation, and HIV status. Data were analyzed from March 15, 2017, through August 8, 2018.
Exposures
Prescribed opioid exposure during the 12 months before the index date was characterized by a composite variable based on timing (none, past, or current); low (<20 mg), medium (20-50 mg), or high (>50 mg) median morphine equivalent daily dose; and opioid immunosuppressive properties (yes vs unknown or no).
Main Outcome and Measure
CAP requiring hospitalization based on VA and Centers for Medicare & Medicaid data.
Results
Among the 25 392 VACS participants (98.9% male; mean [SD] age, 55 [10] years), current medium doses of opioids with unknown or no immunosuppressive properties (adjusted odds ratio [AOR], 1.35; 95% CI, 1.13-1.62) and immunosuppressive properties (AOR, 2.07; 95% CI, 1.50-2.86) and current high doses of opioids with unknown or no immunosuppressive properties (AOR, 2.07; 95% CI, 1.50-2.86) and immunosuppressive properties (AOR, 3.18; 95% CI, 2.44-4.14) were associated with the greatest CAP risk compared with no prescribed opioids or any past prescribed opioid with no immunosuppressive (AOR, 1.24; 95% CI, 1.09-1.40) and immunosuppressive properties (AOR, 1.42; 95% CI, 1.21-1.67), especially with current receipt of immunosuppressive opioids. In stratified analyses, CAP risk was consistently greater among people living with HIV with current prescribed opioids, especially when prescribed immunosuppressive opioids (eg, AORs for current immunosuppressive opioids with medium dose, 1.76 [95% CI, 1.20-2.57] vs 2.33 [95% CI, 1.60-3.40]).
Conclusions and Relevance
Prescribed opioids, especially higher-dose and immunosuppressive opioids, are associated with increased CAP risk among persons with and without HIV.
Introduction
Prescription opioids remain commonly prescribed for pain among people living with HIV (PLWH) and those who are uninfected.1,2 However, accumulating evidence suggests that prescribed opioids increase the risk of infections, including community-acquired pneumonia (CAP).3,4,5,6 Some opioids impair innate and adaptive immune system defenses to bacterial infections,7,8 suppress cough and respirations, and inhibit bronchial mucus secretion and alveolar neutrophil response to Streptococcus pneumoniae.9,10 These findings support biological plausibility for an adverse effect of opioids on CAP risk. In addition, opioids vary in their immunosuppressive properties; for example, morphine sulfate appears to be immunosuppressive, whereas oxycodone hydrochloride does not.3,11
People living with HIV are more commonly prescribed opioids than uninfected comparators despite an increased risk for infectious complications in general and for CAP in particular.2,12 Notably, CAP is a major contributor to morbidity and mortality even in the current HIV treatment era and especially as patients age.12 Few studies have examined the extent to which prescribed opioids affect CAP risk, and none have focused on PLWH.3,4,5,13 Prior studies have been limited to specific populations3,5 and relied on diagnoses and surrogate measures of frailty to adjust for disease severity.5 Thus, using a national sample of PLWH and people without HIV and accounting for overall severity of illness with a validated measure, we conducted the present study to (1) determine the association between prescribed opioids and CAP risk and to examine whether this association differs by (2) prescribed opioid properties, including duration of opioid exposure, average (ie, mean) morphine equivalent daily dose (MEDD), and immunosuppressive properties of prescribed opioids or (3) HIV status. We hypothesized that prescribed opioids would increase CAP risk, with increasing risk at higher doses and with known immunosuppressive properties, and that associations would be stronger among PLWH compared with an uninfected comparison group.
Methods
Study Design and Data Sources
We conducted a nested case-control study using January 1, 2000, through December 31, 2012, data from the Veterans Aging Cohort Study (VACS). VACS, a prospective study of all PLWH receiving care at Veterans Health Administration (VA) medical centers across the United States and an uninfected comparison group, investigates the effect of comorbid disease on outcomes in HIV infection.14 VACS uses data from the VA-based electronic medical record, including demographic data, medical diagnoses (based on International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] codes), laboratory results, and pharmacy records. We additionally linked this VA data with the Center for Medicare & Medicaid Services (CMS) data to capture CAP events outside the VA. The study was approved by the Human Investigations Committee at Yale University, New Haven, Connecticut, and the VA Connecticut Healthcare System, West Haven, and was granted a waiver of informed consent.
Patients were not reimbursed for participation. The time frame during which prescribed opioids would exert an immunosuppressive effect is not known. Therefore, we chose a nested case-control design. This design allowed us to consider factors associated with CAP risk proximal and distal to the event, including proximal exposure to prescribed opioids.15
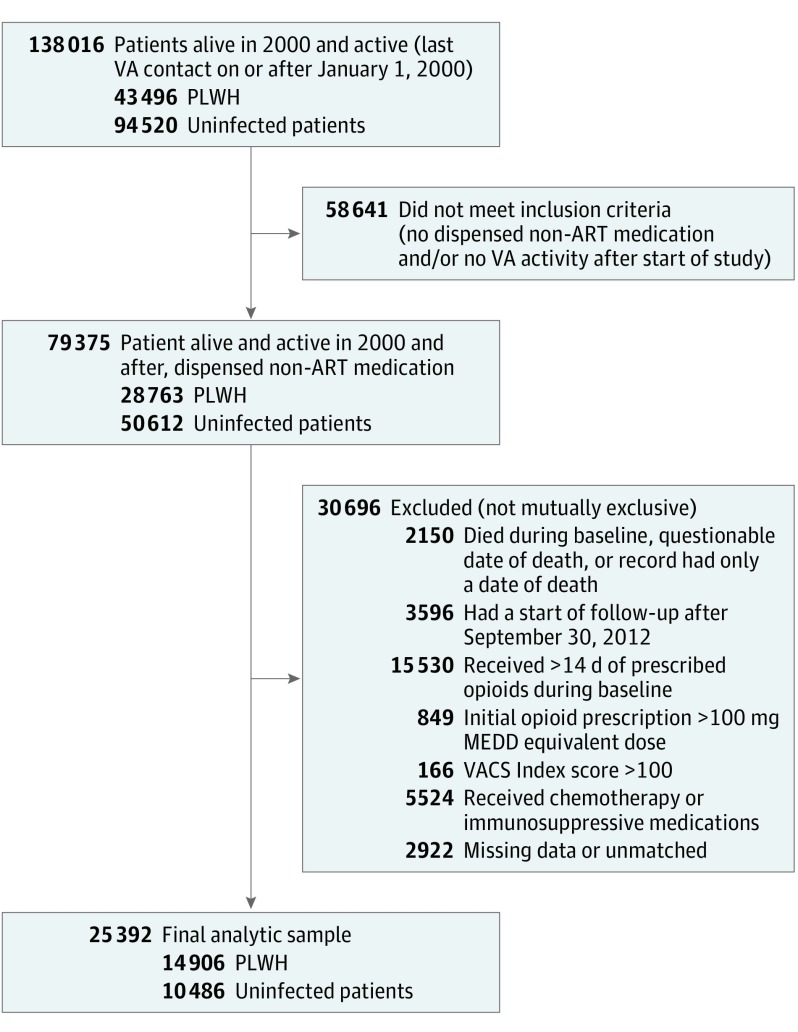
Baseline Cohort
Among VACS participants, we established a base cohort of patients who were alive as of January 1, 2000, and had a subsequent VA visit (Figure 1 and Figure 2). Patients were eligible for cohort entry on receipt of their first VA outpatient nonantiretroviral (non-ARV) prescription (to ensure they received medications from the VA); the next 365 days constituted the baseline period. The baseline period was used to establish preexisting opioid use and other exclusions. Follow-up began at the end of the baseline period. To increase clinical relevance and internal validity, we excluded patients who (1) died during the baseline period, had a questionable date of death, or whose only subsequent record was a death date (n = 2150); (2) started follow-up after September 30, 2012 (n = 3596); (3) received more than 14 days of prescribed opioids during the baseline period (n = 15 530) to ensure that we captured patients who were newly prescribed opioids16,17; (4) had an initial VA opioid dosage greater than 100 mg MEDD (n = 849) to exclude patients likely prescribed opioids before transferring into the VA; (5) had a VACS Index score greater than 100 (n = 166) during the baseline period; (6) received chemotherapy or immunosuppressive medications other than systemic or inhaled corticosteroids (n = 5524)18 because this factor may increase CAP risk; or (7) had missing data (n = 2922). VACS Index is a validated measure of morbidity and mortality in patients with and without HIV that is calculated based on HIV biomarkers (CD4 cell count and HIV viral load, where values are assumed to be normal for uninfected patients), hemoglobin level, Fibrosis 4 Index for Liver Fibrosis score, estimated glomerular filtration rate, and hepatitis C status.19 Scores greater than 100 indicate a 20% risk of death in the next year, a proxy for severe illness.
Figure 1. Overview of Nested Case-Control Design.
The index date for comparing exposure history in cases and controls was the date of community-acquired pneumonia (CAP) admission for cases and the date corresponding to the same length of time in the study for controls. From the base cohort (ie, Veterans Aging Cohort Study patients who met eligibility criteria for this analysis), 5 controls were identified for each case with CAP.
Figure 2. Study Flow Diagram.
MEDD indicates morphine equivalent daily dose; non-ART, nonantiretroviral; PLWH, people living with HIV; VA, Veterans Health Administration; and VACS, Veterans Aging Cohort Study.
Selection of Cases and Controls
We next identified cases and controls. Using VA and CMS data, we identified patients with CAP requiring hospitalization (ie, cases). Incident CAP was defined using ICD-9 codes 480 to 487 and 5073 as the primary diagnosis or secondary to HIV, sepsis, and respiratory failure, similar to other studies of CAP20 and validated in VACS.21 For each case, we matched 5 controls (ie, patients without CAP) at the time of the case event by age (±1 year), sex, race/ethnicity, length of observation, and HIV status. The index date for comparing exposure history in cases and controls was the date of hospital admission for cases and the date corresponding to the same length of time in the study for controls. The analytic sample consisted of 4246 cases and 21 146 controls.
Measures of Prescribed Opioid Exposure
The primary exposure of interest was prescribed opioid use in the 365 days before the index date determined using outpatient VA pharmacy fill and refill data. We determined receipt of oral and transdermal opioids for pain (ie, excluding buprenorphine hydrochloride and methadone hydrochloride for opioid use disorder).18,22 We characterized prescribed opioid exposure based on the timing of the prescription, dose, and immunosuppressive properties to create a composite variable (Table 1).
Table 1. Prescribed Opioid Characteristics.
| Characteristica | Description |
|---|---|
| Timing of prescriptionb | |
| None3 | No fills from 5 to 365 d before the index date |
| Past opioid use3 | Fills from 61 to 365 d before the index date, but no current opioid use |
| Current opioid use3 | Fills from 5 to 60 d before the index date |
| Days supplied | Total of opioids received from 5 to 365 d before the index date |
| Mean MEDDb,c | Total milligrams of morphine equivalents prescribed from 5 to 365 d before the index date divided by the days supplied in the 360-d period, where total morphine equivalents dose equals quantity of each prescription times strength using standard conversion factors |
| Low dose | <20 mg |
| Medium dose | 20-50 mg |
| High dose | >50 mg |
| Immunosuppressive opioidb | |
| Yes3,11,23 | Receipt of ≥1 prescription, including codeine, dihydrocodeine, fentanyl, or morphine |
| Unknown3,11,23 | Receipt of no immunosuppressive opioids but ≥1 prescription of levorphanol, meperidine, methadone, pentazocine, propoxyphene, or tapentadol |
| No3,11,23 | Receipt of only hydrocodone, hydromorphone, oxycodone, or tramadol |
| Days supplied | Total days of opioids received from 5 to 365 d before the index date |
| Long-acting opioid formulation | Receipt of ≥1 prescription of fentanyl patch, sustained-action hydromorphone, levorphanol, methadone, sustained-action morphine, sustained-action oxycodone, sustained-action oxymorphone, or sustained-action tramadol |
Abbreviations: CAP, community-acquired pneumonia; MEDD, morphine equivalent daily dose.
Indicates included in composite variable; each level was considered mutually exclusive.
If a patient only had prescribed opioids during 0 to 4 days before the index date, this was not considered as opioid exposure and was not included in above calculations to decrease risk of indication bias.24 In cases, this duration of prescription could be for treatment of CAP symptoms.
Dose categories defined based on the distribution in study sample.
Covariates
We selected covariates that may confound the association between prescribed opioids and CAP risk.3,4,5,25 Sociodemographic characteristics included age, sex, and race/ethnicity. All other covariates were defined based on data collected between the date of initial non-ARV prescription to 30 days before the index date. We used laboratory values closest to the end of this window. Diagnoses were considered present if assigned before the end of this window. Clinical characteristics, determined based on the presence of 1 inpatient or 2 outpatient ICD-9-CM codes, included HIV, hepatitis C (with positive antibody or detectable RNA), diabetes mellitus (including laboratory results and medications), chronic obstructive pulmonary disease, congestive heart failure, stroke, pain-related diagnoses, alcohol- and other drug–related diagnoses, and prior CAP. Pain-related diagnoses were classified as previously described.2,22 Smoking status was determined based on a clinical reporting system and categorized as current, former, or never.26 Based on pharmacy data, additional medications included long-term benzodiazepine receipt (defined as ≥90 days supplied, allowing for a 30-day gap in fill and refill) and inhaled or oral corticosteroids (defined as current use if filled from 5 to 60 days before the index date and past use if filled 61 to 365 days before the index date). Preventive care measures included past-year influenza vaccination, 23- or 13-valent pneumococcal vaccination to 30 days before the index date, and prophylaxis for opportunistic infections in the prior 12 months for PLWH as previously described.27 Patients were considered to receive ARV therapy if they were prescribed at least 3 ARV agents, excluding boosters. Laboratory measures included VACS Index score19 and, among PLWH, CD4 cell count and HIV viral load.
Statistical Analysis
Data were analyzed from March 15, 2017, through August 8, 2018. We performed descriptive analysis comparing the characteristics of the 4246 CAP cases with those of the 21 146 controls in our sample. We also compared cases and controls by HIV status. We ran bivariable and multivariable conditional (to account for matching of cases and controls) logistic regression to estimate unadjusted and adjusted odds ratios (ORs) and 95% CIs for CAP risk associated with opioid exposure characterized with a composite variable that captured (1) prescribed opioid timing, (2) average MEDD, and (3) receipt of an immunosuppressive opioid (yes vs unknown or no). We also ran models stratified by HIV status and formally checked for an interaction between prescribed opioid characteristics and HIV status. In sensitivity analyses, we censored patients with a cancer diagnosis (excluding skin cancers except melanoma), those who achieved a VACS Index score greater than 100 during follow-up, or those who had a diagnosis of an opioid use disorder (based on ICD-9 code for opioid abuse or dependence). In post hoc analyses, we used a 3-level variable to assess the effects of immunosuppressive opioids (yes, unknown, or no) on CAP risk. We considered statistical significance to be a 2-sided P < .05. Analyses were performed using SAS statistical software (version 9.4; SAS Institute Inc).
Results
Patient Demographic and Clinical Characteristics
Cases and controls totaled 25 392 participants (98.9% male and 1.1% female; mean [SD] age, 55 [10] years). Baseline characteristics of cases and controls are presented in Table 2.
Table 2. Baseline Demographic and Clinical Characteristics of Cases and Controls, Overall and Stratified by HIV Status.
| Characteristic | All Participants (N = 25 392) | Uninfected Participants (n = 10 486) | PLWH (n = 14 906) | |||||
|---|---|---|---|---|---|---|---|---|
| Controls (n = 21 146) | Cases (n = 4246) | Controls (n = 8710) | Cases (n = 1776) | P Value | Controls (n = 12 436) | Cases (n = 2470) | P Value | |
| Age, mean (SD), y | 55 (10) | 55 (10) | 59 (10) | 59 (10) | .41 | 52 (9) | 52 (9) | .70 |
| Sex, No. (%) | ||||||||
| Male | 20 917 (98.9) | 4199 (98.9) | 8652 (99.3) | 1764 (99.3) | .96 | 12 265 (98.6) | 2435 (98.6) | .87 |
| Female | 229 (1.1) | 47 (1.1) | 58 (0.7) | 12 (0.7) | 171 (1.4) | 35 (1.4) | ||
| Race/ethnicity, No. (%) | ||||||||
| White | 7881 (37.3) | 1587 (37.4) | 3723 (42.7) | 762 (42.9) | .96 | 4158 (33.4) | 825 (33.4) | .30 |
| Black | 11 317 (53.5) | 2265 (53.3) | 4235 (48.6) | 857 (48.3) | 7082 (56.9) | 1408 (57.0) | ||
| Hispanic | 1573 (7.3) | 330 (7.8) | 651 (7.5) | 134 (7.5) | 922 (7.4) | 196 (7.9) | ||
| Other | 375 (1.8) | 64 (1.5) | 101 (1.2) | 23 (1.3) | 274 (2.2) | 41 (1.6) | ||
| HIV infection, No. (%) | 12 436 (58.8) | 2470 (58.2) | ||||||
| Hepatitis C, No. (%)a | 6628 (31.3) | 1689 (39.8) | 1412 (16.2) | 408 (23.0) | <.001 | 5216 (41.9) | 1281 (51.9) | <.001 |
| Diabetes mellitus, No. (%)a | 3904 (18.5) | 1029 (24.2) | 2348 (27.0) | 645 (36.3) | <.001 | 1556 (12.5) | 384 (15.5) | <.001 |
| Chronic obstructive pulmonary disease, No. (%)a | 2095 (9.9) | 852 (20.1) | 1061 (12.2) | 433 (24.4) | <.001 | 1034 (8.3) | 419 (17.0) | <.001 |
| Congestive heart failure, No. (%)a | 600 (2.8) | 365 (8.6) | 392 (4.5) | 222 (12.5) | <.001 | 208 (1.7) | 143 (5.8) | <.001 |
| Stroke, No. (%)a | 428 (2.0) | 183 (4.3) | 255 (2.9) | 118 (6.6) | <.001 | 173 (1.4) | 65 (2.6) | <.001 |
| Pain-related diagnosis, No. (%)a | ||||||||
| Acute | 1050 (5.0) | 359 (8.4) | 407 (4.7) | 144 (8.1) | <.001 | 643 (5.2) | 215 (8.7) | <.001 |
| Chronic | 6214 (29.4) | 1450 (34.1) | 3347 (38) | 724 (40.8) | 2867 (23.1) | 726 (29.4) | ||
| Major depression, No. (%)a | 2020 (9.6) | 552 (13.0) | 740 (8.4) | 221 (12.4) | <.001 | 1280 (10.3) | 331 (13.4) | <.001 |
| Alcohol-related diagnosis, No. (%)a | 2223 (10.5) | 630 (14.8) | 931 (10.7) | 254 (14.3) | <.001 | 1292 (10.4) | 376 (15.2) | <.001 |
| Other drug–related diagnosis, No. (%)a | 1798 (8.5) | 532 (12.5) | 575 (6.6) | 185 (10.4) | <.001 | 1223 (9.8) | 347 (14.0) | <.001 |
| Prior CAP, No. (%)a | 260 (1.2) | 208 (4.9) | 25 (0.3) | 40 (2.3) | <.001 | 235 (1.9) | 168 (6.8) | <.001 |
| Smoking, No. (%)a | ||||||||
| Never | 5565 (26.3) | 709 (16.7) | 2392 (27.5) | 324 (18.2) | .004 | 3173 (25.5) | 385 (15.6) | <.001 |
| Current | 10 924 (51.7) | 2604 (61.3) | 4050 (46.5) | 1006 (56.6) | 6874 (55.3) | 1598 (64.7) | ||
| Former | 3933 (18.6) | 641 (15.1) | 1992 (22.9) | 339 (19.1) | 1941 (15.6) | 302 (12.2) | ||
| Missing | 724 (3.4) | 292 (6.9) | 276 (3.2) | 107 (6.0) | 448 (3.6) | 185 (7.5) | ||
| Long-term benzodiazepine receipt, No. (%)a | 1716 (8.1) | 427 (10.1) | 828 (9.5) | 209 (11.8) | <.001 | 888 (7.1) | 218 (8.8) | .004 |
| Prescribed corticosteroids, No. (%)a | ||||||||
| Current oral | 306 (1.4) | 267 (6.3) | 162 (1.9) | 147 (8.3) | <.001 | 144 (1.2) | 120 (4.9) | <.001 |
| Prior oral | 788 (3.7) | 440 (10.4) | 397 (4.6) | 210 (11.8) | <.001 | 391 (3.1) | 230 (9.3) | <.001 |
| Current inhaled | 532 (2.5) | 294 (6.9) | 324 (3.7) | 182 (10.2) | <.001 | 208 (1.7) | 112 (4.5) | <.001 |
| Prior inhaled | 770 (3.6) | 394 (9.3) | 456 (5.2) | 245 (13.8) | <.001 | 314 (2.5) | 149 (6.0) | <.001 |
| Influenza vaccine, No. (%)a | 15 829 (74.9) | 3015 (71.0) | 5980 (68.7) | 1248 (70.3) | 0.18 | 9849 (79.2) | 1767 (71.5) | <.001 |
| Pneumococcal (Pneumovax or Prevnar) vaccine, No. (%)a | 13 428 (63.5) | 2642 (62.2) | 4559 (52.3) | 1056 (59.4) | <.001 | 8869 (71.3) | 1586 (64.2) | <.001 |
| Mycobacterium avium complex prophylaxis, No. (%)b | NA | NA | NA | NA | NA | 432 (76.6) | 329 (76/9) | .92 |
| Pneumocystis pneumonia prophylaxis, No. (%)c | NA | NA | NA | NA | NA | 1879 (83.2) | 845 (82.1) | .42 |
| Prescribed ARV therapy, No. (%)a,d | NA | NA | NA | NA | NA | 10 543 (84.8) | 2017 (81.7) | .001 |
| VACS Index score, median (IQR)a,e | 22 (12-35) | 31 (18-50) | 18 (12-33) | 22 (12-39) | <.001 | 24 (13-39) | 37 (22-58) | <.001 |
| CD4 cell count, median (IQR), cells/mm3a,d | 434 (266-644) | 310 (143-498) | NA | NA | NA | 434 (266-644) | 310 (143-498) | <.001 |
| HIV viral load, median (IQR), copies/mLa,d | 84 (50-2954) | 1057 (59-38 631) | NA | NA | NA | 84 (50-2954) | 1057 (59-38 631) | <.001 |
Abbreviations: ARV, antiretroviral; CAP, community-acquired pneumonia; HIV, human immunodeficiency virus; IQR, interquartile range; NA, not applicable; PLWH, people living with HIV; VACS, Veterans Aging Cohort Study.
Comparison for cases vs controls significant at P < .05 level.
Includes PLWH with CD4 cell count of 50 cells/mm3 or less (n = 992).
Includes PLWH with CD4 cell count of 200 cells/mm3 or less (n = 3286).
Includes PLWH only.
Scores greater than 100 indicate a 20% risk of death in the next year, a proxy for severe illness.
Prescribed Opioid Characteristics
In the year before the index date, cases were more likely than controls to have past (713 [16.8%] vs 2675 [12.7%]) and current (852 [20.1%] vs 2385 [11.3%]) prescribed opioids (P < .001 overall) (eTable 1 in the Supplement). Among those persons prescribed opioids, cases had higher median average MEDD (22 mg [interquartile range {IQR}, 15-39 mg] vs 20 mg [IQR, 14-30 mg]; P < .001) and were more likely to have received an immunosuppressive opioid (646 [41.3%] vs 1672 [33.2%]; P < .001) compared with controls. The pattern of findings did not differ among PLWH and uninfected patients. Compared with uninfected patients, the magnitude of the difference between cases and controls in median average MEDD (uninfected patients, 23 mg [IQR, 15-42 mg] vs 20 mg [IQR, 14-31 mg]; PLWH, 22 mg [IQR, 14-36 mg] vs 20 mg [IQR, 14-31 mg]) and the proportion prescribed immunosuppressive opioids (uninfected patients, 274 [38.5%] vs 697 [30.3%]; PLWH, 372 [43.6%] vs 975 [35.3%]) was similar among PLWH.
Prescribed Opioid Characteristics and CAP
In unadjusted conditional logistic regression, compared with none, prescribed opioids were associated with increased odds of CAP, with the greatest risk observed with current prescribed opioids (OR range, 1.82 [95% CI, 1.54-2.15] to 5.11 [95% CI, 4.02-6.48]) compared with past prescribed opioids (OR range, 1.51 [95% CI, 1.34-1.69] to 1.80 [95% CI, 1.56-2.08]) or none (OR, 1 [reference]) (eTable 2 in the Supplement). In adjusted models, compared with none, prescribed opioids remained associated with CAP for past unknown or no immunosuppressive (adjusted OR [AOR], 1.24; 95% CI, 1.09-1.40) and immunosuppressive opioid use (AOR, 1.42; 95% CI, 1.21-1.67) and current prescribed opioids (AORs, 1.23 [95% CI, 1.03-1.48] to 3.18 [95% CI, 2.44-4.14]). We found evidence of a dose-response effect such that current prescribed high-dose opioids were associated with the greatest CAP risk (AOR for unknown or no immunosuppressive opioids, 2.07 [95% CI, 1.50-2.86]; AOR for immunosuppressive opioids, 3.18 [95% CI, 2.44-4.14]), followed by medium-dose opioids (AOR for unknown or no immunosuppressive opioids, 1.35 [95% CI, 1.13-1.62]; AOR for immunosuppressive opioids, 2.07 [95% CI, 1.50-2.86]) and low-dose opioids (AOR for unknown or no immunosuppressive opioids, 1.23 [95% CI, 1.03-1.48]; AOR for immunosuppressive opioids, 1.35 [95% CI, 1.13-1.62]). Similarly, within each stratum, evidence suggested that the immunosuppressive properties of prescribed opioids were associated with differential CAP risk (AORs for immunosuppressive opioids, 1.35 [95% CI, 1.07-1.70] to 3.18 [95% CI, 2.44-4.14]; AORs for unknown or no immunosuppressive properties, 1.23 [95% CI, 1.03-1.48] to 2.07 [95% CI, 1.50-2.86]).
Prescribed Opioid Characteristics by HIV Status
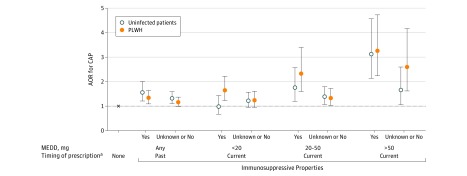
In adjusted analyses stratified by HIV status, CAP risk tended to be greater among PLWH with current prescribed opioids, especially immunosuppressive opioids, compared with uninfected patients (Figure 3 and eTable 2 in the Supplement). For example, among PLWH, compared with no prescribed opioids, the OR of CAP was 3.26 (95% CI, 2.24-4.73) higher with current high-dose immunosuppressive opioids; among uninfected patients, the OR of CAP was 3.13 (95% CI, 2.14-4.57). For current medium-dose immunosuppressive opioids, the OR of CAP was 2.33 (95% CI, 1.60-3.40) for PLWH vs 1.76 (95% CI, 1.20-2.57) for uninfected patients. The overall interaction term for opioid × HIV status, however, was not significant (P = .36).
Figure 3. Prescribed Opioid Characteristics and Community-Acquired Pneumonia Risk by HIV Status, Conditional Multivariable Logistic Regression.
Includes 25 392 cases and controls. Multivariable conditional logistic regression models were stratified by HIV status and adjusted for smoking status, diabetes mellitus, chronic obstructive pulmonary disease, congestive heart failure, stroke, alcohol- and other drug–related diagnoses, prior pneumonia, long-term benzodiazepine receipt, prescribed oral and inhaled corticosteroid receipt, influenza vaccination, pneumococcal vaccination, and Veterans Aging Cohort Study Index score. P = .36 for interaction of immunosuppressive properties × HIV status from unstratified model. Error bars indicate 95% CI. AOR indicates adjusted odds ratio; CAP, community-acquired pneumonia; MEDD, morphine equivalent daily dose; and PLWH, people living with HIV.
aCurrent indicates prescribed opioid receipt 5 to 60 days before the index date; past, 61 to 365 days before the index date.
Sensitivity and Post Hoc Analyses
In sensitivity analyses that excluded patients with a cancer diagnosis (n = 1561), those with a VACS Index score greater than 100 during follow-up (n = 92), or those with an opioid use disorder (n = 1690), results were not substantively different. In post hoc analyses in the overall sample, compared with no prescribed opioids and within each stratum, prescribed opioids with unknown immunosuppressive properties appeared to be associated with lower odds of CAP compared with opioids with immunosuppressive properties or no immunosuppressive properties.
Discussion
In this large, national cohort of patients with detailed prescribed opioid exposure, we found a strong, independent association between prescribed opioids and the risk of CAP requiring hospitalization in PLWH and uninfected patients. This finding was true for current and past-year prescribed opioid receipt. In addition, among those prescribed opioids, we found that the CAP risk increased with a higher current opioid dose and in patients prescribed opioids with known immunosuppressive properties. Notably, PLWH appear to have a greater CAP risk at lower opioid doses and particularly with immunosuppressive opioids compared with uninfected patients. No previous research has compared the association of prescribed opioids with CAP risk among those with and without HIV infection.3,4,5,13
Our findings complement and extend the literature indicating that prescribed opioids have clinically relevant immunosuppressive effects. Three prior studies are most relevant.3,4,5 In a cohort of community-dwelling older adults (1039 cases and 2022 controls),3 CAP risk was greatest immediately after initiation of prescribed opioid treatment, and immunosuppressive opioids were associated with increased risk. That study did not find that past prescribed opioids or average MEDD among those with long-term prescribed opioids were associated with CAP risk. Similarly, in a self-controlled case series of patients with rheumatoid arthritis,4 an adjusted incidence rate ratio of 1.39 (95% CI, 1.19-1.62) was found for serious infections requiring hospitalization. However, although that study found an incidence rate ratio of 1.22 for CAP during periods of prescribed opioids compared with those without, the 95% CI crossed 1.00 (0.99-1.51).4 Finally, in a nested case-control study including 1233 cases with invasive pneumococcal disease, including pneumonia, and 24 399 controls,5 investigators found that cases had greater odds than controls of prescribed opioid use with an AOR of 1.62 (95% CI, 1.36-1.92). These associations were strongest for prescribed opioids that were long acting, high potency, and high dose. As in these studies,3,4,5 we found that current receipt of prescribed opioids and higher doses were associated with greatest CAP risk. Our finding that past prescribed opioid receipt was also associated with CAP risk may be associated with prolonged immunosuppressive effects, stockpiling unused medication, which may enable use of medications longer than prescribed, and/or unmeasured or residual confounding. Consistent with preclinical studies demonstrating differential immunosuppressive effects of specific opioids,11 we observed that CAP risk was greatest with use of immunosuppressive prescribed opioids, including codeine, dihydrocodeine bitartrate, fentanyl, and morphine. Notably, prior work3,5 has found that receipt of long-acting opioids is associated with increased CAP risk. This finding is consistent with ours given that morphine, the most commonly prescribed long-acting opioid in our data, is considered immunosuppressive, and use of long-acting formulations is correlated with receipt of higher-dose opioids.
We observed a differential association of prescribed opioids with CAP risk by HIV status based on opioid dose and immunosuppressive properties as the magnitude of the association between these opioid characteristics, and CAP risk was higher for PLWH compared with uninfected patients. We have reported that prescribed opioids are not associated with CD4 cell count recovery among patients initiating ARV therapy.18 The findings from the present study, however, suggest that HIV infection may make patients more susceptible to the effects of prescribed opioids and are consistent with our findings demonstrating that PLWH have a greater mortality risk at lower opioid doses than uninfected individuals.28 Consistent with our hypothesis, our findings suggest that PLWH may be more susceptible to the effects of immunosuppressive medications and with lower levels of exposure.
Strengths and Limitations
Several strengths of our study deserve mention. First, by excluding patients who received more than 14 days of prescribed opioids during the baseline period, we restricted our sample to individuals who were relatively naive to prescribed opioids to decrease bias.16 Second, we had comprehensive ascertainment of CAP by including data from the VA and CMS and applied validated methods.21 Third, we used a validated measure of morbidity and mortality to account for underlying disease severity and to minimize confounding.19 Fourth, we accounted for other potential confounders in the association between prescribed opioids and CAP risk, including comorbid conditions, such as alcohol use disorder; use of immunosuppressive medications; and preventive care. However, residual confounding may still exist. For example, we adjusted for smoking status but not for the number of cigarettes or pack-years of smoking history for our patients. Fifth, more than 95% of cases and controls had a VA or CMS visit in the 18 months before the index date, indicating that our data comprehensively captured diagnoses and health care services for participants in our sample. Finally, our large sample size allowed us to increase precision of the estimated association of prescribed opioids with CAP risk.
Our study also has limitations. First, although we were able to adjust for confounders proximal to the CAP event, we cannot completely rule out unmeasured confounding. Second, we assumed that patients took opioid medications as prescribed and were unable to account for opioids prescribed outside the VA. However, most VA patients predominantly use VA pharmacies.14,29 Third, we were unable to account for nonmedical use of prescribed opioids; however, a previous study30 found that rates of nonmedical use of prescribed opioids do not differ by HIV status. Fourth, we were unable to include a measure of illicit opioid (eg, heroin) use; however, our findings did not change in a sensitivity analysis that excluded patients with opioid use disorder. Fifth, our sample was predominantly male and with comorbid diseases, potentially limiting generalizability to female and less complex patients. However, opioids are commonly prescribed to medically and psychiatrically complex patients.31,32 Sixth, we cannot prove causality or rule out respiratory depression (vs immunosuppression) as the cause of the increased CAP risk. The observed effects of opioid immunosuppressive properties and CAP risk lend support to our hypothesis that opioids have clinically relevant immunosuppressive properties. Seventh, due to collinearity between long-acting opioid formulations and those that are immunosuppressive, we were unable to separate these effects. Last, we may not have captured CAP events for patients with Medicare Advantage; however, this subgroup reflected less than 10% of our sample, with no difference by HIV status.
Conclusions
This study adds to growing evidence of potential medical harms associated with prescribed opioids. Health care professionals should be aware of this additional CAP risk when they prescribe opioids, and future studies should investigate the effects of opioids prescribed for longer durations and on other immune-related outcomes. Understanding whether mitigating the risk of prescribed opioids for CAP is possible by using a lower dose and nonimmunosuppressive opioids awaits further study. For now, in those settings when prescribed opioids are warranted, care should be implemented to address other risk factors (eg, smoking cessation33 and vaccination) known to modify CAP risk.
eTable 1. Prescribed Opioid Characteristics Among Cases and Controls Prescribed Opioids, Overall and Stratified by HIV Status
eTable 2. Conditional Logistic Regression for Prescribed Opioids and Community-Acquired Pneumonia Risk
References
- 1.Berterame S, Erthal J, Thomas J, et al. Use of and barriers to access to opioid analgesics: a worldwide, regional, and national study. Lancet. 2016;387(10028):1644-1656. doi: 10.1016/S0140-6736(16)00161-6 [DOI] [PubMed] [Google Scholar]
- 2.Becker WC, Gordon K, Edelman EJ, et al. Trends in any and high-dose opioid analgesic receipt among aging patients with and without HIV. AIDS Behav. 2016;20(3):679-686. doi: 10.1007/s10461-015-1197-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dublin S, Walker RL, Jackson ML, et al. Use of opioids or benzodiazepines and risk of pneumonia in older adults: a population-based case-control study. J Am Geriatr Soc. 2011;59(10):1899-1907. doi: 10.1111/j.1532-5415.2011.03586.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wiese AD, Griffin MR, Stein CM, Mitchel EF Jr, Grijalva CG. Opioid analgesics and the risk of serious infections among patients with rheumatoid arthritis: a self-controlled case series study. Arthritis Rheumatol. 2016;68(2):323-331. doi: 10.1002/art.39462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wiese AD, Griffin MR, Schaffner W, et al. Opioid analgesic use and risk for invasive pneumococcal diseases: a nested case-control study. Ann Intern Med. 2018;168(6):396-404. doi: 10.7326/M17-1907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mora AL, Salazar M, Pablo-Caeiro J, et al. Moderate to high use of opioid analgesics are associated with an increased risk of Clostridium difficile infection. Am J Med Sci. 2012;343(4):277-280. doi: 10.1097/MAJ.0b013e31822f42eb [DOI] [PubMed] [Google Scholar]
- 7.Roy S, Ninkovic J, Banerjee S, et al. Opioid drug abuse and modulation of immune function: consequences in the susceptibility to opportunistic infections. J Neuroimmune Pharmacol. 2011;6(4):442-465. doi: 10.1007/s11481-011-9292-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang J, Barke RA, Ma J, Charboneau R, Roy S. Opiate abuse, innate immunity, and bacterial infectious diseases. Arch Immunol Ther Exp (Warsz). 2008;56(5):299-309. doi: 10.1007/s00005-008-0035-0 [DOI] [PubMed] [Google Scholar]
- 9.Rogers DF, Barnes PJ. Opioid inhibition of neurally mediated mucus secretion in human bronchi. Lancet. 1989;1(8644):930-932. doi: 10.1016/S0140-6736(89)92509-9 [DOI] [PubMed] [Google Scholar]
- 10.Wang J, Barke RA, Charboneau R, Roy S. Morphine impairs host innate immune response and increases susceptibility to Streptococcus pneumoniae lung infection. J Immunol. 2005;174(1):426-434. doi: 10.4049/jimmunol.174.1.426 [DOI] [PubMed] [Google Scholar]
- 11.Sacerdote P. Opioid-induced immunosuppression. Curr Opin Support Palliat Care. 2008;2(1):14-18. doi: 10.1097/SPC.0b013e3282f5272e [DOI] [PubMed] [Google Scholar]
- 12.Akgün KM, Huang L, Morris A, Justice AC, Pisani M, Crothers K. Critical illness in HIV-infected patients in the era of combination antiretroviral therapy. Proc Am Thorac Soc. 2011;8(3):301-307. doi: 10.1513/pats.201009-060WR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwacha MG, McGwin G Jr, Hutchinson CB, Cross JM, Maclennan PA, Rue LW III. The contribution of opiate analgesics to the development of infectious complications in burn patients. Am J Surg. 2006;192(1):82-86. doi: 10.1016/j.amjsurg.2006.01.001 [DOI] [PubMed] [Google Scholar]
- 14.Fultz SL, Skanderson M, Mole LA, et al. Development and verification of a “virtual” cohort using the National VA Health Information System. Med Care. 2006;44(8)(suppl 2):S25-S30. doi: 10.1097/01.mlr.0000223670.00890.74 [DOI] [PubMed] [Google Scholar]
- 15.Graham DJ, Campen D, Hui R, et al. Risk of acute myocardial infarction and sudden cardiac death in patients treated with cyclo-oxygenase 2 selective and non-selective non-steroidal anti-inflammatory drugs: nested case-control study. Lancet. 2005;365(9458):475-481. doi: 10.1016/S0140-6736(05)70270-1 [DOI] [PubMed] [Google Scholar]
- 16.Schneeweiss SSS. Advanced Approaches to Controlling Confounding in Pharmacoepidemiologic Studies. Hoboken, NJ: John Wiley & Sons, Ltd; 2013. [Google Scholar]
- 17.Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol. 2003;158(9):915-920. doi: 10.1093/aje/kwg231 [DOI] [PubMed] [Google Scholar]
- 18.Edelman EJ, Gordon KS, Tate JP, et al. The impact of prescribed opioids on CD4 cell count recovery among HIV-infected patients newly initiating antiretroviral therapy. HIV Med. 2016;17(10):728-739. doi: 10.1111/hiv.12377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Justice AC, Modur SP, Tate JP, et al. ; NA-ACCORD and VACS Project Teams . Predictive accuracy of the Veterans Aging Cohort Study index for mortality with HIV infection: a North American cross cohort analysis. J Acquir Immune Defic Syndr. 2013;62(2):149-163. doi: 10.1097/QAI.0b013e31827df36c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Metersky ML, Tate JP, Fine MJ, Petrillo MK, Meehan TP. Temporal trends in outcomes of older patients with pneumonia. Arch Intern Med. 2000;160(22):3385-3391. doi: 10.1001/archinte.160.22.3385 [DOI] [PubMed] [Google Scholar]
- 21.Rodriguez-Barradas MC, Akgün K, Brown ST, et al. Community acquired pneumonia (CAP) requiring hospitalization in HIV-infected (HIV+) and uninfected (HIV−) patients: evaluation of patients identified by ICD-9 codes. Open Forum Infect Dis. 2015;2(suppl 1):1583. doi: 10.1093/ofid/ofv133.1136 [DOI] [Google Scholar]
- 22.Edelman EJ, Gordon K, Becker WC, et al. Receipt of opioid analgesics by HIV-infected and uninfected patients. J Gen Intern Med. 2013;28(1):82-90. doi: 10.1007/s11606-012-2189-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sacerdote P. Opioids and the immune system. Palliat Med. 2006;20(suppl 1):s9-s15. [PubMed] [Google Scholar]
- 24.Faillie JL. Indication bias or protopathic bias? Br J Clin Pharmacol. 2015;80(4):779-780. doi: 10.1111/bcp.12705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crothers K, Huang L, Goulet JL, et al. HIV infection and risk for incident pulmonary diseases in the combination antiretroviral therapy era. Am J Respir Crit Care Med. 2011;183(3):388-395. doi: 10.1164/rccm.201006-0836OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sigel K, Wisnivesky J, Gordon K, et al. HIV as an independent risk factor for incident lung cancer. AIDS. 2012;26(8):1017-1025. doi: 10.1097/QAD.0b013e328352d1ad [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Korthuis PT, Fiellin DA, McGinnis KA, et al. Unhealthy alcohol and illicit drug use are associated with decreased quality of HIV care. J Acquir Immune Defic Syndr. 2012;61(2):171-178. doi: 10.1097/QAI.0b013e31826741aa [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weisberg DF, Gordon KS, Barry DT, et al. Long-term prescription of opioids and/or benzodiazepines and mortality among HIV-infected and uninfected patients. J Acquir Immune Defic Syndr. 2015;69(2):223-233. doi: 10.1097/QAI.0000000000000591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith MW, Joseph GJ. Pharmacy data in the VA health care system. Med Care Res Rev. 2003;60(3)(suppl):92S-123S. doi: 10.1177/1077558703256726 [DOI] [PubMed] [Google Scholar]
- 30.Barry DT, Goulet JL, Kerns RK, et al. Nonmedical use of prescription opioids and pain in veterans with and without HIV. Pain. 2011;152(5):1133-1138. doi: 10.1016/j.pain.2011.01.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seal KH, Shi Y, Cohen G, et al. Association of mental health disorders with prescription opioids and high-risk opioid use in US veterans of Iraq and Afghanistan. JAMA. 2012;307(9):940-947. doi: 10.1001/jama.2012.234 [DOI] [PubMed] [Google Scholar]
- 32.Mudumbai SC, Oliva EM, Lewis ET, et al. Time-to-cessation of postoperative opioids: a population-level analysis of the Veterans Affairs Health Care System. Pain Med. 2016;17(9):1732-1743. doi: 10.1093/pm/pnw015 [DOI] [PubMed] [Google Scholar]
- 33.US Department of Veterans Affairs. Mental health: tobacco and health. https://www.mentalhealth.va.gov/quit-tobacco/. Updated October 15, 2018. Accessed November 27, 2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Prescribed Opioid Characteristics Among Cases and Controls Prescribed Opioids, Overall and Stratified by HIV Status
eTable 2. Conditional Logistic Regression for Prescribed Opioids and Community-Acquired Pneumonia Risk