This cohort study compares the 30-day mortality among hospitalized patients with Enterobacteriaceae bacteremia treated with oral step-down therapy vs intravenous therapy.
Key Points
Question
Is conversion to oral therapy associated with effective treatment of Enterobacteriaceae bloodstream infections?
Findings
In this multicenter cohort study of 1478 unique patients with Enterobacteriaceae bacteremia, no difference in 30-day mortality or recurrent bacteremia was observed between patients converted to early, oral step-down therapy and propensity score–matched patients who continued to receive intravenous antibiotics for the duration of therapy.
Meaning
Oral step-down therapy appears to be a potential treatment approach for adults with Enterobacteriaceae bacteremia after the source is controlled and clinical improvement is observed.
Abstract
Importance
Conversion to oral therapy for Enterobacteriaceae bacteremia has the potential to improve the quality of life of patients by improving mobility, eliminating catheter-associated discomfort, decreasing the risk for noninfectious and infectious catheter-associated adverse events, and decreasing health care costs.
Objective
To compare the association of 30-day mortality with early oral step-down therapy vs continued parenteral therapy for the treatment of Enterobacteriaceae bloodstream infections.
Design, Setting, and Participants
This retrospective multicenter cohort study included a 1:1 propensity score–matched cohort of 4967 unique patients hospitalized with monomicrobial Enterobacteriaceae bloodstream infection at 3 academic medical centers from January 1, 2008, through December 31, 2014. Eligibility criteria included appropriate source control measures, appropriate clinical response by day 5, active antibiotic therapy from day 1 until discontinuation of therapy, availability of an active oral antibiotic option, and ability to consume other oral medications or feeding. Statistical analysis was performed from March 2, 2018, to June 2, 2018.
Exposures
Oral step-down therapy within the first 5 days of treatment of Enterobacteriaceae bacteremia.
Main Outcomes and Measures
The main outcome was 30-day all-cause mortality.
Results
Of the 2161 eligible patients, 1185 (54.8%) were male and 1075 (49.7%) were white; the median (interquartile range [IQR]) age was 59 (48-68) years. One-to-one propensity-score matching yielded 1478 patients, with 739 in each study arm. Sources of bacteremia included urine (594 patients [40.2%]), gastrointestinal tract (297 [20.1%]), central line-associated (272 [18.4%]), pulmonary (58 [3.9%]), and skin and soft tissue (41 [2.8%]). There were 97 (13.1%) deaths in the oral step-down group and 99 (13.4%) in the intravenous (IV) group within 30 days (hazard ratio [HR], 1.03; 95% CI, 0.82-1.30). There were no differences in recurrence of bacteremia within 30 days between the groups (IV, 6 [0.8%]; oral, 4 [0.5%]; HR, 0.82 [0.33-2.01]). Patients transitioned to oral step-down therapy were discharged from the hospital an average of 2 days (IQR, 1-6) sooner than patients who continued to receive IV therapy (5 days [IQR, 3-8 days] vs 7 days [IQR, 4-14 days]; P < .001).
Conclusions and Relevance
In this study, 30-day mortality was not different among hospitalized patients who received oral step-down vs continued parenteral therapy for the treatment of Enterobacteriaceae bloodstream infections. The findings suggest that transitioning to oral step-down therapy may be an effective treatment approach for patients with Enterobacteriaceae bacteremia who have received source control and demonstrated an appropriate clinical response. Early transition to oral step-down therapy may be associated with a decrease in the duration of hospital stay for patients with Enterobacteriaceae bloodstream infections.
Introduction
Gram-negative bacteremia continues to be associated with considerable morbidity and mortality.1 Although national guidelines give general recommendations for durations of antibiotic therapy for gram-negative bloodstream infections, the optimal route of administration remains undefined.2 Most patients with gram-negative bacteremia initially receive parenteral therapy, but it is unclear whether patients can be transitioned to oral therapy after an appropriate clinical response is observed without compromising patient outcomes. Data indicating that conversion to oral therapy for gram-negative bloodstream infections is safe and effective are largely limited to bacteremia secondary to urinary tract sources.3,4,5
Although the comparative efficacy of transitioning patients with gram-negative bloodstream infection to oral step-down therapy vs continued intravenous (IV) therapy is unknown, the benefits associated with oral step-down therapy are generally well recognized. Oral therapy improves the quality of life of patients by reducing length of hospital stay, improving mobility, and eliminating discomfort associated with IV catheters.6,7 Furthermore, oral therapy decreases the risk for noninfectious and infectious catheter-associated adverse events, such as line breakage, venous thrombosis, phlebitis, and catheter-associated bloodstream infections.6,7 In addition, oral therapy is associated with lower health care costs by reducing drug preparation and administration fees, as well as costs associated with the placement and maintenance of central lines.6,8 We sought to compare the clinical outcomes of hospitalized adult patients receiving oral step-down therapy vs those in patients receiving continued parenteral antibiotic therapy after source control and an appropriate clinical response for the treatment of Enterobacteriaceae bacteremia.
Methods
Setting and Participants
We conducted a multicenter retrospective cohort study that included patients 18 years or older who were hospitalized with monomicrobial Enterobacteriaceae bloodstream infections at The Johns Hopkins Hospital (Baltimore, Maryland) (1154 patient beds), the Hospital of the University of Pennsylvania (Philadelphia) (789 patient beds), and the University of Maryland Medical Center (Baltimore) (772 patient beds) from January 1, 2008, through December 31, 2014. Although all 3 institutions had existing antibiotic stewardship programs, none of the institutions had stewardship initiatives to routinely encourage transition from IV to oral therapy for bacteremia. Study participants had a blood culture positive for 1 of the following organisms: Citrobacter species, Enterobacter species, Escherichia coli, Klebsiella species, Proteus mirabilis, or Serratia marcescens. These organisms were selected because they represent common Enterobacteriaceae recovered from bloodstream infections.9 This study was approved by the institutions review boards of each of the 3 participating hospitals. A waiver of patient consent was granted because the study was retrospective, involved no interaction with patients, and was considered minimal risk.
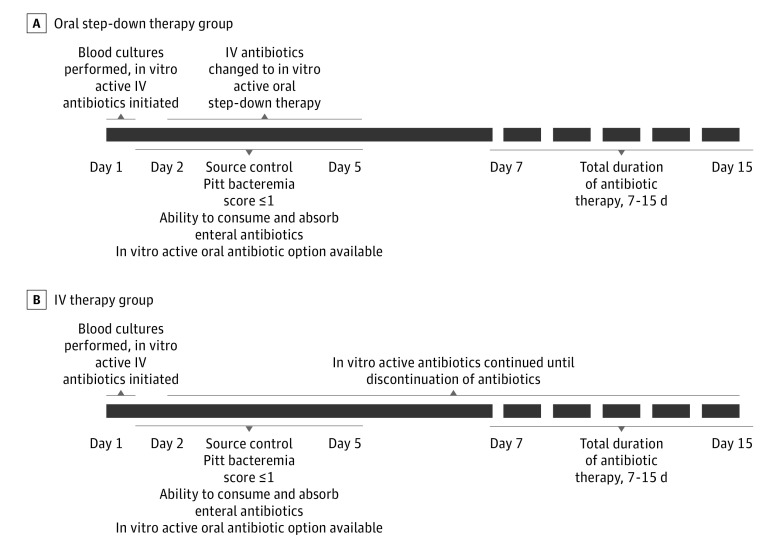
Patients in the exposed group (ie, those whose treatment was converted to oral therapy; hereafter referred to as the oral step-down group) had to be transitioned from IV to oral therapy by day 5 of therapy (Figure 1). Day 1 was defined as the first day of in vitro active antibiotic therapy, and antibiotics had to be administered within 24 hours of the time that the first positive blood culture was collected. Because data suggest that 7 days of antibiotics may be sufficient treatment for uncomplicated gram-negative bacteremia,10 transitioning to oral therapy on day 7 or later may not be meaningful because it is possible that sufficient antibiotic therapy was already administered. Therefore, 5 days was selected as the cut-off for the transition to oral step-down therapy for patients to be included in the oral step-down group. Patients for whom oral antibiotic therapy was initiated on day 1 of bacteremia and those who transitioned to oral therapy after day 5 of therapy were excluded from the study.
Figure 1. General Eligibility Criteria for the Study Sample.
All patients received at least 7 days of treatment, with a range of 7 to 15 days. IV indicated intravenous.
Unexposed patients were those who continued to receive IV therapy for their entire treatment course. All patients who continued IV therapy had to be candidates for oral step-down therapy to be eligible for this study. Eligibility criteria for oral step-down therapy included meeting all of the following: source control (ie, removal of infected hardware, drainage of infected fluid collections, or resolution of obstruction for biliary or urinary sources) achieved within the first 5 days; availability of an in vitro active oral antibiotic agent; receipt of other enteral medications or food by day 5; and a Pitt bacteremia score of 1 or lower by day 5. The Pitt bacteremia score is a measure of severity of illness, with a possible range from 0 to 14 based on patient’s temperature, blood pressure, respiratory, cardiac, and mental status. Higher scores indicate a poorer prognosis. The requirement of a Pitt bacteremia score of 1 or lower by day 5 regardless of the Pitt bacteremia score on day 1 was included to help overcome the likely bias that patients transitioned to oral step-down therapy would have a greater likelihood of an early favorable clinical response to initial management than patients who continued to receive IV therapy. Of note, patients in the oral step-down therapy group also had to meet the same criteria of source control within the initial 5 days and a Pitt bacteremia score of 1 or lower by day 5.
Patients were excluded if they met any of the following criteria: at least 1 in vitro active antibiotic was not initiated within 24 hours of initial collection of a blood sample for culture; receipt of fewer than 7 or more than 16 days of total antibiotic therapy, because durations greater than 16 days generally imply a complicated infection in which source control may not have been obtained (ie, osteomyelitis, endocarditis or endovascular infection, and meningitis); the bacteria recovered were not susceptible to the prescribed antibiotic regimen for the full duration of therapy (ie, day 1 until completion of therapy regardless of route of administration); receipt of a second in vitro active agent beyond 5 days (eg, for the oral step-down group, transitioning cefepime hydrochloride to oral ciprofloxacin but the continuation of gentamicin sulfate); or death within the first 5 days of treatment, because this may have precluded the opportunity to convert to oral therapy.
Outcomes included 30-day all-cause mortality, 30-day recurrent bloodstream infection with the same organism, and duration of hospitalization from day 1 of bacteremia until hospital discharge.
Clinical Data Collection
All data were extracted by manual review of electronic medical records from the 3 facilities by infectious diseases physicians, and data were stored in a secure and standardized REDCap database.11 The following data were collected: demographic information, preexisting medical conditions, presumed source of bacteremia (eg, pulmonary, skin and soft tissue, urinary tract, biliary, gastrointestinal tract, or catheter-associated) and source control measures, Pitt bacteremia score on day 1 through day 5 of bacteremia, microbiologic data, antibiotic treatment information, and patient outcomes. Antibiotics prescribed at discharge were collected for all patients. Oral step-down agents were further stratified by their bioavailability. Fluoroquinolones or trimethoprim-sulfamethoxazole were categorized as moderately to highly bioavailable agents (hereafter referred to as the high-bioavailability group), and oral β-lactams were considered to be low-bioavailability agents, with bioavailability being defined as no significant difference in the absorption of antibiotics administered via the oral or parenteral route.12,13
Microbiology Methods
Blood cultures were processed by the clinical microbiology laboratories at each of the participating institutions according to standard operating procedures. Antibiotic susceptibility data were determined by the BD Phoenix Automated System (BD Diagnostics) for isolates at The Johns Hopkins Hospital and by the Vitek 2 System (bioMérieux) for isolates at the Hospital of the University of Pennsylvania and the University of Maryland Medical Center. Current Clinical and Laboratory Standards Institute breakpoints were used to define isolates susceptible to the antibiotics administered.14
Statistical Analysis
Statistical analysis was performed from March 2, 2018, to June 2, 2018. We anticipated that there would be inherent differences between patients converted to oral step-down therapy and those who remained on parenteral therapy regardless of the timing of the clinical response to the initial IV antibiotic therapy prescribed and source control measures. We hypothesized that patients continuing to receive IV therapy would be more likely to have a greater severity of illness on day 1 of bacteremia, would be more likely to have complex underlying medical conditions, and would be more likely to have frequent exposures to health care facilities.
Propensity scores were calculated using a multivariable logistic regression model in which the dependent variable was a binary indicator of receipt of oral step-down therapy within the first 5 days (the exposed group). Covariates included in generating the propensity score included age, race/ethnicity, preexisting medical conditions (each included separately: end-stage liver disease, end-stage renal disease requiring dialysis, structural lung disease, congestive heart failure with an ejection fraction of <45%, or diabetes), immunocompromised status (each included separately: HIV infection with a CD4 cell count <200/mL, solid organ transplant, hematopoietic stem cell transplant, chemotherapy within 6 months, severe neutropenia15 [ie, absolute neutrophil count <500/mm3 at the time of blood sample obtainment for culture], active immunomodulatory therapy, or at least 20 mg of corticosteroids daily for 14 days or longer), Pitt bacteremia score on day 1 of therapy, intensive care unit stay on day 1 of bacteremia, source of bacteremia, prescription of combination antibiotic therapy beyond the first 48 hours (but not more than 5 days because patients who received combination therapy beyond 5 days were excluded), and total duration of antibiotic therapy.
One-to-one nearest-neighbor matching without replacement with a caliper width of 0.20 SD was conducted. Standardized mean biases were evaluated to ensure balance after propensity score matching between the oral step-down and IV therapy groups, with a difference of less than 10% between the groups considered to be well balanced. Patient characteristics were compared using the χ2 test for categorical variables and the Wilcoxon rank sum test for continuous data. Categorical variables were presented as numbers with percentages, and continuous variables were reported as medians and interquartile ranges (IQRs). A 30-day survival curve was developed, and comparisons between the exposure groups were evaluated using the log-rank test. Cox proportional hazard regression models were constructed to obtain hazard ratios (HRs) and 95% CIs for the outcomes of 30-day mortality, 30-day bacteremic relapse, and length of stay from day 1 of bacteremia until hospital discharge. The proportional hazards assumption was checked for all models. Additional adjustment was planned for any variables with P < .10 on univariable analysis in the propensity score–matched cohort. A subgroup analysis was conducted comparing outcomes between patients in the oral step-down therapy group receiving high-bioavailability vs low-bioavailability agents. Two-sided P values ≤05 were used for statistical significance testing. Analyses were performed using the Stata, version 15.0 (Stata Corp) statistical package.
Results
Study Population
In a cohort of 4967 unique adult patients with Enterobacteriaceae bacteremia, 2161 patients met study eligibility criteria. The median (IQR) age of the eligible cohort was 59 years (IQR, 48-69 years); 1185 (54.8%) were male, and 1075 (49.7%) were white. The oral step-down therapy group consisted of 876 patients (40.5%), and the IV therapy group consisted of 1285 patients (59.5%). Patients with Enterobacteriaceae bloodstream infections at all 3 participating hospitals were more likely to continue to receive IV therapy for their entire treatment courses.
Patient and microbial characteristics of the 2161 patients meeting eligibility criteria are presented in Table 1. There were several notable differences between the 2 groups. Patients who were transitioned to oral step-down therapy were less likely than patients who continued to receive IV therapy to be severely neutropenic (59 patients [6.7%] vs 181 patients [14.1%]) or have received a hematopoietic stem cell transplant in the previous 12 months (30 patients [3.4%] vs 97 patients [7.5%]). Moreover, patients in the oral step-down group were less likely to be severely ill at the onset of their bloodstream infection; as they had a decreased likelihood of requiring care in an intensive care unit (161 patients [18.4%] vs 415 patients [32.3%]) and had lower median Pitt bacteremia scores on day 1 of antibiotic therapy (1 [IQR, 1-3] vs 2 [IQR, 2-4]) than patients who continued to receive IV therapy. Patients in the oral step-down group were less likely to receive combination antibiotic therapy (ie, addition of a fluoroquinolone or aminoglycoside) for greater than 48 hours (54 patients [6.2%] vs 154 patients [12.0%]). There were also differences in the likelihood of transitioning to oral step-down therapy based on the source of bacteremia. Patients with urinary tract sources for bacteremia were significantly more likely to be converted to oral step-down therapy (405 patients [46.2%] vs 383 patients [29.8%]), whereas patients with gastrointestinal tract (160 patients [18.3%] vs 290 patients [22.6%]), pulmonary (29 patients [3.3%] vs 117 patients [9.1%]), or catheter-associated (137 patients [15.6%] vs 282 patients [22.0%]) sources were more likely to continue to receive IV therapy.
Table 1. Baseline Characteristics of Hospitalized Adult Patients With Enterobacteriaceae Bacteremia Transitioned to Oral Step-Down Therapy and Patients Continuing Intravenous Therapya.
| Characteristic | Full Cohort | Propensity Score–Matched Cohort | |||||
|---|---|---|---|---|---|---|---|
| Oral Therapy (n = 876) | Intravenous Therapy (n = 1285) | P Value | Oral Therapy (n = 739) | Intravenous Therapy (n = 739) | P Value | ||
| Age, median (IQR), y | 59 (47-69) | 59 (48-68) | .93 | 59 (47-69) | 59 (49-69) | .46 | |
| Male | 439 (50.1) | 706 (54.9) | .03 | 383 (51.8) | 386 (52,2) | .88 | |
| Race/ethnicity | |||||||
| White | 423 (48.3) | 663 (51.6) | .12 | 363 (49.1) | 363 (49.1) | >.99 | |
| Black | 369 (42.1) | 493 (38.4) | .08 | 312 (42.2) | 309 (41.8) | .87 | |
| Asian | 30 (3.4) | 55 (4.3) | .31 | 30 (4.1) | 27 (3.7) | .68 | |
| Latino | 25 (2.9) | 34 (2.7) | .78 | 20 (2.7) | 20 (2.7) | >.99 | |
| Unknown or multiracial | 27 (3.1) | 35 (2.7) | .63 | 14 (1.9) | 21 (2.8) | .30 | |
| Weight, median (IQR), kg | 74.9 (63.5-88.25) | 73.5 (62.3-88.4) | .60 | 74.7 (63.5-87.6) | 73.9 (62.3-90) | .43 | |
| Preexisting medical conditions | |||||||
| End-stage liver disease | 51 (5.8) | 87 (6.8) | .38 | 44 (6.0) | 41 (5.5) | .74 | |
| End-stage renal disease requiring dialysis | 41 (4.7) | 100 (7.8) | .004 | 40 (5.4) | 40 (5.4) | >.99 | |
| Structural lung disease | 43 (4.9) | 98 (7.6) | .01 | 43 (5.8) | 43 (5.8) | >.99 | |
| Congestive heart failure, ejection fraction <45% | 78 (8.9) | 121 (9.4) | .69 | 68 (9.2) | 63 (8.5) | .65 | |
| Diabetes | 228 (26.0) | 307 (23.9) | .26 | 187 (25.3) | 184 (24.9) | .86 | |
| Immunocompromised | |||||||
| HIV infection | 39 (4.5) | 48 (3.7) | .40 | 32 (4.3) | 25 (3.4) | .34 | |
| Chemotherapy within 6 mo | 246 (28.1) | 355 (27.6) | .82 | 216 (29.2) | 205 (27.7) | .53 | |
| Absolute neutrophil count <500/mL | 59 (6.7) | 181 (14.1) | <.001 | 59 (8.0) | 59 (8.0) | >.99 | |
| Immunomodulatory therapy or steroids within 30 d | 33 (3.8) | 43 (3.3) | .60 | 25 (3.4) | 24 (3.2) | .88 | |
| Solid organ transplant | 103 (11.8) | 114 (8.9) | .03 | 78 (10.6) | 78 (10.6) | >.99 | |
| Hematopoietic stem cell transplant within 12 mo | 30 (3.4) | 97 (7.5) | <.001 | 29 (3.9) | 35 (4.7) | .44 | |
| Total No. of days of antibiotic therapy, median (IQR) | 15 (12-16) | 14 (11-15) | <.001 | 14 (11-16) | 14 (11-16) | .82 | |
| Total No. of days of intravenous therapy, median (IQR) | 3 (2-4) | 14 (11-15) | <.001 | 3 (2-4) | 14 (11-16) | <.001 | |
| Combination antibiotic therapy for >48 h | 54 (6.2) | 154 (12.0) | <.001 | 53 (7.2) | 51 (6.9) | .84 | |
| Source of infection | |||||||
| Pulmonary | 29 (3.3) | 117 (9.1) | <.001 | 29 (3.9) | 29 (3.9) | >.99 | |
| Skin and soft tissue | 22 (2.5) | 50 (3.9) | .08 | 22 (3.0) | 19 (2.6) | .63 | |
| Urinary tract | 405 (46.2) | 383 (29.8) | <.001 | 295 (39.9) | 299 (40.5) | .83 | |
| Biliary | 121 (13.8) | 148 (11.5) | .11 | 103 (13.9) | 107 (14.5) | .77 | |
| Gastrointestinal tract | 160 (18.3) | 290 (22.6) | .02 | 152 (20.6) | 145 (19.6) | .65 | |
| Catheter-associated | 137 (15.6) | 282 (22.0) | <.001 | 135 (18.3) | 137 (18.5) | .84 | |
| Pitt bacteremia score on day 1, median (IQR) | 1 (0-3) | 2 (1-4) | <.001 | 2 (1-3) | 1 (0-3) | .27 | |
| Intensive care unit on day 1 | 161 (18.4) | 415 (32.3) | <.001 | 156 (21.1) | 158 (21.4) | .90 | |
| Enterobacteriaceae isolated from bloodstream | |||||||
| Citrobacter species | 21 (2.4) | 20 (1.6) | .16 | 16 (2.2) | 11 (1.5) | .33 | |
| Enterobacter species | 98 (11.2) | 159 (12.4) | .43 | 91 (12.3) | 82 (11.1) | .47 | |
| Escherichia coli | 420 (48.0) | 513 (39.9) | <.001 | 336 (45.5) | 309 (41.8) | .16 | |
| Klebsiella pneumoniae | 267 (30.4) | 458 (35.6) | .01 | 237 (32.0) | 268 (36.2) | .10 | |
| Klebsiella oxytoca | 17 (1.9) | 19 (1.5) | .50 | 12 (1.6) | 10 (1.3) | .83 | |
| Proteus mirabilis | 29 (3.3) | 63 (4.9) | .07 | 25 (3.4) | 40 (5.4) | .06 | |
| Serratia marcescens | 24 (2.7) | 53 (4.1) | .09 | 22 (3.0) | 19 (2.6) | .63 | |
Abbreviation: IQR, interquartile range.
Data are presented as number (percentage) of patients unless otherwise indicated.
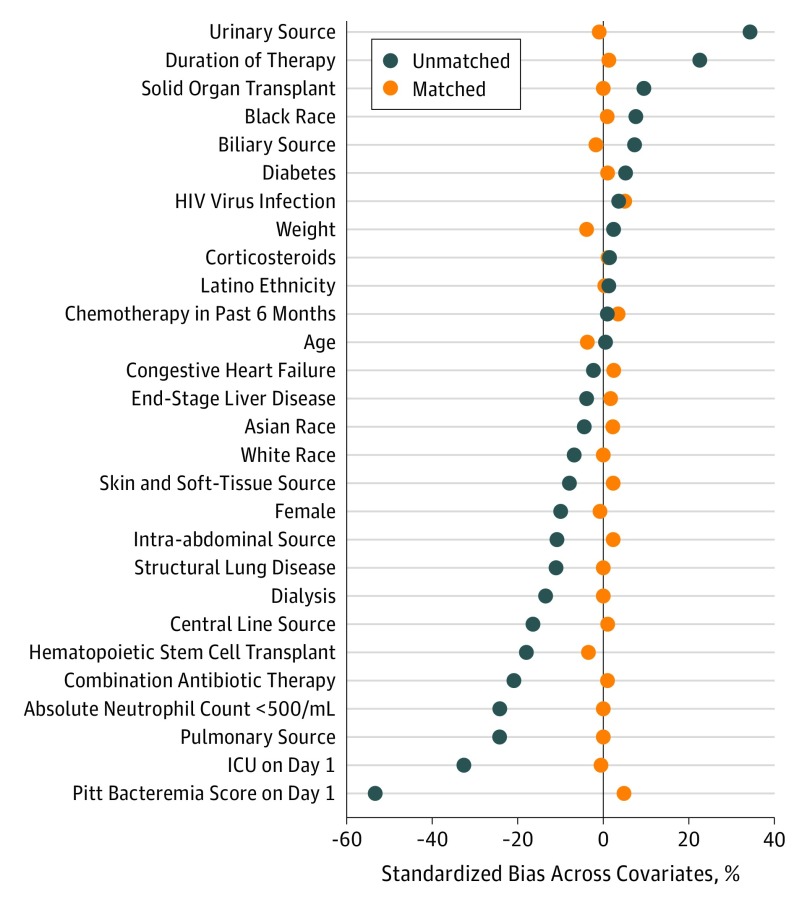
One-to-one propensity-score matching yielded 1478 patients, with 739 in each study arm. Patient characteristics were well balanced between the 2 treatment groups (Table 1). Standardized biases for all variables were 0.05 or less (Figure 2). Patients received a median of 3 days (IQR, 2-4 days) of IV therapy in the oral step-down group and 14 days (IQR, 11-15 days) of IV therapy in the IV group.
Figure 2. Standardized Mean Biases Comparing the Full Unmatched Cohort With the Propensity Score–Matched Cohort.
ICU indicates intensive care unit.
Represented isolates among the 1478 patients in the propensity-matched cohort included E coli (645 patients [43.6%]), Klebsiella species (527 patients [35.7%]), Enterobacter species (173 patients [11.7%]), P mirabilis (65 patients [4.4%]), S marcescens (41 patients [2.8%]), and Citrobacter species (27 patients [1.8%]) (Table 1). Common sources of bacteremia in the matched cohort included urinary tract (594 patients [40.2%]), gastrointestinal tract (297 patients [20.1%]), catheter-associated (272 patients [18.4%]), biliary (210 patients [14.2%]), pulmonary (58 patients [3.9%]), and skin and soft tissue (41 patients [2.8%]).
Outcomes
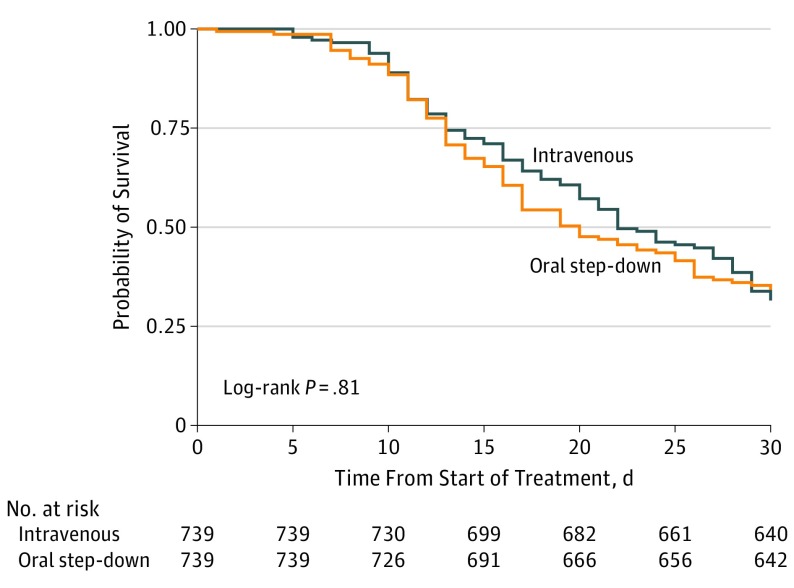
In the propensity score–matched cohort, there were 97 deaths (13.1%) in the oral step-down group within 30 days vs 99 (13.4%) in the IV group (HR, 1.03; 95% CI, 0.82-1.30) (Figure 3). There were 6 episodes (0.8%) of recurrent bacteremia within 30 days in the oral step-down group vs 4 (0.5%) in the IV group (HR, 0.82; 95% CI, 0.33-2.01). The median time from day 1 of bacteremia to hospital discharge was 5 days (IQR, 3-8 days) in the oral step-down group vs 7 days (IQR, 4-14 days) in the IV group (HR, 0.98; 95% CI, 0.97-1.00; P < .001).
Figure 3. Probability of 30-Day Survival in the Propensity Score–Matched Cohort.
Of the 739 patients in the propensity score–matched cohort transitioned to oral step-down therapy, 617 (83.5%) received agents in the high-bioavailability group and 122 (16.5%) received low-bioavailability agents (Table 2). Of patients transitioned to a high-bioavailability agent, 518 (83.9%) received a fluoroquinolone. Sixty-eight (11.0%) patients in the high-bioavailability group vs 15 (12.3%) patients in the low-bioavailability group died within 30 days (HR, 1.05; 95% CI, 0.67-1.66). Four (0.6%) 30-day recurrences of bacteremia with the same organism occurred in the high-bioavailability group, and none occurred in the low-bioavailability group.
Table 2. Antibiotic Therapy Administered to Patients Transitioned to Oral Antibiotic Therapy for Enterobacteriaceae Bacteremia.
| Antibiotic | Common Regimen | Bioavailability | Patients Receiving Treatment, No. (%) (n = 739) |
|---|---|---|---|
| Amoxicillin-clavulanate | 500-1000 mg orally every 8-12 h | Low | 38 (5.1) |
| Cefdinir | 300 mg orally every 12 h | Low | 30 (4.1) |
| Cefixime | 200-400 mg orally every 12-24 h | Low | 21 (2.8) |
| Cephalexin hydrochloride | 500 mg orally every 6 h | Low | 16 (2.2) |
| Cefpodoxime proxetil | 200-400 mg orally every 12 h | Low | 17 (2.3) |
| Ciprofloxacin hydrochloride | 500-750 mg orally every 12 h | High | 337 (45.6) |
| Levofloxacin | 500-750 mg orally every 24 h | High | 171 (23.1) |
| Moxifloxacin hydrochloride | 400 mg orally every 24 h | High | 10 (1.3) |
| Trimethoprim-sulfamethoxazole | 160-320 mg orally every 6-12 h | High | 99 (13.4) |
Discussion
In this study, 30-day mortality was not different among hospitalized patients who received oral step-down vs continued parenteral therapy for the treatment of Enterobacteriaceae bloodstream infections. Our study suggests that early oral step-down therapy is an effective treatment approach for patients with Enterobacteriaceae bacteremia who have undergone appropriate source control measures and have demonstrated favorable clinical responses to initial parenteral therapy. More specifically, we found that there was no difference in all-cause mortality or recurrent bacteremia within 30 days for patients converted to oral step-down therapy vs those who continued to receive parenteral therapy for the remainder of their treatment course. However, patients who were transitioned to oral step-down therapy were discharged from the hospital approximately 2 days earlier than those who continued to receive IV therapy. Although not evaluated in our study, this delay could perhaps be a result of the wait time associated with peripherally inserted central catheter placement, a reluctance of clinicians to send patients home with peripherally inserted central catheters, delays associated with arranging outpatient parenteral antibiotic therapy, or impediments associated with placement of patients in post–acute care facilities.
To our knowledge, there have been no randomized clinical trials designed to specifically address the role of oral step-down therapy for the treatment of Enterobacteriaceae bacteremia. However, the effectiveness of early oral step-down therapy for Enterobacteriaceae bacteremia was previously investigated in an observational study conducted at the Medical University of South Carolina by Rieger and colleagues.3 These investigators limited their evaluation to patients with Enterobacteriaceae bloodstream infections from urinary tract sources. They compared proportions of treatment failure between 135 patients transitioned to oral step-down therapy (at a median of 4 days) vs 106 patients who continued to receive IV therapy. Owing to their relatively small sample size, the investigators were unable to adjust for the notable differences in severity of illness between the 2 groups. Although the proportion of treatment failure was similar between patients transitioned to oral step-down therapy and those continuing with IV therapy, as with our study, these investigators found that patients transitioned to oral step-down therapy were discharged from the hospital approximately 2 days earlier than those who continued to receive IV therapy.
We presumed that patients converted to oral fluoroquinolones or trimethoprim-sulfamethoxazole would be less likely to experience treatment failure than patients transitioned to oral β-lactam agents because of both the high bioavailability and the favorable pharmacokinetic and pharmacodynamic profile of the former agents.12,13 However, we found no differences in clinical outcomes between patients converted to high-bioavailability vs low-bioavailability agents. Others have found similar results. Kutob and colleagues16 conducted a retrospective cohort study including 362 patients with gram-negative bloodstream infection to define the role of the bioavailability of oral agents for the treatment of gram-negative bacteremia predominantly from urinary tract sources. As with our study, treatment failures were no different between patients receiving fluoroquinolones or trimethoprim-sulfamethoxazole and patients receiving oral β-lactams. Similarly, Mercuro and colleagues17 compared 140 adult patients at the Maine Medical Center receiving fluoroquinolones with 84 patients receiving oral β-lactams as step-down therapy for Enterobacteriaceae bacteremia and found no difference in clinical outcomes. Reported success with oral step-down therapy exceeded 85% in both of these studies, as well as in the study by Rieger and colleagues described above.3 We conducted a post hoc power calculation comparing 30-day mortality between the high-bioavailability and low-bioavailability groups in our cohort but had only 11% power to detect the difference that we observed with our study sample size. All studies to date, including ours, remain underpowered to determine whether the bioavailability of oral agents is less important for uncomplicated Enterobacteriaceae bacteremia after an appropriate clinical response has been observed and source control has been achieved. Therefore, it remains unclear whether no difference exists in clinical outcomes between high-bioavailability and low-bioavailability agents because we had insufficient power to detect a difference if one exists; after the bacterial burden is sufficiently reduced, the bioavailability of the agent becomes less important, or the duration of therapy typically prescribed for gram-negative bloodstream infection is simply too long.
Broom and colleagues18 attempted to further elucidate reasons that clinicians cite when transitioning patients to oral therapy vs continuing IV therapy for bacterial infections. After conducting semistructured interviews with 20 physicians, they identified 3 key issues driving the decision-making process: concern about potential litigation if patients or family members were not satisfied with the treatment course after transition to oral therapy, discomfort with suggesting to senior clinicians that conversion to oral step-down therapy was a reasonable option, and the perception that continued IV antibiotics were more potent than oral step-down therapy.18 Further education of clinicians is needed to demystify perceptions related to the relative efficacy of oral step-down therapy after appropriate clinical responses are observed.
Limitations
A number of limitations should be accounted for when interpreting our findings. First, owing to the retrospective nature of our study, we most likely were unable to account for all variables that are considered in the decision to transition patients to oral step-down therapy. For example, the decision to convert to oral step-down therapy could be somewhat arbitrary across clinicians and influenced by their general comfort level with prescribing oral agents to treat bloodstream infections. We were unable to address this with our available data. We attempted to overcome some of the confounding by indication with the development and incorporation of propensity scores. The use of stringent propensity score matching led to the exclusion of large numbers of patients in the overall cohort; however, it ensured that the 2 comparator groups were similar with regard to demographic characteristics, preexisting medical conditions, source control measures, severity of illness at the onset of bacteremia, use of combination therapy, and overall duration of therapy prescribed. Furthermore, we limited the cohort to patients who received adequate source control, had an appropriate clinical response in the early ensuing days of bacteremia, and received antibiotic therapy that was active against the pathogen for their entire course of therapy. However, we cannot exclude the possibility of additional unmeasured residual factors in the association between transitioning to oral step-down therapy and mortality that we did not address. Second, the outcome of bacteremic relapse was rare and experienced by less than 1% of patients in the cohort. Although it occurred with the same frequency in both the oral step-down and IV therapy groups, the study was underpowered to detect differences in this outcome. Another limitation is the lack of granular data on patient-specific absorption of enteral antibiotics. For example, enteral feeding coadministered with fluoroquinolones has been shown to reduce the antibiotics’ absorption by more than 50%.19,20 This interference is minimized by refraining from administering specific medications (eg, antacids, multivitamins) and tube feeding for a few hours before and after ingestion of fluoroquinolones.19,20 We were unable to account for differences in enteral absorption for patients who received oral therapy. However, all 3 institutions in the study have in-house and discharge paperwork guidance that emphasizes not combining fluoroquinolones with interacting agents. Similarly, we did not evaluate compliance with filling prescriptions and consuming oral medications after hospital discharge. Because clinical failures were similar between the oral step-down and IV therapy groups, this information is likely more relevant in comparisons between patients receiving high-bioavailability vs low-bioavailability oral step-down therapy.17
Conclusions
Until a clinical trial is performed, our findings suggest that oral step-down therapy is not associated with inferior clinical outcomes for patients with Enterobacteriaceae bacteremia who have received appropriate source control and demonstrated an appropriate clinical response compared with patients who continue to receive IV therapy for the duration of their treatment course. Furthermore, early transition to oral step-down therapy may be associated with decreased duration of hospital stay for patients with Enterobacteriaceae bloodstream infection.
References
- 1.Suljagić V, Cobeljić M, Janković S, et al. Nosocomial bloodstream infections in ICU and non-ICU patients. Am J Infect Control. 2005;33(6):333-340. doi: 10.1016/j.ajic.2005.03.010 [DOI] [PubMed] [Google Scholar]
- 2.Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the Infectious Diseases Society of America [published correction appears in Clin Infect Dis. 2010;50(7):1079]. Clin Infect Dis. 2009;49(1):1-45. doi: 10.1086/599376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rieger KL, Bosso JA, MacVane SH, Temple Z, Wahlquist A, Bohm N. Intravenous-only or intravenous transitioned to oral antimicrobials for Enterobacteriaceae-associated bacteremic urinary tract infection. Pharmacotherapy. 2017;37(11):1479-1483. doi: 10.1002/phar.2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Talan DA, Stamm WE, Hooton TM, et al. Comparison of ciprofloxacin (7 days) and trimethoprim-sulfamethoxazole (14 days) for acute uncomplicated pyelonephritis pyelonephritis in women: a randomized trial. JAMA. 2000;283(12):1583-1590. doi: 10.1001/jama.283.12.1583 [DOI] [PubMed] [Google Scholar]
- 5.Talan DA, Klimberg IW, Nicolle LE, Song J, Kowalsky SF, Church DA. Once daily, extended release ciprofloxacin for complicated urinary tract infections and acute uncomplicated pyelonephritis. J Urol. 2004;171(2 Pt 1):734-739. doi: 10.1097/01.ju.0000106191.11936.64 [DOI] [PubMed] [Google Scholar]
- 6.Keller SC, Dzintars K, Gorski LA, Williams D, Cosgrove SE. Antimicrobial agents and catheter complications in outpatient parenteral antimicrobial therapy. Pharmacotherapy. 2018;38(4):476-481. doi: 10.1002/phar.2099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keller SC, Williams D, Gavgani M, et al. Rates of and risk factors for adverse drug events in outpatient parenteral antimicrobial therapy. Clin Infect Dis. 2018;66(1):11-19. doi: 10.1093/cid/cix733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lau BD, Pinto BL, Thiemann DR, Lehmann CU. Budget impact analysis of conversion from intravenous to oral medication when clinically eligible for oral intake. Clin Ther. 2011;33(11):1792-1796. doi: 10.1016/j.clinthera.2011.09.030 [DOI] [PubMed] [Google Scholar]
- 9.Al-Hasan MN, Eckel-Passow JE, Baddour LM. Impact of healthcare-associated acquisition on community-onset gram-negative bloodstream infection: a population-based study: healthcare-associated gram-negative BSI. Eur J Clin Microbiol Infect Dis. 2012;31(6):1163-1171. doi: 10.1007/s10096-011-1424-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chotiprasitsakul D, Han JH, Cosgrove SE, et al. ; Antibacterial Resistance Leadership Group . Comparing the outcomes of adults with Enterobacteriaceae bacteremia receiving short-course versus prolonged-course antibiotic therapy in a multicenter, propensity score–matched cohort. Clin Infect Dis. 2018;66(2):172-177. doi: 10.1093/cid/cix767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Béïque L, Zvonar R. Addressing concerns about changing the route of antimicrobial administration from intravenous to oral in adult inpatients. Can J Hosp Pharm. 2015;68(4):318-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chui D, Cheng L, Tejani AM. Clinical equivalency of ciprofloxacin 750 mg enterally and 400 mg intravenously for patients receiving enteral feeding: systematic review. Can J Hosp Pharm. 2009;62(2):127-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clinical and Laboratory Standards Institute Performance Standards for Antimicrobial Susceptibility Testing, 28th ed Wayne, PA: Clinical and Laboratory Standards Institute; 2018. CLSI document M100. [Google Scholar]
- 15.Taplitz RA, Kennedy EB, Bow EJ, et al. Outpatient management of fever and neutropenia in adults treated for malignancy: American Society of Clinical Oncology and Infectious Diseases Society of America Clinical Practice Guideline Update. J Clin Oncol. 2018;36(14):1443-1453. doi: 10.1200/JCO.2017.77.6211 [DOI] [PubMed] [Google Scholar]
- 16.Kutob LF, Justo JA, Bookstaver PB, Kohn J, Albrecht H, Al-Hasan MN. Effectiveness of oral antibiotics for definitive therapy of gram-negative bloodstream infections. Int J Antimicrob Agents. 2016;48(5):498-503. doi: 10.1016/j.ijantimicag.2016.07.013 [DOI] [PubMed] [Google Scholar]
- 17.Mercuro NJ, Stogsdill P, Wungwattana M. Retrospective analysis comparing oral stepdown therapy for Enterobacteriaceae bloodstream infections: fluoroquinolones versus β-lactams. Int J Antimicrob Agents. 2018;51(5):687-692. doi: 10.1016/j.ijantimicag.2017.12.007 [DOI] [PubMed] [Google Scholar]
- 18.Broom J, Broom A, Adams K, Plage S. What prevents the intravenous to oral antibiotic switch? a qualitative study of hospital doctors’ accounts of what influences their clinical practice. J Antimicrob Chemother. 2016;71(8):2295-2299. doi: 10.1093/jac/dkw129 [DOI] [PubMed] [Google Scholar]
- 19.Healy DP, Brodbeck MC, Clendening CE. Ciprofloxacin absorption is impaired in patients given enteral feedings orally and via gastrostomy and jejunostomy tubes. Antimicrob Agents Chemother. 1996;40(1):6-10. doi: 10.1128/AAC.40.1.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams NT. Medication administration through enteral feeding tubes. Am J Health Syst Pharm. 2008;65(24):2347-2357. doi: 10.2146/ajhp080155 [DOI] [PubMed] [Google Scholar]