This case series study assesses the preoperative and postoperative findings of recipients of an electronic retinal prosthesis using optical coherence tomography.
Key Points
Question
What are the postoperative changes shown on macular optical coherence tomography after implantation of an electronic retinal prosthesis?
Findings
In this case series study of the preoperative and postoperative optical coherence tomography findings of 33 eyes among 33 retinal prothesis implant recipients, 50% of the analyzed patients developed a fibrosislike hyperreflective tissue limited to the interface between the array and the retina, a significant finding. In most of these cases, the area of fibrosis progressed to retinal schisis without a change noted in visual performance, a result reported as statistically significant.
Meaning
These findings suggest that optical coherence tomography may help to identify retinal anatomic changes after retinal implantation.
Abstract
Importance
The postoperative retinal changes at the interface between an implant electrode array and the retina and whether these anatomic changes have an association with the patient visual performance are unknown.
Objective
To report morphologic changes in recipients of an Argus II Retinal Prosthesis.
Design, Setting, and Participants
This consecutive, noncomparative case series study included a retrospective review of the preoperative and postoperative optical coherence tomography of 33 eyes among 33 individuals who underwent Argus II Retinal Prosthesis System implantation between October 28, 2011, and June 8, 2017, at 2 different centers, by the same surgeon (S.R.). Thirteen patients received an implant at Azienda Ospedaliero Universitaria Pisana, Pisa, Italy, between October 28, 2011, and October 27, 2014, and 20 patients underwent surgery at Azienda Ospedaliera Universitaria Careggi, Florence, Italy, between December 20, 2014, and June 8, 2017. Patients were excluded if they did not reach the 6-month follow-up.
Main Outcomes and Measures
All patients were evaluated before surgery, during the first postoperative day, and at 1, 3, 6, 12, and 24 months (subsequently once a year, except for patient-related adverse events), with a comprehensive ophthalmic examination, retinal fundus photography, spectral-domain optical coherence tomography, and visual function tests to evaluate the stability or improvement of their visual performance.
Results
Of the 20 patients included in the analysis, all were of white race/ethnicity, 12 (60%) were male, and the mean (SD) age was 57.4 (11.6) years. Optical coherence tomography revealed the development of a fibrosislike hyperreflective tissue limited at the interface between the array and retina in 10 eyes (50%). In 9 of 10 patients (90%), fibrosis evolved and progressed to retinal schisis. Despite the development of the fibrosis and schisis, there was no deterioration in the patient’s visual performance evaluated prospectively with visual function tests (square localization and direction of motion).
Conclusions and Relevance
Optical coherence tomography may be used to observe the retinal anatomic changes in patients with an Argus II Prothesis. This analysis revealed the development of a fibrosislike hyperreflective tissue limited at the interface between array and retina that progressed to retinal schisis but with no deterioration in the patients’ visual performance.
Introduction
A variety of approaches to restoring sight are currently under investigation, opening new possibilities for patients with blindness and retinal pathologies. These possibilities include optogenetic techniques,1 gene therapy,2,3 stem cell therapy,4 and various artificial vision prostheses.5,6 An electronic retinal prosthesis represents a novel paradigm proposed to restore vision in patients with blindness due to retinitis pigmentosa.7,8 The Argus II Retinal Prosthesis System (Second Sight Medical Products Inc) is the first and only epiretinal device with commercial approval in Europe and North America, receiving the European Conformity stamp in 2011 and US Food and Drug Administration clearance in 2013 for the humanitarian use in patients with blindness due to retinitis pigmentosa.
Retinitis pigmentosa leads to blindness by causing photoreceptor death9; however, the inner retina cells are preserved.10,11,12 The Argus II Retinal Prostheses System takes advantage of the surviving ganglion cells by delivering small pulses of electricity to the inner retina, thereby creating a new sensation of vision.
The Argus II implant electrode array (9 × 5.5 mm) is composed of 60 platinum electrodes each of which is 200 µm in diameter, where electrical stimulation pulses are emitted. The electrode array is centered over the macula and is anchored to the retinal surface with a retinal tack. The array stimulates the residual ganglion cells to create visual percepts in the central 20° visual field.13
There are few articles that analyze the interface between the array and the retinal surface. Optical coherence tomography (OCT) is described in the literature as a useful tool for the detection of preoperative retinal abnormalities during candidate screening, such as staphylomas and macular epiretinal membranes, that can potentially affect Argus II performance after implantation, intraoperative monitoring of correct array positioning,14,15,16 and postoperative analysis of the association between the electrode array-retina distance and the electrical threshold amplitude.17
The aim of this study was to describe retinal changes after Argus II implantation, to investigate the causes of these modifications, and to evaluate their possible influence on the patient’s visual performance.
Methods
This consecutive, retrospective, noncomparative case series study was carried out based on the approval of the institutional review boards of Careggi University Hospital, Florence, Italy and of Pisa University Hospital, Pisa, Italy, and in accordance with the tenets of the Declaration of Helsinki.18 All patients signed a written informed consent to participate. All patients were screened preoperatively and were included or excluded according to the Argus II European Conformity–approved indications. Full inclusion and exclusion criteria are listed in the eMethods in the Supplement.
A total of 33 patients underwent Argus II implantation between October 28, 2011, and June 8, 2017, at 2 different centers, by the same surgeon (S.R.). Thirteen patients (all with end-stage retinitis pigmentosa) received implants at Azienda Ospedaliero Universitaria Pisana, Pisa, Italy, between October 28, 2011, and October 27, 2014, and 20 patients (19 with end-stage retinitis pigmentosa and 1 with Stargardt maculopathy) underwent surgery at Azienda Ospedaliera Universitaria Careggi, Florence, Italy, between December 20, 2014, and June 8, 2017.
In this study, we included only patients with more than 6 months of follow-up. Twenty of the 33 Argus II recipients met this inclusion criteria. Among the 13 excluded patients, 4 received an implant less than 6 months before this retrospective study. Nine patients were lost to follow-up for various reasons, including rehabilitation carried out in their own country (n = 1), patient death not associated with the implant (n = 1), inability to monitor the patient because of noncompliance with scheduled examinations and rehabilitation sessions (n = 3), and the onset of postoperative, serious adverse events requiring further surgery (n = 4), of which 2 were severe cases of hypotony associated with choroidal detachment that did not resolve with medical treatment and 2 were cases of retinal detachment.
During the surgical procedure, in all patients, a core vitrectomy was performed and 0.5 to 1.0 mL of triamcinolone acetate solution was injected with a 25-gauge needle into the mid-vitreous cavity to help the surgeon perform a more thorough removal of the vitreous and the posterior hyaloid. Meticulous removal of minute amounts of vitreous and hyaloid membrane is important because postoperative residual hyaloid may provide scaffolding for fibrovascular proliferation or may provide a foundation of traction. The vitreous cortex is usually very adherent in eyes with retinitis pigmentosa (especially in young patients) and normally does not detach further than the mid-periphery. If the epiretinal membrane was present and documented by a preoperative OCT scan, it was removed through macular peeling. In all cases, the intention was to completely remove epiretinal membranes. Without performing a previous fluid-air exchange, membrane blue-dual was applied onto the macula (while the vitreous cavity was completely filled with fluid) and all excess dye was immediately aspirated with a blunt backflush instrument. The stained epiretinal membrane was removed using routine surgical techniques by engaging the tissue with a pick or hooked needle, peeling the tissue from the underlying retina and removing it from the eye with intraocular forceps.
Approximately 2 weeks after the implantation, patients returned to the clinic to have the Argus II device fitted, or custom programmed, before the camera could be turned on. A video configuration file was generated that defined how the video signal from the camera was mapped to the electrical signal for the electrodes.
All patients were evaluated before surgery, at the first postoperative day, and at 1, 3, 6, 12, and 24 months (subsequently once a year except for patient-related adverse events) after the surgical procedure as per protocol. At each visit, a comprehensive ophthalmic examination, retinal fundus photography, spectral-domain OCT, and visual function tests to evaluate the stability or improvement of their visual performance were documented. In addition to the protocol visits, other additional visits were completed at the request of the patient.
All spectral-domain–OCT images were obtained using Heidelberg Spectralis HRA + OCT (Heidelberg Engineering Inc) for the 13 patients who underwent a surgical procedure and were followed up at Pisa University Hospital and using Nidek RS-3000 Advance (Nidek Co) for the 20 patients who underwent a procedure and were followed up at Careggi University Hospital in Florence. A single experienced technician at each institution who was not involved in the data analysis acquired all of the OCT images. The OCT images were reviewed and analyzed independently by 2 of us (L.C. and L.F.). Any discrepancies were resolved by discussion with the principal investigator (S.R.) until a consensus was reached. To include the entire electrode array and a small border of surrounding retina, radial scan, cross scan, line scan, and macular cube scan were captured for all patients at the planned control visits. In some cases, the presence of nystagmus and the inability of the patient to maintain ocular fixation prevented the acquisition of clear OCT images; thus, the multiple OCT scan methods allowed the acquisition of a good number of OCT data. For the patients in Pisa, the electrode-to-retina distance measurements were made using the caliper measurement tool on the Heidelberg Eye Explorer software (Heidelberg Engineering), and for the patients in Florence, the measurements were made using the caliper measurement tool on Nidek NAVIS-EX software (Nidek Co). For each of the 60 electrodes of the 20 implants, the cursor was positioned on the center of the electrode visualized on the infrared image showing the scan line location. On the associated raster OCT scans, the distance (micrometers) between each electrode and the inner limiting membrane of the retina was then measured vertically using calipers. The electrode-to-retina distance measurement method has been described previously.19
For each electrode underneath which fibrosis was observed, the distance (micrometers) between the upper surface of the fibrosis and the lower surface of the fibrosis (thickness of the fibrosis underneath the electrode) was measured vertically using calipers.
Results
Of the 20 patients who met the inclusion criteria of reaching at least the 6-month follow-up after surgery, all were of white race/ethnicity and 12 (60%) were male; the mean (SD) age was 57.4 (11.6) years. Additional demographic and preoperative ophthalmologic characteristics of the enrolled participants are summarized in Table 1.
Table 1. Demographic and Ophthalmologic Characteristics and Postoperative Optical Coherence Tomography Findingsa.
| Characteristic | Patients (N = 20) |
|---|---|
| Age, mean (SD), y | 57.4 (11.6) |
| Male sex | 12 (60) |
| Axial length, mean (range), mm | 23.41 (20.50-25.73) |
| Right eye selected for surgery | 14 (70) |
| Lens status of eyes before surgery, No. | |
| Phakic | 7 (35) |
| Pseudophakic | 13 (65) |
| Preoperative presence of epiretinal membrane | 4 (20) |
| Perioperative removal of epiretinal membrane | 4 (20) |
| Presence of posterior hyaloid | 8 (40) |
| Removal of posterior hyaloid | 8 (40) |
| Postoperative optical coherence tomography finding | |
| Follow-up, mean (SD), mo | 36.8 (19.4) |
| With fibrosis | 10 (50) |
| Onset of fibrosis, mean (SD) [range], mo | 11.0 (9.7) [2-33] |
| With schisis | 9 (45) |
| Onset of schisis, mean (SD) [range], mo | 21.3 (10.1) [6-36] |
Data are presented as number (percentage) of patients unless otherwise indicated.
Development of Preretinal Fibrosis and Schisis
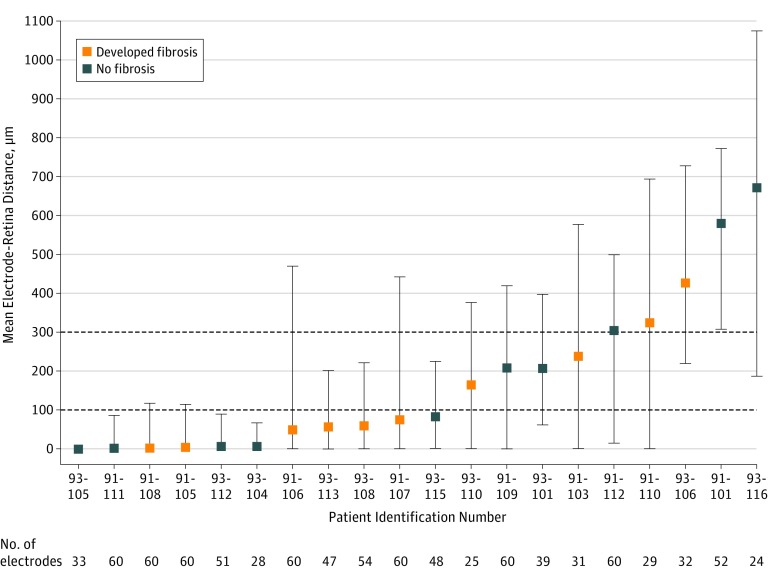
With use of OCT, it was possible to observe the retinal anatomic modifications in patients with an Argus II implant. The OCT image of the 20 patients revealed the development in 10 eyes (50%) of a fibrosislike hyperreflective tissue at the interface between the array and retina, with an onset period ranging from 2 months to 33 months after implantation (mean [SD] onset time, 11.0 [9.7] months). We observed that, in all 10 patients, fibrosis initially appeared as a thin hyperreflective band that was not present in the immediate postoperative follow-up. This fibrosis tended to thicken over time, becoming a real hyperreflective fibrotic plaque that was always limited to below the array and to the contact points between the array and retina (Figure 1A and B). Figure 2 shows the correlation of the electrode-to-retina distance of each of the 20 patients included (measured on the first OCT image available after implantation and before the appearance of fibrosis) with the presence or absence of fibrosis developed subsequently. We observed that 6 of 11 patients (54%) who had a mean electrode-to-retina distance less than 100 μm (measured on the first available OCT images after implantation before the observation of fibrosis) developed fibrosis. Two of 4 patients (50%) who had a mean electrode-to-retina distance more than 100 μm and less than 300 μm developed fibrosis. Two of 5 patients (40%) who had a mean electrode-to-retina distance of more than 300 μm developed fibrosis.
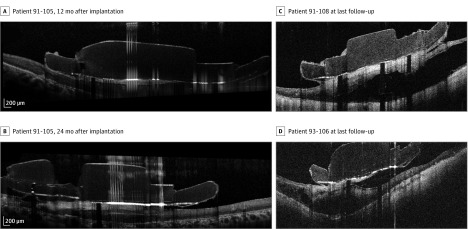
Figure 1. Fibrosis Evolution and Schisis at Follow-up.
For patient 91-105 on optical coherence tomography, fibrosis initially appeared as a thin hyperreflective line that thickened over time, becoming a real hyperreflective fibrotic plaque 12 months after implantation (A) and 24 months after implantation (B). C and D, Images of schisis at the last available follow-ups for patient 91-108 and patient 93-106.
Figure 2. Mean Electrode-to-Retina Distance for All 20 Patients.
Mean electrode-to-retina distance for each patient was placed in ascending order, and error bars represent data distribution. Of 1200 electrodes (60 electrodes of 20 implants), the distance could be measured for 913 electrodes (76.1%). For the remaining 287 electrodes (23.9%), optical coherence tomography scans did not cover all of the implanted area and this measure could not be assessed.
In 9 of 10 patients (90%), fibrosis evolved and progressed to retinal schisis with an onset ranging from 6 months to 36 months after implantation (mean [SD] onset time, 21.3 [10.1] months). Schisis remained localized below the surface of the array in 8 eyes. In 1 patient, it extended beyond the array area to involve the temporal region. In addition, the retinal schisis had a different evolution from patient to patient, even in the thickness present at the last available follow-up (Figure 1C and D). Once formed, schisis tended to remain stable (if the thickness of the schisis was compared between the first and last follow-up for each patient). Postoperative anatomic changes in the implanted retina and the fibrosis and schisis onset for each patient are shown in Table 1.
The presence of the posterior hyaloid was determined after the injection of 0.5 to 1.0 mL of triamcinolone acetate solution in the midvitreous cavity to stain and enhance visualization. In 3 of 10 patients (30%), the posterior hyaloid was present and required maneuvers for induction of posterior vitreous detachment in eyes with firmly adherent posterior hyaloid. In 1 of 10 patients (10%), the posterior hyaloid and the epiretinal membrane were present. For these 4 patients (40%), the posterior hyaloid and/or the epiretinal membrane were removed at the time of the surgical procedure.
Table 2 shows the onset date (in months) of fibrosis and schisis for each of the 10 patients and reports the mean (SD) fibrosis thickness at each evaluation point (when the patient returned for imaging). For each evaluation point, the table shows the number of electrodes underneath which fibrosis was observed and where it was possible to perform the fibrosis thickness measurement (eg, patient identification number, 91-107: 38 measurements at month 12, 16 measurements at month 24, and 15 measurements at month 36).
Table 2. Mean Fibrosis Thickness at Evaluation Time Points.
| Patient Identification Number | Fibrosis Onset, mo | Schisis Onset, mo | Fibrosis Thickness, Mean (SD), μm [Measurement Points] | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 mo | 3 mo | 6 mo | 12 mo | 15 mo | 18 mo | 21 mo | 24 mo | 33 mo | 36 mo | |||
| 93-110 | 2 | 15 | 44.1 (5.1) [8] | 50.9 (10.2) [22] | 75.0 (15.7) [16] | 83.8 (15.7) [20] | 95.7 (19.7) [15] | NA | NA | NA | NA | NA |
| 93-106 | 3 | 24 | NA | 20.0 (6.3) [5] | 20.2 (7.2) [9] | 29.8 (8.6) [11] | NA | 93.2 (24.0) [12] | NA | 102.9 (26.3) [42] | NA | NA |
| 93-113 | 3 | 6 | NA | 68.3 (9.5) [12] | 99.3 (15.0) [26] | NA | NA | NA | NA | NA | NA | NA |
| 93-108 | 6 | NA | NA | NA | 53.1 (19.3) [16] | 79.3 (21.1) [40] | NA | 86.8 (22.9) [36] | NA | NA | NA | NA |
| 91-108 | 6 | 12 | NA | NA | 84.3 (15.7) [29] | 103.9 (20.0) [53] | NA | NA | NA | NA | NA | NA |
| 91-105 | 12 | 24 | NA | NA | NA | 92.6 (32.0) [23] | NA | NA | NA | 120.1 (41.2) [21] | NA | NA |
| 91-106 | 12 | 18 | NA | NA | NA | 81.9 (35.4) [51] | NA | 100.7 (16.0) [9] | NA | NA | NA | NA |
| 91-107 | 12 | 36 | NA | NA | NA | 94.4 (22.6) [38] | NA | NA | NA | 131.6 (30.8) [16] | NA | 157.5 (26.6) [15] |
| 91-103 | 21 | 21 | NA | NA | NA | NA | NA | NA | 144.9 (23.3) [10] | NA | NA | NA |
| 91-110 | 33 | 36 | NA | NA | NA | NA | NA | NA | NA | NA | 110.2 (26.9) [13] | 121.5 (15.1) [12] |
Abbreviation: NA, not applicable.
At each evaluation point, the number of measurements was different because of many factors: OCT scans did not cover all the implanted area, and this measure could not be assessed. Fibrosis was not observed under each single electrode, and the OCT image low contrast did not permit a valuable measurement of the fibrosis thickness underneath the electrode.
The retinal schisis appeared when the mean thickness of the fibrosis was equal to or greater than approximately 100 μm as if this were the limit beyond which fibrosis exerted a tractional force that generated the onset of schisis.
Visual functions test results (square localization and direction of motion) were analyzed to evaluate whether the development of the fibrosis or the retinal schisis provoked a deterioration in the patient’s visual performance. If the patient was available for the testing session, visual function tests were performed for each patient at each evaluation point (before implantation and at 3, 6, 12, and 24 months after implantation). For the 10 patients who developed fibrosis, the video configuration files were maintained unchanged during the 24 months when visual function tests were performed.
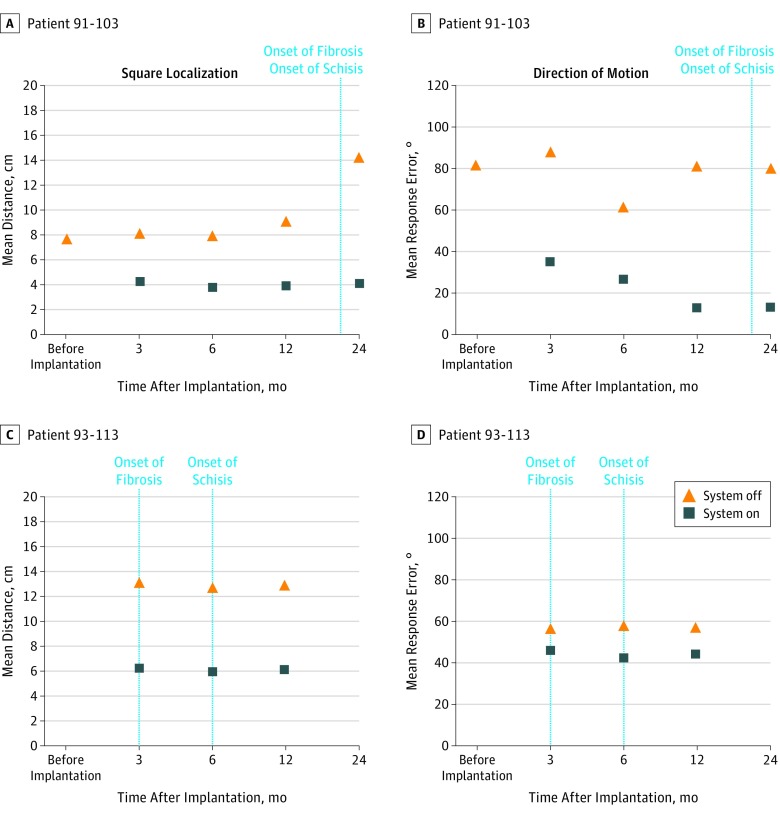
For the square localization test, Figure 3 shows the mean distance from the target in centimeters (accuracy) with the system on and off for 2 patients who developed fibrosis (the shorter the mean distance, the closer the patient’s response was to the target). For the direction of motion test, a more challenging assessment, Figure 3 shows the observed mean response error (stimulus angle – response angle) with the system on and off for 2 patients who developed fibrosis (the smaller the mean response error, the closer the patient’s response was to the stimulus direction).
Figure 3. Visual Function Tests Results for 2 Patients.
The square localization test reports the mean distance from the target in centimeters (accuracy) for the system on and off, and the direction of motion test reports the observed mean response error (stimulus angle – response angle) with the system on and off.
Because the visual function tests were performed until a maximum follow-up of 24 months, we were only able to assess the visual performance of patients who had developed fibrosis and schisis within the 24 months after implantation. Only for 9 patients (91-103, 91-105, 91-106, 91-107, 91-108, 93-106, 93-108, 93-110, and 93-113) was it possible to correlate the development of the fibrosis with the visual function test results, and only for 4 patients (91-103, 91-106, 91-108, and 93-113) was it possible to correlate the development of the retinal schisis with the visual function test results.
Figure 3 reports the square localization and the direction of motion results for patients 91-103 and 93-113. Despite the development of fibrosis and schisis, there was no deterioration in the patient’s visual performance evaluated using visual function tests. Analysis of variance test showed no significant difference along evaluation points for the 10 patients who developed fibrosis for the square localization test (F3,9: 1.55; P = .23) and for the direction of motion test (F3,9: 2.91; P = .06). In addition, all 10 patients who developed fibrosis and schisis self-reported that there was no deterioration of their visual perception.
Discussion
In our study, we described the development of a preretinal fibrosislike tissue between the array and the retina that led to the development of a macular retinal schisis in some cases. Gliosis phenomena and epiretinal membranes have been described after epiretinal implantations.19,20 A recent study of 18 eyes from France also reported retinal fibrosis in some patients with the Argus II implant.21 To our knowledge, our study is the first observational study with a large number of included patients that analyzes in details the development of this anomalous tissue and its association with the patient functional vision.
Of all participants, 50% developed a fibrotic tissue limited to below the electrode array and the retina that thickened over time. In 90% of patients, the fibrosis progressed to retinal schisis.
In all patients who developed fibrosis and schisis, there was no deterioration of their visual performance. This might be explained by the fact that the Argus II Retinal Prosthesis System can be custom programmed to optimize performance, and the changes in the patient perception may be counterbalanced with custom electrical stimulation measures (frequency, interphase gap, and pulse width). Further studies will need to analyze the association of fibrosis and schisis with perceptual thresholds.
The highlighted hyperreflective tissue may be characterized by a fibrotic nature, as previously described by de Juan et al22 in 1 of the 4 patients analyzed when they were forced to extract the custom-built titanium alloy retinal tack from the posterior coats of 4 patients after prolonged epiretinal Argus II prosthesis implantation (up to 19 months). We can hypothesize that the development of epiretinal fibrosis may be induced by direct array-to-retina contact. Nevertheless, the percentage of patients with an array-to-retina distance less than 100 μm who develop fibrosis (54%) was not different from those who did not develop fibrosis (46%).
Another hypothesis is that the fibrosis developed from vitreous residuals or from epiretinal membranes or hyaloid residuals that progressed into hyperproliferation (abnormal growth) because of the stimulation of the electric current. We observed that the dissection of the residual posterior cortical vitreous from the retina in patients with advanced outer retinal degenerative disease may be challenging because of its strong adhesion to the superficial retina, and some nondetectable hyaloid residue may involuntarily remain on the retina.
Another hypothesis could be the development of the fibrosislike membrane due to tack irritation of the retina, which was previously reported when retinal tacks were initially used for the treatment of retinal detachment and were found to be associated with glial reactions.23,24 However, in our analysis of OCT, we observed that fibrosis emerged at a location far from the tack in all 10 patients who developed fibrosis.
The role that electrical stimulation plays in the retinal changes was investigated by Colodetti et al.25 The study focused on the retinal changes in normal-sighted, adult, Long Evans pigmented rats implanted with a single platinum microelectrode. They concluded that mechanical pressure alone and mechanical pressure with excessive electrical stimulation cause retinal damage.
We can assume that the origin of the schisis could have been tractional from the array-to-retina fibrosis and that it did not originate from the insertion of the tack into the entire retina. The retinal schisis seemed to appear when the mean thickness of the fibrosis was equal to or greater than approximately 100 μm as if this were the limit beyond which there may be a tractional force capable of inducing the onset of schisis.
Limitations
The limitations of our study were the retrospective data collection, the imprecision of only a few cases, the relatively short follow-up, and the absence of a histopathologic analysis of the fibrosislike tissue.
Conclusion
Optical coherence tomography may be used to observe the retinal anatomic changes in patients with an Argus II Prothesis implant. This analysis revealed the development of a fibrosislike hyperreflective tissue limited at the interface between array and retina that progressed to retinal schisis but no deterioration in the patients’ visual performance. Further studies are needed to evaluate and investigate the morphologic features and pathogenesis of retinal changes secondary to the Argus II implant.
eMethods
References
- 1.Busskamp V, Picaud S, Sahel JA, Roska B. Optogenetic therapy for retinitis pigmentosa. Gene Ther. 2012;19(2):169-175. doi: 10.1038/gt.2011.155 [DOI] [PubMed] [Google Scholar]
- 2.Petrs-Silva H, Linden R. Advances in gene therapy technologies to treat retinitis pigmentosa. Clin Ophthalmol. 2014;8:127-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McClements ME, MacLaren RE. Gene therapy for retinal disease. Transl Res. 2013;161(4):241-254. doi: 10.1016/j.trsl.2012.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Shamekh S, Goldberg JL. Retinal repair with induced pluripotent stem cells. Transl Res. 2014;163(4):377-386. doi: 10.1016/j.trsl.2013.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lewis PM, Ackland HM, Lowery AJ, Rosenfeld JV. Restoration of vision in blind individuals using bionic devices: a review with a focus on cortical visual prostheses. Brain Res. 2015;1595:51-73. doi: 10.1016/j.brainres.2014.11.020 [DOI] [PubMed] [Google Scholar]
- 6.Ghezzi D. Retinal prostheses: progress toward the next generation implants. Front Neurosci. 2015;9:290. doi: 10.3389/fnins.2015.00290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zrenner E. Will retinal implants restore vision? Science. 2002;295(5557):1022-1025. doi: 10.1126/science.1067996 [DOI] [PubMed] [Google Scholar]
- 8.Weiland JD, Liu W, Humayun MS. Retinal prosthesis. Annu Rev Biomed Eng. 2005;7:361-401. doi: 10.1146/annurev.bioeng.7.060804.100435 [DOI] [PubMed] [Google Scholar]
- 9.Marc RE, Jones BW, Watt CB, Strettoi E. Neural remodeling in retinal degeneration. Prog Retin Eye Res. 2003;22(5):607-655. doi: 10.1016/S1350-9462(03)00039-9 [DOI] [PubMed] [Google Scholar]
- 10.Stone JL, Barlow WE, Humayun MS, de Juan E Jr, Milam AH. Morphometric analysis of macular photoreceptors and ganglion cells in retinas with retinitis pigmentosa. Arch Ophthalmol. 1992;110(11):1634-1639. doi: 10.1001/archopht.1992.01080230134038 [DOI] [PubMed] [Google Scholar]
- 11.Santos A, Humayun MS, de Juan E Jr, et al. Preservation of the inner retina in retinitis pigmentosa: a morphometric analysis. Arch Ophthalmol. 1997;115(4):511-515. doi: 10.1001/archopht.1997.01100150513011 [DOI] [PubMed] [Google Scholar]
- 12.Humayun MS, Prince M, de Juan E Jr, et al. Morphometric analysis of the extramacular retina from postmortem eyes with retinitis pigmentosa. Invest Ophthalmol Vis Sci. 1999;40(1):143-148. [PubMed] [Google Scholar]
- 13.da Cruz L, Dorn JD, Humayun MS, et al. ; Argus II Study Group . Five-year safety and performance results from the Argus II Retinal Prosthesis System clinical trial. Ophthalmology. 2016;123(10):2248-2254. doi: 10.1016/j.ophtha.2016.06.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rachitskaya AV, Yuan A, Marino MJ, Reese J, Ehlers JP. Intraoperative OCT imaging of the Argus II Retinal Prosthesis System. Ophthalmic Surg Lasers Imaging Retina. 2016;47(11):999-1003. doi: 10.3928/23258160-20161031-03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seider MI, Hahn P. Argus II retinal prosthesis malrotation and repositioning with intraoperative optical coherence tomography in a posterior staphyloma. Clin Ophthalmol. 2015;9:2213-2216. doi: 10.2147/OPTH.S96570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grewal DS, Carrasco-Zevallos OM, Gunther R, Izatt JA, Toth CA, Hahn P. Intra-operative microscope-integrated swept-source optical coherence tomography guided placement of Argus II retinal prosthesis. Acta Ophthalmol. 2017;95(5):e431-e432. doi: 10.1111/aos.13123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parmeggiani F, De Nadai K, Piovan A, Binotto A, Zamengo S, Chizzolini M. Optical coherence tomography imaging in the management of the Argus II retinal prosthesis system. Eur J Ophthalmol. 2017;27(1):e16-e21. doi: 10.5301/ejo.5000852 [DOI] [PubMed] [Google Scholar]
- 18.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 19.Roessler G, Laube T, Brockmann C, et al. Implantation and explantation of a wireless epiretinal retina implant device: observations during the EPIRET3 prospective clinical trial. Invest Ophthalmol Vis Sci. 2009;50(6):3003-3008. doi: 10.1167/iovs.08-2752 [DOI] [PubMed] [Google Scholar]
- 20.Menzel-Severing J, Laube T, Brockmann C, et al. Implantation and explantation of an active epiretinal visual prosthesis: 2-year follow-up data from the EPIRET3 prospective clinical trial. Eye (Lond). 2012;26(4):501-509. doi: 10.1038/eye.2012.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Delyfer MN, Gaucher D, Govare M, et al. Adapted surgical procedure for Argus II retinal implantation: feasibility, safety, efficiency, and postoperative anatomical findings. Ophthalmol Retina. 2018;2(4):276-287. doi: 10.1016/j.oret.2017.08.010 [DOI] [PubMed] [Google Scholar]
- 22.de Juan E Jr, Spencer R, Barale PO, da Cruz L, Neysmith J. Extraction of retinal tacks from subjects implanted with an epiretinal visual prosthesis. Graefes Arch Clin Exp Ophthalmol. 2013;251(10):2471-2476. doi: 10.1007/s00417-013-2452-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ando F, Kondo J. A plastic tack for the treatment of retinal detachment with giant tear. Am J Ophthalmol. 1983;95(2):260-261. doi: 10.1016/0002-9394(83)90029-6 [DOI] [PubMed] [Google Scholar]
- 24.de Juan E Jr, Hickingbotham D, Machemer R. Retinal tacks. Am J Ophthalmol. 1985;99(3):272-274. doi: 10.1016/0002-9394(85)90355-1 [DOI] [PubMed] [Google Scholar]
- 25.Colodetti L, Weiland JD, Colodetti S, et al. Pathology of damaging electrical stimulation in the retina. Exp Eye Res. 2007;85(1):23-33. doi: 10.1016/j.exer.2007.02.014 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods