Key Points
Question
What is the pathophysiology of abnormal dorsal movements that occur after upper spine instrumentation?
Findings
In this case series, dorsal afferent nerve injury during upper spine instrumentation was associated with prolonged neuropathic pain and abnormal movements predominantly affecting dorsal quadrilateral muscles (trapezius and rhomboids). The dancing dorsal quadrilaterals syndrome consists of repetitive, semirhythmic, writhing, and jerky movements with distinctive rotatory motions that occur when upright and disappear when lying down and with sensory stimulation, voluntary muscle activation, and sleep.
Meaning
Similar to other peripherally induced movement disorders, the dancing dorsal quadrilaterals syndrome is produced by peripheral induction of abnormal central sensorimotor reorganization.
Abstract
Importance
Recognized peripherally induced movement disorders include the painful legs moving toes syndrome, postamputation dyskinesias, and belly dancer dyskinesias.
Objective
To introduce and characterize the dancing dorsal quadrilaterals, a novel peripherally induced movement disorder that predominantly affects dorsal quadrilateral muscles (trapezius and rhomboids) after upper spine instrumentation.
Design, Setting, and Participants
Between 1990 and 2015, a total of 4 patients who developed abnormal movements of the dorsal quadrilateral muscles after upper spine instrumentation were referred to movement disorders clinics at 3 academic medical centers in the United States, Canada, and Argentina. A prospective and retrospective analysis of the clinical and electrophysiologic characteristics of their abnormal movements is presented in this brief report. Data were analyzed between July 2015 and January 2018.
Exposures
Extensive upper spine instrumentation complicated with misalignment and prolonged postsurgical neuropathic pain.
Main Outcomes and Measures
Video documentation of clinical and electrophysiologic characteristics of dancing dorsal quadrilaterals.
Results
Four patients with upper spine disease (2 women and 2 men, ranging in age from early 30s to early 70s) required extensive surgical manipulation and instrumentation that was complicated by misalignment, prolonged dorsal neuropathic pain, and unusual abnormal movements. These movements consisted of semirhythmic, repetitive writhing, and jerky movements of the scapular region with distinctive rotatory motions. They are referred to as the dancing dorsal quadrilaterals because they predominantly affected the bilateral trapezius and rhomboids (dorsal quadrilateral muscles) but could spread to adjacent muscles, and they are similar in appearance and possibly pathogenesis to “belly dancer” dyskinetic movements. The movements of the dancing dorsal quadrilaterals occur when upright but not when lying down or during voluntary muscle activation. Sensory stimulation also diminishes the movements. Long-duration bursts of normal motor unit potentials with normal recruitment pattern were evidenced.
Conclusions and Relevance
The dancing dorsal quadrilaterals syndrome represents a further example of a peripherally induced movement disorder characterized by neuropathic pain preceding a regional movement disorder following soft-tissue or nerve injury.
This study introduces and characterizes the dancing dorsal quadrilaterals syndrome, a novel peripherally induced movement disorder that predominantly affects dorsal quadrilateral muscles (trapezius and rhomboids) after upper spine instrumentation.
Introduction
The trapezius and rhomboids are referred to as dorsal quadrilaterals (DQ). Focal dyskinesias involving DQ have been rarely reported following soft-tissue and/or nerve injury.1,2,3,4,5,6 Similar to other peripherally induced movement disorders (PIMD), they are usually preceded by neuropathic symptoms. Their irregular jerky components imitate myoclonus; however, slow and sustained contractions can resemble dystonia with overflow to adjacent muscles, positional variation, and changes with cutaneous stimulation.1,2,3,4,5,6
Methods
Clinical and electrophysiologic description of PIMD involving DQ after spinal instrumentation in 4 patients. Institutional review board approval was not applicable, and written informed consent was obtained from all 4 patients.
Results
Case 1
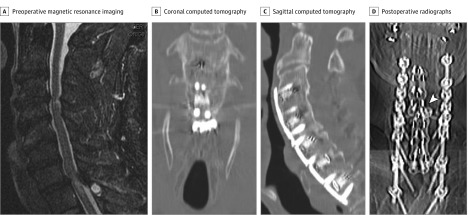
A man in his 70s with cervical spondylosis underwent C2 to T3 laminectomy, discectomy, posterior instrumentation, and fusion. During the next 8 months, he developed dorsal neuropathic pain followed by abnormal movements. Examination revealed slow, semirhythmic, rippling contractions producing semicircular dorsal motions when upright. The movements disappeared when recumbent, with muscle contraction, stretching, touch, and during sleep. There was left scapular winging, dorsal allodynia, and hyperpathia. Cervical spine imaging studies suggested hardware instability (Figure). Needle electromyography (EMG) while upright demonstrated semirhythmic, 0.2- to 0.3-second bursts of grouped, normal motor unit potentials (MUP) firing at 1 to 2 Hz with a crescendo-decrescendo recruitment pattern in left DQ (Video 1). Multiple medications and botulinum toxin A injected into each DQ bilaterally were not effective.
Figure. Imaging Studies for Case 1.
A, Preoperative sagittal T2-weighted magnetic resonance imaging of the cervical spine demonstrates cervical spondylosis associated with myelopathy. Postoperative coronal (B) and sagittal (C) computed tomography studies of the cervical spine show normal vertebral and hardware alignment. D, Postoperative anterior-posterior cervical spine radiographs suggest hardware instability (arrowhead).
Video 1. Semirhythmic, Semicircular Movements of Head, Neck, Left Shoulder, and Scapular Regions.
Resolution with voluntary activation, stretching, tactile stimulation and decubitus position. Needle electromyography of the left upper trapezius showing semirhythmic, 1-Hz to 2-Hz, 0.2- to 0.3-second bursts of grouped, normal motor unit potentials recruited in a crescendo-decrescendo pattern while standing but not while lying down.
Case 2
A man in his 30s with congenital kyphodextroscoliosis underwent posterior thoracic spine instrumentation. The hardware was removed several years later owing to misalignment. Over the next 2 years, he developed right dorsal neuropathic pain followed by abnormal movements. Examination revealed semirhythmic, jerky, and sustained right DQ contractions that disappeared during sleep and improved when recumbent and with muscle contraction, stretching, and touch. Needle EMG of the right rhomboids showed intermittent, semirhythmic, 0.1- to 0.2-second bursts of grouped, normal MUP firing at 3 to 4 Hz in a crescendo-decrescendo recruitment pattern. Multichannel surface EMG showed simultaneous bursts in right DQ and infraspinatus (Video 2). Botulinum toxin A injections into each right DQ provided subjective improvement that progressively declined during the following year.
Video 2. Semirhythmic, Jerky, and Sustained Movements of the Right Scapula, Neck, and Head.
Needle electromyography of the right rhomboids demonstrating semirhythmic, 3- to 4-Hz, 0.1- to 0.2-second bursts of grouped, normal motor unit potentials recruited in a crescendo-decrescendo pattern while sitting. Surface electromyography showing similar activity in the right rhomboids/midtrapezius and infraspinatus regions.
Case 3
A woman in her 50s with idiopathic kyphosis underwent posterior T4 to T10 spinal instrumentation, which was removed 1 year later owing to left dorsal neuropathic pain. Over the next 2 years, persistent neuropathic pain was followed by abnormal movements that disappeared when recumbent and during sleep. Examination revealed slow, semirhythmic, rippling muscle contractions producing semicircular dorsal motions when upright. They disappeared while recumbent and with muscle contraction, stretching, and touch. Needle EMG revealed tonic activity of lower trapezii with superimposed 0.4- to 0.6-second bursts of alternating, grouped, normal MUP firing semirhythmically at 0.5 to 2 Hz with a crescendo-decrescendo recruitment pattern. These contractions decreased approximately 0.6 seconds after cutaneous stimulation (Video 3). Pharmacologic left spinal accessory nerve blockade distal to the sternocleidomastoid temporarily stopped the movements with concomitant left shoulder droop. Multiple medications were ineffective, and botulinum toxin A was injected into both lower trapezii with improvement in pain and abnormal movements over several days. She was subsequently lost to follow-up.
Video 3. Semirhythmic, Semicircular, Writhing Contractions of the Dorsal Region While Standing.
Resolution with voluntary muscle contraction and stretching, decubitus position, and tactile stimulation. Needle electromyography of the bilateral trapezii showing (1) tonic activity with superimposed alternating, semirhythmic, 0.5- to 2-Hz, 0.4- to 0.6-second bursts of grouped, normal motor unit potentials recruited in a crescendo-decrescendo pattern and (2) attenuation of muscle activity approximately 0.6 seconds after tactile stimulation.
Case 4
A woman in her 60s with thoracic scoliosis underwent posterior spinal instrumentation. Over the following 4 months, she developed hardware instability and neuropathic dorsal pain. She underwent surgical repair, but neuropathic pain worsened. Over the next 2 years, she noted abnormal dorsal movements when upright, which disappeared during recumbence and sleep. Examination revealed slow, semirhythmic, rippling contractions producing semi-circular dorsal motions. They disappeared with muscle contraction, stretching, touch, and recumbence (Video 4). Needle EMG of left DQ demonstrated intermittent, semirhythmic, 0.2- to 0.5-second bursts of grouped, normal MUP firing at 1 to 3 Hz with a crescendo-decrescendo recruitment pattern. Botulinum toxin A improved the movements at 300 units into the bilateral DQ; however, pain was still significant, and movements eventually recurred.
Video 4. Semirhythmic, Semicircular, Writhing, and Jerky Contractions of the Dorsal Region While Standing.
Resolution with voluntary muscle contraction and stretching.
Discussion
The dancing dorsal quadrilateral (DDQ) syndrome consists of repetitive, semirhythmic writhing and jerky contractions of DQ and neighboring muscles. Sequential and/or alternating semirhythmic contractions of the trapezii, tethered between their occipital, cervical, and thoracic spinous process origins and scapular insertions, explain the unusual semicircular motions of the head, neck, and scapular regions. In a comparable case after spinal instrumentation, the movements were attributed to paraspinal muscle contraction,6 although surface EMG electrodes in this region would have also captured activity from the more superficial DQ. The appearance of these movements is similar to the belly dancer dyskinesia following surgical abdominal wall injury.7
Shoulder girdle dyskinesia with slow, sinuous DQ and latissimus dorsi movements that disappeared in sleep was reported following thoracotomy with presumed damage to muscle sensory innervation.5 Sinuous and jerky movements accompanied by sustained posturing, the occurrence in upright truncal postures, and disappearance when lying down or after sensory stimulation is similar to dystonia. However, in contrast to dystonia, the DDQ and other PIMD are abolished or suppressed during voluntary movement.
Other reports have described jerky back movements as myoclonus, affecting the trapezii after spinal accessory nerve lesion,2 latissimus dorsi following thoracodorsal nerve lesion,3 and serratus anterior after long thoracic nerve lesion.4 These abnormal movements diminished with voluntary movement, relaxation and sleep. Abnormal trapezius contractions resolved after removal of the spinal accessory nerve lesion but recurred 3 months later,2 indicating peripheral induction of self-sustaining central mechanisms. Surgical resection of injured nerves was curative.2,3
The DDQ followed extensive posterior spinal instrumentation with potential damage to cutaneous and intramuscular afferents and cervical and thoracic dorsal rami leading to neuropathic pain. The main spinal accessory nerve trunk appeared intact as evidenced by vigorous muscle contractions.
Alterations in peripheral sensory input following injury and leading to central sensorimotor reorganization over several months could account for the delayed onset of PIMD after trauma and possibly the spread to adjacent muscles.1,2,3,4,5,6 The DDQ occurred when sitting or standing, diminished with voluntary muscle contraction and cutaneous stimulation, and disappeared when supine or during sleep. These characteristics, along with bilateral movements and long duration bursts of normal MUP, support a central origin. Central changes are documented at many levels following peripheral injury, but it is not known at what level the abnormal sensory information generates PIMD. Discussion has focused on supraspinal rather than segmental reorganization, despite the fact that PIMD are segmental disorders.
In animal models, persistent, nonsectional nerve injury induces hyperesthetic neuropathic-like changes mediated by chronic hyperglutamatergic activity from partially injured afferents that reduce postsynaptic inhibition in the dorsal horn. These initial changes are followed by activation of tumor necrosis factor and caspase pathways leading to slow apoptosis of inhibitory spinal interneurons.8,9,10
Partial but persistent nerve damage after spinal instrumentation, especially when complicated with hardware instability,6,11 could induce a hyperglutamatergic state, loss of inhibitory interneurons at the corresponding spinal levels and hyperexcitable segmental and suprasegmental sensorimotor reorganization predisposing to hyperkinetic movements. In the DDQ, sensory-induced segmental inhibitory interneuronal loss at C3 to C5 could lead to overflow of abnormal activation to adjacent muscles innervated by these segments such as supraspinatus and infraspinatus.4,5,6 Persistent nerve damage might trigger similar changes in contralateral circuits and bilateral movements as seen in other PIMD.2,7,12
Proprioceptive information relayed to segmental spinal reflex networks is paramount for axial tone control during upright postures.13 In the context of hyperexcitability, normal postural proprioceptive input might switch on dormant segmental and/or suprasegmental generators that could be modulated or switched off by other inputs during tactile stimulation, while lying down, or during sleep.14,15
Limitations
The main limitation of this study is the small number of cases collected from 3 large movement disorders clinics over a period of 15 years. Given the presumed rarity of the DDQ, it is possible that other important factors predisposing to its occurrence have not been uncovered in this small case series. Another limitation is the lack of use of advanced neurophysiologic techniques, such as transcranial magnetic stimulation, to further study the posited abnormal central sensorimotor reorganization underlying this PIMD.
Conclusions
Recognized PIMDs include the painful legs moving toes syndrome, postamputation dyskinesias, and belly dancer dyskinesias. The DDQ syndrome represents a further example of a PIMD characterized by neuropathic pain preceding a regional movement disorder following soft-tissue or nerve injury.7,15
References
- 1.Aggarwal A, Thompson PD. Unusual focal dyskinesias. Handb Clin Neurol. 2011;100:617-628. doi: 10.1016/B978-0-444-52014-2.00044-6 [DOI] [PubMed] [Google Scholar]
- 2.Glocker FX, Deuschl G, Volk B, Hasse J, Lücking CH. Bilateral myoclonus of the trapezius muscles after distal lesion of an accessory nerve. Mov Disord. 1996;11(5):571-575. doi: 10.1002/mds.870110514 [DOI] [PubMed] [Google Scholar]
- 3.Carnero-Pardo C, Sánchez-Alvarez JC, Gómez-Camello A, Minguez-Castellanos A, Hernández-Ramos FJ, Garcia-Gómez T. Myoclonus associated with thoracodorsal neuropathy. Mov Disord. 1998;13(6):971-972. doi: 10.1002/mds.870130620 [DOI] [PubMed] [Google Scholar]
- 4.Camerota F, Celletti C, Paoloni M, et al. Myoclonus of the scapula after acute long thoracic nerve lesion: a case report. Mov Disord. 2006;21(1):71-73. doi: 10.1002/mds.20647 [DOI] [PubMed] [Google Scholar]
- 5.Wali GM. Shoulder girdle dyskinesia following local surgery. Mov Disord. 1999;14(6):1051-1053. doi: [DOI] [PubMed] [Google Scholar]
- 6.Caviness JN, Gabellini A, Kneebone CS, Thompson PD, Lees AJ, Marsden CD. Unusual focal dyskinesias: the ears, the shoulders, the back, and the abdomen. Mov Disord. 1994;9(5):531-538. doi: 10.1002/mds.870090505 [DOI] [PubMed] [Google Scholar]
- 7.Iliceto G, Thompson PD, Day BL, Rothwell JC, Lees AJ, Marsden CD. Diaphragmatic flutter, the moving umbilicus syndrome, and “belly dancer’s” dyskinesia. Mov Disord. 1990;5(1):15-22. doi: 10.1002/mds.870050105 [DOI] [PubMed] [Google Scholar]
- 8.Scholz J, Broom DC, Youn DH, et al. Blocking caspase activity prevents transsynaptic neuronal apoptosis and the loss of inhibition in lamina II of the dorsal horn after peripheral nerve injury. J Neurosci. 2005;25(32):7317-7323. doi: 10.1523/JNEUROSCI.1526-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foster E, Wildner H, Tudeau L, et al. Targeted ablation, silencing, and activation establish glycinergic dorsal horn neurons as key components of a spinal gate for pain and itch. Neuron. 2015;85(6):1289-1304. doi: 10.1016/j.neuron.2015.02.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garraway SM, Woller SA, Huie JR, et al. Peripheral noxious stimulation reduces withdrawal threshold to mechanical stimuli after spinal cord injury: role of tumor necrosis factor alpha and apoptosis. Pain. 2014;155(11):2344-2359. doi: 10.1016/j.pain.2014.08.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cervellati S, Bettini N, Bianco T, Parisini P. Neurological complications in segmental spinal instrumentation: analysis of 750 patients. Eur Spine J. 1996;5(3):161-166. doi: 10.1007/BF00395507 [DOI] [PubMed] [Google Scholar]
- 12.Dressler D, Thompson PD, Gledhill RF, Marsden CD. The syndrome of painful legs and moving toes. Mov Disord. 1994;9(1):13-21. doi: 10.1002/mds.870090104 [DOI] [PubMed] [Google Scholar]
- 13.Gurfinkel V, Cacciatore TW, Cordo P, Horak F, Nutt J, Skoss R. Postural muscle tone in the body axis of healthy humans. J Neurophysiol. 2006;96(5):2678-2687. doi: 10.1152/jn.00406.2006 [DOI] [PubMed] [Google Scholar]
- 14.Brown P, Rothwell JC, Thompson PD, Marsden CD. Propriospinal myoclonus: evidence for spinal “pattern” generators in humans. Mov Disord. 1994;9(5):571-576. doi: 10.1002/mds.870090511 [DOI] [PubMed] [Google Scholar]
- 15.Nugent MM, Milner TE. Segmental specificity in belly dance mimics primal trunk locomotor patterns. J Neurophysiol. 2017;117(3):1100-1111. doi: 10.1152/jn.00693.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]