Abstract
Importance
Racial differences in molecular biomarkers for Alzheimer disease may suggest race-dependent biological mechanisms.
Objective
To ascertain whether there are racial disparities in molecular biomarkers for Alzheimer disease.
Design, Setting, and Participants
A total of 1255 participants (173 African Americans) were enrolled from January 1, 2004, through December 31, 2015, in longitudinal studies at the Knight Alzheimer Disease Research Center at Washington University and completed a magnetic resonance imaging study of the brain and/or positron emission tomography of the brain with Pittsburgh compound B (radioligand for aggregated amyloid-β) and/or cerebrospinal fluid (CSF) assays for the concentrations of amyloid-β42, total tau, and phosphorylated tau181. Independent cross-sectional analyses were conducted from April 22, 2016, to August 27, 2018, for each biomarker modality with an analysis of variance or analysis of covariance including age, sex, educational level, race, apolipoprotein E (APOE) ε4 allele status, and clinical status (normal cognition or dementia). All biomarker assessments were conducted without knowledge of the clinical status of the participants.
Main Outcomes and Measures
The primary outcomes were hippocampal volumes adjusted for differences in intracranial volumes, global cerebral amyloid burden as transformed into standardized uptake value ratios (partial volume corrected), and CSF concentrations of amyloid-β42, total tau, and phosphorylated tau181.
Results
Of the 1255 participants (707 women and 548 men; mean [SD] age, 70.8 [9.9] years), 116 of 173 African American participants (67.1%) and 724 of 1082 non-Hispanic white participants (66.9%) had normal cognition. There were no racial differences in the frequency of cerebral ischemic lesions noted on results of brain magnetic resonance imaging, mean cortical standardized uptake value ratios for Pittsburgh compound B, or for amyloid-β42 concentrations in CSF. However, in individuals with a reported family history of dementia, mean (SE) total hippocampal volumes were lower for African American participants than for white participants (6418.26 [138.97] vs 6990.50 [44.10] mm3). Mean (SE) CSF concentrations of total tau were lower in African American participants than in white participants (293.65 [34.61] vs 443.28 [18.20] pg/mL; P < .001), as were mean (SE) concentrations of phosphorylated tau181 (53.18 [4.91] vs 70.73 [2.46] pg/mL; P < .001). There was a significant race by APOE ε4 interaction for both CSF total tau and phosphorylated tau181 such that only APOE ε4–positive participants showed the racial differences.
Conclusions and Relevance
The results of this study suggest that analyses of molecular biomarkers of Alzheimer disease should adjust for race. The lower CSF concentrations of total tau and phosphorylated tau181 in African American individuals appear to reflect a significant race by APOE ε4 interaction, suggesting a differential effect of this Alzheimer risk variant in African American individuals compared with white individuals.
This cohort study examines whether there are disparities between African American and white individuals in molecular biomarkers for Alzheimer disease.
Key Points
Question
Do African American individuals differ from non-Hispanic white individuals regarding molecular biomarkers of Alzheimer disease?
Findings
This cohort study of 1255 participants in a study of healthy aging and Alzheimer disease found significant differences in the cerebrospinal fluid concentrations of tau protein (and its phosphorylated isoform) between African American and white individuals.
Meaning
Racial differences in Alzheimer biomarkers suggest possible race-dependent biological mechanisms that contribute to expression of disease.
Introduction
Potential racial differences have been examined in Alzheimer disease (AD), particularly for African American individuals compared with non-Hispanic white individuals,1 but the evidence is often conflicting. For example, some studies suggest an increased incidence and prevalence for dementia and AD in African American individuals compared with non-Hispanic white individuals,2,3,4 but other studies find no racial differences in the risk for AD.5,6 There are similar discrepancies as to whether AD-related neuropathologic differences do7 or do not8,9 exist between African American and white individuals and whether there are racial differences in hippocampal volumes.10,11
The mixed results regarding the risk for and expression of AD in African American vs white individuals may be associated, at least in part, with whether there was adjustment for factors that may affect expression of disease. Socioeconomic disparities (including in educational quality)12; psychosocial factors (including the stress of lifelong discrimination)13; and comorbid diseases such as cardiovascular disease and its risk factors,14 all may interact to influence racial differences in AD.15 Finally, although African American individuals represent 13.3% of the population in the United States,16 they are often underrepresented in AD clinical cohorts such that AD research largely has been informed by white research volunteers. For example, only 33 of 2129 participants (1.6%) in a phase 3 trial of solanezumab were African American.17 Furthermore, as of August 2018, the database representing participants from all Alzheimer’s Disease Centers, as maintained by the National Alzheimer’s Coordinating Center,18 had neuropathologic data from 5283 brains, of which only 321 (6.1%) were from African American individuals; the autopsy rate for African American individuals entered into the National Alzheimer’s Coordinating Center database is 25.3% compared with 62.1% for white individuals.
Molecular biomarkers for AD refer to the misaggregated proteins amyloid-β42 (Aβ42) and tau as identified by positron emission tomography (PET) of the brain with radioligands for amyloid plaques and for tau deposition or by the concentrations of these proteins in cerebrospinal fluid (CSF). Biological markers of AD permit the in vivo study of Alzheimer pathophysiologic characteristics in humans. Few studies, however, have compared molecular biomarkers of AD in African American and white individuals to determine whether or not there are potential disparities in underlying AD mechanisms.19,20,21 There also could be important practical considerations should there be racial differences in AD biomarkers. For example, differences would require race-dependent thresholds for biomarker positivity to be used in AD research studies, including screening for clinical trials of experimental therapies.22 We thus reviewed molecular biomarker results in African American and white participants enrolled in the clinical cohorts of the Knight Alzheimer Disease Research Center (ADRC) at Washington University, St Louis, Missouri, to explore potential molecular biomarker differences. Although there are reported differences for racial and ethnic groups other than African American individuals,2,4,23 African American individuals are the largest minority group in St Louis, representing 18% of the population in the metropolitan statistical area24 and 13% of those aged 65 years or older. Hispanic individuals comprise only 3% of the population in the metropolitan statistical area. African American individuals are thus the focus of this report.
Methods
Participants
Community-living adults with normal cognition and those with symptomatic AD (encompassing both mild cognitive impairment due to AD and AD dementia) aged 43 years or older were enrolled from January 1, 2004, through December 31, 2015, as volunteers in the longitudinal clinical studies at the Knight ADRC. The Knight ADRC is not clinic based, and all volunteers are seen for research purposes only. Recruitment primarily is through word of mouth, supplemented by community outreach events and referrals by community physicians. Since 2004, new enrollees have been eligible for and willing in principle to participate in studies of longitudinal molecular biomarkers, including amyloid PET imaging and lumbar puncture (LP) to obtain CSF. Biomarker procedures are performed at study entry and then at a mean interval of every 3 years, with the exception that LP is optional for African American participants at baseline and subsequently (mandating LP for African American participants produced a precipitous drop in their enrollment). Individuals self-report their race at their baseline assessment. All participants aged 65 years or older are clinically assessed annually; individuals aged 43 to 64 years are clinically assessed every 3 years. Eligibility criteria for this study are (1) the absence of conditions, such as renal failure requiring dialysis, that could interfere with longitudinal participation (less severe conditions are permitted, including type 1 and 2 diabetes, affective disorders, and cerebral infarcts); (2) completion of at least 1 amyloid PET scan and/or 1 LP and/or 1 brain magnetic resonance imaging (MRI) study from January 1, 2004, through December 31, 2015; and (3) no known deterministic mutation for AD. All procedures were approved by Washington University’s Human Research Protection Office. Written informed consent was obtained from all participants and their study partners (collateral sources).
Evaluation
Experienced clinicians conducted independent, semistructured interviews with the collateral source and the participant to assess possible decline in cognitive and functional abilities relative to the participant’s previously attained levels. The assessment protocol since 2005 has included the Uniform Data Set of the National Alzheimer’s Coordinating Center.25,26 In addition to clinical and cognitive evaluations, the Uniform Data Set protocol obtains demographic, medication, and health information and includes behavioral and depression inventories. A history of dementia in first-degree relatives is self-reported or, in individuals with cognitive impairment, is reported by the collateral source. Measurements of height and weight allow calculation of body mass index (BMI). A nonfasting blood sample is obtained to measure hemoglobin A1c (HbA1c) and to determine apolipoprotein E (APOE) ε4 allele status.
After the participant undergoes a neurologic examination, the clinician synthesizes all information from the semistructured interviews to determine cognitive and/or functional loss as operationalized by the Clinical Dementia Rating (CDR),27 in which a CDR of 0 indicates normal cognition and a CDR greater than 0 indicates cognitive impairment (CDR of 0.5 indicates very mild dementia, CDR of 1 indicates mild dementia, CDR of 2 indicates moderate dementia, and CDR of 3 indicates severe dementia). The etiologic diagnosis of the cause(s) of dementia is made by the clinician in accordance with standard criteria and methods.25 The diagnosis and CDR determination are made without reference to the participant’s performance on neuropsychological tests or the results of prior assessments.
Within weeks of the clinical assessment, a neuropsychological test battery26 is administered to each participant. The psychometricians are not informed of the results of the clinical assessment or results from prior psychometric evaluations. Similarly, all biomarker assessments are conducted without knowledge of the clinical status of the participants.
CSF Collection and Analysis
Participants underwent LP at 8 am after overnight fasting; typically, 20 to 30 mL of CSF was collected under gravity flow. The CSF was gently inverted to disrupt potential gradient effects, centrifuged at low speed to pellet any cellular debris, aliquoted into polypropylene tubes, and stored at –80°C as previously described.28 Concentrations of Aβ42, phosphorylated tau181 (p-tau181), and total tau (t-tau) were measured by enzyme-linked immunosorbent assay (INNOTEST, Fujirebio [formerly Innogenetics]).
MRI Acquisition and Processing
Structural, magnetization-prepared, rapid gradient-echo images were collected using either a 1.5-T or 3-T Siemens scanner. Scans had a resolution of either 1 × 1 × 1.25 mm or 1 × 1 × 1 mm. Scans were processed with Freesurfer29 to parcellate the cortex using the Desikan atlas.30 For each hemisphere, volumes were obtained for all subcortical regions. The volumes of subcortical structures were adjusted for differences in intracranial volume using a regression approach.31 Current analyses focus on the volume of the hippocampus, as this region has previously been shown to be affected in AD.32,33
PET Acquisition and Processing
Amyloid-β PET imaging was completed using carbon 11–labeled [11C] Pittsburgh compound B (PiB).34 Positron emission tomographic data from the 30- to 60-minute postinjection window were analyzed using FreeSurfer regions of interest.35 Regional values were transformed into standardized uptake value ratios (SUVRs) using the cerebellar cortex as the reference region. Data were partial-volume corrected using a regional spread function.36 Regions known to be sensitive to AD pathologic characteristics were averaged together to represent global amyloid-β burden.35
Sequencing and Genotyping
Apolipoprotein E genotype was determined for all individuals. Briefly, APOE ε2, ε3, and ε4 isoforms were determined by genotyping rs7412 and rs429358 using Taqman genotyping technology as previously described.37
Ascertainment of Cerebral Ischemic Lesions
Research brain MRI scans were reviewed by a neuroradiologist to ascertain incidental findings that may be clinically actionable. These findings include ischemic lesions, such as lacunes and infarcts. The number of neuroradiological reports of such ischemic lesions was noted for both African American and white participants.
Statistical Analysis
Statistical analysis was conducted from April 22, 2016, to August 27, 2018. The analysis sample included all individuals from Knight ADRC who had cross-sectional data for at least 1 biomarker modality (amyloid-β PET, CSF, and/or brain MRI) from January 1, 2004, through December 31, 2015. There were too few completed longitudinal biomarker procedures to date in African American individuals to permit analysis. Independent cross-sectional analyses were conducted for each modality because some individuals chose to participate in some biomarker studies but not the others. Analysis of variance or analysis of covariance, as appropriate, was used to assess the association between biomarker values (CSF and imaging) and race (African American vs white) jointly with other covariates including sex, APOE ε4 status, age, educational level, clinical status (CDR of 0 vs CDR >0), family history of AD, and BMI (eTables 1-3 in the Supplement provide the unadjusted values). The presence of ischemic lesions on MRI findings and HbA1c values were initially considered as covariates in the adjusted analyses, but because of the fact that the initial adjusted analyses indicated no significant effects of these covariates on any of the biomarkers under analysis, they were not included in the final adjusted analyses. All analyses first examined the interactive effect between race and each of the other covariates. If the interaction was significant, the differential race effect on the biomarkers was reported depending on the level of the other covariate. If the interaction was not significant, the race effect on the biomarkers was reported as the main effect regardless of the levels of the other covariates. Because values of some CSF biomarkers as measured by the INNOTEST assay have drifted over time,38 we adjusted for the effect of assay drift by including assay date and type as covariates in all analyses of CSF biomarkers. All analyses were done by SAS PROC/GLM, version 9.4 (SAS Institute Inc). All P values were from 2-sided tests, and results were deemed statistically significant at P < .05.
Results
Clinical
The characteristics of the sample at the clinical assessment closest in time to the participant’s biomarker acquisition are shown in Table 1. The mean (SD) intervals between the closest clinical assessment to biomarker acquisition ranged from 94.4 (52.8) days for LP to 172.3 (180.9) days for amyloid PET. These intervals did not differ significantly between African American and white participants. A total of 1255 participants underwent at least 1 biomarker study (brain MRI, PiB PET, and/or CSF); of these, 173 (13.8%) were African American. African American participants were less likely than white participants to be men (61 [35.3%] vs 487 [45.0%]), had slightly less educational attainment (mean [SD], 14.7 [2.9] vs 15.4 [2.9] years), and were less likely to report a family history of dementia (63 [36.4%] vs 562 [51.9%]) (Table 1). Also, African American participants had greater mean (SD) BMI (calculated as weight in kilograms divided by height in meters squared) than white participants (30.1 [5.8] vs 27.2 [5.0]) as well as higher mean (SD) HbA1c levels (5.9% [0.8%] vs 5.7% [0.7%] [to convert to proportion of total hemoglobin, multiply by 0.01]). There were no racial differences in the frequency of ischemic lesions noted on the brain MRI findings (Table 1). Two-thirds of both African American and white participants had normal cognition (CDR of 0). The Knight ADRC does not follow participants who have a CDR of 2 or greater; thus, almost all individuals with symptomatic AD were in the earliest stages (CDR of 0.5 and CDR of 1).
Table 1. Sample Characteristics.
| Characteristic | Valuea | P Value | |
|---|---|---|---|
| African American Participants (n=173) | Non-Hispanic White Participants (n=1082) | ||
| Age, mean (SD) [range], y | 70.8 (9.6) [43-95] | 70.8 (9.9) [43-104] | .96 |
| Male sex | 61 (35.3) | 487 (45.0) | .02 |
| Educational level, mean (SD), y | 14.7 (2.9) | 15.4 (2.9) | .002 |
| Family history of dementia | 63 (36.4) | 562 (51.9) | <.001 |
| 1 or 2 APOE4 alleles | 77 (45.6) | 451 (41.7) | .36 |
| Body mass index, mean (SD)b | 30.1 (5.8) | 27.2 (5.0) | <.001 |
| Hemoglobin A1c, mean (SD), % | 5.9 (0.8) | 5.7 (0.7) | .02 |
| Ischemic lesions on MRI findings | 14 (10.5) | 18 (13.5) | .45 |
| CDR | .02 | ||
| 0 | 116 (67.1) | 724 (66.9) | |
| 0.5 | 33 (19.1) | 277 (25.6) | |
| 1 | 23 (13.3) | 78 (7.2) | |
| 2 | 1 (0.6) | 3 (0.3) | |
| MMSE scores, mean (SD) | .10 | ||
| CDR 0 | 28.8 (1.5) | 29.0 (1.4) | |
| CDR 0.5 | 25.9 (2.8) | 26.1 (3.3) | |
| CDR 1 | 20.4 (4.2) | 22.2 (3.9) | |
| CDR 2 | 16.0 (NA) | 17.7 (4.0) | |
Abbreviations: APOE4, apolipoprotein E ε4 allele; CDR, Clinical Dementia Rating; MMSE, Mini-Mental State Examination; MRI, magnetic resonance imaging, NA, not applicable.
SI conversion factor: To convert hemoglobin A1c to proportion of total hemoglobin, multiply by 0.01.
Data are presented as number (percentage) of participants unless otherwise indicated. The reported percentages are weighted by the entire sample used in the computations (ie, the percentages are not a derivative of the 2 cells).
Calculated as weight in kilograms divided by height in meters squared.
MRI Findings
When total hippocampal volume as seen on MRI findings was jointly analyzed as a function of race, age, sex, APOE ε4 status, educational level, clinical status (CDR of 0 vs CDR >0), family history of AD, and BMI, African American participants had smaller mean (SE) total volumes than did white participants (6503.05 [93.39] vs 6919.41 [34.10] mm3; P < .001) (Table 2). However, this difference was influenced by family history of dementia. African American participants reporting a family history of dementia had smaller total hippocampal volumes than did white participants with a family history of dementia (6418.26 [138.97] vs 6990.50 [44.10] mm3); no racial differences were noted for individuals without a family history of dementia. The adjusted analyses revealed that the 2 races shared effects of age and of CDR for smaller mean (SE) total hippocampal volumes. In the combined sample, increased age was associated with smaller total mean (SE) hippocampal volume (–59.20 [4.68] mm3 per year; P < .001); this association did not differ by race (African American individuals, –64.42 [8.77] mm3 per year vs white individuals, –53.99 [3.25] mm3 per year; P = .27). Also, in the combined sample, those with a CDR greater than 0 had smaller mean (SE) total hippocampal volumes compared with those with a CDR of 0 (6296.57 [83.19] vs 7185.22 [55.87] mm3; P < .001). There was no effect of severity of dementia (ie, CDR of 0.5 vs CDR of ≥1) on hippocampal volume.
Table 2. Hippocampal Volumes Adjusting for Sex, APOE4 Status, Age, Educational Level, Clinical Status, Family History of AD, and BMI.
| Characteristic | African American Participants (n=143) | Non-Hispanic White Participants (n = 889) | P Value |
|---|---|---|---|
| Age, mean (SD), y | 71.2 (10.0) | 70.3 (10.2) | .36 |
| Male sex, No. (%) | 49 (34.3) | 388 (43.6) | .04 |
| Educational level, mean (SD), y | 14.6 (2.8) | 15.5 (2.9) | .001 |
| CDR, No. (%) | .05 | ||
| 0 | 95 (66.4) | 628 (70.6) | |
| 0.5 | 29 (20.3) | 200 (22.5) | |
| 1 | 19 (13.3) | 60 (6.7) | |
| 2 | 0 | 1 (0.1) | |
| APOE4, No. (%) | .67 | ||
| Negative | 78/139 (56.1) | 514/886 (58.0) | |
| Positive | 61/139 (43.9) | 372/886 (42.0) | |
| Hippocampal Volume, Mean (SE), mm3 | |||
| Right | 3302.57 (49.34) | 3527.10 (18.01) | <.001 |
| CDR | |||
| 0 | 3523.41 (55.56) | 3748.50 (19.94) | .001 |
| >0 | 3115.78 (81.69) | 3334.80 (32.44) | .06 |
| APOE4 | |||
| Negative | 3373.08 (62.50) | 3558.62 (24.03) | .03 |
| Positive | 3266.11 (65.54) | 3524.68 (25.38) | .001 |
| Family history of dementia | |||
| Negative | 3400.65 (57.41) | 3526.19 (25.08) | .19 |
| Positive | 3238.54 (73.42) | 3557.11 (23.29) | <.001 |
| Left | 3200.49 (50.37) | 3392.32 (18.39) | <.001 |
| CDR | |||
| 0 | 3470.48 (56.72) | 3628.06 (20.36) | .04 |
| >0 | 2959.45 (83.39) | 3183.10 (33.12) | .06 |
| APOE4 | |||
| Negative | 3257.68 (63.81) | 3435.27 (24.53) | .05 |
| Positive | 3172.25 (66.91) | 3375.90 (25.91) | .02 |
| Family history of dementia | |||
| Negative | 3250.22 (58.61) | 3377.78 (25.60) | .19 |
| Positive | 3179.71 (74.95) | 3433.39 (23.78) | .007 |
| Total | 6503.05 (93.39) | 6919.41 (34.10) | <.001 |
| CDR | |||
| 0 | 6993.89 (105.18) | 7376.56 (37.75) | .004 |
| >0 | 6075.23 (154.64) | 6517.90 (61.41) | .04 |
| APOE4 | |||
| Negative | 6630.76 (118.32) | 6993.89 (45.49) | .02 |
| Positive | 6438.36 (124.07) | 6900.58 (48.04) | .003 |
| Family history of dementia | |||
| Negative | 6650.86 (108.68) | 6903.96 (47.48) | .14 |
| Positive | 6418.26 (138.97) | 6990.50 (44.10) | <.001 |
Abbreviations: AD, Alzheimer disease; APOE4, apolipoprotein E ε4 allele; BMI, body mass index; CDR, Clinical Dementia Rating.
Amyloid PET
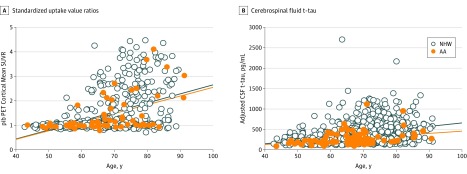
No racial difference was observed on partial volume-corrected mean cortical PiB SUVR. However, in the combined sample, higher mean (SE) PiB SUVR was associated with older age (0.02 [0.005] pg/mL per year; P < .001); this association did not differ by race (African American individuals, 0.02 [0.01] pg/mL per year vs white individuals, 0.03 [0.003] pg/mL per year; P = .48) (Figure, A). Higher mean (SE) PiB SUVR was also associated in the combined sample with CDR greater than 0 (CDR >0, 2.14 [0.12] vs CDR of 0, 1.35 [0.06]; P < .001) and the presence of an APOE ε4 allele (carriers, 1.97 [0.08] vs noncarriers, 1.52 [0.09]; P < .001) (Table 3). When the amyloid-β PET data were converted to the Centiloid scale as previously described,39 the results were consistent with those reported as SUVRs (Table 3).
Figure. Pittsburgh Compound B (PiB) Positron Emission Tomography (PET) Mean Cortical Standardized Uptake Value Ratios (SUVRs) and Cerebrospinal Fluid (CSF) Total Tau (t-tau) as a Function of Age by Race.
A, Mean SUVRs. B, CSF t-tau. Values were adjusted for apolipoprotein E ε4 status, sex, educational level, Clinical Dementia Rating, body mass index, and family history of Alzheimer disease. For non-Hispanic white (NHW) and African American (AA) individuals, points are circles and regression lines are solid lines.
Table 3. Sample Demographics and Adjusted PET PiB SUVR Values Adjusting for Sex, APOE4 Status, Age, Educational Level, Clinical Status, Family History of AD, and BMI.
| Characteristic | African American Participants (n=65) | Non-Hispanic White Participants (n=504) | P Value |
|---|---|---|---|
| Age, mean (SD), y | 67.5 (10.7) | 68.2 (10.2) | .06 |
| Male sex, No. (%) | 23 (35.4) | 202 (40.1) | .47 |
| Educational level, mean (SD), y | 14.9 (2.8) | 15.8 (2.8) | .01 |
| CDR, No. (%) | .64 | ||
| 0 | 54 (83.1) | 425 (84.3) | |
| 0.5 | 8 (12.3) | 66 (13.1) | |
| 1 | 3 (4.6) | 13 (2.6) | |
| 2 | 0 | 0 | |
| Family history of dementia, No. (%) | .02 | ||
| Negative | 41 (63.1) | 239 (47.4) | |
| Positive | 24 (36.9) | 265 (52.6) | |
| APOE4, No. (%) | .21 | ||
| Negative | 35/64 (54.7) | 316 (62.7) | |
| Positive | 29/64 (45.3) | 188 (37.3) | |
| Cortical SUVR, mean (SE) | 1.69 (0.12) | 1.80 (0.04) | .38 |
| CDR | |||
| 0 | 1.29 (0.11) | 1.41 (0.03) | .71 |
| 0.5 or 1 | 2.09 (0.22) | 2.19 (0.08) | .97 |
| APOE4 | |||
| Negative | 1.46 (0.15) | 1.57 (0.05) | .90 |
| Positive | 1.91 (0.14) | 2.02 (0.05) | .89 |
| Family history of dementia | |||
| Negative | 1.78 (0.13) | 1.79 (0.05) | >.99 |
| Positive | 1.60 (0.16) | 1.80 (0.05) | .63 |
| Centiloid scale, mean (SE) | 28.51 (5.37) | 33.49 (1.91) | .38 |
| CDR | |||
| 0 | 10.47 (4.84) | 15.88 (1.55) | .71 |
| 0.5 or 1 | 46.38 (9.80) | 50.89 (3.59) | .97 |
| APOE4 | |||
| Negative | 18.37 (6.72) | 23.30 (2.27) | .90 |
| Positive | 38.48 (6.43) | 43.47 (2.47) | .89 |
| Family history of dementia | |||
| Negative | 32.40 (5.99) | 33.10 (2.46) | >.99 |
| Positive | 24.45 (7.38) | 33.67 (2.22) | .63 |
Abbreviations: AD, Alzheimer disease; APOE4, apolipoprotein E ε4 allele; BMI, body mass index; CDR, Clinical Dementia Rating; PET, positron emission tomography; PiB, Pittsburgh compound B; SUVR, standardized uptake value ratio (partial volume corrected).
CSF Concentrations
There was no difference between African American and white participants for CSF concentrations of Aβ42 (Table 4). In the combined sample, there was an APOE ε4 effect, as APOE ε4 carriers had lower mean (SE) CSF Aβ42 concentrations (carriers, 634.28 [28.29] vs noncarriers, 802.79 [27.12] pg/mL; P < .001) and an effect of CDR, as those with a CDR greater than 0 had lower CSF Aβ42 concentrations (CDR >0, 641.92 [35.83] vs CDR of 0, 795.15 [23.31]; P = .001).
Table 4. Sample Demographics and Adjusted CSF Values Adjusting for Sex, APOE4 Status, Age, Educational Level, Clinical Status, Family History of AD, BMI, and CSF Drift Variables.
| Characteristic | African American Participants (n=87) | Non-Hispanic White Participants (n=816) | P Value |
|---|---|---|---|
| Age, mean (SD), y | 67.0 (9.4) | 69.4 (9.7) | .03 |
| Male sex, No. (%) | 40 (46.0) | 370 (45.3) | .91 |
| Educational level, mean (SD), y | 15.0 (3.1) | 15.6 (2.8) | .07 |
| CDR, No. (%) | .14 | ||
| 0 | 67 (77.0) | 597 (73.2) | |
| 0.5 | 11 (12.6) | 166 (20.3) | |
| 1 | 8 (9.2) | 51 (6.3) | |
| 2 | 1 (1.1) | 2 (0.2) | |
| Family history of dementia, No. (%) | .02 | ||
| Negative | 53 (60.9) | 391 (47.9) | |
| Positive | 34 (39.1) | 425 (52.1) | |
| APOE4, No. (%) | .76 | ||
| Negative | 50 (57.5) | 483 (59.2) | |
| Positive | 37 (42.5) | 333 (40.8) | |
| Aβ42, mean (SE), pg/mL | 717.19 (37.98) | 707.54 (19.05) | .79 |
| CDR | |||
| 0 | 796.61 (37.95) | 793.69 (18.76) | >.99 |
| >0 | 649.56 (63.82) | 634.29 (24.27) | >.99 |
| APOE4 | |||
| Negative | 816.18 (45.80) | 789.41 (20.36) | .93 |
| Positive | 629.99 (48.11) | 638.57 (21.48) | >.99 |
| Family history of dementia | |||
| Negative | 700.59 (44.62) | 715.51 (21.70) | .99 |
| Positive | 745.58 (49.53 | 712.47 (19.91) | .91 |
| Total tau, mean (SE), pg/mL | 293.65 (34.61) | 443.28 (18.20) | <.001 |
| CDR | |||
| 0 | 230.03 (34.52) | 337.67 (18.10) | .01 |
| >0 | 357.59 (58.01) | 548.61 (22.85) | .01 |
| APOE4 | |||
| Negative | 317.96 (41.74) | 422.75 (19.53) | .06 |
| Positive | 269.67 (43.73) | 463.54 (20.32) | <.001 |
| Family history of dementia | |||
| Negative | 288.54 (40.65) | 428.63 (20.67) | .003 |
| Positive | 299.09 (45.03) | 457.65 (18.96) | .003 |
| p-tau181, mean (SE), pg/mL | 53.18 (4.91) | 70.73 (2.46) | <.001 |
| CDR | |||
| 0 | 44.73 (4.91) | 59.35 (2.43) | .01 |
| >0 | 61.91 (8.26) | 82.34 (3.14) | .07 |
| APOE4 | |||
| Negative | 57.86 (5.93) | 66.71 (2.63) | .45 |
| Positive | 48.77 (6.23) | 74.98 (2.78) | <.001 |
| Family history of dementia | |||
| Negative | 52.87 (5.78) | 70.09 (2.81) | .02 |
| Positive | 53.77 (6.41) | 71.60 (2.58) | .03 |
Abbreviations: Aβ42, the 1-42 amino acid isoform of amyloid-β; AD, Alzheimer disease; APOE4, apolipoprotein E ε4 allele; BMI, body mass index; CDR, Clinical Dementia Rating; CSF, cerebrospinal fluid; p-tau181, phosphorylated tau181.
As a function of age, there was a similar degree of increase between the 2 races in CSF t-tau and p-tau181, but African American participants had lower mean (SE) CSF t-tau compared with white participants (293.65 [34.61] vs 443.28 [18.20] pg/mL; P < .001) as well as lower mean (SE) CSF p-tau181 (53.18 [4.91] vs 70.73 [2.46]; P < .001) (Figure, B; Table 4). Further adjustments of other covariates (APOE ε4 status, sex, educational level, CDR, BMI, and family history of AD, in addition to age and race) on CSF t-tau confirmed these racial differences and an additional CDR effect (those with a CDR >0 had higher t-tau levels). Further adjustments of these same covariates on CSF t-tau and p-tau181 also revealed a race by APOE ε4 interaction. Among individuals carrying an APOE ε4 allele, mean (SE) concentrations of both CSF t-tau and p-tau181 were lower in African American participants compared with white participants (t-tau, 269.67 [43.73] vs 463.54 [20.32] pg/mL; P < .001; p-tau181, 48.77 [6.23] vs 74.98 [2.78] pg/mL; P < .001) but there were no racial differences for individuals without an APOE ε4 allele, although a trend for lower mean (SE) CSF t-tau was seen for African American participants compared with white participants in APOE ε4 noncarriers (317.96 [41.74] vs 422.75 [19.53] pg/mL; P = .06).
Discussion
Compared with white individuals, we found that African American individuals (1) have reduced CSF levels of t-tau and p-tau181, perhaps as a function of the presence of APOE ε4; (2) have lower hippocampal volumes for those reporting a family history of dementia; (3) have equivalent amyloid-β burden as determined by global PiB SUVRs and CSF Aβ42 concentrations; and (4) share an identical AD biosignature, such that amyloid burden and CSF t-tau and p-tau181 concentrations increase as a function of age and clinical status (CDR >0). Moreover, the presence of an APOE ε4 allele is associated with increased amyloid PET SUVR and with decreased CSF Aβ42 levels in both African American and white individuals.
Our findings that, compared with white individuals, African American individuals have lower levels of CSF t-tau and p-tau181 is consistent with results from a study of 65 African American individuals and 70 white individuals as recently reported by Howell and colleagues.21 We found that the lower levels of CSF t-tau and p-tau181 for African American individuals was largely a function of carrying an APOE ε4 allele; African American noncarriers of an APOE ε4 allele did not have significantly different concentrations of CSF t-tau and p-tau181 when compared with white individuals, although there was a trend for lower CSF t-tau in African American noncarriers. These findings suggest that the racial differences in CSF t-tau and p-tau181 may, at least in part, reflect a differential effect of APOE ε4, perhaps similar to the lack of an APOE ε4 effect for the risk of cerebral hemorrhage in African American individuals compared with white individuals.40
The lower absolute levels of CSF t-tau and p-tau181 in African American individuals are not readily explained by the presence of comorbid cerebrovascular disease, at least as suggested by the proxy of ischemic lesions on MRI findings. Given recent evidence that APOE ε4 influences tau pathogenesis and tau-mediated neurodegeneration independent of Aβ pathologic characteristics,41 it is possible that the interactions of APOE ε4 with tau in African American individuals differs from its interactions with tau in white individuals, perhaps similar to the observed weaker association in African American individuals of APOE ε4 with AD.42
Our results showing no racial differences for amyloid as seen on PET scan differ from those of the Atherosclerosis Risk in Communities Study (ARIC),19 in which African American individuals without dementia (N = 141) showed higher florbetapir uptake than did white individuals without dementia (N = 188). The discrepant results may result from our use of partial volume-corrected SUVR with [11C] PiB as the amyloid radioligand, whereas ARIC used [18F] florbetapir and treated it as a continuous variable (compared with the dichotomized SUVR). Also, the ARIC sample was approximately 5 years older, on average, than our cohort and included individuals who had mild cognitive impairment in the cohort of participants without dementia. African American individuals had to score below 19 on the Mini-Mental State Examination43 to warrant a diagnosis of dementia, whereas white individuals were diagnosed with dementia when scoring less than 21 on the Mini-Mental State Examination. This differential classification of dementia may have allowed African American individuals with more advanced symptomatic AD to be included in the sample, possibly contributing to the observed racial differences in florbetapir uptake.19
Although our finding that African American individuals who were APOE ε4 carriers had higher PiB uptake on amyloid PET scans is consistent with the ARIC study,19 in general, the association of APOE ε4 and AD in African American individuals is weaker for African American individuals than for white individuals.42 Osuntokun and colleagues44 found no correlation of APOE ε4 with the prevalence of AD in community-dwelling older Yoruba individuals in the city of Ibadan, Nigeria. Similarly, a population-based study in New York City found an increased frequency of AD in African American individuals and Hispanic individuals regardless of their APOE genotype, whereas the risk of AD for white individuals increased significantly in those with 1 or 2 copies of APOE ε4.45 Reported risk variants for AD in African American individuals include ABCA7 (OMIM 604001),46 AKAP9 (OMIM 605414),47 TREM2 (OMIM 605086),48 and COBL (OMIM 610317) and SLC10A2 (OMIM 601295).49 In addition to the potential risk-modifying effects of these variants on environmental factors important for AD, it may be that 1 or more of these variants attenuates the effect of APOE ε4 such that African American individuals have less APOE ε4–associated risk for AD than do white individuals.
Limitations
To our knowledge, our study is the first to examine racial differences in molecular biomarkers of AD in which the cohort contributed data for both amyloid concentrations as seen on PET scan and CSF concentrations of Aβ42, t-tau, and p-tau181. Caution is needed in interpreting our results until they can be confirmed (or refuted) with subsequent analyses in larger cohorts to carefully explore the influences of socioeconomic status, comorbid diseases, and other factors that may contribute to racial differences. Another limitation is that our examination was restricted to African American and white individuals. It will be important to study the expression of molecular biomarkers of AD across all racial and ethnic groups to identify factors that may differentially affect AD risk and expression. Individuals who agree to participate in AD biomarker studies are almost certainly not representative of the overall population; thus, these results may not be generalizable. Also, our assessment of socioeconomic status was limited to educational level, and our assessment of cerebrovascular disease was limited to ischemic lesions on the brain MRI findings; thus, we may have failed to capture other aspects of these important risk factors. Finally, this study is limited by its cross-sectional nature that precludes correlating the biomarker values with progression of AD.
Conclusions
Despite these limitations, this study indicates that racial differences are present in some biomarkers of AD, as African American individuals have lower levels of CSF t-tau and p-tau181 compared with white individuals. Diagnostic algorithms that incorporate AD biomarker data22 must account for potential racial differences in how the AD biomarkers are expressed. For example, the National Institute on Aging-Alzheimer’s Association research framework50 uses abnormal AD biomarkers as proxy measures for AD neuropathologic change to provide a biological definition of disease. Normal vs abnormal values of CSF p-tau181 (proposed as a marker of pathologic tau) and t-tau (proposed as a marker of neurodegeneration) must be race adjusted when African American individuals are considered for the framework’s amyloid/tau/neurodegeneration (A/T/N) classification scheme for AD. Understanding how race may modify the risk and expression of AD may yield new insights into race-dependent biological mechanisms that in turn can inform future diagnostic and therapeutic advances.
eTable 1. Unadjusted Hippocampal Volumes
eTable 2. Unadjusted Amyloid PET Variables
eTable 3. Sample Demographics and Unadjusted CSF Values
References
- 1.Schoenberg BS, Anderson DW, Haerer AF. Severe dementia: prevalence and clinical features in a biracial US population. Arch Neurol. 1985;42(8):740-743. doi: 10.1001/archneur.1985.04210090004002 [DOI] [PubMed] [Google Scholar]
- 2.Tang MX, Cross P, Andrews H, et al. Incidence of AD in African-Americans, Caribbean Hispanics, and Caucasians in northern Manhattan. Neurology. 2001;56(1):49-56. doi: 10.1212/WNL.56.1.49 [DOI] [PubMed] [Google Scholar]
- 3.Green RC, Cupples LA, Go R, et al. ; MIRAGE Study Group . Risk of dementia among white and African American relatives of patients with Alzheimer disease. JAMA. 2002;287(3):329-336. doi: 10.1001/jama.287.3.329 [DOI] [PubMed] [Google Scholar]
- 4.Mayeda ER, Glymour MM, Quesenberry CP, Whitmer RA. Inequalities in dementia incidence between six racial and ethnic groups over 14 years. Alzheimers Dement. 2016;12(3):216-224. doi: 10.1016/j.jalz.2015.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fillenbaum GG, Heyman A, Huber MS, et al. The prevalence and 3-year incidence of dementia in older black and white community residents. J Clin Epidemiol. 1998;51(7):587-595. doi: 10.1016/S0895-4356(98)00024-9 [DOI] [PubMed] [Google Scholar]
- 6.Fitzpatrick AL, Kuller LH, Ives DG, et al. Incidence and prevalence of dementia in the Cardiovascular Health Study. J Am Geriatr Soc. 2004;52(2):195-204. doi: 10.1111/j.1532-5415.2004.52058.x [DOI] [PubMed] [Google Scholar]
- 7.Graff-Radford NR, Besser LM, Crook JE, Kukull WA, Dickson DW. Neuropathologic differences by race from the National Alzheimer’s Coordinating Center. Alzheimers Dement. 2016;12(6):669-677. doi: 10.1016/j.jalz.2016.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilkins CH, Grant EA, Schmitt SE, McKeel DW, Morris JC. The neuropathology of Alzheimer disease in African American and white individuals. Arch Neurol. 2006;63(1):87-90. doi: 10.1001/archneur.63.1.87 [DOI] [PubMed] [Google Scholar]
- 9.Riudavets MA, Rubio A, Cox C, Rudow G, Fowler D, Troncoso JC. The prevalence of Alzheimer neuropathologic lesions is similar in blacks and whites. J Neuropathol Exp Neurol. 2006;65(12):1143-1148. doi: 10.1097/01.jnen.0000248548.20799.a3 [DOI] [PubMed] [Google Scholar]
- 10.Brickman AM, Schupf N, Manly JJ, et al. Brain morphology in older African Americans, Caribbean Hispanics, and whites from northern Manhattan. Arch Neurol. 2008;65(8):1053-1061. doi: 10.1001/archneur.65.8.1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeCarli C, Reed BR, Jagust W, Martinez O, Ortega M, Mungas D. Brain behavior relationships among African Americans, whites, and Hispanics. Alzheimer Dis Assoc Disord. 2008;22(4):382-391. doi: 10.1097/WAD.0b013e318185e7fe [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manly JJ, Touradji P, Tang MX, Stern Y. Literacy and memory decline among ethnically diverse elders. J Clin Exp Neuropsychol. 2003;25(5):680-690. doi: 10.1076/jcen.25.5.680.14579 [DOI] [PubMed] [Google Scholar]
- 13.Szanton SL, Rifkind JM, Mohanty JG, et al. Racial discrimination is associated with a measure of red blood cell oxidative stress: a potential pathway for racial health disparities. Int J Behav Med. 2012;19(4):489-495. doi: 10.1007/s12529-011-9188-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yaffe K, Falvey C, Harris TB, et al. ; Health ABC Study . Effect of socioeconomic disparities on incidence of dementia among biracial older adults: prospective study. BMJ. 2013;347:f7051. doi: 10.1136/bmj.f7051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barnes LL, Bennett DA. Alzheimer’s disease in African Americans: risk factors and challenges for the future. Health Aff (Millwood). 2014;33(4):580-586. doi: 10.1377/hlthaff.2013.1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.United States Census Bureau QuickFacts: population estimates, July 1, 2017. https://www.census.gov/quickfacts/fact/table/US/PST045217#PST045217. Accessed November 21, 2018.
- 17.Honig LS, Vellas B, Woodward M, et al. Trial of solanezumab for mild dementia due to Alzheimer’s disease. N Engl J Med. 2018;378(4):321-330. doi: 10.1056/NEJMoa1705971 [DOI] [PubMed] [Google Scholar]
- 18.The National Alzheimer’s Coordinating Center (NACC) https://www.alz.washington.edu/. Accessed November 21, 2018.
- 19.Gottesman RF, Schneider ALC, Zhou Y, et al. The ARIC-PET amyloid imaging study: brain amyloid differences by age, race, sex, and APOE. Neurology. 2016;87(5):473-480. doi: 10.1212/WNL.0000000000002914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McDonough IM. Beta-amyloid and cortical thickness reveal racial disparities in preclinical Alzheimer’s disease. Neuroimage Clin. 2017;16:659-667. doi: 10.1016/j.nicl.2017.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Howell JC, Watts KD, Parker MW, et al. Race modifies the relationship between cognition and Alzheimer’s disease cerebrospinal fluid biomarkers. Alzheimers Res Ther. 2017;9(1):88. doi: 10.1186/s13195-017-0315-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wolz R, Schwarz AJ, Gray KR, Yu P, Hill DL; Alzheimer’s Disease Neuroimaging Initiative . Enrichment of clinical trials in MCI due to AD using markers of amyloid and neurodegeneration. Neurology. 2016;87(12):1235-1241. doi: 10.1212/WNL.0000000000003126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Noble JM, Manly JJ, Schupf N, Tang MX, Luchsinger JA. Type 2 diabetes and ethnic disparities in cognitive impairment. Ethn Dis. 2012;22(1):38-44. [PMC free article] [PubMed] [Google Scholar]
- 24.Data USA. St Louis, MO-IL metro area. https://datausa.io/profile/geo/st.-louis-mo-il-metro-area/#intro. Accessed November 21, 2018.
- 25.Morris JC, Weintraub S, Chui HC, et al. The Uniform Data Set (UDS): clinical and cognitive variables and descriptive data from Alzheimer Disease Centers. Alzheimer Dis Assoc Disord. 2006;20(4):210-216. doi: 10.1097/01.wad.0000213865.09806.92 [DOI] [PubMed] [Google Scholar]
- 26.Weintraub S, Salmon D, Mercaldo N, et al. The Alzheimer’s Disease Centers’ Uniform Data Set (UDS): the neuropsychologic test battery. Alzheimer Dis Assoc Disord. 2009;23(2):91-101. doi: 10.1097/WAD.0b013e318191c7dd [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412-2414. doi: 10.1212/WNL.43.11.2412-a [DOI] [PubMed] [Google Scholar]
- 28.Fagan AM, Mintun MA, Mach RH, et al. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Aβ42 in humans. Ann Neurol. 2006;59(3):512-519. doi: 10.1002/ana.20730 [DOI] [PubMed] [Google Scholar]
- 29.Fischl B. FreeSurfer. Neuroimage. 2012;62(2):774-781. doi: 10.1016/j.neuroimage.2012.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Desikan RS, Ségonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968-980. doi: 10.1016/j.neuroimage.2006.01.021 [DOI] [PubMed] [Google Scholar]
- 31.Buckner RL, Head D, Parker J, et al. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. Neuroimage. 2004;23(2):724-738. doi: 10.1016/j.neuroimage.2004.06.018 [DOI] [PubMed] [Google Scholar]
- 32.Dickerson BC, Goncharova I, Sullivan MP, et al. MRI-derived entorhinal and hippocampal atrophy in incipient and very mild Alzheimer’s disease. Neurobiol Aging. 2001;22(5):747-754. doi: 10.1016/S0197-4580(01)00271-8 [DOI] [PubMed] [Google Scholar]
- 33.Gordon BA, Blazey T, Su Y, et al. Longitudinal β-amyloid deposition and hippocampal volume in preclinical Alzheimer disease and suspected non-Alzheimer disease pathophysiology. JAMA Neurol. 2016;73(10):1192-1200. doi: 10.1001/jamaneurol.2016.2642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol. 2004;55(3):306-319. doi: 10.1002/ana.20009 [DOI] [PubMed] [Google Scholar]
- 35.Su Y, D’Angelo GM, Vlassenko AG, et al. Quantitative analysis of PiB-PET with FreeSurfer ROIs. PLoS One. 2013;8(11):e73377. doi: 10.1371/journal.pone.0073377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rousset OG, Ma Y, Evans AC. Correction for partial volume effects in PET: principle and validation. J Nucl Med. 1998;39(5):904-911. [PubMed] [Google Scholar]
- 37.Cruchaga C, Kauwe JS, Mayo K, et al. ; Alzheimer’s Disease Neuroimaging Initiative . SNPs associated with cerebrospinal fluid phospho-tau levels influence rate of decline in Alzheimer’s disease. PLoS Genet. 2010;6(9):e1001101. doi: 10.1371/journal.pgen.1001101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schindler SE, Sutphen CL, Teunissen C, et al. Upward drift in cerebrospinal fluid amyloid β 42 assay values for more than 10 years. Alzheimers Dement. 2018;14(1):62-70. doi: 10.1016/j.jalz.2017.06.2264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Su Y, Flores S, Hornbeck RC, et al. Utilizing the Centiloid scale in cross-sectional and longitudinal PiB PET studies. Neuroimage Clin. 2018;19:406-416. doi: 10.1016/j.nicl.2018.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sawyer RP, Sekar P, Osborne J, et al. Racial/ethnic variation of APOE alleles for lobar intracerebral hemorrhage. Neurology. 2018;91(5):e410-e420. doi: 10.1212/WNL.0000000000005908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shi Y, Yamada K, Liddelow SA, et al. ; Alzheimer’s Disease Neuroimaging Initiative . ApoE4 markedly exacerbates tau-mediated neurodegeneration in a mouse model of tauopathy. Nature. 2017;549(7673):523-527. doi: 10.1038/nature24016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Farrer LA, Cupples LA, Haines JL, et al. ; APOE and Alzheimer Disease Meta Analysis Consortium . Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease: a meta-analysis. JAMA. 1997;278(16):1349-1356. doi: 10.1001/jama.1997.03550160069041 [DOI] [PubMed] [Google Scholar]
- 43.Folstein MF, Folstein SE, McHugh PR. ‘Mini-mental state’: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198. doi: 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 44.Osuntokun BO, Sahota A, Ogunniyi AO, et al. Lack of an association between apolipoprotein E epsilon 4 and Alzheimer’s disease in elderly Nigerians. Ann Neurol. 1995;38(3):463-465. doi: 10.1002/ana.410380319 [DOI] [PubMed] [Google Scholar]
- 45.Maestre G, Ottman R, Stern Y, et al. Apolipoprotein E and Alzheimer’s disease: ethnic variation in genotypic risks. Ann Neurol. 1995;37(2):254-259. doi: 10.1002/ana.410370217 [DOI] [PubMed] [Google Scholar]
- 46.Reitz C, Jun G, Naj A, et al. ; Alzheimer Disease Genetics Consortium . Variants in the ATP-binding cassette transporter (ABCA7), apolipoprotein E ϵ4, and the risk of late-onset Alzheimer disease in African Americans. JAMA. 2013;309(14):1483-1492. doi: 10.1001/jama.2013.2973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Logue MW, Schu M, Vardarajan BN, et al. ; Alzheimer Disease Genetics Consortium . Two rare AKAP9 variants are associated with Alzheimer’s disease in African Americans. Alzheimers Dement. 2014;10(6):609-618. doi: 10.1016/j.jalz.2014.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jin SC, Carrasquillo MM, Benitez BA, et al. TREM2 is associated with increased risk for Alzheimer’s disease in African Americans. Mol Neurodegener. 2015;10:19. doi: 10.1186/s13024-015-0016-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mez J, Chung J, Jun G, et al. ; Alzheimer’s Disease Genetics Consortium . Two novel loci, COBL and SLC10A2, for Alzheimer’s disease in African Americans. Alzheimers Dement. 2017;13(2):119-129. doi: 10.1016/j.jalz.2016.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jack CR Jr, Bennett DA, Blennow K, et al. NIA-AA Research Framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018;14(4):535-562. doi: 10.1016/j.jalz.2018.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Unadjusted Hippocampal Volumes
eTable 2. Unadjusted Amyloid PET Variables
eTable 3. Sample Demographics and Unadjusted CSF Values